Abstract
The poly (lactic-co-glycolic acid) (PLGA) based nanoparticles have been applied for drug delivery due to their simple preparation, biodegradability, and ideal biocompatibility. In this study, the factors affecting the degradation of PLGA-based nanoparticles are reviewed, encompassing the ratio of PLA to PGA, relative molecular weight, crystallinity, and preparation process of PLGA nanoparticles. The drug release behavior of PLGA-based nanoparticles, such as the degradation environment, encapsulated drug properties of polymers, and drug loading rates, are also discussed. Since gastrointestinal cancer is one of the major global threats to human health, this paper comprehensively summarizes the application of PLGA nanoparticles in gastrointestinal cancers from diagnosis, chemotherapy, radiotherapy, and novel tumor treatment methods (immunotherapy, gene therapy, and photothermal therapy). Finally, the future application of PLGA-based drug delivery systems in treating gastrointestinal cancers is discussed. The bottleneck of application status and the prospect of PLGA-nanoparticles in gastrointestinal tumor application are presented. To truly realize the great and wide application of PLGA-based nanoparticles, collaborative progress in the field of nanomaterials and life sciences is needed.
Keywords: PLGA nanoparticles, Gastrointestinal cancer, Drug release, Chemotherapy, Encapsulation
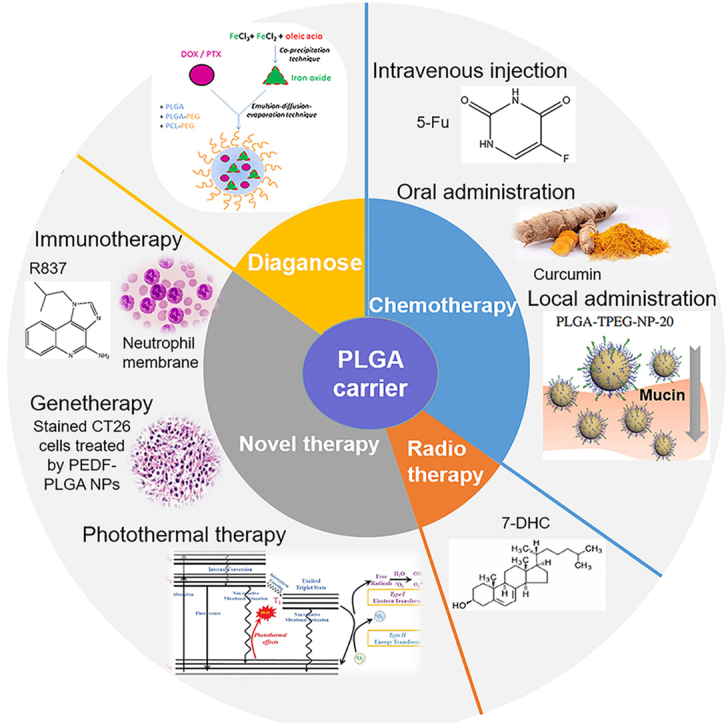
Graphical abstract
A graph displaying the application of PLGA nanoparticle carriers in gastrointestinal cancers from the points of diagnosis, chemotherapy, radiotherapy and novel tumor treatment methods (immunotherapy, gene therapy, and photothermal therapy).
Highlights
-
•
Factors on degradation and drug release of PLGA are Mw, crystallinity and drugs.
-
•
PLGA applications of diagnosis and therapy are reviewed in gastrointestinal cancer.
-
•
The clinical application of PLGA-based drug delivery system in future is discussed.
1. Introduction
In China, gastrointestinal cancer ranks second among malignant tumors of the gastrointestinal tract and poses a major threat to public health [1]. Despite the possibility of a complete cure through early surgical treatment, most patients have insidious symptoms and seek medical attention only when the cancer has progressed to an advanced stage. Without chemotherapy, the probability of a complete cure through curative surgery is limited. However, due to the low blood drug concentration in the digestive and circulatory system, as well as tumor cell resistance to traditional chemotherapy drugs, conventional chemotherapy regimens are often limited by various toxic side effects [2]. Therefore, it is imperative to develop novel drug-delivery nanosystems and drug types to alleviate the toxic side effects of traditional chemotherapy drugs and improve the antitumor effect.
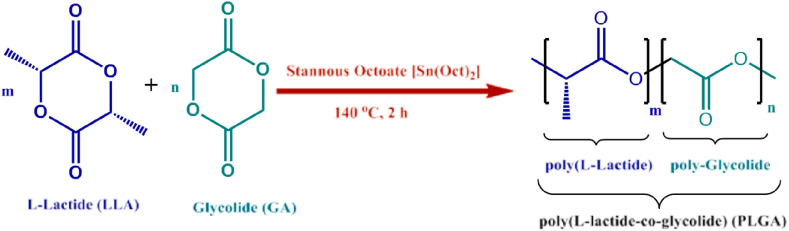
In the biomedical field, poly (lactic-co-glycolic acid) (PLGA) is the only biodegradable polymer approved by the US Food and Drug Administration (FDA) as well as the European Medicines Quality Agency (EMA) [3]. PLGA is a linear aliphatic polyester-based copolymer formed by the polymerization of lactic acids and glycolic acids in a particular proportion (see Fig. 1). In addition to lipid nanoparticles, PLGA particles in the nano and micron range have emerged as the predominant drug carrier polymers extensively investigated for biomedical applications [4]. The US FDA has approved 12 PLGA-based nanoparticle drug products [5]. The biodegradable aliphatic amorphous polymer PLGA is formed from acetic acid and glycolic acid polymerization. The classical methods of PLGA synthesis are ring-opening polymerization addition and direct polycondensation reactions [3]. The emulsification-solvent evaporation technique is the most common method for preparing PLGA particle formulation. Other preparation methods encompass spray-drying, phase separation, microfluidics, and nanoprecipitation [6]. The PLGA nanoparticle has been developed for sustained release (from weeks to months) of different active pharmaceutical ingredients such as small molecules, peptides, and proteins. It overcomes the limitations of chemotherapeutic drug therapy to protect them from degradation in the gastrointestinal tract, achieve high load capacity, and provide a variety of surface modifications. A wide range of diverse biomedical applications involving PLGA-based drug delivery systems are being investigated in preclinical and clinical studies encompassing the treatment of cardiovascular and inflammatory diseases as well as wound healing and tissue regeneration [7].
Fig. 1.
Structure and synthesis of PLGA. Reprinted with permission [14].
Nevertheless, the development of new drug-delivery products poses significant challenges due to potential dose dumping and associated risks to patient safety arising from high drug loads intended for sustained release. Therefore, a comprehensive understanding of key parameters affecting the drug release behavior of PLGA-based nanoparticles is essential to ensure their performance and safety. This study summarizes information about drug-loaded PLGA-based nanoparticles, including factors affecting drug release and their applications in treating gastrointestinal cancers. This study aims to provide reference ideas for experts in the field of gastrointestinal cancer and to prospect the future application of PLGA-based nanoparticles in the clinical treatment of gastrointestinal cancers.
2. The drug release of PLGA-based nanoparticles
As a nano drug loaded nanoparticle carrier, the degradation behavior of PLGA polymer directly affects the release behavior of sustained-release preparations [8]. Therefore, by adjusting the relevant factors affecting the PLGA degradation behavior [9], the release degree of sustained-release drugs can be adjusted [10]. The factors affecting the PLGA degradation behavior include the ratio of PLA (polylactic acid) monomer to PGA (polyglycolic acid), relative molecular weight (Mw), crystallinity, degradation environment (pH value, temperature, etc.), drug type, and preparation type after matrix encapsulation of the drug.
2.1. Factors influencing the degradation of PLGA-based nanoparticles
2.1.1. Ratio of PLA to PGA
The PLGA composition is usually determined by the ratio of its monomer PLA to PGA. As known to all, the molar ratio of L-lactic acid (LA)/glycolic acid (GA) affects the PLGA crystallinity, thereby affecting the swelling and water absorptivity of the polymer. Specifically, the introduction of glycolic acid reduces the crystallinity of the polymer and accelerates the water absorption rate of the nanomaterial. Therefore, by changing the ratio of LA to GA in amorphous polymers, the degradation rate of PLGA can be adjusted. Research has proven that when the GA content is below 50 %, PLGA is more inclined to the properties of PLA as the GA content decreases, namely an enhanced hydrophobicity and reduced degradation rate. The higher the PGA content, the stronger the hydrophilicity, wettability, and amorphous nature of PLGA, and the faster the degradation rate, resulting in faster drug release [11]. PLGA copolymers undergo degradation in both in vivo and in vitro aquatic environments, and the degradation time of PLGA can vary from several months to years depending on the polymer composition and its relative molecular weight. Petposri et al. compared the degradation rates of PLGA polymer membranes with different compositions (PLA to PGA ratios of 10:90 and 70:30) and same relative molecular weight. As the PGA ratio decreased and the PLA ratio increased, the PLGA degradation rate decreased [12]. Essa et al. found that higher GA content and more amorphous regions in PLGA polymers resulted in faster degradation of PLGA polymers due to stronger hydrophilic [13]. Due to this property of PLGA, drug release can be flexibly regulated in the drug delivery systems.
2.1.2. Relative molecular weight
The molecular weight (Mw) and the distribution of polymerization degree (Mw/Mn, PDI) of PLGA are important factors affecting the PLGA degradation rate. With low polymer's Mw and a short PLGA chain, the content of terminal carboxylic groups is higher comparing to that of high Mw PLGA. Due to the fact that carboxylic groups can cause self-catalysis of polymers, the degradation rate of low Mw PLGA is faster [15]. Therefore, when low Mw PLGA is used to prepare drug loaded particles, the sustained release period of the delivery system is short. Brauner et al. investigated the release rates of low relative Mw PLGA 2300 and PLGA 503H. The results showed that low relative Mw PLGA can produce water-soluble oligomers more quickly than high relative Mw PLGA, ultimately releasing drugs faster [16]. Ochi et al. used leuprorelin acetate as the active carrier drug and increased the relative Mw of PLGA (75:25) from 6500 to 15400 [17]. They found that with the increase of the PLGA relative Mw, the particle size of the nanoparticles gradually increased, and the drug loading and release significantly decreased. The above research results indicate that as the relative Mw of PLGA decreases, both the polymer degradation and drug release rates will increase. The drug release of low Mw PLGA nanoparticles is usually regulated by drug diffusion, while the drug release of high molecular weight PLGA nanoparticles is usually controlled by both drug diffusion and polymer erosion [9].
2.1.3. Crystallinity
The crystallinity of PLGA polymers affects their mechanical strength, swelling behavior, biodegradation rate, and drug release behavior. The PLGA polymers crystallinity depends on the crystallinity of the units formed during the copolymerization process of glycolic acid (GA) and lactic acid (LA) [18]. Lactic acid is crystalline, while glycolic acid exhibits more amorphous characteristics. A higher content of GA will cause the proportion of crystalline and amorphous phases within PLGA particles to shift towards the amorphous region, resulting in faster hydrolysis of polymer particles. When the ratio of LA to GA is 50:50, the degradation rate is the fastest [6]. Therefore, the ratio of LA to GA can affect the crystallinity of PLGA copolymers. PLGA is generally an amorphous polymer. Studies have proven that the X-ray diffraction peak intensity of its crystalline phases gradually increases with the enhancement of LA content in the PLGA copolymer. When the amount of LA in the copolymer reaches or exceeds 90 %, the PLGA copolymer has a strong crystalline diffraction peak, and the increase of crystallinity reduces the degradation rate of PLGA. In addition, the reason why the degradation rate slows down with a higher PLA proportion may also be due to the presence of methyl groups in PLA, which increases the PLGA hydrophobicity and thus reduces its degradation rate [19]. Frank et al. [20] applied lidocaine as an active drug and PLGA as a highly crystalline form of L-lactic acid. When the crystallinity of PLGA increased from 40 % to 50 %, the diffusion coefficient of the drug reduced from 6.95 × 10−14 cm2/s to 3.84 × 10−14 cm2/s, leading to a decreased drug release rate.
2.1.4. Preparation process of PLGA nanoparticles
The preparation method, conditions, and nanoparticle properties of PLGA nanoparticles can all affect the burst release rate of drugs [9]. There are various preparation methods of PLGA nanoparticles, among which the emulsification-solvent evaporation method is the most simple. It can be divided into two parts: the emulsion preparation, and the solvent evaporation. Colostrum is the product of the emulsion preparation, and also an important intermediate during the nanoparticles preparation. The stability of colostrum is a significant prerequisite for ensuring the nanoparticles quality. Firstly, different emulsifiers applied in the emulsion preparation display different drug release behaviors. For example, the emulsifiers can be made into water-in-oil (W/O), oil-in-water (O/W), water-in-oil-in-water (W/O/W) or solid-in-oil (S/O) emulsion and so on. W/O/W double emulsion solvent evaporation method (i.e. double emulsification-solvent evaporation method) is usually used to prepare protein peptide drug loaded PLGA nanoparticles [21,22]. Secondly, the drug release behavior of drug loaded PLGA nanoparticles varies with the emulsification conditions. Changing the shear force (e.g. using a homogenizer to prepare colostrum) or ultrasound intensity (e.g. using probe ultrasound to prepare colostrum) during the W/O colostrum preparation can finally result in the PLGA nanoparticles with different physicochemical properties and drug release behaviors. Qi et al. [23] prepared drug loaded PLGA nanoparticles by selecting exenatide, a type II diabetes treatment drug, as a model polypeptide. They found that the nanoparticles synthesized by ultrasonic emulsification method released slowly in vivo with a low hypoglycemic degree, while the nanoparticles prepared by homogenization method released rapidly in the first two weeks, leading to a better hypoglycemic effect. Thirdly, the stirring rate of colostrum during the emulsion preparation can affect the drug release behavior.
2.2. Factors influencing the drug release behavior of drug loaded PLGA nanoparticles
2.2.1. Degradation Environment
Environmental factors can also affect the degradation of PLGA polymers, thereby affecting drug release behavior. For example, high temperature can accelerate the drugs release controlled by nanoparticles corrosion via enhancing the hydration and degradation of PLGA polymers. High temperature can also increase the migration rate of PLGA polymers in the nanoparticles matrix, thus improve the polymers fluidity and the PLGA degradation rate, and finally accelerate drug diffusion effectively. Therefore, high temperature is also the most commonly used parameter in accelerating drug release experiments [24]. Generally speaking, PLGA nanoparticles can remain stable for 12 months below the glass transition temperature (Tg) [8]. At temperatures close to the glass transition temperature, the drug diffusion coefficient can increase by up to three orders of magnitude. Due to changes in the release mechanism at temperatures above Tg, it is recommended that the ambient temperature should not be higher than the Tg of the PLGA polymers. The plasticization of polymers at high temperatures can lead to changes in particle morphology (such as surface pore closure and particle aggregation), thereby reducing the drugs release rate [25]. Wang et al. [26] proposed that humid conditions and elevated temperatures can affect the mobility of PLGA polymer chains, leading to their Tg changes, furthermore resulting in undesirable PLGA degradation and drug release behavior.
The pH value of the surrounding environment has a significant impact on the degradation rate, stability, and drug release of PLGA nanoparticles. The degradation of PLGA polymers is accelerated under both acidic and alkaline conditions, especially under alkaline conditions. This is because PLGA belongs to ester polymers, and its degradation mainly occurs through the hydrolysis of ester bonds. Under alkaline conditions, OH− in the environment can attack ester bonds, causing them to break [27]. At the same time, excessive alkali will neutralize the acidic products produced by PLGA hydrolysis, producing salt substances that enhance the hydrophilic of the polymer and accelerate degradation [28]. In Xu's study [10], the degradation rate constant of PLGA nanoparticles with a relative Mw of 25000 at pH 2.4 was 1.63 times higher than that at pH 7.4. It takes 21 days to fully release the drug in a neutral environment with 7.4 pH level, while it only takes 2 days to release the entire drug in an alkaline environment with 10.8 pH level. This property of PLGA can help to construct pH-responsive drug delivery systems [29].
2.2.2. Drug types, drug distribution, and drug loading rates
The drug types affect the drug release behavior of drug loaded PLGA nanoparticles. This is because different physicochemical properties of drugs can affect the PLGA degradation rate and the release parameters of selected drug [30]. Due to the association between hydrophilic and drug release, it is necessary to explain the drug release mechanism based on drugs and polymer types [31]. The Naidu research group [32] investigated the effect of hydrophilic or hydrophobic drugs on the degradation rate and drug release of PLGA carriers. The results showed that hydrophilic drugs significantly accelerated the degradation rate of PLGA polymers, thereby accelerating drug release. It may be due to the high water solubility of hydrophilic drugs, leading to a great sustained driving force for drug diffusion and a high initial rupture degree of nanoparticles [33].
The drug distribution in PLGA polymer matrix can also affect the drug release performance. Under normal circumstances, the drug release in nanoparticles is from the surface to the inner layer, so the drug diffusion distance can significantly affect the drug release behavior from the inner nanoparticles. The uniform distribution of drugs in the carrier provides a sustained driving force for drug diffusion, which may lead to early burst release. Kakish et al. [34] regulated the drug concentration distribution in PLGA nanoparticles to form a gradient distribution with higher center drug concentration. This nanoparticle structure can reduce early burst release and enable drug release at a steady rate.
The drug loading rate of PLGA nanoparticles affects the burst release efficiency, thus the drug loading rate plays a vital role in the drug release process. The higher the drug loading rate of PLGA nanoparticles, the higher the concentration gradient of drug diffusion in the internal environment, and furthermore the higher the drug burst release rate. A low drug loading rate can lead to a decrease in drug burst release but requires a longer time to release the drug [30].
3. Application of PLGA nanoparticles in the treatment of gastrointestinal cancers
Gastrointestinal cancer is a major disease threatening human health worldwide. Surgery combined with radiotherapy and chemotherapy is still the most popular treatment for this disease [[35], [36], [37]]. In recent years, the advancing comprehension of malignant tumors has led to significant breakthroughs in novel treatment modalities within clinical practice, thereby enhancing patients’ quality of life. Despite this, the overall prognosis of patients with gastrointestinal tumors is still unsatisfactory. For example, colon cancer is the third most common cancer and the fourth most common cause of death in the world [38,39], gastric cancer is the fifth most common cancer and the third most common cause of cancer death globally [40].
3.1. Chemotherapy
Chemotherapy inhibits tumor growth or eliminates tumor by systemic or local administration of chemotherapeutic drugs, which plays an irreplaceable role in the treatment of malignant tumors. There are primarily three ways of administration: intravenous injection, oral administration and local administration [[41], [42], [43]]. As a widely applied chemotherapeutic drug in the treatment of gastrointestinal cancer, 5-fluorouracil's (5-FU) side effects include the unspecific damage of normal cells, the short half-life and wide distribution [44].
To prolong its plasma circulation life and improve its therapeutic activity, a magnetic PLGA nanocapsule loaded with 5-FU was prepared and displayed great anticancer ability against colon cancer allografts in mice [45]. There was little difference in the drug release between in vitro and in vivo studies. The 5-Fu-loaded magnetic nanocapsules hindered tumor growth effectively at week 3, in accordance with sharply rising of the in vitro release plot from day 20. Intravenous injection of drugs loaded with magnetic PLGA nanocapsules supports the idea that magnetic drug targeting processes may be an effective delivery strategy. When the drug loaded PLGA nanoparticles are exploited as intravenous injection in the treatment of gastrointestinal cancers, the polymeric nanoparticles must avoid non-specific interactions with mononuclear phagocyte system and survive in circulation until reaching tumors [46]. Additionally, once polymeric nanoparticles reach the tumors, it is essential to establish interactions with tumor cells to avoid the nanoparticles drain from the tissues [47]. To improve PLGA nanoparticles retention in tumors, Park et al. [48] constructed PLGA nanoparticles coated with zwitterionic chitosans as the surface and dopamine as a mediator. Zwitterionic chitosans (ZWC) can change charges along with pH level and allow PLGA nanoparticles to interact with cells in acidic tumor microenvironment (TME) [49]. Magnetic resonance imaging showed that ZWC-coated PLGA nanoparticles were more persistent in tumors of bearing LS174T-xenografts mice than polyethylene glycol (PEG)-coated nanoparticles, in agreement with the in vitro results. The results demonstrate that zwitterionic chitosans coated PLGA nanoparticles exhibited pH-dependent cell interactions and longer retention in LS174T human colon tumors.
The advantage of oral drugs delivery over injection or infusion chemotherapy is that it can expose cancer cells to a relatively lower and safer concentrations of anticancer drugs for a long time, leaving tumor blood vessels little chance to grow [50,51]. Oral chemotherapy can finally provide an innovative concept of chemotherapy: “chemotherapy at home” [52,53]. In terms of oral drug delivery materials for treatment of gastrointestinal diseases, PLGA nanoparticles have been widely studied to improve the relative oral bioavailability of curcumin and other polyphenols drugs recently. Curcumin is a polyphenol compound extracted from the root of Curcuma longa. It's anti-inflammatory, antioxidant, antibacterial etc., and possesses large pharmacological potential. Nevertheless, due to its low intestine absorption capacity and its easy metabolism by the liver, the therapeutic application of curcumin has been widely limited. Shaikh et al. [54] uncovered that the oral bioavailability of curcumin was significantly improved after PLGA encapsulation via pharmacokinetic studies. And the curcumin concentration in plasma was maintained longer after oral administration of nanoparticles. These results indicate that curcumin can be constantly released from PLGA nanoparticles. In vitro drug release of PLGA nanoparticles occurs through diffusion and degradation. In this case, a biphasic release was observed, following the square root plot of Higuchi. It was observed from in vivo pharmacokinetic studies in rats that PLGA nanoparticles can improve the relative bioavailability of curcumin to 9.2 times, compared with piperine as an absorption enhancer for curcumin release. To boost the physical stabilization of nanoemulsions, Miele et al. proposed ionic amphiphilic chitosan derivative, chitosan oleate salt (CS-OA), to prepare curcumin loaded PLGA nanoparticles [55]. Previous tests validated the positive interaction between CS-OA coated PLGA nanoparticles and mucin, which helped to increase drugs absorption by prolonging the residence time of nanoparticles. Xie et al. [56] illustrated that one of the main reasons why PLGA nanoparticles can enhance the oral bioavailability in rats is that PLGA can prevent the curcumin efflux mediated by a biological transporter P-glycoprotein (P-gp). Vitamin E succinated polyethylene glycol 1000 (vitamin E TPGS) is an excellent emulsifier/solubilizer/absorption enhancer with great emulsification efficacy and cellular adhesion [57]. It can also be exploited as a reversal agent of P-gp mediated multidrug resistance and inhibit P-gp mediated drug transport [58]. It was shown that the curcumin was slowly released from the constructed curcumin (CUR)-PLGA nanoparticles in the artificial intestinal environment. Oral curcumin released from the CUR-PLGA nanoparticles reached the maximum concentration significantly longer compared with native CUR. Considering this, a fluorescent marker coumarin-6 encapsulated in PLGA nanoparticles manufactured by a modified solvent extraction/evaporation technique with vitamin E TPGS as emulsifier were prepared. The results of using Caco-2 cells as an in vitro model illustrated that vitamin E TPGS coated PLGA nanoparticles exhibited considerably higher cellular uptake compared with polystyrene nanoparticles and polyvinyl-alcohol-coated PLGA nanoparticles [59]. A Mucinous gastric carcinoma is known for its deeper invasion, more frequent lymph node metastasis, more progressive pathologic stage, and lower survival [60]. One uppermost reason is that therapeutic drugs can't penetrate the mucus barrier to reach the underlying epithelial cells [61]. It has been found that reducing hydrophobic and electrostatic interactions can minimize mucoadhesion [62]. In view of this, Lin et al. manufactured PEG-PLGA nanoparticles (PEG-PLGA-NPs) with hydrophilic and near-neutrally-charged surfaces. In their study, HGC-27 cells, a mucin-producing cell line derived from the metastatic lymph node of gastric tumor, took in 1.6- to 2.1-fold higher amount of PEG-PLGA-NPs than pure PLGA nanoparticles incubated for the same time [63]. It illustrated that with the help of a low Mw PEG and a high density of PEG-surface coverage, PEG-PLGA-NPs can quickly penetrate the mucus.
As to local administration materials for treatment of gastrointestinal cancers, Khalili et al. have modified the surfaces of PLGA-PEG nanoparticles with a low-density, second PEG layer (PLGA-TPEG-NPs-20) to decrease protein binding affinity, thus enhancing the delivery of therapeutics into dense mucinous cancer barriers [2]. Via in vitro, ex vivo, and in vivo LS174T murine xenograft subcutaneous cancer models, they showed that PLGA-TPEG-NPs-20 can effectively reduce nanocarrier entrapment and act as a possible platform for regional intraperitoneal chemotherapy. Local administration can attain high concentration of PLGA nanomaterials in the ideal position, while it is hard to touch many deep cancers, hence there are few application scenarios [64].
Different from traditional chemotherapy, target therapy targets to already defined oncogenic sites from cellular molecular level. It first emerged in the late 1990's, having a significant impact on the treatment of certain types of tumors. Small molecule targeted therapeutic agents can be taken orally and are often enzyme domain inhibitors of mutations, overexpression, or other key proteins within cancer cells. Jena et al. constructed capecitabine loaded Eudragit S100 and PLGA based nanoparticles [65]. The pH-sensitive polymer Eudragit S100 could prevent the release of capecitabine at the top of the gastrointestinal tract and can directly target the colon. In vivo pharmacokinetic studies applied a rabbit model, and in vivo study exhibited enhanced oral bioavailability. This better targeted PLGA based nanoparticles can improve the therapeutic effect of colorectal cancer.
3.2. Radiotherapy
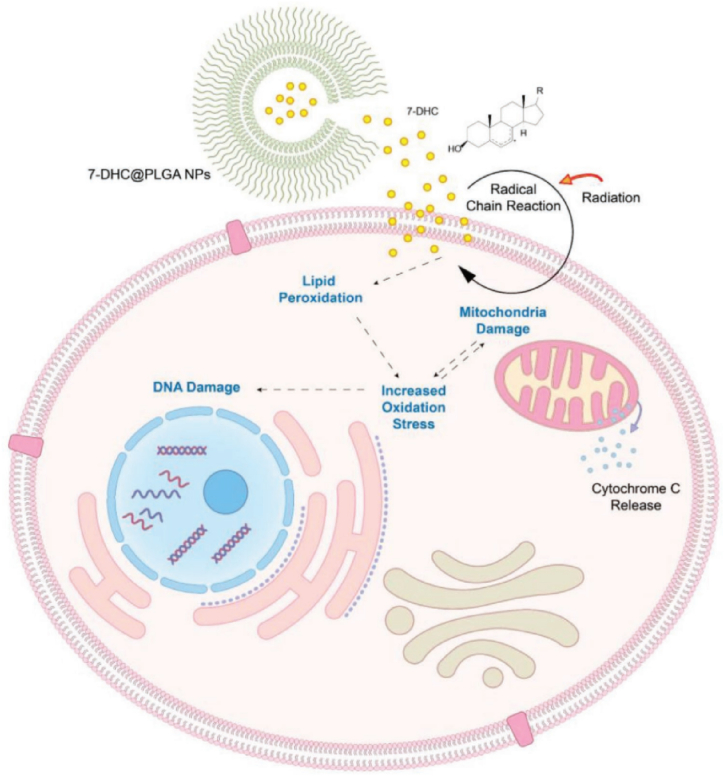
Radiotherapy is still a typical treatment option for gastrointestinal cancer, and approximately 50 % patients have received radiotherapy at least once in the treatment process [66,67]. It may cause morbidities such as scars, rectal irritation and sexual issues, and its lifetime dose is limited to below 60 Gy [68]. Therefore, developing radiation-activatable radiosensitizing agents to improve the radiotherapy efficacy is extremely urgent. Inspired by the fact that the accumulation of low toxic 7-dehydrocholesterol (7-DHC) can cause a buildup of the cellular reactive oxygen species and lead to cholesterol-rich tissues damage [[69], [70], [71], [72], [73], [74]], Delahunty et al. [75] explored 7-DHC as a radiosensitizer (Fig. 2). Poorly hydrosoluble 7-DHC was encapsulated into PLGA nanoparticles for efficient delivery [76]. 7-DHC@PLGA nanoparticles were intravenously injected into CT26 tumor bearing mice, followed by ionizing radiation. By the end of the study on Day 43, 60 % of the mice remained alive, half of which were tumor-free. It revealed that radiosensitizing effects of 7-DHC@PLGA nanoparticles accumulated in tumors through the enhanced permeability and retention effect and improved the radiotherapy efficacy [75].
Fig. 2.
Schematic illustration of radiosensitization with 7-DHC@PLGA-NPs. Reprinted with permission [75].
Additionally, it has been found that tumor hypoxia and dysregulation of histone deacetylase (HDAC) in cancers are another two vital factors of radiation resistance [77,78]. Given these, Zhang et al. provided an alliance strategy to overcome radiation resistance through simultaneous hypoxia relief and chromatin remodeling. They established a multifunctional radiosensitizer CAT-SAHA@PLGA by the double emulsion method. The PLGA nanoparticles encapsulated catalase (a biological peroxidase) and SAHA (an US FDA approved HDAC inhibitor) with protective catalytic activity of catalase, and extended the pharmacokinetic exposure time of the HDAC inhibitor [79]. When combined with radiotherapy, the CAT-SAHA@PLGA treatment displayed obvious tumor inhibition in CT26-bearing mice, and there was no observable damage in the CAT-SAHA@PLGA group compared to other control groups in the presence of radiation. It confirmed that CAT-SAHA@PLGA combined with radiation displayed improved therapeutic efficiency in CT26 cells. The synergistic strategy has been proven obvious sensitization effect on tumor radiotherapy, which possesses great capability for further research in cancer treatment.
3.3. Novel tumor treatment methods
Classical chemotherapy and radiotherapy are prone to cause serious systemic adverse reactions, poor compliance, and tumor recurrence and metastasis. Therefore, the development of novel tumor treatment strategies has become a significant problem urging to be solved. With the in-depth understanding of tumor tissues, some new emerging tumor treatment methods such as immunotherapy [80], gene therapy [81], and photothermal therapy [82] have attracted more and more attention, and achieved good therapeutic effects.
Stimulating the host's immune system to attack cancer cells is called immunotherapy. It is a promising method to fight cancer and is considered to be one of the four pillars of tumor treatment [83]. Recent studies have illustrated that specific chemotherapeutic drugs such as doxorubicin (DOX) can cause immunogenic cell death (ICD) of cancer cells [[84], [85], [86]]. Yet, the short circulating half-life, low cancer accumulation and off-target toxicity of DOX lessened the ICD efficiency. In view of PLGA polymeric nanoparticles constricted by low drug loading ability, Zhang et al. prepared lipid/PLGA nanoparticles to deliver DOX and peptide-based programmed cell death ligand-1 (PD-L1) inhibitor P-12 concurrently for chemo-immunotherapy of tumor [[87], [88], [89]]. The optimal DOX and P-12 co-loaded nanocomposite exhibited improved tumor cellular uptake and effective ICD and PD-L1 blockade in in vitro CT26 tumor cells, and the in vivo anticancer effect was also verified by tumor-bearing mice model [90]. Lee et al. attached the Fc truncated F(ab) portion of α-PD-L1 monoclonal antibody (α-PD-L1 mAb) to a PEG-PLGA polymer [91]. They concluded that α-PD-L1 based nanoparticles has no apparent toxicity in vitro or in vivo. There is no evidence of enhanced efficacy in mice treated with α-PD-L1 nanoparticles. Authors ascribed it to the insufficient immune activation to increase the regression of insensitive MC38 tumors. Given the restriction of local immunotherapy, systemic delivery of immune-activating combinations without systemic side effects is necessary [92]. To realize this, Kwon et al. developed adenosine triphosphate (ATP)-modified paclitaxel (PTX) loaded PLGA nanoparticles [93]. The designed PLGA nanoparticles could codeliver the ICD attractant (PTX) and the inducer of antigen-presenting cells (ATP) to solid tumors. By producing an immunoactive TME, the PTX-loaded NPpD-ATP weakened CT26 growth, and the designed PLGA nanoparticles combined with anti-PD-1 antibody realized complete tumor regression and specific antitumor immune memory in 6 out of 8 mice [93]. Besides ICD, immune checkpoint blocking (ICB) therapy is also a broadly applied immunotherapy in the treatment of tumors [94,95]. Increasing evidence proves that cancer acidity can promote tumor immunosuppression meanwhile damaging the anticancer effects of cytotoxic CD8+ T cells [[96], [97], [98]]. Considering this, Hao et al. reported that calcium carbonate encapsulated by PLGA and PLGA-PEG copolymers could function as a proton nanosponge to neutralize cancer acidity by reacting with protons after intravenous administration [99]. They demonstrated that CaCO3@PLGA nanoparticles can enhance the therapeutic effect of ICB therapies on CT26 tumor xenografts by reinvigorating exhausted CD8+ T cells and innate anticancer immunity. In recent years, with the extensive employment of ICB therapy in tumor, radiotherapy has been verified to promote anticancer immunity by adjusting the immune system [100]. Lu et al. assembled PLGA and R837 (Toll-like receptor 7 agonist) and then coated them with neutrophil membrane obtaining R837@PLGA@Neu nanoparticle [101,102]. Results suggested that the nanoparticle owned strong uptake capacity, low cytotoxicity, and a high tumor targeting efficacy [103]. Combining with radiotherapy, it possessed an excellent effect in CT26-bearing mice through immune cell regulation, providing a promising strategy for colorectal tumor treatment.
Gene therapy targeting at the level of cellular gene expression provides another new route for gastrointestinal tumor treatment [104]. As reported, pigment epithelial-derived factor (PEDF) gene possessed excellent therapeutic effects on many tumors [[105], [106], [107], [108], [109]]. Based on the low-toxicity, biodegradability and biocompatibility of PLGA, Cui et al. loaded PEDF gene in PLGA nanoparticles and found that PEDF gene loaded in PLGA nanoparticles could efficiently impede CT26 cancer growth [110]. The hindering effect is related to CT26s apoptosis, and the inhibition of angiogenesis required for cancer growth. The animal results exhibited that the number of apoptotic CT26s from the PEDF-PLGA nanoparticles treated mice was apparently higher than that of normal saline (NS) treated group. Adopting PEDF gene and PLGA nanoparticles is an effective and safe gene delivery system for primary colorectal cancer treatment. Yet, its effect on late stage tumor growth and metastasis tumor are still unknown. Due to the high specificity of RNAi technology for target sequences, it possesses an ability to silence therapy-related genes in multiple cancer models. Moreover, evidence has accumulated over the years suggesting that the effector function of CD8+ T cells can be improved by siRNA-mediated silencing of programmed cell death protein-1 (PD-1) or PD-L1. Based on this, Kwak et al. synthesized PLGA nanoparticles loaded with PD-1 and PD-L1 siRNA (i.e., siRNA@PLGA NPs) [111]. Investigating by in vitro and in vivo colon cancer models, they found that in contrast to silencing PD-1 alone or PD-L1 alone, cosilencing of PD-1 and PD-L1 by siRNA@PLGA NPs more significantly encouraged the immune response of tumor-specific CD8+ T cells. Furthermore, systemic administration of PD-1 and PD-L1 siRNA@PLGA NPs in MC38 tumor-bearing mice also led to a more pronounced antitumor effect. Moreover, synergistic therapy based on combination of gene and small-molecule chemotherapeutic medicine can be expected both high anticancer efficiency and low side effects [112]. Xu et al. fabricated a truncated basic fibroblast growth factor (tbFGF)-mediated targeting delivery system concurrently loaded with PEDF gene and PTX in PLGA nanoparticles (T-NPs) [113]. The improved anticancer capacity of T-NPs principally because the specific interaction between tbFGF on T-NPs and FGFR1 overexpressed on tumor cells and tumor neovascular endothelial cells elevated the nanoparticles distribution in tumor tissues [114]. The experiment results demonstrated that this combination therapy showed significantly improved anticancer effects in a C26 subcutaneous tumor model. It was also indicated that nanoparticle treated mice had no obvious side effects compared to the group treated by NS. Hence, T-NPs were safe formulations by systemic administration of intravenous injection.
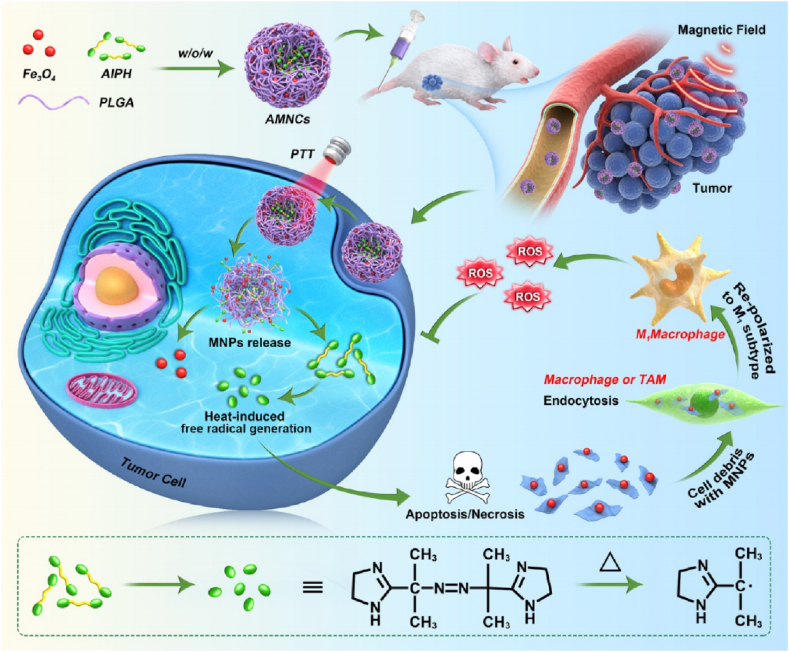
Photothermal therapy (PTT) refers to the adopt of photothermal agents (PTAs) to eliminate cancer cells under laser irradiation. PTT is considered to be one of the most effective and hopeful clinical treatments for carcinoma owing to its noninvasive, controllable and repeatable character [[115], [116], [117]]. Thermodynamic therapy (TDT) is a novel type of dynamic therapy developed in recent years, characterized by applied of thermodynamic agents to produce oxygen-independent free radicals to kill tumors. At present, the main thermodynamic agent used is 2,2′-azobis[2-(2-imidazolin-2-yl) propane]-dihydrochloride (AIPH). The compound decomposes to release free radicals but is thermally unstable (half-life temperature is 44 °C), which generally needs to be loaded on the carrier and enters the body. Combined PTT, TDT and Fe3O4 magnetic nanoparticles mediated macrophage polarization strategies, Li et al. constructed PLGA-based nanocomposites (AMNCs) that efficiently co-delivered AIPH and magnetic nanoparticles (MNPs) (Fig. 3). In the CT26 xenograft mouse model, AMNCs were highly gathered at the tumor site, and the encapsulated MNPs preserved their property as contrast agents for enhanced magnetic resonance imaging (MRI). Under near-infrared light irradiation, Fe3O4 and AIPH were released and produced oxygen-independent free radicals, which expediting the apoptosis and necrosis of cancer cells. Afterwards, fragments of tumor cells bond to undegraded Fe3O4 and were arrested by macrophages through endocytosis. At last, Fe3O4 induced macrophages to repolarize into pro-inflammatory subtypes, which further created free radicals, and thus improved the immune response to prevent cancer growth [118]. It offers an alternative strategy for MRI guided PTT/TDT combined therapy to produce oxygen-independent free-radical cascade. Scientists also adopt synergistic chemo-photothermal therapy for cancer treatment [119]. Banstola et al. reported on paclitaxel (PTX)-loaded PLGA microspheres coated with gold nanoparticles for pancreatic cancer treatment [120]. The developed nanosystem and NIR-treated Panc-1 cells displayed the highest cytotoxicity with meaningfully decreased viability compared with photothermal or chemotherapeutic agents alone. It suggested that PLGA microspheres coated with gold nanoparticles could provide synergistic chemical-PTT for cancer treatment of alimentary system.
Fig. 3.
Illustration of the AMNC-mediated free-radical cascade for enhancing MRI-guided chemo/thermodynamic hypoxic tumor therapy. Reprinted with permission [118].
Radiodynamic therapy (RDT), sometimes also referred to as X-photodynamic therapy (PDT), is a modification of conventional PDT in that the external activating light source is replaced by high-energy X-rays. Clement et al. designed a drug delivery system for RDT comprising a photosensitizer Verteporfin incorporated into PLGA nanoparticles that were surface-functionalized with a cell-penetrating HIV trans-activator of transcription (TAT) peptide [121]. Cytotoxicity tests in pancreatic cancer cells in vitro under 4 Gy X-ray exposure from a clinical 6 MV linear accelerator revealed that TAT targeting of the PLGA nanoparticles markedly increases the RDT effectiveness.
3.4. Diagnosis
Gastrointestinal cancer dose not exhibit obvious symptoms in the early stage, and vast majority of patients are in the middle and late stage at the first diagnosis. This is also the main reason for the low five-year survival rate of these patients. Hence, early diagnosis and timely treatment are crucial to improve the clinical prognosis of patients with gastrointestinal cancers. For gastrointestinal cancers, early screening and diagnosis are also very important [[122], [123], [124]]. Currently, clinical endoscopy primarily applies white light imaging technology, whose disadvantage is the high rate of missed detection, owing to the subtle lesions of many gastrointestinal diseases difficultly to recognize by naked eyes. To solve this problem, many studies have focused on new imaging technologies, such as fluorescence imaging [[125], [126], [127], [128]] and magnetic resonance imaging [129].
Fluorescent labeled probes are employed to bind to receptors with specific molecular structures at the lesion site, and special light sources and camera systems are adopted for visualization, thus impressively improving diagnostic accuracy. As mentioned above, curcumin is anti-inflammatory, antioxidant, antibacterial. Moreover, curcumin can exhibit fluorescence spectra properties but poor photochemical stability in some solvents due to its hydrophobicity [[130], [131], [132]]. To improve its solubility and luminous property, Wang et al. encapsulated curcumin into PLGA nanoparticles (Cur@PLGA-NPs) as a novel aggregation-induced emission (AIE) formulation for cell bioimaging, in which curcumin plays two vital roles: AIE emission and drug [128]. The results indicated that Cur@PLGA-NPs had raised effectiveness on CT26 cancer cells compared with pure curcumin. Furthermore, Cur@PLGA-NPs can light up in cells via a concentration-dependent way, thus assisting the exploration of fluorescence probes for clinical gastrointestinal applications.
As to magnetic resonance imaging (MRI), Schleich et al. [129] have successfully co-encapsulated superparamagnetic iron oxide (SPIO, as MRI contrast agent) and PTX in PLGA based nanoparticles. The in vivo antitumor efficacy of the multifunctional PTX/SPIO loaded PLGA NPs was evaluated in CT26 tumor-bearing mice. Consistent with the in vitro cytotoxicity data, the PTX/SPIO loaded NPs brought a higher delay of tumor growth than the other treatments. These PLGA NPs were proven to constrain the growth of CT26 colon carcinoma cells and simultaneously can be adopted as potential tumor targeted MRI contrast agents due to their high absorption by tumor cells and their magnetism. The PLGA based nanoparticles may become an encouraging candidate as an effective codelivery system for both the diagnosis and therapeutic applications for gastrointestinal diseases.
3.5. The clinical application of PLGA-based drug delivery systems
Currently, more than 20 PLGA particle-based formulations are approved for commercialization, and most of them are used as chemotherapeutic agents to treat malignancies [6]. Actually, there are two unavoidable problems in the way of clinical application of PLGA-based drug delivery systems [133]. The first problem is scale-up productions of PLGA-based systems. Although several well-established methods for producing PLGA nanoparticles on a laboratory-scale have been developed, it can induce changes in particle properties (e.g. drug release profiles) when the process is scaled up to industrial batch sizes. The second is the efficacy and safety assessment, which is the critical step during the ways of clinical translation. As summarized in Table 1, there are still works cited in this paper without animal experiments. And most of them conducted animal experiments on mice or rabbits rather than large animals. Pilot studies in large animal models are urgently needed, where protection and effectiveness can be investigated over long periods. Clinical trials enlisted in clinicaltrials.gov website for marketed PLGA-based therapeutics have been checked, and major tumor-related clinical trials focus on prostate cancer, oral cancer, breast cancer and so on. There are few clinical trials about advanced solid tumor, and none of them is investigating gastrointestinal cancers as to now [133]. Hence, a huge effort in investigating different PLGA platforms and evaluating their safety and efficacy on clinical trials of gastrointestinal cancers is really needed. Based on the generalization of studies in laboratories, we found that majority of them adopted the administration pathway of intravenous injection. More varied administration pathway may need to be investigated in the future. PLGA-based clinical probes are also needed to be noticed, since the small quantity of PLGA-based delivery systems for gastrointestinal cancers diagnosis [128,129].
Table 1.
Comparisons of several PLGA-based nanoparticles for the treatment and diagnosis of gastrointestinal cancers. (Uploaded as a single file).
| No. | Formulation | PLGA | Methods | Therapeutic agents | Loading techniques | Entrapment efficiency wt./% | Release rate/time | Treatments | Target tumor cell lines | Animals | Differences between in vitro and in vivo studies | References |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 5-Fu loaded magnetic PLGA nanocapsules | 50:50, MW 12000 |
w/o/w | 5-fluorouracil (5-Fu) | Entrapped | 63.8 | 100%-33 d | Chemotherapy | CT26 | Mice | No | [45] |
| 2 | NP-pD-ZWC0.1 (Zwitterionic chitosans coated PLGA NPs) | LA:GA = 85:15, 150 kDa | w/o/w | Hydrophobic iron oxide (IO) Indocyanine green (ICG) |
Entrapped | – | 90%-24 h (ICG) | Chemotherapy | LS174T 4T1 NIH/3T3 |
Mice | No | [48] |
| 3 | Curcumin loaded PLGA NPs | 50:50, 0.41 dL/g | o/w | Curcumin | Entrapped | 84.6 | 42%-20 d | Chemotherapy | – | Rats (oral) | – | [54] |
| 4 | Cur-CS-OA (Chitosan oleate) PLGA | Resomer RG 503H | w/o/w | Curcumin | Entrapped | 68.8 | – | Chemotherapy | Caco-2 | – | – | [55] |
| 5 | CUR-PLGA-NPs | 50:50, MW 30000 |
s/o/w | Curcumin | Entrapped | 92.0 | 77%-168 h (pH 7) 48%-168 h (pH 2) |
Chemotherapy | – | Rats (oral) | – | [56] |
| 6 | Coumarin 6-loaded PLGA NPs coated with vitamin E TPGS | 50:50, MW 40000–75000 |
o/w | Coumarin-6 | Entrapped | – | 3.5%-24 h | Chemotherapy | Caco-2 | – | – | [59] |
| 7 | 10%-PEG2000-PLGA-NPs | LA:GA = 75:25, 18 kDa | o/w | Coumarin-6 | Entrapped | 91.0 | 14%-24 h | Chemotherapy | HGC-27 | – | – | [63] |
| 8 | PLGA-TPEG-NPs-20 | MW 5000–20000 |
Nanoprecipitation method | DiD (fluorochrome) | Entrapped | – | – | Chemotherapy | LS174T | Mice | No | [2] |
| 9 | Cap loaded PLGA based Nanoparticles | 50:50, Sigma | – | Capecitabine | Entrapped | 66.5 | – | Target therapy | HT-29 | Rabbits (oral) | – | [65] |
| 10 | 7-DHC@PLGA NPs | – | Nanoprecipitation method | 7-dehydrocholesterol (7-DHC) | Entrapped | 21.9 | 35%-24 h (pH 6.5) | Radiotherapy | CT26 | Mice | No | [75] |
| 11 | CAT-SAHA@PLGA | Sigma-Aldrich | w/o/w | Catalase SAHA (HDAC inhibitor) |
Entrapped | 66.9 (Catalase) 18.8 (SAHA) |
40%-24 h (Catalase with H2O2) 40%-24 h (SAHA) |
Radiotherapy | CT26 | Mice | No | [79] |
| 12 | LPN-30-R82K@DP | 50:50 | w/o/w | Doxorubicin (DOX) P-12 (PD-L1 inhibitor) |
Entrapped | – | 35%-70 h (DOX) 72%-12 h (P-12) |
Chemo-immunotherapy | CT26 | Mice | No | [90] |
| 13 | ɑ-PD-L1 NP | PEG-PLGA, MW = 5 kDa | – | ɑ-PD-L1 monoclonal antibodies | Attached | – | – | Immunotherapy | MC38 | Mice | Yes | [91] |
| 14 | PTX@NPpD-ATP | LA:GA = 85:15, ester end, 0.55−0.75 dL/g | o/w | Paclitaxel (PTX) | Entrapped | 3.4 | 70%-24 h 83%-96 h |
Immunotherapy | CT26 | Mice | No | [93] |
| 15 | CaCO3@PLGA NPs | MW 7000–17000 |
w/o/w | CaCO3 | Entrapped | 15.5 | – | Immunotherapy | CT26 | Mice | No | [99] |
| 16 | R837@PLGA@Neu | PEG-PLGA 50:50 (w/w), Mw∼5000:10000 Da | o/w | R837 (Toll-like receptor 7 agonist) | Entrapped | – | 41%-100 h | Radio-immunotherapy | CT26 | Mice | No | [103] |
| 17 | PEDF-PLGANPs | LA: GA = 75:25, MW 15000 Da |
w/o/w | PEDF gene | Entrapped | 88.0 | – | Genetherapy | CT26 | Mice | No | [110] |
| 18 | siRNA@PLGA NPs | LA: GA = 75:25, MW 20000 Da |
w/o/w | PD-1 and PD-L1 siRNA | Entrapped | 80.2 | – | Genetherapy | MC38 | Mice | No | [111] |
| 19 | T-NPs | PEG-PLGA MW = 15 kDa; LA/GA = 75:25 | w/o/w | Paclitaxel (PTX) PEDF gene |
Entrapped | 86.3 (PTX) 83.7 (PEDF) |
65%-48 h (PTX) 95%-48 h (PEDF) |
Gene-chemo-therapy | CT26 A549 LL2 |
Mice | No | [113] |
| 20 | AMNCs | Rui'xi Biotechnology Co. Ltd (Xi'an, China) | w/o/w | AIPH Fe3O4 |
Entrapped | – | 95%-after laser 25 h (AIPH) | MRI guided PTT/TDT | CT26 | Mice | No | [118] |
| 21 | GNPs-pD-PTX-PLGA-Ms | 50:50 DLG 5 E from Lakeshore Biomaterials Inc. (Birmingham, AL, USA) | – | PTX | Entrapped | 70.0 | 65%-24 d | Chemical-PTT | PANC-1 | – | – | [120] |
| 22 | PLGA-VP-TAT NPs | 50:50, MW 38000–54000 |
w/o/w | Verteporfin | Entrapped | – | 5%-30 h | RDT | PANC-1 | – | – | [121] |
| 23 | Cur@PLGA-NPs | 50:50, MW 40000 |
w/o/w | Curcumin | Entrapped | 70.0 | – | Fluorescence probes | CT26 | – | – | [128] |
| 24 | SPIO/PTX-NP | 50:50, MW 7000–17000 |
o/w | SPI (superparamagnetic iron oxide) PTX |
Entrapped | 24.8 (PTX) | – | MRI contrast agents | CT26 | Mice | No | [129] |
4. Conclusions and perspectives
This study summarizes drug-loaded PLGA-based nanoparticles, elucidates the factors influencing drug release, and explores their application in treating gastrointestinal cancers. In the field of biomedicine, PLGA is the only biodegradable polymer approved by the US FDA. The drug release behavior is primarily affected by the degradation of PLGA-based nanoparticles, which is determined by various factors, including the ratio of PLA to PGA, Mw, crystallinity, and preparation process of PLGA nanoparticles. Moreover, the degradation environment and drug parameters, such as drug types, drug distribution, and drug loading rates, also significantly influence drug release behavior. Due to its facile functionalization, PLGA can potentially be applied in targeted drug delivery and diagnostic imaging for the treatment of gastrointestinal cancers. In chemotherapy of gastrointestinal cancers, PLGA nanoparticles are commonly employed as hydrophilic drug carriers to load chemotherapeutic drugs and subsequently functionalize with targeted ligands. The functionalized PLGA nanoparticles interact with tumor cells, enhancing their retention within tumors and ultimately increasing their therapeutic activity. In radiotherapy of gastrointestinal tumors, PLGA nanoparticles are designed to load various radiation-activatable radiosensitizing agents to enhance the radiotherapy efficacy of the delivery system. As to novel tumor treatment methods (immunotherapy, gene therapy, or photothermal therapy), loading antibodies, genes, or thermodynamic agents on PLGA nanoparticles to construct delivery systems has become a general consensus. These novel drug delivery systems based on PLGA nanoparticles have also achieved satisfactory therapeutic effects in the clinical treatment of gastrointestinal cancer. When PLGA-based nanoparticles are exploited for gastrointestinal diagnostics, these particles are utilized for encapsulating or loading luminescent nanomaterials or magnetic Fe3O4 nanocrystal-based T2 contrast agents.
However, due to the relative hydrophobicity of PLGA polymers, they cannot load hydrophilic drugs with higher encapsulation efficiencies [134]. The issue of burst release from PLGA nanoparticles arises due to the positioning of drugs on the surface or the formation of pores inside the particles [135,136]. In addition, using emulsion-based solvent evaporation technology to prepare hydrophilic drug-loaded nanoparticles exacerbates drug leaching in the water continuous phase during preparation, leading to low drug encapsulation efficiency [137]. These outstanding problems also directly impact the performance of PLGA polymer applications in the treatment of gastrointestinal cancers. Breaking through the above bottlenecks requires the design and modification of PLGA polymers from the perspective of microstructure. Moreover, the advantages of radiosensitizers largely depend on the radiation resistance mechanism behind them. Similarly, there are limitations in understanding the inherent mechanism of immunotherapy and gene therapy, making it difficult to develop innovative drug-loaded PLGA nanoparticles. Finally, the synergistic development of nanomaterials and life science fields is needed to realize the wide application of PLGA-based nanoparticles in the clinical treatment of gastrointestinal tumors.
This study provides a comprehensive overview of the application of drug-loaded PLGA-based nanoparticles in the treatment of gastrointestinal cancers. However, due to the limited availability of studies on drug loading polymeric nanoparticles specifically for gastrointestinal cancer treatment, a more extensive literature review comparing PLGA with other biodegradable polymers used in this context could not be included. Additionally, given the scarcity of research on different drug loading techniques employed with PLGA nanoparticles, a section discussing different drug loading techniques could not be incorporated.
Data availability statement
This is a review, and all of the data come from references.
CRediT authorship contribution statement
Rui Sun: Writing – original draft, Visualization, Resources, Conceptualization. Yanfei Chen: Writing – review & editing, Visualization, Conceptualization. Yanjiang Pei: Writing – review & editing, Methodology, Conceptualization. Wenbin Wang: Resources, Conceptualization. Zhi Zhu: Visualization, Resources. Zhaohua Zheng: Resources, Conceptualization. Limeng Yang: Writing – original draft, Funding acquisition. Li Sun: Writing – review & editing, Resources, Conceptualization.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:Limeng Yang reports financial support was provided by Xi'an Polytechnic University. Limeng Yang reports a relationship with Xi'an Polytechnic University that includes: employment. If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported financially by Natural Science Basic Research Program of Shaanxi Province (No. 2022JQ-525).
References
- 1.Wang G., Wang H., Ji X., Wang T., Zhang Y., Jiang W., Meng L., Wu H.J., Xing X., Ji J. Intratumoral microbiome is associated with gastric cancer prognosis and therapy efficacy. Gut Microb. 2024;16 doi: 10.1080/19490976.2024.2369336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khalili M., Zhou H., Thadi A., Daniels L., Fan Z., Morano W.F., Ang J., Goldstein E., Polyak B., Mapow B.C., Cheng H., Bowne W.B. Slippery nanoparticles as a diffusion platform for mucin producing gastrointestinal tumors. Ann. Surg Oncol. 2020;27:76–84. doi: 10.1245/s10434-019-07493-7. [DOI] [PubMed] [Google Scholar]
- 3.Lu Y., Cheng D., Niu B., Wang X., Wu X., Wang A. Properties of poly (lactic-co-glycolic acid) and progress of poly (lactic-co-glycolic acid)-based biodegradable materials in biomedical research. Pharmaceuticals. 2023;16 doi: 10.3390/ph16030454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kapoor D.N., Bhatia A., Kaur R., Sharma R., Kaur G., Dhawan S. PLGA: a unique polymer for drug delivery. Ther. Deliv. 2015;6:41–58. doi: 10.4155/tde.14.91. [DOI] [PubMed] [Google Scholar]
- 5.Wan B., Bao Q., Burgess D. Long-acting PLGA microspheres: advances in excipient and product analysis toward improved product understanding. Adv. Drug Deliv. Rev. 2023;198 doi: 10.1016/j.addr.2023.114857. [DOI] [PubMed] [Google Scholar]
- 6.Horvath D., Basler M. PLGA particles in immunotherapy. Pharmaceutics. 2023;15 doi: 10.3390/pharmaceutics15020615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sun X., Xu C., Wu G., Ye Q., Wang C. Poly (lactic-co-glycolic acid): applications and future prospects for periodontal tissue regeneration. Polymers. 2017;9 doi: 10.3390/polym9060189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hu F., Qi J., Lu Y., He H., Wu W. PLGA-based implants for sustained delivery of peptides/proteins: current status, challenge and perspectives. Chin. Chem. Lett. 2023;34 doi: 10.1016/j.cclet.2023.108250. [DOI] [Google Scholar]
- 9.Rahmani F., Naderpour S., Nejad B.G., Rahimzadegan M., Ebrahimi Z.N., Kamali H., Nosrati R. The recent insight in the release of anticancer drug loaded into PLGA microspheres. Med. Oncol. 2023;40:229. doi: 10.1007/s12032-023-02103-9. [DOI] [PubMed] [Google Scholar]
- 10.Xu Y., Kim C.S., Saylor D.M., Koo D. Polymer degradation and drug delivery in PLGA-based drug-polymer applications: a review of experiments and theories. J. Biomed. Mater. Res. B. 2017;105:1692–1716. doi: 10.1002/jbm.b.33648. [DOI] [PubMed] [Google Scholar]
- 11.Sanchez A., Mejia S.P., Orozco J. Recent advances in polymeric nanoparticle-encapsulated drugs against intracellular infections. Molecules. 2020;25:3760. doi: 10.3390/molecules25163760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Petposri S., Thuaksuban N., Buranadham S., Suwanrat T., Punyodom W., Supphaprasitt W. Physical characteristics and biocompatibility of 3D-printed polylactic-co-glycolic acid membranes used for guided bone regeneration. J. Funct. Biomater. 2023;14:275. doi: 10.3390/jfb14050275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Essa D., Kondiah P.P., Choonara Y.E., Pillay V. The design of poly(lactide-co-glycolide) nanocarriers for medical applications. Front. Bioeng. Biotechnol. 2020;8:48. doi: 10.3389/fbioe.2020.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Butreddy A., Gaddam R.P., Kommineni N., Dudhipala N., Voshavar C. PLGA/PLA-based long-acting injectable depot microspheres in clinical use: production and characterization overview for protein/peptide delivery. Int. J. Mol. Sci. 2021;22:8884. doi: 10.3390/ijms22168884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Witschi C., Doelker E. Influence of the microencapsulation method and peptide loading on poly(lactic acid) and poly(lactic-co-glycolic acid) degradation during in vitro testing. J. Contr. Release. 1998;51:327–341. doi: 10.1016/s0168-3659(97)00188-0. [DOI] [PubMed] [Google Scholar]
- 16.Brauner B., Schuster C., Wirth M., Gabor F. Trimethoprim-loaded microspheres prepared from low-molecular-weight PLGA as a potential drug delivery system for the treatment of urinary tract infections. ACS Omega. 2020;5:9013–9022. doi: 10.1021/acsomega.0c00981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ochi M., Wan B., Bao Q., Burgess D.J. Influence of PLGA molecular weight distribution on leuprolide release from microspheres. Int. J. Pharm. 2021;599 doi: 10.1016/j.ijpharm.2021.120450. [DOI] [PubMed] [Google Scholar]
- 18.Zhu B., Xue H., Liu Q., Zhang S., Tang Y. Research progress on the effect of degradation behavior of polylactic acid glycolic acid copolymer on drug release behavior of sustained-release formulations. Moder. Med. Clin. Pract. 2020;39:2496–2500. doi: 10.7501/j.issn.1674-5515.2020.12.041. [DOI] [Google Scholar]
- 19.Makadia H.K., Siegel S.J. Poly lactic-co-glycolic acid (PLGA) as biodegradable controlled drug delivery carrier. Polymers. 2011;3:1377–1397. doi: 10.3390/polym3031377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Frank A., Rath S.K., Venkatraman S.S. Controlled release from bioerodible polymers: effect of drug type and polymer composition. J. Contr. Release. 2005;102:333–344. doi: 10.1016/j.jconrel.2004.10.019. [DOI] [PubMed] [Google Scholar]
- 21.Park H. Exploring the effects of process parameters during W/O/W emulsion preparation and supercritical fluid extraction on the protein encapsulation and release properties of PLGA microspheres. Pharmaceutics. 2024;16:302. doi: 10.3390/pharmaceutics16030302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yagi Y., Liu Y., Li J., Shimada S., Ohkouchi M., Taguchi Y., Nii T., Mori T., Katayama Y. Oral administration of PLGA nanoparticles to deliver antisense oligonucleotides to inflammatory lesions in the gastrointestinal tract. Biol. Pharm. Bull. 2024;47:848–855. doi: 10.1248/bpb.b23-00769. [DOI] [PubMed] [Google Scholar]
- 23.Qi F., Wu J., Hao D., Yang T., Ren Y., Ma G., Su Z. Comparative studies on the influences of primary emulsion preparation on properties of uniform-sized exenatide-loaded PLGA microspheres. Pharm. Res. (N. Y.) 2014;31:1566–1574. doi: 10.1007/s11095-013-1262-6. [DOI] [PubMed] [Google Scholar]
- 24.Shen J., Burgess D.J. Accelerated in vitro release testing of implantable PLGA microsphere/PVA hydrogel composite coatings. Int. J. Pharm. 2012;422:341–348. doi: 10.1016/j.ijpharm.2011.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kang J., Schwendeman S.P. Pore closing and opening in biodegradable polymers and their effect on the controlled release of proteins. Mol. Pharm. 2007;4:104–118. doi: 10.1021/mp060041n. [DOI] [PubMed] [Google Scholar]
- 26.Wang Y., Burgess D.J. Influence of storage temperature and moisture on the performance of microsphere/hydrogel composites. Int. J. Pharm. 2013;454:310–315. doi: 10.1016/j.ijpharm.2013.06.012. [DOI] [PubMed] [Google Scholar]
- 27.Park T.G. Degradation of poly(lactic-co-glycolic acid) microspheres: effect of copolymer composition. Biomaterials. 1995;16:1123–1130. doi: 10.1016/0142-9612(95)93575-x. [DOI] [PubMed] [Google Scholar]
- 28.Cohn D., Younes H. Compositional and structural analysis of PELA biodegradable block copolymers degrading under in vitro conditions. Biomaterials. 1989;10:466–474. doi: 10.1016/0142-9612(89)90088-4. [DOI] [PubMed] [Google Scholar]
- 29.Huang F., Yang S., Wang H., Zhao P., Zhou B., Cheng B., Dong S., Yang J., Li B., Wang X. pH-responsive PLGA/gelatin porous microspheres containing paclitaxel used for inhibition of cancer cell proliferation. J. Drug Deliv. Sci. Technol. 2023;86 doi: 10.1016/j.jddst.2023.104735. [DOI] [Google Scholar]
- 30.Zhang Z. Wuhan University of Technology; Wuhan, China: 2019. Preparation and Characterization of PLGA Copolymer and the Antitumor Activity and Mechanism Exploration of Paclitaxel Loaded PLGA Microspheres. [DOI] [Google Scholar]
- 31.Han F.Y., Thurecht K.J., Whittaker A.K., Smith M.T. Bioerodable PLGA-based microparticles for producing sustained-release drug formulations and strategies for improving drug loading. Front. Pharmacol. 2016;7:185. doi: 10.3389/fphar.2016.00185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Naidu P.S.R., Norret M., Dunlop S.A., Fitzgerald M., Clemons T., Iyer K.S. Novel hydrophilic copolymer-based nanoparticle enhances the therapeutic efficiency of doxorubicin in cultured MCF-7 cells. ACS Omega. 2019;4:17083–17089. doi: 10.1021/acsomega.8b02894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gasmi H., Siepmann F., Hamoudi M.C., Danede F., Verin J., Willart J.F., Siepmann J. Towards a better understanding of the different release phases from PLGA microparticles: dexamethasone-loaded systems. Int. J. Pharm. 2016;514:189–199. doi: 10.1016/j.ijpharm.2016.08.032. [DOI] [PubMed] [Google Scholar]
- 34.Kakish H.F., Tashtoush B., Ibrahim H.G., Najib N.M. A novel approach for the preparation of highly loaded polymeric controlled release dosage forms of diltiazem HCl and diclofenac sodium. Eur. J. Pharm. Biopharm. 2002;54:75–81. doi: 10.1016/s0939-6411(02)00035-8. [DOI] [PubMed] [Google Scholar]
- 35.Shakeri-Zadeh A., Kamrava S.K., Farhadi M., Hajikarimi Z., Maleki S., Ahmadi A. A scientific paradigm for targeted nanophotothermolysis; the potential for nanosurgery of cancer, Laser. Med. Sci. 2014;29:847–853. doi: 10.1007/s10103-013-1399-x. [DOI] [PubMed] [Google Scholar]
- 36.Shakeri-Zadeh A., Shiran M.B., Khoee S., Sharifi A.M., Ghaznavi H., Khoei S. A new magnetic nanocapsule containing 5-fluorouracil: in vivo drug release, anti-tumor, and pro-apoptotic effects on CT26 cells allograft model. J. Biomater. Appl. 2014;29:548–556. doi: 10.1177/0885328214536940. [DOI] [PubMed] [Google Scholar]
- 37.Khoei S., Mahdavi S.R., Fakhimikabir H., Shakeri-Zadeh A., Hashemian A. The role of iron oxide nanoparticles in the radiosensitization of human prostate carcinoma cell line DU145 at megavoltage radiation energies. Int. J. Radiat. Biol. 2014;90:351–356. doi: 10.3109/09553002.2014.888104. [DOI] [PubMed] [Google Scholar]
- 38.Thomas A.M., Kapanen A.I., Hare J.I., Ramsay E., Edwards K., Karlsson G., Bally M.B. Development of a liposomal nanoparticle formulation of 5-Fluorouracil for parenteral administration: formulation design, pharmacokinetics and efficacy. J. Contr. Release. 2011;150:212–219. doi: 10.1016/j.jconrel.2010.11.018. [DOI] [PubMed] [Google Scholar]
- 39.Haggar F.A., Boushey R.P. Colorectal cancer epidemiology: incidence, mortality, survival, and risk factors, Clin. Colon Rect. Surgery (St Louis) 2009;22:191–197. doi: 10.1055/s-0029-1242458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Smyth E.C., Nilsson M., Grabsch H.I., van Grieken N.C.T., Lordick F. Gastric cancer. Lancet. 2020;396:635–648. doi: 10.1016/s0140-6736(20)31288-5. [DOI] [PubMed] [Google Scholar]
- 41.Zhang P., Li Y., Tang W., Zhao J., Jing L., McHugh K.J. Theranostic nanoparticles with disease-specific administration strategies. Nano Today. 2022;42 doi: 10.1016/j.nantod.2021.101335. [DOI] [Google Scholar]
- 42.Zhang L., Xu L., Wang Y., Liu J., Tan G., Huang F., He N., Lu Z. A novel therapeutic vaccine based on graphene oxide nanocomposite for tumor immunotherapy. Chin. Chem. Lett. 2022;33:4089–4095. doi: 10.1016/j.cclet.2022.01.071. [DOI] [Google Scholar]
- 43.Ahmad R., Deng Y., Singh R., Hussain M., Shah M.A.A., Elingarami S., He N., Sun Y. Cutting edge protein and carbohydrate-based materials for anticancer drug delivery. J. Biomed. Nanotechnol. 2018;14:20–43. doi: 10.1166/jbn.2018.2476. [DOI] [PubMed] [Google Scholar]
- 44.Li S., Wang A., Jiang W., Guan Z. Pharmacokinetic characteristics and anticancer effects of 5-Fluorouracil loaded nanoparticles. BMC Cancer. 2008;8:103. doi: 10.1186/1471-2407-8-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shakeri-Zadeh A., Khoee S., Shiran M.-B., Sharifi A.M., Khoei S. Synergistic effects of magnetic drug targeting using a newly developed nanocapsule and tumor irradiation by ultrasound on CT26 tumors in BALB/c mice. J. Mater. Chem. B. 2015;3:1879–1887. doi: 10.1039/c4tb01708k. [DOI] [PubMed] [Google Scholar]
- 46.Hatakeyama H., Akita H., Harashima H. A multifunctional envelope type nano device (MEND) for gene delivery to tumours based on the EPR effect: a strategy for overcoming the PEG dilemma. Adv. Drug Deliv. Rev. 2011;63:152–160. doi: 10.1016/j.addr.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 47.Wilhelm S., Tavares A.J., Dai Q., Ohta S., Audet J., Dvorak H.F., Chan W.C.W. Analysis of nanoparticle delivery to tumours. Nat. Rev. Mater. 2016;1 doi: 10.1038/natrevmats.2016.14. [DOI] [Google Scholar]
- 48.Park J., Pei Y., Hyun H., Castanares M.A., Collins D.S., Yeo Y. Small molecule delivery to solid tumors with chitosan-coated PLGA particles: a lesson learned from comparative imaging. J. Contr. Release. 2017;268:407–415. doi: 10.1016/j.jconrel.2017.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Helmlinger G., Yuan F., Dellian M., Jain R.K. Interstitial pH and pO2 gradients in solid tumors in vivo: high-resolution measurements reveal a lack of correlation. Nat. Med. 1997;3:177–182. doi: 10.1038/nm0297-177. [DOI] [PubMed] [Google Scholar]
- 50.Ajani J.A., Takiuchi H. Recent developments in oral chemotherapy options for gastric carcinoma. Drugs. 1999;58:85–90. doi: 10.2165/00003495-199958003-00012. [DOI] [PubMed] [Google Scholar]
- 51.Feng S.S., Chien S. Chemotherapeutic engineering: application and further development of chemical engineering principles for chemotherapy of cancer and other diseases. Chem. Eng. Sci. 2003;58:4087–4114. doi: 10.1016/S0009-2509(03)00234-3. [DOI] [Google Scholar]
- 52.Bottomley A. The cancer patient and quality of life. Oncol. 2002;7:120–125. doi: 10.1634/theoncologist.7-2-120. [DOI] [PubMed] [Google Scholar]
- 53.DeMario M.D., Ratain M.J. Oral chemotherapy: rationale and future directions. J. Clin. Oncol. 1998;16:2557–2567. doi: 10.1200/jco.1998.16.7.2557. [DOI] [PubMed] [Google Scholar]
- 54.Shaikh J., Ankola D.D., Beniwal V., Singh D., Kumar M.N.V.R. Nanoparticle encapsulation improves oral bioavailability of curcumin by at least 9-fold when compared to curcumin administered with piperine as absorption enhancer. Eur. J. Pharmaceut. Sci. 2009;37:223–230. doi: 10.1016/j.ejps.2009.02.019. [DOI] [PubMed] [Google Scholar]
- 55.Miele D., Rossi S., Sandri G., Vigani B., Sorrenti M., Giunchedi P., Ferrari F., Bonferoni M.C. Chitosan oleate salt as an amphiphilic polymer for the surface modification of poly-lactic-glycolic acid (PLGA) nanoparticles. preliminary studies of mucoadhesion and cell interaction properties. Mar. Drugs. 2018;16:447. doi: 10.3390/md16110447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xie X., Tao Q., Zou Y., Zhang F., Guo M., Wang Y., Wang H., Zhou Q., Yu S. PLGA nanoparticles improve the oral bioavailability of curcumin in rats: characterizations and mechanisms. J. Agric. Food Chem. 2011;59:9280–9289. doi: 10.1021/jf202135j. [DOI] [PubMed] [Google Scholar]
- 57.Mu L., Feng S.S. Vitamin E TPGS used as emulsifier in the solvent evaporation/extraction technique for fabrication of polymeric nanospheres for controlled release of paclitaxel (Taxol) J. Contr. Release. 2002;80:129–144. doi: 10.1016/s0168-3659(02)00025-1. [DOI] [PubMed] [Google Scholar]
- 58.Rege B.D., Kao J.P.Y., Polli J.E. Effects of nonionic surfactants on membrane transporters in Caco-2 cell monolayers. Eur. J. Pharmaceut. Sci. 2002;16:237–246. doi: 10.1016/s0928-0987(02)00055-6. [DOI] [PubMed] [Google Scholar]
- 59.Win K.Y., Feng S.S. Effects of particle size and surface coating on cellular uptake of polymeric nanoparticles for oral delivery of anticancer drugs. Biomaterials. 2005;26:2713–2722. doi: 10.1016/j.biomaterials.2004.07.050. [DOI] [PubMed] [Google Scholar]
- 60.Choi J.S., Kim M.A., Lee H.E., Lee H.S., Kim W.H. Mucinous gastric carcinomas clinicopathologic and molecular analyses. Cancer. 2009;115:3581–U3581. doi: 10.1002/cncr.24422. [DOI] [PubMed] [Google Scholar]
- 61.Khanvilkar K., Donovan M.D., Flanagan D.R. Drug transfer through mucus. Adv. Drug Deliv. Rev. 2001;48:173–193. doi: 10.1016/s0169-409x(01)00115-6. [DOI] [PubMed] [Google Scholar]
- 62.Lai S.K., O'Hanlon D.E., Harrold S., Man S.T., Wang Y.Y., Cone R., Hanes J. Rapid transport of large polymeric nanoparticles in fresh undiluted human mucus. Proc. Natl. Acad. Sci. USA. 2007;104:1482–1487. doi: 10.1073/pnas.0608611104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lin D., Li G., Qin L., Wen Z., Wang J., Sun X. Preparation, characterization and uptake of PEG-coated, muco-inert nanoparticles in HGC-27 cells, a mucin-producing, gastric-cancer cell line. J. Biomed. Nanotechnol. 2013;9:2017–2023. doi: 10.1166/jbn.2013.1708. [DOI] [PubMed] [Google Scholar]
- 64.Ji T., Kohane D.S. Nanoscale systems for local drug delivery. Nano Today. 2019;28 doi: 10.1016/j.nantod.2019.100765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jena G.K., Patra C.N., Dixit P.K. Cytotoxicity and pharmacokinetic studies of PLGA based capecitabine loaded nanoparticles. Indian J. Pharm. Educ. 2020;54:349–356. doi: 10.5530/ijper.54.2.40. [DOI] [Google Scholar]
- 66.Begg A.C., Stewart F.A., Vens C. Genomic instability in cancer strategies to improve radiotherapy with targeted drugs. Nat. Rev. Cancer. 2011;11:239–253. doi: 10.1038/nrc3007. [DOI] [PubMed] [Google Scholar]
- 67.Delaney G., Jacob S., Featherstone C., Barton M. The role of radiotherapy in cancer treatment: estimating optimal utilization from a review of evidence-based clinical guidelines. Cancer. 2005;104:1129–1137. doi: 10.1002/cncr.21324. [DOI] [PubMed] [Google Scholar]
- 68.Bentzen S.M. Preventing or reducing late side effects of radiation therapy: radiobiology meets molecular pathology. Nat. Rev. Cancer. 2006;6:702–713. doi: 10.1038/nrc1950. [DOI] [PubMed] [Google Scholar]
- 69.Meljon A., Watson G.L., Wang Y., Shackleton C.H.L., Griffiths W.J. Analysis by liquid chromatography-mass spectrometry of sterols and oxysterols in brain of the newborn Dhcr7Δ3-5/T93M mouse: a model of Smith-Lemli-Opitz syndrome. Biochem. Pharmacol. 2013;86:43–55. doi: 10.1016/j.bcp.2013.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Windsor K., Genaro-Mattos T.C., Kim H.Y.H., Liu W., Tallman K.A., Miyamoto S., Korade Z., Porter N.A. Probing lipid-protein adduction with alkynyl surrogates: application to Smith-Lemli-Opitz syndrome. J. Lipid Res. 2013;54:2842–2850. doi: 10.1194/jlr.M041061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Xu L., Sheflin L.G., Porter N.A., Fliesler S.J. 7-Dehydrocholesterol-derived oxysterols and retinal degeneration in a rat model of Smith-Lemli-Opitz syndrome. BBA-Mol. Cell. Bio. L. 2012;1821:877–883. doi: 10.1016/j.bbalip.2012.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Xu L., Liu W., Sheflin L.G., Fliesler S.J., Porter N.A. Novel oxysterols observed in tissues and fluids of AY9944-treated rats: a model for Smith-Lemli-Opitz syndrome. J. Lipid Res. 2011;52:1810–1820. doi: 10.1194/jlr.M018366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Xu L., Korade Z., Rosado D.A., Jr., Liu W., Lamberson C.R., Porter N.A. An oxysterol biomarker for 7-dehydrocholesterol oxidation in cell/mouse models for Smith-Lemli-Opitz syndrome. J. Lipid Res. 2011;52:1222–1233. doi: 10.1194/jlr.M014498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Korade Z., Folkes O.M., Harrison F.E. Behavioral and serotonergic response changes in the Dhcr7-HET mouse model of Smith-Lemli-Opitz syndrome. Pharmacol., Biochem. Behav. 2013;106:101–108. doi: 10.1016/j.pbb.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 75.Delahunty I., Li J., Jiang W., Lee C., Yang X., Kumar A., Liu Z., Zhang W., Xie J. 7-Dehydrocholesterol encapsulated polymeric nanoparticles as a radiation-responsive sensitizer for enhancing radiation therapy. Small. 2022;18 doi: 10.1002/smll.202200710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sun F., Ju C., Chen J., Liu S., Liu N., Wang K., Liu C. Nanoparticles based on hydrophobic alginate derivative as nutraceutical delivery vehicle: vitamin D3 loading. Artif. Cell. Blood Sub. 2012;40:113–119. doi: 10.3109/10731199.2011.597759. [DOI] [PubMed] [Google Scholar]
- 77.Semenza G.L. Intratumoral hypoxia, radiation resistance, and HIF-1. Cancer Cell. 2004;5:405–406. doi: 10.1016/s1535-6108(04)00118-7. [DOI] [PubMed] [Google Scholar]
- 78.Li Y., Seto E. HDACs and HDAC inhibitors in cancer development and therapy. CSH Perspect. Med. 2016;6:a026831. doi: 10.1101/cshperspect.a026831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhang Z., Wang L., Ding Y., Wu J., Hu Y., Yuan A. Synergy of hypoxia relief and chromatin remodeling to overcome tumor radiation resistance. Biomater. Sci. 2020;8:4739–4749. doi: 10.1039/d0bm00119h. [DOI] [PubMed] [Google Scholar]
- 80.Zhang Y., Liu Q., Zhang X., Huang H., Tang S., Chai Y., Xu Z., Li M., Chen X., Liu J., Yang C. Recent advances in exosome-mediated nucleic acid delivery for cancer therapy. J. Nanobiotechnol. 2022;20:279. doi: 10.1186/s12951-022-01472-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Qu Y., Chen L., Lowe D.B., Storkus W.J., Taylor J.L. Mol. Ther. 2012;20:644–651. doi: 10.1038/mt.2011.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Guo B., Huang Z., Shi Q., Middha E., Xu S., Li L., Wu M., Jiang J., Hu Q., Fu Z., Liu B. Organic small molecule based photothermal agents with molecular rotors for malignant breast cancer therapy. Adv. Funct. Mater. 2020;30 doi: 10.1002/adfm.201907093. [DOI] [Google Scholar]
- 83.Murciano-Goroff Y.R., Warner A.B., Wolchok J.D. The future of cancer immunotherapy: microenvironment-targeting combinations. Cell Res. 2020;30:507–519. doi: 10.1038/s41422-020-0337-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hu L., Cao Z., Ma L., Liu Z., Liao G., Wang J., Shen S., Li D., Yang X. The potentiated checkpoint blockade immunotherapy by ROS-responsive nanocarrier-mediated cascade chemo-photodynamic therapy. Biomaterials. 2019;223 doi: 10.1016/j.biomaterials.2019.119469. [DOI] [PubMed] [Google Scholar]
- 85.Wen Y., Chen X., Zhu X., Gong Y., Yuan G., Qin X., Liu J. Photothermal-chemotherapy integrated nanoparticles with tumor microenvironment response enhanced the induction of immunogenic cell death for colorectal cancer efficient treatment. ACS Appl. Mater. Interfaces. 2019;11:43393–43408. doi: 10.1021/acsami.9b17137. [DOI] [PubMed] [Google Scholar]
- 86.Liu Q., Sun Y., Yin X., Li J., Xie J., Xie M., Wang K., Wu S., Li Y., Hussain M., Jiang B., Liu Y., Huang C., Tao J., Zhu J. Hyaluronidase-functionalized silica nanocarrier for enhanced chemo-immunotherapy through inducing immunogenic cell death. ACS Appl. Bio Mater. 2020;3:3378–3389. doi: 10.1021/acsabm.0c00299. [DOI] [PubMed] [Google Scholar]
- 87.Chan J.M., Zhang L., Yuet K.P., Liao G., Rhee J.W., Langer R., Farokhzad O.C. PLGA-lecithin-PEG core-shell nanoparticles for controlled drug delivery. Biomaterials. 2009;30:1627–1634. doi: 10.1016/j.biomaterials.2008.12.013. [DOI] [PubMed] [Google Scholar]
- 88.Zheng M., Yue C., Ma Y., Gong P., Zhao P., Zheng C., Sheng Z., Zhang P., Wang Z., Cai L. Single-step assembly of DOX/ICG loaded lipid-polymer nanoparticles for highly effective chemo-photothermal combination therapy. ACS Nano. 2013;7:2056–2067. doi: 10.1021/nn400334y. [DOI] [PubMed] [Google Scholar]
- 89.Chang H.N., Liu B.Y., Qi Y.K., Zhou Y., Chen Y.P., Pan K.M., Li W.W., Zhou X.M., Ma W.W., Fu C.Y., Qi Y.M., Liu L., Gao Y.F. Blocking of the PD-1/PD-L1 interaction by a D-peptide antagonist for cancer immunotherapy. Angew. Chem. Int. Ed. 2015;54:11760–11764. doi: 10.1002/anie.201506225. [DOI] [PubMed] [Google Scholar]
- 90.Zhang N., Li J., Gao W., Zhu W., Yan J., He Z., Li L., Wu F., Pu Y., He B. Co-delivery of doxorubicin and anti-PD-L1 peptide in lipid/PLGA nanocomplexes for the chemo-Immunotherapy of cancer. Mol. Pharm. 2022;19:3439–3449. doi: 10.1021/acs.molpharmaceut.2c00611. [DOI] [PubMed] [Google Scholar]
- 91.Lee C.K., Atibalentja D.F., Yao L.E., Park J., Kuruvilla S., Felsher D.W. Anti-PD-L1 F(ab) conjugated PEG-PLGA nanoparticle enhances immune checkpoint therapy. Nanotheranostics. 2022;6:243–255. doi: 10.7150/ntno.65544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Angela Aznar M., Tinari N., Rullan A.J., Sanchez-Paulete A.R., Rodriguez-Ruiz M.E., Melero I. Intratumoral delivery of immunotherapy-act locally, think globally. J. Immunol. 2017;198:31–39. doi: 10.4049/jimmunol.1601145. [DOI] [PubMed] [Google Scholar]
- 93.Kwon S., Meng F., Tamam H., Gadalla H.H., Wang J., Dong B., Hopf Jannasch A.S., Ratliff T.L., Yeo Y. Systemic delivery of paclitaxel by Find-Me nanoparticles activates antitumor immunity and eliminates tumors. ACS Nano. 2024;18:3681–3698. doi: 10.1021/acsnano.3c11445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Riley R.S., June C.H., Langer R., Mitchell M.J. Delivery technologies for cancer immunotherapy. Nat. Rev. Drug Discov. 2019;18:175–196. doi: 10.1038/s41573-018-0006-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ribas A., Wolchok J.D. Cancer immunotherapy using checkpoint blockade. Science. 2018;359:1350–1355. doi: 10.1126/science.aar4060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Huber V., Camisaschi C., Berzi A., Ferro S., Lugini L., Triulzi T., Tuccitto A., Tagliabue E., Castelli C., Rivoltini L. Cancer acidity: an ultimate frontier of tumor immune escape and a novel target of immunomodulation. Semin. Cancer Biol. 2017;43:74–89. doi: 10.1016/j.semcancer.2017.03.001. [DOI] [PubMed] [Google Scholar]
- 97.Fischer K., Hoffmann P., Voelkl S., Meidenbauer N., Ammer J., Edinger M., Gottfried E., Schwarz S., Rothe G., Hoves S., Renner K., Timischl B., Mackensen A., Schughart L.K., Andreesen R., Krause S.W., Kreutz M. Inhibitory effect of tumor cell-derived lactic acid on human T cells. Blood. 2007;109:3812–3819. doi: 10.1182/blood-2006-07-035972. [DOI] [PubMed] [Google Scholar]
- 98.Lardner A. The effects of extracellular pH on immune function. J. Leukoc. Biol. 2001;69:522–530. doi: 10.1189/jlb.69.4.522. [DOI] [PubMed] [Google Scholar]
- 99.Hao Y., Chen M., Wu Y., Dong Z., Zhu Y., Wang C., Li Q., Yang Z., Liu Z., Feng L. CaCO3 based proton nanosponge to potentiate immune checkpoint blockade therapy by synergistically reversing tumor immunosuppression. Chem. Eng. J. 2023;462 doi: 10.1016/j.cej.2023.142206. [DOI] [Google Scholar]
- 100.Donlon N.E., Power R., Hayes C., Reynolds J.V., Lysaght J. Radiotherapy, immunotherapy, and the tumour microenvironment: turning an immunosuppressive milieu into a therapeutic opportunity. Cancer Lett. 2021;502:84–96. doi: 10.1016/j.canlet.2020.12.045. [DOI] [PubMed] [Google Scholar]
- 101.Miller R.L., Gerster J.F., Owens M.L., Slade H.B., Tomai M.A. Imiquimod applied topically: a novel immune response modifier and new class of drug. Int. J. Immunopharm. 1999;21:1–14. doi: 10.1016/s0192-0561(98)00068-x. [DOI] [PubMed] [Google Scholar]
- 102.Walshaw R.C., Honeychurch J., Choudhury A., Illidge T.M. Toll-like receptor agonists and radiation therapy combinations: an untapped opportunity to induce anticancer immunity and improve tumor control. Int. J. Radiat. Oncol. 2020;108:27–37. doi: 10.1016/j.ijrobp.2020.04.020. [DOI] [PubMed] [Google Scholar]
- 103.Lu D., Xu Y., Yang X., Li Y., Li M., Zheng Y., Wang Y., Wang W., Wang S., Gao J., Liu Y. Cancer Nanotechnol. 2023;14:39. doi: 10.1186/s12645-023-00193-8. [DOI] [Google Scholar]
- 104.LaVan D.A., McGuire T., Langer R. Small-scale systems for in vivo drug delivery. Nat. Biotechnol. 1998;392:25–30. doi: 10.1038/nbt876. [DOI] [PubMed] [Google Scholar]
- 105.Matsumoto K., Ishikawa H., Nishimura D., Hamasaki K., Nakao K., Eguchi K. Antiangiogenic property of pigment epithelium-derived factor in hepatocellular carcinoma. Hepatology. 2004;40:252–259. doi: 10.1002/hep.20259. [DOI] [PubMed] [Google Scholar]
- 106.Doll J.A., Stellmach V.M., Bouck N.P., Bergh A.R.J., Lee C., Abramson L.P., Cornwell M.L., Pins M.R., Borensztajn J., Crawford S.E. Pigment epithelium-derived factor regulates the vasculature and mass of the prostate and pancreas. Nat. Med. 2003;9:774–780. doi: 10.1038/nm870. [DOI] [PubMed] [Google Scholar]
- 107.Abe R., Shimizu T., Yamagishi S., Shibaki A., Amano S., Inagaki Y., Watanabe H., Sugawara H., Nakamura H., Takeuchi M., Imaizumi T., Shimizu H. Overexpression of pigment epithelium-derived factor decreases angiogenesis and inhibits the growth of human malignant melanoma cells in vivo. Am. J. Pathol. 2004;164:1225–1232. doi: 10.1016/s0002-9440(10)63210-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Yang L.P., Cheng P., Peng X.C., Shi H.S., He W.H., Cui F.Y., Luo S.T., Wei Y.Q., Yang L. Anti-tumor effect of adenovirus-mediated gene transfer of pigment epithelium-derived factor on mouse B16-F10 melanoma. J. Exp. Clin. Cancer Res. 2009;28:75. doi: 10.1186/1756-9966-28-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Dawson D.W., Volpert O.V., Gillis P., Crawford S.E., Xu H., Benedict W., Bouck N.P. Pigment epithelium-derived factor: a potent inhibitor of angiogenesis. Science. 1999;285:245–248. doi: 10.1126/science.285.5425.245. [DOI] [PubMed] [Google Scholar]
- 110.Cui F.Y., Song X.R., Li Z.Y., Li S.Z., Mu B., Mao Y.Q., Wei Y.Q., Yang L. The pigment epithelial-derived factor gene loaded in PLGA nanoparticles for therapy of colon carcinoma. Oncol. Rep. 2010;24:661–668. doi: 10.3892/or_00000905. [DOI] [PubMed] [Google Scholar]
- 111.Kwak S.Y., Lee S., Han H.D., Chang S., Kim K.P., Ahn H.J. PLGA nanoparticles codelivering siRNAs against programmed cell death protein-1 and its ligand gene for suppression of colon tumor growth. Mol. Pharm. 2019;16:4940–4953. doi: 10.1021/acs.molpharmaceut.9b00826. [DOI] [PubMed] [Google Scholar]
- 112.Han K., Chen S., Chen W.H., Lei Q., Liu Y., Zhuo R.X., Zhang X.Z. Synergistic gene and drug tumor therapy using a chimeric peptide. Biomaterials. 2013;34:4680–4689. doi: 10.1016/j.biomaterials.2013.03.010. [DOI] [PubMed] [Google Scholar]
- 113.Xu B., Xia S., Wang F., Jin Q., Yui T., He L., Chen Y., Liu Y., Li S., Tan X., Ren K., Yao S., Zeng J., Song X. Polymeric nanomedicine for combined gene/chemotherapy elicits enhanced tumor suppression. Mol. Pharm. 2016;13:663–676. doi: 10.1021/acs.molpharmaceut.5b00922. [DOI] [PubMed] [Google Scholar]
- 114.Xu B., Jin Q., Zeng J., Yu T., Chen Y., Li S., Gong D., He L., Tan X., Yang L., He G., Wu J., Song X. Combined tumor- and neovascular-"Dual Targeting" gene/chemo-therapy suppresses tumor growth and angiogenesis. ACS Appl. Mater. Interfaces. 2016;8:25753–25769. doi: 10.1021/acsami.6b08603. [DOI] [PubMed] [Google Scholar]
- 115.Liu Y., Bhattarai P., Dai Z., Chen X. Photothermal therapy and photoacoustic imaging via nanotheranostics in fighting cancer. Chem. Soc. Rev. 2019;48:2053–2108. doi: 10.1039/c8cs00618k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Peng J., Xiao Y., Li W., Yang Q., Tan L., Jia Y., Qian Z. Photosensitizer micelles together with Ido inhibitor enhance cancer photothermal therapy and immunotherapy. Adv. Sci. 2018;5 doi: 10.1002/advs.201700891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Sun X., Wang C., Gao M., Hu A., Liu Z. Remotely controlled red blood cell carriers for cancer targeting and near-infrared light-triggered drug release in combined photothermal-chemotherapy. Adv. Funct. Mater. 2015;25:2386–2394. doi: 10.1002/adfm.201500061. [DOI] [Google Scholar]
- 118.Li W., Li B., Wu B., Tian B., Chen X., Wang C., Hong W., Peng J. Free-radical cascade generated by AIPH/Fe3O4-coloaded nanoparticles enhances MRI-guided chemo/thermodynamic hypoxic tumor therapy. ACS Appl. Mater. Interfaces. 2022;14:29563–29576. doi: 10.1021/acsami.2c05748. [DOI] [PubMed] [Google Scholar]
- 119.Ibarra J., Basurto D.E., Almada M., Juarez J., Valdez M.A., Barbosa S., Taboada P. Gold half-shell-coated paclitaxel-loaded plga nanoparticles for the targeted chemo-photothermal treatment of cancer. Micromachines. 2023;14 doi: 10.3390/mi14071390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Banstola A., Pham T.T., Jeong J.H., Yook S. Polydopamine-tailored paclitaxel-loaded polymeric microspheres with adhered NIR-controllable gold nanoparticles for chemo-phototherapy of pancreatic cancer. Drug Deliv. 2019;26:629–640. doi: 10.1080/10717544.2019.1628118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Clement S., Anwer A.G., Pires L., Campbell J., Wilson B.C., Goldys E.M. Radiodynamic therapy using TAT peptide-targeted verteporfin-encapsulated PLGA nanoparticles. Int. J. Mol. Sci. 2021;22 doi: 10.3390/ijms22126425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Guo Z., Liu Y., He N., Deng Y., Jin L. Discussion of the protein characterization techniques used in the identification of membrane protein targets corresponding to tumor cell aptamers. Chin. Chem. Lett. 2021;32:40–47. doi: 10.1016/j.cclet.2020.11.061. [DOI] [Google Scholar]
- 123.Liu M., Xi L., Tan T., Jin L., Wang Z., He N. A novel aptamer-based histochemistry assay for specific diagnosis of clinical breast cancer tissues. Chin. Chem. Lett. 2021;32:1726–1730. doi: 10.1016/j.cclet.2020.11.072. [DOI] [Google Scholar]
- 124.Sekiguchi M., Oda I., Matsuda T., Saito Y. Epidemiological trends and future perspectives of gastric cancer in eastern Asia. Digestion. 2022;103:22–28. doi: 10.1159/000518483. [DOI] [PubMed] [Google Scholar]
- 125.Barberio M., Pizzicannella M., Spota A., Ashoka A.H., Agnus V., Taher M.A., Winkeln B.J., Gockel I., Marescaux J., Swanstrom L., Kong S.H., Felli E., Klymchenko A., Diana M. Preoperative endoscopic marking of the gastrointestinal tract using fluorescence imaging: submucosal indocyanine green tattooing versus a novel fluorescent over-the-scope clip in a survival experimental study. Surg. Endosc. 2021;35:5115–5123. doi: 10.1007/s00464-020-07999-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Stibbe J.A., Hoogland P., Achterberg F.B., Holman D.R., Sojwal R.S., Burggraaf J., Vahrmeijer A.L., Nagengast W.B., Rogalla S. Highlighting the undetectable-fluorescence molecular imaging in gastrointestinal endoscopy. Mol. Imag. Biol. 2023;25:18–35. doi: 10.1007/s11307-022-01741-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Yang Y., Zhang J., Xia F., Zhang C., Qian Q., Zhi X., Yue C., Sun R., Cheng S., Fang S., Jin W., Yang Y., Cui D. Human CIK cells loaded with Au nanorods as a theranostic platform for targeted photoacoustic imaging and enhanced immunotherapy and photothermal therapy. Nanoscale Res. Lett. 2016;11:285. doi: 10.1186/s11671-016-1468-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Wang R., Zou L., Yi Z., Zhang Z., Zhao M., Shi S. PLGA nanoparticles loaded with curcumin produced luminescence for cell bioimaging. Int. J. Pharm. 2023;639 doi: 10.1016/j.ijpharm.2023.122944. [DOI] [PubMed] [Google Scholar]
- 129.Schleich N., Sibret P., Danhier P., Ucakar B., Laurent S., Muller R.N., Jérôme C., Gallez B., Préat V., Danhier F. Dual anticancer drug/superparamagnetic iron oxide-loaded PLGA-based nanoparticles for cancer therapy and magnetic resonance imaging. Int. J. Pharm. 2013;447:94–101. doi: 10.1016/j.ijpharm.2013.02.042. [DOI] [PubMed] [Google Scholar]
- 130.Abdelkader H., Wertheim D., Pierscionek B., Alany R.G. Curcumin in situ gelling polymeric insert with enhanced ocular performance. Pharmaceutics. 2020;12:1158. doi: 10.3390/pharmaceutics12121158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Dwivedi P., Yuan S., Han S., Mangrio F.A., Zhu Z., Lei F., Ming Z., Cheng L., Liu Z., Si T., Xu R.X. Core-shell microencapsulation of curcumin in PLGA microparticles: programmed for application in ovarian cancer therapy. Artif. Cell. Nanomed. B. 2018;46:S481–S491. doi: 10.1080/21691401.2018.1499664. [DOI] [PubMed] [Google Scholar]
- 132.Wang X., Gu H., Zhang H., Xian J., Li J., Fu C., Zhang C., Zhang J. Oral core-shell nanoparticles embedded in hydrogel microspheres for the efficient site-specific delivery of magnolol and enhanced antiulcerative colitis therapy. ACS Appl. Mater. Interfaces. 2021;13:33948–33961. doi: 10.1021/acsami.1c09804. [DOI] [PubMed] [Google Scholar]
- 133.Alsaab H.O., Alharbi F.D., Alhibs A.S., Alanazi N.B., Alshehri B.Y., Saleh M.A., Alshehri F.S., Algarni M.A., Almugaiteeb T., Uddin M.N., Alzhrani R.M. PLGA-based nanomedicine: history of advancement and development in clinical applications of multiple diseases. Pharmaceutics. 2022;14 doi: 10.3390/pharmaceutics14122728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Vrignaud S., Benoit J.P., Saulnier P. Strategies for the nanoencapsulation of hydrophilic molecules in polymer-based nanoparticles. Biomaterials. 2011;32:8593–8604. doi: 10.1016/j.biomaterials.2011.07.057. [DOI] [PubMed] [Google Scholar]
- 135.Yeo Y., Park K.N. Control of encapsulation efficiency and initial burst in polymeric microparticle systems. Arch Pharm. Res. (Seoul) 2004;27:1–12. doi: 10.1007/bf02980037. [DOI] [PubMed] [Google Scholar]
- 136.Ye M., Kim S., Park K. Issues in long-term protein delivery using biodegradable microparticles. J. Contr. Release. 2010;146:241–260. doi: 10.1016/j.jconrel.2010.05.011. [DOI] [PubMed] [Google Scholar]
- 137.McGinity J.W., O'Donnell P.B. Preparation of microspheres by the solvent evaporation technique. Adv. Drug Deliv. Rev. 1997;28:25–42. doi: 10.1016/s0169-409x(97)00049-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This is a review, and all of the data come from references.