Abstract
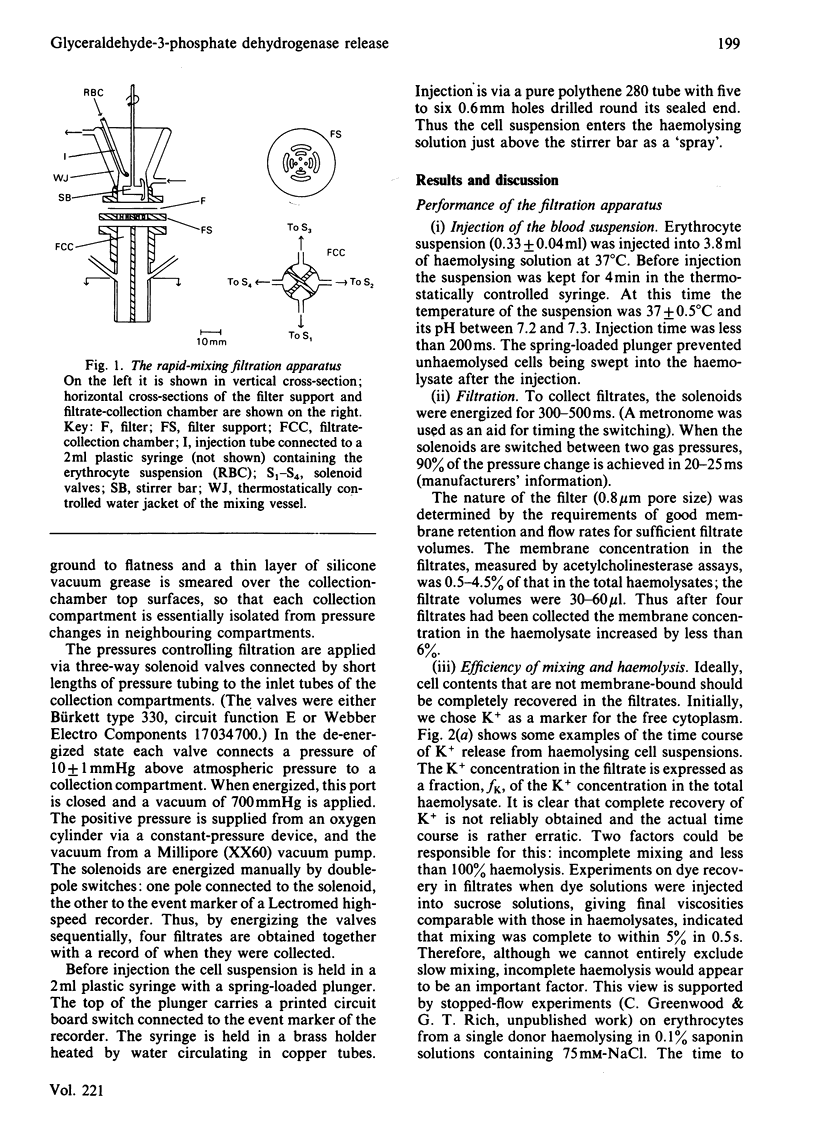
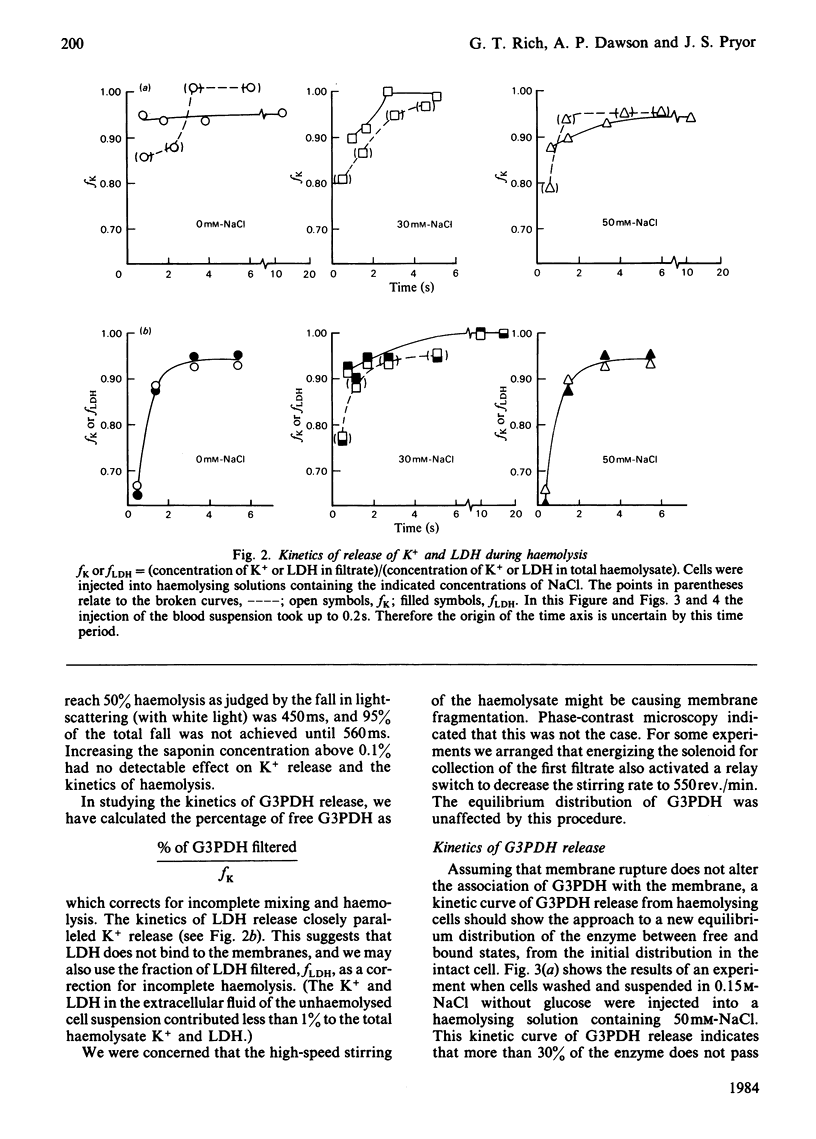
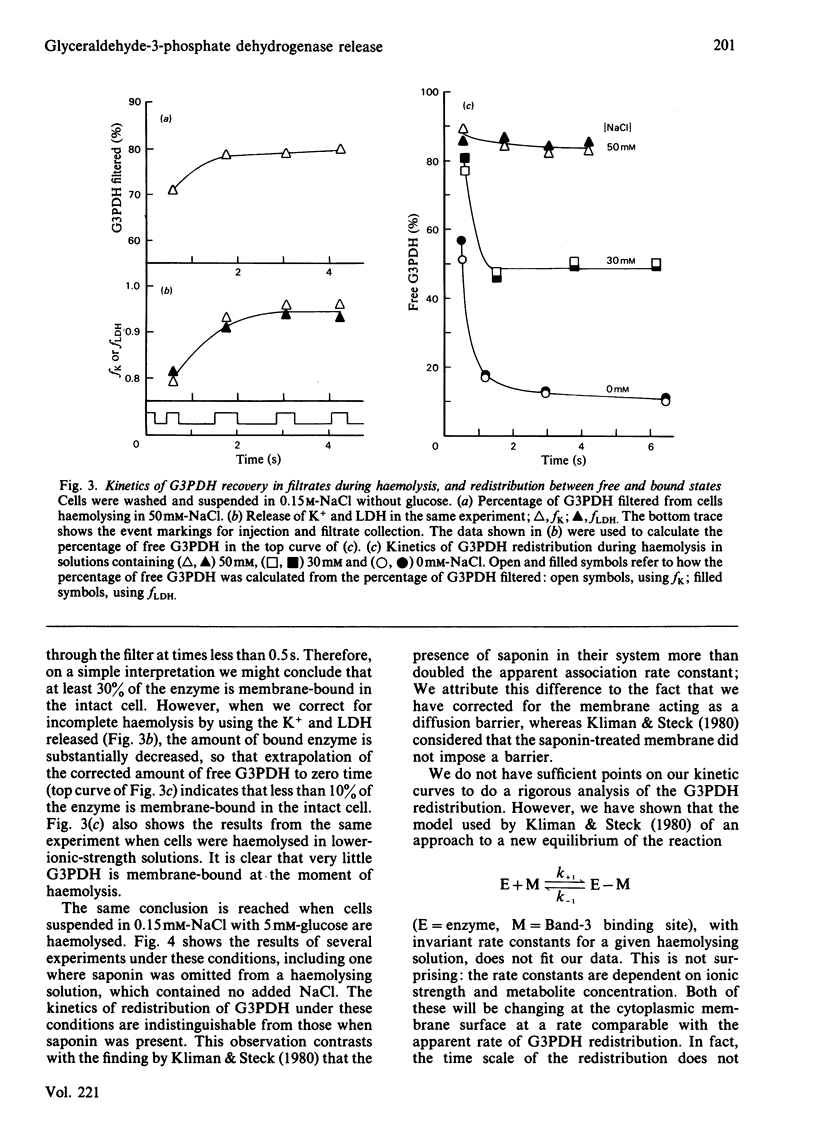
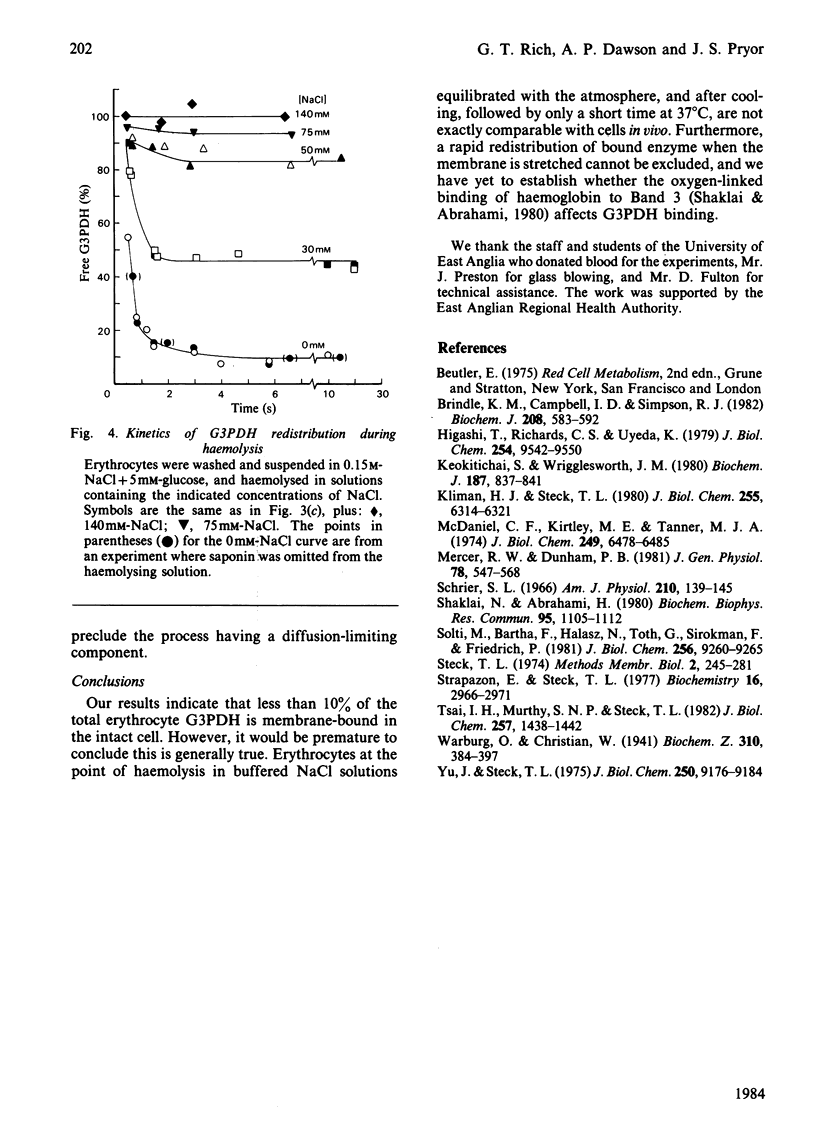
Glyceraldehyde-3-phosphate dehydrogenase (G3PDH, EC 1.2.1.12) release from haemolysing erythrocytes, and its redistribution between free and membrane-bound states, were studied with a new type of rapid-mixing filtration apparatus. The apparatus is described. The results indicate that the rate of G3PDH appearance in filtrates is determined not only by the enzyme redistribution but also by the kinetics of haemolysis. We have quantified the extent of haemolysis as a function of time, by measuring the amounts of filterable K+ and lactate dehydrogenase. These are cytoplasmic components that are not membrane-bound. When we correct for incomplete haemolysis, extrapolation to zero times indicates that very little G3PDH is membrane-bound in the intact cell.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brindle K. M., Campbell I. D., Simpson R. J. A 1H n.m.r. study of the kinetic properties expressed by glyceraldehyde phosphate dehydrogenase in the intact human erythrocyte. Biochem J. 1982 Dec 15;208(3):583–592. doi: 10.1042/bj2080583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higashi T., Richards C. S., Uyeda K. The interaction of phosphofructokinase with erythrocyte membranes. J Biol Chem. 1979 Oct 10;254(19):9542–9550. [PubMed] [Google Scholar]
- Keokitichai S., Wrigglesworth J. M. Association of glyceraldehyde 3-phosphate dehydrogenase with the membrane of the intact human erythrocyte. Biochem J. 1980 Jun 1;187(3):837–841. doi: 10.1042/bj1870837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliman H. J., Steck T. L. Association of glyceraldehyde-3-phosphate dehydrogenase with the human red cell membrane. A kinetic analysis. J Biol Chem. 1980 Jul 10;255(13):6314–6321. [PubMed] [Google Scholar]
- McDaniel C. F., Kirtley M. E., Tanner M. J. The interaction of glyceraldehyde 3-phosphate dehydrogenase with human erythrocyte membranes. J Biol Chem. 1974 Oct 25;249(20):6478–6485. [PubMed] [Google Scholar]
- Mercer R. W., Dunham P. B. Membrane-bound ATP fuels the Na/K pump. Studies on membrane-bound glycolytic enzymes on inside-out vesicles from human red cell membranes. J Gen Physiol. 1981 Nov;78(5):547–568. doi: 10.1085/jgp.78.5.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrier S. L. Organization of enzymes in human erythrocyte membranes. Am J Physiol. 1966 Jan;210(1):139–145. doi: 10.1152/ajplegacy.1966.210.1.139. [DOI] [PubMed] [Google Scholar]
- Shaklai N., Abrahami H. The interaction of deoxyhemoglobin with the red cell membrane. Biochem Biophys Res Commun. 1980 Aug 14;95(3):1105–1112. doi: 10.1016/0006-291x(80)91586-7. [DOI] [PubMed] [Google Scholar]
- Solti M., Bartha F., Halász N., Tóth G., Sirokmán F., Friedrich P. Localization of glyceraldehyde-3-phosphate dehydrogenase in intact human erythrocytes. Evaluation of membrane adherence is autoradiographs at low grain density. J Biol Chem. 1981 Sep 10;256(17):9260–9265. [PubMed] [Google Scholar]
- Strapazon E., Steck T. L. Interaction of the aldolase and the membrane of human erythrocytes. Biochemistry. 1977 Jun 28;16(13):2966–2971. doi: 10.1021/bi00632a025. [DOI] [PubMed] [Google Scholar]
- Tsai I. H., Murthy S. N., Steck T. L. Effect of red cell membrane binding on the catalytic activity of glyceraldehyde-3-phosphate dehydrogenase. J Biol Chem. 1982 Feb 10;257(3):1438–1442. [PubMed] [Google Scholar]


