Abstract
Simian virus 40 small t antigen (st) is required for optimal transformation and replication properties of the virus. We find that in certain cell types, such as the human osteosarcoma cell line U2OS, st is capable of inducing apoptosis, as evidenced by a fragmented nuclear morphology and positive terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling staining of transfected cells. The cell death can be p53 independent, since it also occurs in p53-deficient H1299 cells. Genetic analysis indicates that two specific mutants affect apoptosis induction. One of these (C103S) has been frequently used as a PP2A binding mutant. The second mutant (TR4) lacks the final four amino acids of st, which have been reported to be unimportant for PP2A binding in vitro. However, TR4 unexpectedly fails to bind PP2A in vivo. Furthermore, a long-term colony assay reveals a potent colony inhibition upon st expression, and the behavior of st mutants in this assay reflects the relative frequency of nuclear fragmentation observed in transfections using the same mutants. Notably, either Bcl-2 coexpression or broad caspase inhibitor treatment could restore normal nuclear morphology. Finally, fluorescence-activated cell sorting analysis suggests a correlation between the ability of st to modulate cell cycle progression and apoptosis. Taken together, these observations underscore that st does not always promote proliferation but may, depending on conditions and cell type, effect a cell death response.
The early region of simian virus 40 (SV40) encodes three gene products: large T (LT) antigen, 17k, and small t antigen (st). Because of the splicing arrangement, LT and st share the amino-terminal 82 amino acids encoded within the first exon (referred to as the T/t common region). Previous work has demonstrated that this segment encompasses a bona fide DnaJ domain capable of binding Hsc70 (11, 74, 75; W. L. Kelley and S. J. Landry, Letter, Trends Biochem. Sci. 19:277–278, 1994). The DnaJ domain is important for several functions of cell growth regulation as well as viral replication (11, 74, 75, 88). LT and 17k share a region responsible for interactions with the pRB tumor suppressor protein and related family members, but they differ in their carboxyl termini. st contains at its carboxy terminus an additional 92 unique amino acids coded for in a portion of the early transcript spliced out of the LT and 17k messages.
While the major transforming protein of SV40 is LT (reviewed in reference 37), st also contributes. The function of st appears to be auxiliary in both transformation and viral replication, often apparent only under limiting conditions (6, 7, 41, 60, 70, 76). In certain cell types, such as primary human diploid fibroblasts, focus formation requires st as well as LT (55, 61). Furthermore, anchorage-independent growth in certain cell lines also depends on both st and LT (31, 48). One activity commonly attributed to st is the induction of cell cycle progression in otherwise quiescent cells (16, 28, 29, 72). Transgenic animal models also suggest a requirement for st in tumor formation within certain nondividing tissues; LT is not sufficient (15). In addition, st can complement LT for transformation when the latter is expressed at low levels (7). Notably, in the context of the viral life cycle, st promotion of cell cycling is likely to benefit viral replication, since efficient replication takes place only when the host cell is in S phase (16, 78).
The deregulation of the cell cycle caused by st appears complex. So far the only known cellular target besides Hsc70 is protein phosphatase 2A (PP2A) (52). Although the association appears to be stoichiometric in nature, it is unclear if this interaction serves only to inhibit PP2A activity, as suggested by in vitro experiments, or whether st also redirects PP2A to a different set of substrates (52, 86). Notably, the interaction with and inhibition of PP2A affects signal transduction pathways. It was demonstrated that the binding of st to PP2A in CV-1 cells causes activation of the MEK and ERK family kinases with concomitant stimulation of cell growth (72). In addition, st can activate other kinases, such as Jun N-terminal kinase (JNK) and protein kinase Cζ (PKCζ); activation of the latter was reported to enhance NF-κB activity (73). The activation of PKCζ and NF-κB appears to depend somehow on phosphatidylinositol 3-kinase, since specific inhibitors of this pathway, such as wortmannin, LY294002, and dominant-negative p85α, can block it (73). In light of these effects of st on signal transduction pathways, it is perhaps not surprising that st is also a rather promiscuous modulator of transcription. While st is reported to transcriptionally activate most of its target promoters, such as cyclin D1 (83), cyclin A (55), adenovirus E2 (48), and c-fos (47), it can also, albeit less often, repress transcription. In one report, Wang et al. demonstrated that st represses c-fos- and AP-1-dependent transcription in CV1-P cells (81), whereas it was previously reported that in CV-1 cells AP-1 transcription is activated by st (25). These seemingly contradictory observations are likely to be attributable to cell type variations in the st response.
While the transcriptional induction of the cyclin D1 promoter requires binding of st to PP2A (83), the corresponding induction of cyclin A or adenovirus E2 promoter activity depends instead on a functional J domain, as suggested by the fact that a double mutation at residues 43 and 45 disrupts this function (48, 55). Experiments in which st expression is accomplished via recombinant adenovirus infection demonstrate a clear up-regulation of cellular cyclin A mRNA and protein levels (55, 56). In addition, it was observed that the cyclin-dependent kinase (cdk) inhibitor p27KIP1 was down-regulated by induced protein degradation (56). For SV40 st, it is not yet clear which function is required for this; however, in the case of the related polyomavirus st, p27KIP1 degradation correlated with PP2A binding (66). Since LT down-regulates the related cdk inhibitor p21CIP1, this may well explain why the concerted action of st and LT is required for the observed increase in cyclin A and cdk2 activity upon their coexpression (56). st may also influence the p53 growth-suppressive pathway, since it can complement a pRB binding mutant (K1) of LT for override of a p53-induced growth arrest in the T64-7B cell line (27).
Although only two functions of st have been identified so far, more are likely to be unraveled. Evidence from the related polyomavirus st points to the possible existence of other SV40 st functions (40). In fact, it was originally reported that stable lines expressing polyomavirus st could not be established due to an uncharacterized cytotoxicity of st (5, 40). In contrast to the positive, stimulatory role st plays in many aspects of cell proliferation, we find that in certain cell types, such as the human lines U2OS and H1299, st causes cell death by apoptosis. The induction of cell death is p53 independent and depends on PP2A binding, as demonstrated by two different types of binding mutants. Cell death is manifested both in the disrupted nuclear morphology and in positive terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling (TUNEL) staining of st-expressing cells as well as potent colony inhibition in a long-term assay for puromycin-selected cells. Consistent with death by apoptosis, we find that either Bcl-2 or the broad caspase inhibitor z-VAD-FMK partially protects against cell death. Hence, st not only positively affects cell proliferation but can induce cell death in select cell types and perhaps under certain cellular conditions.
MATERIALS AND METHODS
Cells and transfections.
The human osteosarcoma cell line U2OS and the human lung carcinoma cell line H1299 were grown in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal calf serum (FCS) (JRH Biosciences) and penicillin-streptomycin at 37°C under 5% CO2. The monkey kidney cell line CV1-P was cultured under identical conditions. Cells were transfected by using Fugene-6 according to the manufacturer's instructions (Roche). For the colony inhibition assays, 5 μg of pLPCX (Clontech) vector or 5 μg of pLPCX expressing the wild-type or mutant st was used. Briefly, 235 μl of DMEM (without penicillin, streptomycin, or serum) was incubated with 15 μl of Fugene-6 reagent for 5 min. Subsequently, all of this mixture was transferred to another tube with 5 μg of plasmid DNA, mixed, and incubated for another 15 min. Finally, this mixture was added dropwise to cells on 6-cm-diameter tissue culture dishes. Approximately 24 h later, these cells were trypsinized and transferred into a 10-cm-diameter dish. After another 24 h, selection was initiated with 1.5 μg of puromycin/ml. After 2 to 3 weeks the colonies were visualized by staining with 0.5% crystal violet.
Some of the flow cytometry experiments employed BES (N,N-bis[2-hydroxyethyl]-2-aminoethanesulfonic acid, sodium salt)-buffered calcium phosphate transfection, which was performed according to previously detailed protocols (13, 27).
Plasmids and mutagenesis.
Transfections for apoptosis assays such as TUNEL and 4′,6′-diamidino-2-phenylindole (DAPI) staining were conducted with st constructs or mutants thereof expressed from the SV40 early promoter in pSG5 (Stratagene). All the st constructs are cDNA versions obtained by PCR in order to contain only the minimal st coding sequence and cloned into pSG5 with BamHI sites on either end. The design of the st expression vector ensures that there are no spurious splice products deriving from downstream LT splice acceptor sites. For construction of the TR4 mutant, a downstream oligonucleotide was designed which causes the PCR product to lack coding for the final four amino acids. In all cases in which PCR was involved, the entire st cDNA was verified by automated sequencing. The mutants D44N, C103S, and C103S/TR4 were all derived by QuikChange mutagenesis following guidelines of the manufacturer (Stratagene). Mutations were confirmed by sequencing. For colony assays, st wild-type or mutant cDNAs were cloned into the BglII site of pLPCX (Clontech), which carries a puromycin resistance gene. The st cDNA was also cloned into the BamHI site of pIRES2-EGFP (Clontech) to generate pIRES2-EGFP st, thus allowing st to be expressed bicistronically with enhanced green fluorescent protein (EGFP). The pCMV Bcl-2 and pcDNA3 baculovirus p35 expression vectors have been previously described (54). Furthermore, the LT and 17k expression vectors pCMV-TAg and pSG5 17k have been previously described (11, 27).
Western blots.
U2OS cells transiently transfected with 10 μg of pSG5 st or mutants thereof by the Fugene-6 method on 10-cm-diameter dishes were washed once and then collected in phosphate-buffered saline (PBS) at 48 h posttransfection. Subsequently, extracts were prepared in T antigen extraction buffer (TEB) (50 mM Tris [pH 7.5], 150 mM NaCl, 1.0% Nonidet P-40, 2 mM EDTA, 5 μg of leupeptin/ml, 5 μg of pepstatin/ml, 0.5 mM phenylmethylsulfonyl fluoride) for 15 min on ice. The lysates was obtained by spinning out the debris in a microcentrifuge at 10,000 × g for 10 min at 4°C. Finally, the lysates were boiled with an equal volume of 2× sodium dodecyl sulfate (SDS) sample buffer (5% SDS, 25% glycerol, 62.5 mM Tris [pH 6.8], 0.0075% bromophenol blue, 0.7 M β-mercaptoethanol) for 4 min. Approximately 100 μg of each lysate was loaded on an SDS–12% polyacrylamide gel electrophoresis (SDS–12% PAGE) system. After resolution by SDS-PAGE, the proteins were transferred by Western blotting to a nitrocellulose membrane, which was probed overnight with a PAb419 tissue culture hybridoma supernatant diluted 1:10. After appropriate washes and incubation with a secondary anti-mouse, horseradish peroxidase-linked antibody, the signal was visualized by enhanced chemiluminescence according to the protocol provided by the supplier (Amersham-Pharmacia).
[35S]methionine labeling and immunoprecipitation.
Transiently transfected U2OS cells on 10-cm-diameter dishes were washed twice with DMEM minus methionine (Gibco Life Sciences) and then incubated in this medium for 30 min. A 2-ml mixture of DMEM minus methionine, 2% dialyzed FCS (Gibco Life Sciences), and 1 mCi of [35S]methionine Express label (New England Nuclear) was added to each dish. Metabolic labeling proceeded for 4 h in a 37°C incubator with 5% CO2. The cells were washed twice with PBS and then extracted in the dish with 1 ml of TEB for 15 min on ice. Lysates were obtained after centrifugation at 10,000 × g for 10 min at 4°C to remove cell debris. For immunoprecipitation, 2 μl of PAb430 ascites fluid (kindly provided by M. Imperiale, University of Michigan Medical School) was added to the lysate, and mixing at 4°C proceeded for 2 h, at which point 30 μl of protein A-Sepharose (Pharmacia) was added for incubation for another 45 min. The immunoprecipitates were washed three times with TEB, and finally the beads were eluted by boiling in 2× SDS sample buffer for 4 min. Radiolabeled, immunoprecipitated proteins were analyzed by SDS-PAGE on a 12% gel.
Immunofluorescence/TUNEL.
Cells were seeded on either coverslips coated with poly-l-lysine (Sigma) or Labtek Chamberslides II (Nunc) and transfected with Fugene-6 reagent. After 24 or 48 h, cells were fixed in 4% paraformaldehyde (catalog no. 15710; EM Biosciences) for 20 min at 4°C. The cells were then gently washed twice with PBS and permeabilized with 0.2% Triton X-100 for 5 min. Subsequent to two PBS washes, TUNEL staining was carried out with the Promega kit (Apoptosis Detection System, fluorescein, catalog no. G3250) according to the manufacturer's protocol. After TUNEL staining, the cells were processed for immunofluorescence staining for st. Blocking of nonspecific binding with 10% goat serum in PBS was carried out for 20 min. Cells were then incubated for 1 h with PAb419 monoclonal anti-T/t (recognizes an epitope within the 82-amino-acid common region between st and LT) hybridoma supernatant diluted 1:5 in PBS with 1% goat serum. After three washes in PBS, secondary anti-mouse Cy3-conjugated antibody (Jackson Sciences) at 1:1,000 was applied for 30 min. Finally, the cells were stained with DAPI (Sigma) at a concentration of 1 μg/ml and mounted with Fisher Scientific mounting medium. Microscopy was carried out on a Nikon Eclipse TE300 fluorescence microscope setup, where cell pictures can be captured with a digital camera using a Uniblitz shutter and the Metamorph program.
For protection experiments, the cells were treated with the broad caspase inhibitor z-VAD-Fmk (Calbiochem) (50 μM) or the specific MEK inhibitor U0126 (Promega) (10 μM) overnight and compared to vehicle (dimethyl sulfoxide)-treated cells.
FACS.
Dual-color fluorescence-activated cell sorting (FACS) was performed to determine the cell cycle profile of the transfected cell population. Briefly, the H1299 cells on 10-cm-diameter dishes were transfected with 2 μg of pCMV CD20 and 8 μg of pSG5 st expression vector by using Fugene-6. The cells were detached at 72 h posttransfection (at this time point the cycling of H1299 cells was minimal and the cell cycle effects of st were maximized) with 0.1% EDTA in PBS and then incubated with 20 μl of anti-CD20–fluorescein isothiocyanate conjugate (BD PharMingen) for 30 min on ice. Subsequently, the cells were washed in PBS containing 1% FCS and permeabilized in 70% ethanol for a minimum of 30 min on ice. Finally, the cells were washed again with PBS–1% FCS and stained for 30 min at 37°C with 50 μg of propidium iodide/ml in the presence of 10 μg of RNase A/ml. Cell cycle profiles were obtained on a Becton Dickinson flow cytometer using FACScan software. A minimum of 10,000 CD20- and FITC-positive cells were gated for calculation of cell cycle distribution. The relative proportion of cells in G0/G1, S, and G2/M was estimated using the program ModFitLT.
RESULTS
Induction of apoptosis by SV40 st but not LT in U2OS cells.
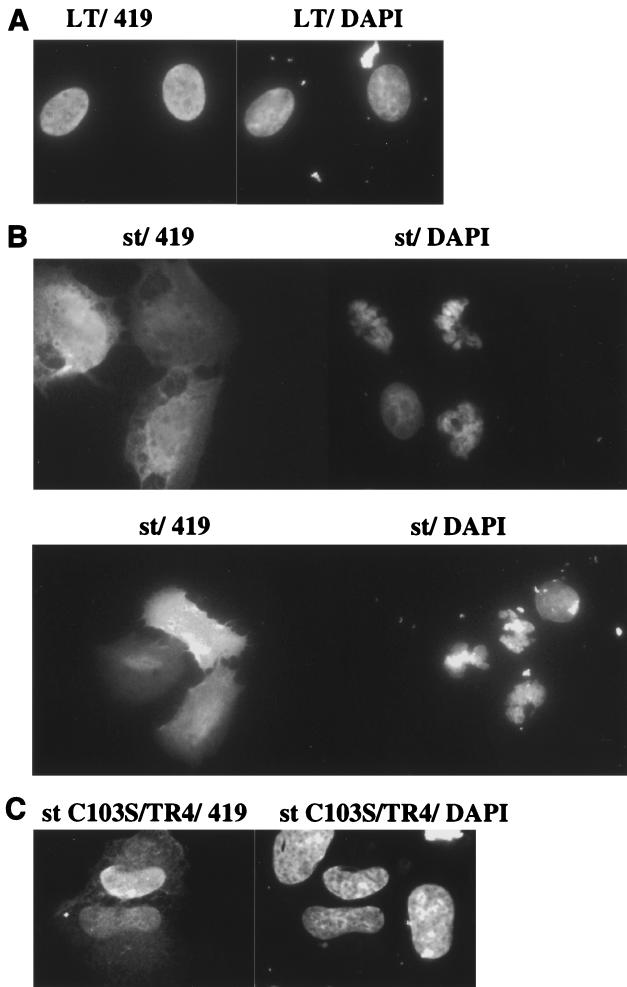
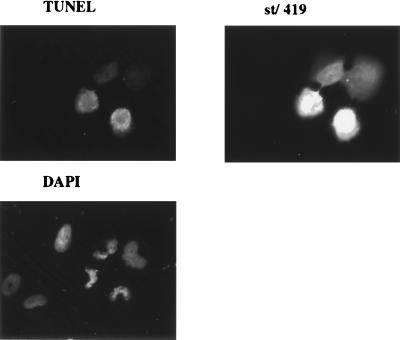
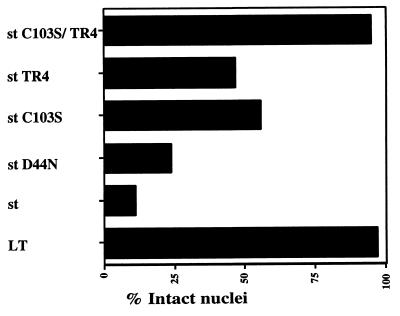
Several lines of past experimental evidence have documented the important helper function for st in both replication and transformation mediated by SV40. Given the duality of many viral oncoproteins in contributing both positively and negatively to cell survival, we set out to investigate if st might have proapoptotic activities under certain conditions (see Fig. 1 for a schematic drawing of st functions). In order to examine signs of cell death, we transiently transfected the human osteosarcoma cell line U2OS and studied the nuclear morphology of expressing cells using DAPI staining of the DNA. Although LT in some cell systems reportedly can induce apoptosis (14, 18, 33), we found that U2OS cells were refractory to apoptosis by LT, and LT therefore served as a suitable negative control. We identified transfected cells by immunofluorescence using the PAb419 antibody, which recognizes an epitope contained within the first exon of LT; hence the same antibody was used for st cell staining. The vast majority of LT-expressing cells had intact nuclear morphology as visualized by DAPI staining (Fig. 2A). In striking contrast, we found that the st-expressing U2OS cells stained with DAPI displayed the typical features of apoptotic cells, such as chromatin condensation, extensive rounding, membrane blebbing, and, most importantly, nuclear fragmentation (Fig. 2B). Cell death could be detected as early as 24 h but was typically measured at 48 h, when it was more pronounced. As a confirmation of cell death by apoptosis, we also performed TUNEL staining on st transfectants (Fig. 3). TUNEL staining quantitates apoptotic chromosome fragmentation by labeling the free 3′-OH of fragmented DNA with terminal deoxynucleotidyltransferase and fluorescein-12-dUTP, hence the name deoxynucleotidyltransferase-mediated dUTP nick-end labeling. A majority of the cells expressing st were also TUNEL positive, thus verifying death by apoptosis. Quantitative data from a minimum of two independent experiments, each counting a total of 100 st-expressing cells, demonstrated that an average of 89% of the nuclei were fragmented, thus showing clear signs of programmed cell death (Fig. 4).
FIG. 1.
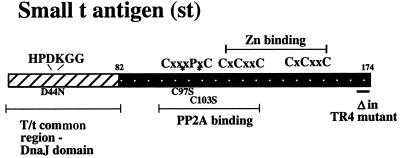
Schematic drawing of st features. This diagram outlines the salient features of SV40 st. The first 82 amino acids of st constitute the first exon and are shared with LT. Previous work has demonstrated that this region contains a bona fide DnaJ domain capable of binding Hsc70, depending on an intact HPDK motif (11, 74, 75; Kelley and Landry, letter). One frequently used mutant, D44N, disables DnaJ domain function (11). The central region of st appears to contain the major binding determinants for PP2A (42, 48, 72). Two frequently used PP2A binding-deficient mutants, C97S and C103S, are indicated (48). Furthermore, the diagram depicts two cysteine clusters responsible for binding Zn. Also indicated is the TR4 mutant lacking the final four amino acids of st.
FIG. 2.
St, but not LT, induces apoptosis in U2OS cells. (A) U2OS cells seeded on Chamberslides were transiently transfected with a pCMV LT expression vector and processed for immunofluorescence staining with PAb419 antibody (left panel). The right panel outlines the nucleus after visualization of the DNA with DAPI. Note the preserved integrity of the nucleus, thus showing no signs of cell death. (B) U2OS cells were transfected as for panel A but with a pSG5 st expression vector. The left panel shows staining for st expression with PAb419 (two different fields), and the right one demonstrates DAPI staining of the cells in the same field. Strikingly, the st-expressing cells have a nuclear morphology very different from that of the nontransfected cell. The cells with fragmented nuclei show signs of cell death by apoptosis. (C) U2OS cells were transfected as for panel A but with the pSG5 st C103S/TR4 expression vector. The left panel shows staining for the st C103S/TR4 protein, and the right panel shows the DAPI staining of the same field. Interestingly, the nuclei are preserved, in contrast with the nuclei of wild-type st-expressing cells. Also note the predominantly nuclear localization pattern of this double mutant.
FIG. 3.
U2OS cells expressing st undergo apoptosis as determined by TUNEL. U2OS cells were transfected, as described for Fig. 2B, with the pSG5 st expression vector. The cells were subsequently processed for TUNEL (Promega) to detect the fragmented nucleosomal DNA ends indicative of apoptosis. Finally, the cells were processed for st immunofluorescence to identify the successfully transfected cells and DAPI to visualize the DNA.
FIG. 4.
Quantitative analysis of cell death induction by st mutants as measured by nuclear fragmentation. U2OS cell were transfected as described for Fig. 2B with an expression vector encoding either wild-type st or the mutant indicated. Immunofluorescence staining for st and DAPI staining of the DNA were performed. Every cell that expressed the desired mutant was counted as having either a damaged or an intact nucleus based on DAPI visualization of the nuclear morphology. The graph contains data from a minimum of two independent experiments (for some mutants more than two), and in each experiment 100 positive (expressing) cells were scored. Finally, the data were averaged and plotted as the percentage of cells with intact nuclei. LT was used as a negative control, since it did not affect nuclear integrity in U2OS cells.
Genetic analysis of the SV40 st death response.
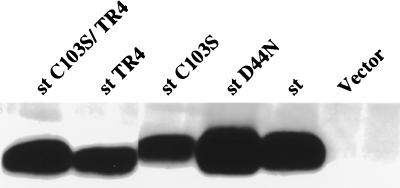
st is known to possess at least two functions, one attributed to its J domain and the second to its PP2A binding capacity (illustrated in the diagram in Fig. 1). We tested the possible contribution of each of these two st functions in induction of death using the D44N and C103S mutants, which are defective in J domain function and PP2A binding, respectively (27, 48). While the J domain did play a minor role (77% of the nuclei were disrupted with the D44N mutant), we found the PP2A binding function to be a more major player (45% of the nuclei disrupted for the C103S mutant) (Fig. 4). Consistently, however, we found that the C103S mutant showed a significant ability to promote cell death. Furthermore, the double mutant D44N/C103S showed the same propensity to induce cell death as the single C103S mutant (data not shown). Hence, we concluded that another function might be required in concert with PP2A binding to induce efficient cell death, or, alternatively, the C103S mutant is not fully defective for PP2A binding. Interestingly, the tail region of st is relatively conserved among the polyomavirus st antigens, although for mouse polyomavirus st, this homologous region is not located at the immediate carboxy terminus. Given the potential significance of the tail sequence, we created a truncation mutant of SV40 st lacking the carboxy-terminal four amino acids, henceforth referred to as TR4. When tested in a cell death induction assay based on apoptotic morphology of nuclei, we found the TR4 mutant to be partially defective (54% of the nuclei disrupted), thus confirming that the st function affected by the TR4 deletion contributes to cell killing (Fig. 4). The double mutant C103S/TR4 was totally defective for cell death induction, since virtually all the nuclei analyzed were intact (Fig. 2C and 4). Interestingly, the subcellular localization of the C103S/TR4 double mutant also appeared to be altered from the uniform distribution throughout the cells of wild-type st to a predominantly nuclear pattern (compare Fig. 2C and B). Cell staining, when performed after transfections with the various mutants, did not reveal major variations in expression levels. Nevertheless, the expression levels were assessed in a more quantitative manner by transient transfection into U2OS cells followed by Western blot analysis of the lysates using the PAb419 antibody. As shown in Fig. 5, the expression levels were quite comparable between wild-type st and the various mutants. Notably, the truncation mutants TR4 and C103S/TR4 run with a faster mobility on an SDS-polyacrylamide gel, consistent with their smaller size. It should also be mentioned that the expression levels upon transient transfection with our SV40 early promoter-driven construct were approximately equivalent on a per-cell basis to the levels of st found in COS-1 or COS-7 cells (data not shown). Western analysis with PAb419 followed by quantitation on a FluorImager suggested that the endogenous expression of LT in COS or ectopic expression of LT in U2OS was much higher than that of st, yet no apoptosis ensued.
FIG. 5.
Western blot analysis after transient expression of wild-type st and mutants in U2OS cells. Wild-type st and mutant st expression vectors were transfected into U2OS cells, and then lysates were prepared and assayed for st expression by Western blotting using the PAb419 antibody. Notice the slightly faster mobility of the truncation mutants st TR4 and st C103S/TR4.
The TR4 deletion unexpectedly diminishes PP2A binding in vivo.
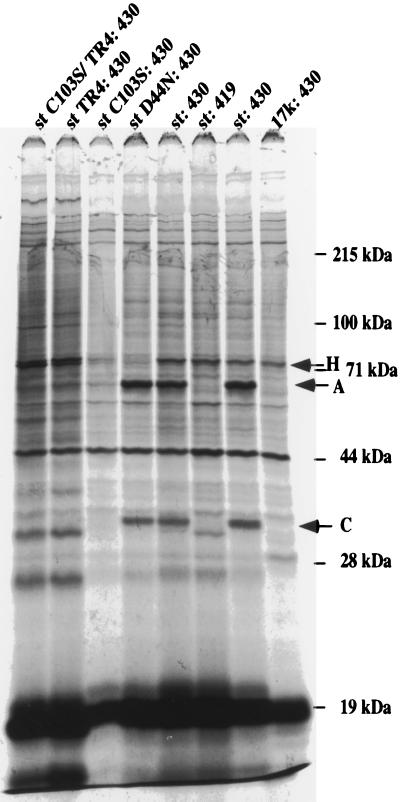
Since the C103S and TR4 functions each partially affected induction of apoptosis, it became important to distinguish whether the TR4 deletion mutant defines a novel function or whether it also affects PP2A binding. For this purpose, we obtained PAb430 antibody, which is capable of coprecipitating PP2A in a complex with st, whereas the PAb419 antibody obscures the interaction (Fig. 6). In order to analyze the interaction in vivo, we metabolically labeled wild-type or mutant st-transfected U2OS cells with [35S]methionine and subsequently immunoprecipitated the st with PAb430 antibody. In parallel, an expression vector for 17k was also transfected as a control. As shown in Fig. 6, it was observed that wild-type as well as the D44N mutant of st coprecipitated both the 63-kDa A subunit and the 36-kDa C subunit of PP2A. As expected, the binding of the C103S mutant to PP2A was reduced. We consistently observed in several experiments that the level of C103S was somewhat lower than that of wild-type st or other mutants; this has been previously noted (48). Importantly, the TR4 mutant was also quite defective for PP2A binding, suggesting that the carboxy terminus might make contributions in vivo to an efficient interaction. This result was unexpected given previous demonstrations that several carboxy-terminal truncation mutants of st still bound the A subunit of PP2A in vitro. It should be emphasized that binding assays in vitro consistently show that both the C103S and TR4 mutants retain considerable residual binding to PP2A (reference 48 and our unpublished data).
FIG. 6.
Interaction of st mutants with PP2A in vivo. Wild-type or mutant st expression vectors and a 17k expression plasmid were each transfected into U2OS cells. At 48 h posttransfection, the transfected cells were metabolically labeled with [35S]methionine for 4 h, after which the cells were lysed and the lysates were immunoprecipitated with PAb430 antibody. The thoroughly washed immunoprecipitates were analyzed on an SDS–12% polyacrylamide gel. For comparison, an immunoprecipitation performed using PAb419 was included; this antibody fails to bring down the st-PP2A complex. Arrows with letters indicate the bands representing the 63-kDA A subunit and the 36-kDa C subunit of PP2A. Furthermore, an arrow indicates the band corresponding to Hsc70 (labeled H).
Inhibition of colony formation by SV40 st and mutants.
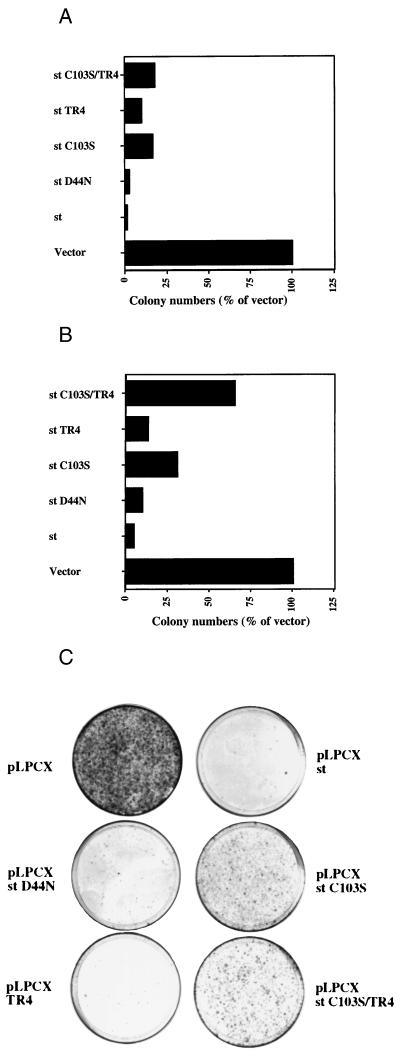
Although the evidence for apoptotic cell death obtained by microscopy techniques described above was quite extensive, we pursued further evidence and quantitative data for cell death using a colony suppression assay which has been successfully applied before in similar contexts. Basically, the colony-forming ability of an empty vector (pLPCX) and that of the same vector directing expression of st from the CMV promoter (pLPCX st) were compared when transfecting U2OS cells and selecting for puromycin resistance inherent in the pLPCX vector (Clontech). We observed, in at least five independent experiments, a dramatic reduction in colony formation upon st expression (Fig. 7A). Subsequently, we tested the st mutants D44N, C103S, TR4, and C103S/TR4 in the colony inhibition assay. While the J domain was found to affect st colony inhibition only marginally (Fig. 7A, D44N mutant), the PP2A binding-defective mutant C103S was impaired in st-mediated colony suppression (Fig. 7A). The TR4 mutant also displayed a reduced ability to inhibit colony formation. The double mutant C103S/TR4 appeared to be slightly more defective for st colony suppression than the C103S mutant alone. Collectively, the colony inhibition data for mutant st's are quite consistent with the data obtained in transient transfections using miscroscopic examination of nuclear integrity (Fig. 4). The data from the colony suppression assay, while largely correlating with data from inspection of nuclear morphology, did demonstrate some residual induction of cell death, even by the most defective C103S/TR4 mutant (Fig. 7A). This observation may suggest that a long-term clonogenic assay could reveal additional functions in st that might be detrimental to cell growth.
FIG. 7.
Suppression of colony formation by st and mutants. (A) U2OS cells were transfected with either the pLPCX (Clontech) parental vector or the pLPCX vector directing expression of st or its mutants. After approximately 2 weeks of selection for puromycin resistance (encoded on the pLPCX plasmid), individual drug-resistant colonies appeared. These colonies were visualized by crystal violet staining and counted. For each experiment, the number of colonies was normalized to that obtained with the pLPCX vector alone and plotted in this graph as a percentage of vector value. For most of the mutants at least five independent experiments were conducted, but for the st C103S/TR4 mutant the graph represents data from two independent experiments. (B) A colony assay was performed as outlined in the legend to panel A but with H1299 cells, which are p53 deficient. The colony numbers were once again normalized to that obtained with the pLPCX vector alone and plotted in the graph as percentages. (C) A typical H1299-based colony assay conducted as described in the legend to panel B is shown. Note the dramatic reduction in colony-forming potential upon st expression, probably due to a cell death response.
Colony inhibition by SV40 st occurs in p53-deficient H1299 cells.
One frequently studied tumor suppressor, the p53 protein, has been implicated in several apoptotic pathways induced by a wide variety of agents. It was of interest to determine if the cell death response we observed with st was p53 dependent or independent. For this purpose, we performed a colony suppression assay with the p53-deficient lung carcinoma cell line H1299 (Fig. 7B and C). Notably, we observed an equally dramatic decrease in colony formation upon st expression as was seen in U2OS cells, which are wild type for p53 (compare Fig. 7A with B and C). It was also noted that st expression in H1299 caused a nuclear fragmentation characteristic of apoptosis that was similar to that seen in U2OS (data not shown). Furthermore, induction of cell death in H1299 cells by the mutants of st as judged from the colony suppression assay largely reflected the pattern observed in U2OS (Fig. 7A versus B and C). Significantly, cell killing was markedly diminished by the C103S mutation but was almost unchanged in the TR4 mutant. Although we cannot exclude the possibility that some part of the st death response involves p53, st can induce substantial cell death in a p53 null background.
Induction of cell death by SV40 st is cell type dependent.
Given our observation that st can induce apoptotic cell death in some human cancer lines, such as U2OS and H1299, it was important to establish how universal this observation is in the context of other cell lines from different species. Since the natural host of SV40 for a lytic infection is the monkey, we investigated whether st expression in the monkey kidney cell line CV1-P could cause cell death. As shown in Fig. 8, the CV1-P cells expressing st displayed no signs of fragmented nuclei, and we did not notice any other indications of compromised viability. This is in agreement with previous studies that made use of CV-1 cells stably expressing st. Also, we did not observe apoptosis after transient transfection in NIH 3T3 cells (data not shown). Hence, we conclude that the ability of st to elicit a cell death response is cell type and/or species dependent.
FIG. 8.
Failure of st to induce apoptosis in CV1-P cells. The monkey kidney cell line CV1-P was transfected as outlined in the legend to Fig. 2. Cell staining with PAb419 was carried out to visualize st expression (right panel), and DAPI staining was done for visualization of the nuclear morphology (left panel). In contrast to U2OS cells (Fig. 2), the transfected CV1-P cells expressing st have largely intact nuclear morphology.
SV40 st-induced apoptosis can be partially suppressed by Bcl-2 or broad caspase inhibitors.
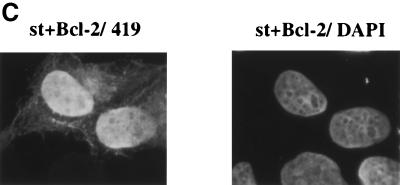
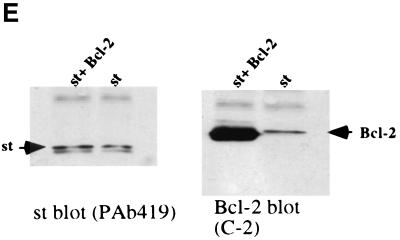
One of the most thoroughly characterized suppressors of apoptosis is the Bcl-2 protein. Hence, we tested whether Bcl-2 might suppress the death response mediated by st in U2OS cells. For these studies, we made use of a construct expressing st coupled bicistronically via an internal ribosome entry site (IRES) to EGFP, hence the name pIRES2 EGFP-st. This allows us to visualize cells that express both st and EGFP as a marker. Indeed, we found that st when expressed from this construct caused extensive cell rounding and condensed chromatin as visualized by staining of the DNA with Hoechst 33258 (Fig. 9A). When st was coexpressed with Bcl-2 in a 1:4 ratio of expression plasmids, the morphology of GFP-expressing cells reverted to being largely normal (Fig. 9B). Significantly, the majority of the nuclei also looked intact (Fig. 9C). In fact, upon Bcl-2 coexpression, 67% of the nuclei appeared normal whereas of the cells expressing st alone, only 11% were normal (Fig. 9D). We conclude that Bcl-2 does have a protective effect, at least short term, in largely preserving nuclear integrity and overall morphology of the cell. As a control to demonstrate that Bcl-2 cotransfection did not lower the level of expression of st, a Western blot was performed (Fig. 9E). Transfection of 1 μg of st vector plus 4 μg of pcDNA3 vector was directly compared to 1 μg of st vector plus 4 μg of pCMV Bcl-2. As shown in Fig. 9E, coexpression with Bcl-2 did not reduce the expression of st; in fact, quantitation on a FluorImager indicated that there was twofold more st upon coexpression of Bcl-2. The right panel in Fig. 9E shows a Western blot using the same samples but probing for Bcl-2. The apparent increase in st production upon Bcl-2 coexpression may be due to better survival of cells expressing a large amount of st.
FIG. 9.
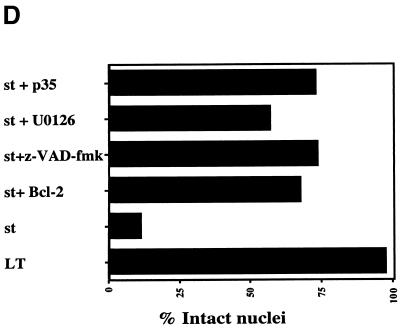
Protection from apoptosis by Bcl-2, caspase inhibitor, or MEK inhibitor. (A) U2OS cells were transfected with the pIRES2 EGFP-st vector, which expresses st bicistronically with EGFP as a marker (left panel). The right panel shows staining of the same field of cells with Hoechst 33258, thus visualizing cellular DNA. Note the rounded shape of st-expressing cells (left panel) as well as the condensed chromatin (right panel). (B) U2OS cells were transfected with pIRES2 EGFP-st either alone (left panel) or together with a CMV Bcl-2 expression vector in a 1:4 ratio (right panel). The rounded shape of dying cells is reversed by Bcl-2 coexpression to resemble normal cell shape. (C) U2OS cells were cotransfected with pSG5 st and CMV Bcl-2 in a 1:4 ratio. Subsequently, the cells were stained for st with PAb419 and with DAPI to visualize the DNA as described in the legend to Fig. 2A. Note the intact nuclear morphology, in comparison to Fig. 2B. (D) U2OS cells were transfected with st alone or, alternatively, coexpressed with either Bcl-2 or the broad caspase inhibitor p35. In some instances, the st-transfected cells were treated with potential modulators of apoptosis such as the broad caspase inhibitor z-VAD-Fmk (50 μM) or the MEK inhibitor U0126 (10 μM), and results were compared to that obtained with vehicle (DMSO) alone. Transfected cells expressing st were identified by cell staining with PAb419, and nuclear morphology was assessed as intact or disrupted. The percentage of disrupted nuclei was plotted in the graph and compared to that obtained with LT (negative control). At least two independent experiments, each time counting 100 st-expressing cells, were conducted; for some of the combinations several more experiments were performed. Average values are displayed in the graph. (E) U2OS cells transfected with either 1 μg of st vector plus 4 μg pcDNA3 vector or 1 μg of st vector plus 4 μg of pCMV Bcl-2 were lysed after 48 h and assayed by Western blotting for st expression using the PAb419 antibody. In the right panel, the same samples were analyzed for Bcl-2 expression using a Bcl-2 monoclonal antibody.
Many apoptotic processes converge at the level of executioner proteases, termed caspases. We tested whether caspases are involved in the st-mediated death response by using either treatment with the broad caspase inhibitor z-VAD-Fmk (final concentration, 50 mM) or coexpression with the baculovirus-encoded caspase inhibitor p35 (54). With z-VAD-Fmk treatment, 73% of the nuclei appeared normal (Fig. 9D). Similarly, we found that p35 coexpression yielded 75% intact nuclei, consistent with the conclusion that caspases are indeed involved in the execution step of st-mediated cell death (Fig. 9D).
As mentioned previously, one major route for st mitogenic activity involves the activation of the MEK/ERK pathway via PP2A inhibition (72). Since the induction of cell death also involves PP2A binding (Fig. 4 and 7), it was important to probe the possible contribution of MEK/ERK activity to st-mediated cell death. For that purpose, we used the specific MEK inhibitor U0126 (Promega) and found that this treatment could protect the cells partially (56.5% of the nuclei appeared intact [Fig. 9D]). Consequently, we conclude that the MEK/ERK pathway contributes to the st cell death response but that there are other players involved.
Induction of apoptosis by SV40 st correlates with its cell cycle modulatory activities.
Because apoptosis is often linked to deregulation of cell cycle transitions, we examined the effects of st on the cell cycle using dual-color FACS analysis with H1299 cells. Briefly, st expression plasmids were cotransfected in a 5:1 ratio with an expression vector for CD20, which was then used as a surface marker for transfected cells. The transfection marker allowed us to use flow cytometry to obtain the cell cycle profile of the transfected cell population. Cell cycle profiles were obtained at 72 h posttransfection because this time point gave the lowest level of cell cycling in untransfected H1299 cells. We also compared two different transfection methods: one involving the Fugene-6 reagent and the other based on BES-buffered calcium phosphate coprecipitation. We found that using either transfection protocol, a significant increase (always greater than 10%) in the S-phase population was obtained when wild-type st was transfected (Table 1). Interestingly, either mutation of the PP2A binding motif (C103S) or truncation of the last four amino acids (TR4) totally abolished the S-phase increase; the J domain mutation had only a minor effect. These results suggest that there may be connections between cell cycle effects and induction of apoptosis by st. Interestingly, we found that wild-type st, especially when transfected into H1299 cells by the calcium phosphate method (Table 1, experiments III and IV), elicited an accumulation of cells in G2/M. One possibility remains, that st causes a G2 block (62). It may have been revealed only with the calcium phosphate transfection protocol because these cells are switched to fresh medium with 10% FCS on the first day posttransfection whereas the Fugene-6-transfected cells are not. Consistent with this hypothesis, Rundell and Parakati found that expression of st in human diploid fibroblasts using a recombinant adenovirus induced significant G2/M-phase accumulation only in the presence of serum (62).
TABLE 1.
Cell cycle distribution of H1299 cells transiently expressing st or mutantsa
| Vector | Growth stage | % Increase in cell population for expt:
|
|||
|---|---|---|---|---|---|
| I. Fugene | II. Fugene | III. CaPO4 | IV. CaPO4 | ||
| pcDNA3 | G0/G1 | 61.8 | 66.5 | 64.6 | 61.4 |
| S | 26.1 | 27.8 | 27.6 | 25.7 | |
| G2/M | 12.0 | 5.8 | 7.8 | 12.9 | |
| st | G0/G1 | 48.5 | 50.4 | 37.5 | 33.3 |
| S | 36.2 | 39.5 | 37.9 | 37.3 | |
| G2/M | 15.3 | 10.1 | 24.7 | 29.4 | |
| st D44N | G0/G1 | 53.2 | 56.2 | 47.5 | |
| S | 38.9 | 32.6 | 34.6 | ||
| G2/M | 7.9 | 11.1 | 17.9 | ||
| st C103S | G0/G1 | 62.0 | 71.5 | 60.1 | |
| S | 28.9 | 22.7 | 28.7 | ||
| G2/M | 9.1 | 5.8 | 11.2 | ||
| st TR4 | G0/G1 | 59.2 | 70.8 | 62.0 | |
| S | 29.6 | 21.3 | 27.6 | ||
| G2/M | 11.2 | 7.9 | 10.4 | ||
| st C103S/TR4 | G0/G1 | 62.6 | 77.3 | 65.5 | |
| S | 26.0 | 16.4 | 25.9 | ||
| G2/M | 11.4 | 6.3 | 8.6 | ||
H1299 cells were transiently transfected with an expression vector for st, or mutant st, together with a cell surface marker, CD20. After 72 h, the cell cycle distribution of the st-expressing population was assessed using FACS and gating on the CD20-positive cells. This table shows cell cycle distributions from four independent experiments which produced consistent results. Experiments I and II used Fugene-6 for the transfection protocol, while experiments III and IV employed calcium phosphate.
DISCUSSION
This study reveals the existence of a novel proapoptotic activity of SV40 st based on an assessment of nuclear morphology, TUNEL staining, colony reduction, and suppression of cell death by Bcl-2 or caspase inhibitors (Fig. 2, 3, 4, 7, and 9A, B, and C). Most previous reports have characterized the positive role st plays in promoting cell proliferation pathways. Notably, st can induce cell cycle progression in certain scenarios and activate signal transduction pathways involving MEK/ERK and JNK via PP2A binding (16, 28, 29, 47, 56, 72, 73) (Fig. 10 and Table 1). With this demonstration of st participation in cell death regulation, st joins the already impressive list of cellular and viral oncoproteins implicated in apoptosis signaling (for a review, see reference 59). Regulation of cell survival, growth, and proliferation is often multifaceted, showing complexities even within one given protein (for a review, see reference 23). For example, the cellular oncoproteins c-Myc and E2F-1 induce both proliferation and apoptosis (reviewed in reference 23). It is likely that this characteristic acts as a fail-safe mechanism to prevent tumorigenesis in the absence of a function protective against cell death. Certain proteins have even evolved to possess both proapoptotic and antiapoptotic activities, e.g., activated H-Ras, which turns on several downstream targets with opposing effects on cell death. In this case, the final outcome of its overexpression is likely to depend on the cell type in conjunction with other factors and may determine whether the cell undergoes DNA synthesis, cell death by apoptosis, or withdrawal from the cell cycle as in differentiation or senescence.
FIG. 10.
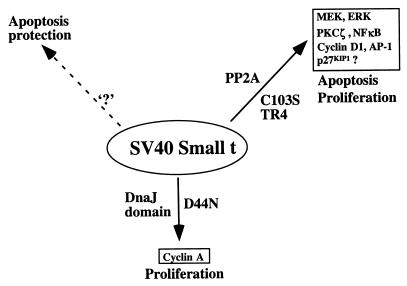
Schematic overview of st activities in proliferation and apoptosis. This figure attempts to summarize what we currently know about activities of st involved in proliferation and apoptosis. The binding and inhibition of PP2A by st can affect both proliferation and apoptosis. Indeed, there are reports that it is required for the st helper function in oncogenic transformation (29, 48, 55). This may in part be mediated by activation of the MEK/ERK, PKCζ/NF-κB, cyclin D1, and AP1 pathways and down-regulation of p27 KIP1 (25, 56, 72, 73, 83). This report suggests that st activation of the MEK/ERK pathways also under some conditions may be involved in promoting the apoptotic response, since the MEK1 inhibitor partially protects. The DnaJ domain of st has been previously implicated in cyclin A promoter activation as well as transformer helper function, at least under certain conditions (55). We did not find a functional DnaJ domain to contribute significantly to the cell death response elicited by st. Finally, we surmise, based on published evidence, the existence of an activity in st conferring increased survival in certain contexts (5, 33). This function is indicated by ‘?,’ since we do not know if it represents a novel function or, alternatively, if one of the known functions under specific conditions may effect a cellular survival response.
Several viral oncoproteins mimic their cellular counterparts in their duality to induce both cell cycle progression and apoptosis; examples include adenovirus E1A, SV40 LT, human papillomavirus E7, and polyomavirus middle and large T antigens (2, 20–22, 24, 30, 36, 47, 53, 57, 65, 67). For a subset of these proteins, the cell cycle-promoting and apoptosis-inducing functions may well be inherently linked, as appears to be the case for adenovirus E1A (21, 57, 64). In the case of st, there is anecdotal as well as published evidence to support the notion of it being cytotoxic, at least in the case of the homologous polyomavirus st (5, 40; Roberts laboratory, unpublished observations). The observed cytotoxicity precluded the isolation of stable lines expressing st (40). Interestingly, there is also a report suggesting that st can protect rat embryo fibroblasts from LT-induced apoptosis (33). Furthermore, polyomavirus st can protect against the middle T-induced tumor necrosis factor alpha hypersensitivity (5). We do not view these findings as contradictory to ours. One protein, especially when multifunctional, can be both proapoptotic and antiapoptotic depending on expression level, cell type, and environment. For example, the human immunodeficiency virus type 1 Vpr and Tat proteins are each known to regulate apoptosis both positively and negatively (1, 3, 9, 17, 26, 44). SV40 LT is yet another example of a viral protein with opposing activities in induction of cell survival and death (14, 18, 33, 43, 71, 77, 79). Apoptosis often occurs as a consequence of stimulation of proliferative pathways in an unregulated, nonphysiological manner or in the absence of balancing cell survival signaling (23). Thus, apoptosis constitutes part of the host cell defense against viral infection. For some viral gene products, apoptosis can also serve the purpose of releasing progeny virus and thus can play an important role in the viral life cycle during a lytic infection (59). In the case of st, we find this to be the less plausible scenario, since st does not induce apoptosis in its natural host cell (e.g., CV1-P) (Fig. 8).
Notably, induction of apoptosis as measured by either nuclear fragmentation or inhibition of colony formation is affected by mutation of the PP2A binding site in st C103S (Fig. 2C, 4, and 7). PP2A is involved in a multitude of signaling pathways contributing to diverse cellular responses, such as proliferation, apoptosis, cell cycle progression, transcription, and replication (reviewed in references 45 and 80). PP2A may be regulated at many levels, since the B subunit is believed to target the enzyme to proper substrates or localization (reviewed in references 45 and 80). st replaces the B subunit and inhibits the phosphatase activity on most substrates in vitro (52, 72, 73, 86). It is conceivable that st may be doing more than simply inactivating PP2A enzyme activity, since, as mentioned above, the B subunit that st replaces has important functions in regulating substrate specificity as well as subcellular localization of PP2A. At least in the case of polyomavirus middle T and st, cellular localization in addition to PP2A binding determines the biological consequences (47). For example, both polyomavirus middle T and st bind PP2A, but in different cellular compartments. Interestingly, st induces cell cycle progression as well as the c-fos promoter in a PP2A-binding-dependent manner, whereas middle T, which is membrane localized, does not. Conversely, middle T, but not st, activates JNK dependent on PP2A binding. Finally, there are also reports that polyomavirus st imparts specificity of PP2A toward phosphotyrosine substrates (12).
An important part of this study is represented by the discovery that deletion of the last four amino acids (the tail) in the mutant TR4 strongly decreases binding of PP2A to st in vivo in U2OS cells. This finding was unexpected, given that previous reports found several carboxy-terminal truncation mutants of st to bind the A subunit of PP2A in vitro based on glutathione S-transferase (GST)-A fusion pull-downs. A primary interaction site in st was reported to be from amino acids 105 to 122 (42), and carboxy-terminal truncations until amino acid 131 were reported not to affect binding significantly in vitro (72). It remains a distinct possibility that our TR4 mutant directs the attention to a previously ignored role of the st carboxy terminus for efficient binding in vivo. Indeed, the other carboxy-terminal truncation mutants used in previous reports were not tested for binding to PP2A in vivo (42, 72). We cannot, however, entirely discount the possibility that our TR4 mutant is unfolded while all the other published carboxy-terminal truncations are not, but this seems unlikely, since TR4 does bind to GST-A under in vitro conditions previously reported (42, 72) (data not shown). It seems a plausible scenario that C103S and TR4 are both partial PP2A binding-defective mutants (Fig. 6) (48), whereas the double mutant C103S/TR4 is more binding defective. This would be consistent with the conclusion that the induction of apoptosis is entirely PP2A binding dependent; nevertheless, we cannot entirely discount the possibility that the TR4 deletion affects another, as yet uncharacterized function of st. The idea that the TR4 deletion is altering something in addition to PP2A binding is bolstered by the fact that the C103S and TR4 mutations have different effects on cell survival in H1299 cells (Fig. 7B and C).
Consistent with our data implicating PP2A binding in cell death pathways, several reports over the years have presented evidence that the specific PP2A inhibitor okadaic acid can cause apoptosis in a wide variety of cell types (4, 8, 19, 35, 46, 49, 58, 85). It remains unclear what the critical substrates are in eliciting cell death by okadaic acid, but in some scenarios, at least, transformed cells are more susceptible to death induction (58). This is certainly consistent with our findings that transformed human cells are quite sensitive to st. Okadaic acid-induced apoptosis is reported to be both p53 dependent (85) and independent (8, 46, 58), possibly due to cell type variations. PP2A has been implicated in both positive and negative regulation of a multitude of cellular kinases, perhaps accounting for the pleiotropic responses observed upon its inhibition (reviewed in reference 45).
Association of st with PP2A has been previously connected to ERK activation (72) (Fig. 10). Although ERK activation is traditionally considered a survival signal, there is a precedent for activated MEK/ERK playing a proapoptotic role under specific conditions, e.g., cisplatin-induced, p53-independent cell death (82) or p53-dependent induction of NF-κB (63). This may explain why treatment with the specific MEK inhibitor U0126 was found to result in partial protection against st-mediated cell death (Fig. 9D). Certain other viral oncoproteins known to bind PP2A are also involved in induction of apoptosis. For example, polyomavirus middle T under certain conditions induces apoptosis in a PP2A-dependent manner (X. Cullere and B. Schaffhausen, personal communication). Furthermore, adenovirus E4orf4 is capable of inducing apoptosis in a manner that requires binding of PP2A (38, 69). Although E4orf4 binds the B subunit of PP2A and, as preliminary evidence suggests, activates it, this observation nevertheless links PP2A to apoptosis regulation. Interestingly, E4orf4-induced apoptosis is likewise p53 independent (34, 38, 39, 68).
Deregulation of the cell cycle has been frequently associated with apoptosis induction and, in some instances, tumorigenesis (reviewed in reference 32). In some models, apoptosis may be viewed as a consequence of conflicting growth signals. Interestingly, st can affect cell cycle transitions at several levels. For instance, st is reported to promote exit from G0, transit through G1, and entry into S phase, at least in some cell types (16, 29, 47, 72) (Table 1). Analysis of transiently transfected H1299 cells by dual-color flow cytometry demonstrates that the C103S and TR4 mutants in st fail to affect cell cycle progression (Table 1). Since the same two mutants are also involved in apoptosis induction (Fig. 4 and 7), it remains possible that apoptosis is mechanistically linked to cell cycle progression. Although we believe there may be a link between cell cycle transitions and apoptosis induced by st, we do not wish to imply that the latter is simply a consequence of the former. Arguing for a more complex relationship, we find that LT also induces cell cycle progression in H1299 but fails to induce apoptosis (Fig. 2; also data not shown).
Consistent with our cell cycle results, a previous study using CV-1 cells infected with an adenovirus encoding st also observed increased G1 exit and active cell cycling dependent on PP2A binding but not an intact DnaJ domain (29). The ability of st to perturb these cell cycle transitions is perhaps linked to its upregulation of cyclin D1 and cyclin A concomitant with downregulation of the cdk inhibitor p27KIP1 (55, 56, 66). st may also affect cell cycle transitions other than the G1-S transition, as suggested by studies showing accumulation in G2/M phase upon st expression (50, 62) (Table 1, experiments III and IV). These observations may provide evidence for a G2 or M phase cell cycle block (62). A delay or block preventing mitosis would be consistent with the ability of st to increase endoreduplication during lytic infection in CV1 cells (84). The ability of st to manipulate the cell cycle machinery for optimal replication activity during a lytic infection may in other nonpermissive cell types lead to a cell death response.
Traditionally, apoptosis is often cathegorized mechanistically into either of two cellular pathways (reviewed in reference 10). One leads from cell surface death receptors, such as Fas and TNF-R1, via adapter proteins, such as FADD or TRADD, to the proteolytic processing of initiator caspase 8 followed by activation of the effector caspases 3, 6, and 7. The second pathway is frequently initiated by cellular stress signals, such as DNA damage, and invariably involves release of cytochrome c from the mitochondria. This released cytochrome c activates Apaf-1, thus generating active caspase 9, which in turn activates the effector caspases 3, 6, and 7. At this point we do not have definitive evidence for which pathway is involved in st-induced apoptosis; however, the partial protective effect of Bcl-2 (Fig. 9B and C) suggests that mitochondrial regulation may be involved in the death response. Interestingly, Bcl-2 only reverses the nuclear morphology toward that of a normal cell in the transient assay. In a long-term colony assay, Bcl-2 failed to allow the cells to grow into visible colonies (data not shown). Perhaps other death pathways not suppressible by Bcl-2 prevail long term, or, alternatively, Bcl-2 may only delay the cell death. It remains possible that st, due to its PP2A inhibition activity, maintains Bcl-2 in a phosphorylated state, which in some contexts promotes apoptosis (51). Consistent with apoptosis being the prevalent death pathway, we find that the broad caspase inhibitor z-VAD-Fmk as well as the baculovirus p35 caspase inhibitor can protect (Fig. 9D). Future studies will address which specific caspases are involved in induction of death by st and whether reactive oxygen species may participate.
Interestingly, several viruses, including adenovirus, have been determined to utilize a sophisticated and complex network of pro- as well as antiapoptotic mechanisms, presumably for the most efficient manipulation of the host cell, thus maximizing viral replication. With our present demonstration of one or more proapoptotic activities of st, it appears that SV40 LT and st can modulate the apoptotic machinery both positively and negatively depending on the cell context (5, 14, 18, 33, 43, 71, 77, 79; this study). Our present study emphasizes the importance of PP2A binding for efficient induction of a cell death response as well as cell cycle progression mediated by st. Since the death response is p53 independent, it may involve other p53 family members, such as p73. While the genetic basis for the proapoptotic response is characterized in this paper, we do not yet understand the nature of the st antiapoptotic function reported previously in the literature (5, 33). It may be that this function is inseparable from the proapoptotic one(s) and thus depends on the cellular context. Alternatively, apoptosis in human cancer cell lines, such as U2OS and H1299, may result from the lack of this protective function or its override by dominant proapoptotic signals. To distinguish between these intriguing possibilities, we await the further characterization of both pro- and antiapoptotic activities contained in st, work that is currently in progress.
ACKNOWLEDGMENTS
This work was supported by NIH grants to T.M.R. (PO1-CA50661 and CA30002).
We thank Brian Schaffhausen and Karen Vousden for Bcl-2 and baculovirus p35 plasmids, respectively. We are further indebted to Brian Schaffhausen, Sorab Dalal, and Lei Zhang for critical reading of the manuscript. Finally, we acknowledge Michael Imperiale for his kind gift of PAb430 antibody. In compliance with Harvard Medical School guidelines on possible conflicts of interest, we disclose that one of the authors (T.M.R.) has consulting relationships with Upstate Biotechnology and Novartis Pharmaceuticals, Inc.
ADDENDUM
While this manuscript was under review, an article appeared in the Journal of Virology concluding that polyomavirus st, when expressed in HL-60 cells under differentiation conditions, can redirect this response into apoptosis (87).
REFERENCES
- 1.Ayyavoo V, Mahboubi A, Mahalingam S, Ramalingam R, Kudchodkar S, Williams W V, Green D R, Weiner D B. HIV-1 Vpr suppresses immune activation and apoptosis through regulation of nuclear factor kappa B. Nat Med. 1997;3:1117–1123. doi: 10.1038/nm1097-1117. [DOI] [PubMed] [Google Scholar]
- 2.Banks L, Edmonds C, Vousden K H. Ability of the HPV16 E7 protein to bind RB and induce DNA synthesis is not sufficient for efficient transforming activity in NIH3T3 cells. Oncogene. 1990;5:1383–1389. [PubMed] [Google Scholar]
- 3.Bartz S R, Emerman M. Human immunodeficiency virus type 1 Tat induces apoptosis and increases sensitivity to apoptotic signals by up-regulating FLICE/caspase-8. J Virol. 1999;73:1956–1963. doi: 10.1128/jvi.73.3.1956-1963.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bergqvist A, Magnusson G. Apoptosis of Spodoptera frugiperda cells induced by okadaic acid is abrogated by baculovirus infection. Exp Cell Res. 1994;215:223–227. doi: 10.1006/excr.1994.1335. [DOI] [PubMed] [Google Scholar]
- 5.Bergqvist A, Soderbarg K, Magnusson G. Altered susceptibility to tumor necrosis factor alpha-induced apoptosis of mouse cells expressing polyomavirus middle and small T antigens. J Virol. 1997;71:276–283. doi: 10.1128/jvi.71.1.276-283.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bikel I, Mamon H, Brown E L, Boltax J, Agha M, Livingston D M. The t-unique coding domain is important to the transformation maintenance function of the simian virus 40 small t antigen. Mol Cell Biol. 1986;6:1172–1178. doi: 10.1128/mcb.6.4.1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bikel I, Montano X, Agha M E, Brown M, McCormack M, Boltax J, Livingston D M. SV40 small t antigen enhances the transformation activity of limiting concentrations of SV40 large T antigen. Cell. 1987;48:321–330. doi: 10.1016/0092-8674(87)90435-1. [DOI] [PubMed] [Google Scholar]
- 8.Boe R, Gjertsen B T, Vintermyr O K, Houge G, Lanotte M, Doskeland S O. The protein phosphatase inhibitor okadaic acid induces morphological changes typical of apoptosis in mammalian cells. Exp Cell Res. 1991;195:237–246. doi: 10.1016/0014-4827(91)90523-w. [DOI] [PubMed] [Google Scholar]
- 9.Borgatti P, Zauli G, Colamussi M L, Gibellini D, Previati M, Cantley L L, Capitani S. Extracellular HIV-1 Tat protein activates phosphatidylinositol 3- and Akt/PKB kinases in CD4+ T lymphoblastoid Jurkat cells. Eur J Immunol. 1997;27:2805–2811. doi: 10.1002/eji.1830271110. [DOI] [PubMed] [Google Scholar]
- 10.Budihardjo I, Oliver H, Lutter M, Luo X, Wang X. Biochemical pathways of caspase activation during apoptosis. Annu Rev Cell Dev Biol. 1999;15:269–290. doi: 10.1146/annurev.cellbio.15.1.269. [DOI] [PubMed] [Google Scholar]
- 11.Campbell K S, Mullane K P, Aksoy I A, Stubdal H, Zalvide J, Pipas J M, Silver P A, Roberts T M, Schaffhausen B S, DeCaprio J A. DnaJ/hsp40 chaperone domain of SV40 large T antigen promotes efficient viral DNA replication. Genes Dev. 1997;11:1098–1110. doi: 10.1101/gad.11.9.1098. [DOI] [PubMed] [Google Scholar]
- 12.Cayla X, Ballmer-Hofer K, Merlevede W, Goris J. Phosphatase 2A associated with polyomavirus small-T or middle-T antigen is an okadaic acid-sensitive tyrosyl phosphatase. Eur J Biochem. 1993;214:281–286. doi: 10.1111/j.1432-1033.1993.tb17922.x. [DOI] [PubMed] [Google Scholar]
- 13.Chen C, Okayama H. High-efficiency transformation of mammalian cells by plasmid DNA. Mol Cell Biol. 1987;7:2745–2752. doi: 10.1128/mcb.7.8.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen S L, Tsao Y P, Chen Y L, Huang S J, Chang J L, Wu S F. The induction of apoptosis by SV40 T antigen correlates with c-jun overexpression. Virology. 1998;244:521–529. doi: 10.1006/viro.1998.9109. [DOI] [PubMed] [Google Scholar]
- 15.Choi Y W, Lee I C, Ross S R. Requirement for the simian virus 40 small tumor antigen in tumorigenesis in transgenic mice. Mol Cell Biol. 1988;8:3382–3390. doi: 10.1128/mcb.8.8.3382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cicala C, Avantaggiati M L, Graessmann A, Rundell K, Levine A S, Carbone M. Simian virus 40 small-t antigen stimulates viral DNA replication in permissive monkey cells. J Virol. 1994;68:3138–3144. doi: 10.1128/jvi.68.5.3138-3144.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Conti L, Matarrese P, Varano B, Gauzzi M C, Sato A, Malorni W, Belardelli F, Gessani S. Dual role of the HIV-1 vpr protein in the modulation of the apoptotic response of T cells. J Immunol. 2000;165:3293–3300. doi: 10.4049/jimmunol.165.6.3293. [DOI] [PubMed] [Google Scholar]
- 18.Conzen S D, Snay C A, Cole C N. Identification of a novel antiapoptotic functional domain in simian virus 40 large T antigen. J Virol. 1997;71:4536–4543. doi: 10.1128/jvi.71.6.4536-4543.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.D'Ambrosio C, Valentinis B, Prisco M, Reiss K, Rubini M, Baserga R. Protective effect of the insulin-like growth factor I receptor on apoptosis induced by okadaic acid. Cancer Res. 1997;57:3264–3271. [PubMed] [Google Scholar]
- 20.Debbas M, White E. Wild-type p53 mediates apoptosis by E1A, which is inhibited by E1B. Genes Dev. 1993;7:546–554. doi: 10.1101/gad.7.4.546. [DOI] [PubMed] [Google Scholar]
- 21.de Stanchina E, McCurrach M E, Zindy F, Shieh S Y, Ferbeyre G, Samuelson A V, Prives C, Roussel M F, Sherr C J, Lowe S W. E1A signaling to p53 involves the p19ARF tumor suppressor. Genes Dev. 1998;12:2434–2442. doi: 10.1101/gad.12.15.2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dickmanns A, Zeitvogel A, Simmersbach F, Weber R, Arthur A K, Dehde S, Wildeman A G, Fanning E. The kinetics of simian virus 40-induced progression of quiescent cells into S phase depend on four independent functions of large T antigen. J Virol. 1994;68:5496–5508. doi: 10.1128/jvi.68.9.5496-5508.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Evan G, Littlewood T. A matter of life and cell death. Science. 1998;281:1317–1322. doi: 10.1126/science.281.5381.1317. [DOI] [PubMed] [Google Scholar]
- 24.Fromm L, Shawlot W, Gunning K, Butel J S, Overbeek P A. The retinoblastoma protein-binding region of simian virus 40 large T antigen alters cell cycle regulation in lenses of transgenic mice. Mol Cell Biol. 1994;14:6743–6754. doi: 10.1128/mcb.14.10.6743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Frost J A, Alberts A S, Sontag E, Guan K, Mumby M C, Feramisco J R. Simian virus 40 small t antigen cooperates with mitogen-activated kinases to stimulate AP-1 activity. Mol Cell Biol. 1994;14:6244–6252. doi: 10.1128/mcb.14.9.6244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fukumori T, Akari H, Iida S, Hata S, Kagawa S, Aida Y, Koyama A H, Adachi A. The HIV-1 Vpr displays strong anti-apoptotic activity. FEBS Lett. 1998;432:17–20. doi: 10.1016/s0014-5793(98)00824-2. [DOI] [PubMed] [Google Scholar]
- 27.Gjoerup O, Chao H, DeCaprio J A, Roberts T M. pRB-dependent, J domain-independent function of simian virus 40 large T antigen in override of p53 growth suppression. J Virol. 2000;74:864–874. doi: 10.1128/jvi.74.2.864-874.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hiscott J B, Defendi V. Simian virus 40 gene A regulation of cellular DNA synthesis. II. In nonpermissive cells. J Virol. 1981;37:802–812. doi: 10.1128/jvi.37.2.802-812.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Howe A K, Gaillard S, Bennett J S, Rundell K. Cell cycle progression in monkey cells expressing simian virus 40 small t antigen from adenovirus vectors. J Virol. 1998;72:9637–9644. doi: 10.1128/jvi.72.12.9637-9644.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Howe J A, Mymryk J S, Egan C, Branton P E, Bayley S T. Retinoblastoma growth suppressor and a 300-kDa protein appear to regulate cellular DNA synthesis. Proc Natl Acad Sci USA. 1990;87:5883–5887. doi: 10.1073/pnas.87.15.5883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jog P, Joshi B, Dhamankar V, Imperiale M J, Rutila J, Rundell K. Mutational analysis of simian virus 40 small-t antigen. J Virol. 1990;64:2895–2900. doi: 10.1128/jvi.64.6.2895-2900.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.King K L, Cidlowski J A. Cell cycle regulation and apoptosis. Annu Rev Physiol. 1998;60:601–617. doi: 10.1146/annurev.physiol.60.1.601. [DOI] [PubMed] [Google Scholar]
- 33.Kolzau T, Hansen R S, Zahra D, Reddel R R, Braithwaite A W. Inhibition of SV40 large T antigen induced apoptosis by small T antigen. Oncogene. 1999;18:5598–5603. doi: 10.1038/sj.onc.1202942. [DOI] [PubMed] [Google Scholar]
- 34.Lavoie J N, Nguyen M, Marcellus R C, Branton P E, Shore G C. E4orf4: a novel adenovirus death factor that induces p53-independent apoptosis by a pathway that is not inhibited by zVAD-fmk. J Cell Biol. 1998;140:637–645. doi: 10.1083/jcb.140.3.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lerga A, Richard C, Delgado M D, Canelles M, Frade P, Cuadrado M A, Leon J. Apoptosis and mitotic arrest are two independent effects of the protein phosphatases inhibitor okadaic acid in K562 leukemia cells. Biochem Biophys Res Commun. 1999;260:256–264. doi: 10.1006/bbrc.1999.0852. [DOI] [PubMed] [Google Scholar]
- 36.Lowe S W, Ruley H E. Stabilization of the p53 tumor suppressor is induced by adenovirus 5 E1A and accompanies apoptosis. Genes Dev. 1993;7:535–545. doi: 10.1101/gad.7.4.535. [DOI] [PubMed] [Google Scholar]
- 37.Manfredi J J, Prives C. The transforming activity of simian virus 40 large tumor antigen. Biochim Biophys Acta. 1994;1198:65–83. doi: 10.1016/0304-419x(94)90006-x. [DOI] [PubMed] [Google Scholar]
- 38.Marcellus R C, Chan H, Paquette D, Thirlwell S, Boivin D, Branton P E. Induction of p53-independent apoptosis by the adenovirus E4orf4 protein requires binding to the Bα subunit of protein phosphatase 2A. J Virol. 2000;74:7869–7877. doi: 10.1128/jvi.74.17.7869-7877.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marcellus R C, Lavoie J N, Boivin D, Shore G C, Ketner G, Branton P E. The early region 4 orf4 protein of human adenovirus type 5 induces p53-independent cell death by apoptosis. J Virol. 1998;72:7144–7153. doi: 10.1128/jvi.72.9.7144-7153.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Martens I, Nilsson S A, Linder S, Magnusson G. Mutational analysis of polyomavirus small-T-antigen functions in productive infection and in transformation. J Virol. 1989;63:2126–2133. doi: 10.1128/jvi.63.5.2126-2133.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Martin R G, Chou J Y. Simian virus 40 functions required for the establishment and maintenance of malignant transformation. J Virol. 1975;15:599–612. doi: 10.1128/jvi.15.3.599-612.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mateer S C, Fedorov S A, Mumby M C. Identification of structural elements involved in the interaction of simian virus 40 small tumor antigen with protein phosphatase 2A. J Biol Chem. 1998;273:35339–35346. doi: 10.1074/jbc.273.52.35339. [DOI] [PubMed] [Google Scholar]
- 43.McCarthy S A, Symonds H S, Van Dyke T. Regulation of apoptosis in transgenic mice by simian virus 40 T antigen-mediated inactivation of p53. Proc Natl Acad Sci USA. 1994;91:3979–3983. doi: 10.1073/pnas.91.9.3979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McCloskey T W, Ott M, Tribble E, Khan S A, Teichberg S, Paul M O, Pahwa S, Verdin E, Chirmule N. Dual role of HIV Tat in regulation of apoptosis in T cells. J Immunol. 1997;158:1014–1019. [PubMed] [Google Scholar]
- 45.Millward T A, Zolnierowicz S, Hemmings B A. Regulation of protein kinase cascades by protein phosphatase 2A. Trends Biochem Sci. 1999;24:186–191. doi: 10.1016/s0968-0004(99)01375-4. [DOI] [PubMed] [Google Scholar]
- 46.Morimoto Y, Ohba T, Kobayashi S, Haneji T. The protein phosphatase inhibitors okadaic acid and calyculin A induce apoptosis in human osteoblastic cells. Exp Cell Res. 1997;230:181–186. doi: 10.1006/excr.1996.3404. [DOI] [PubMed] [Google Scholar]
- 47.Mullane K P, Ratnofsky M, Cullere X, Schaffhausen B. Signaling from polyomavirus middle T and small T defines different roles for protein phosphatase 2A. Mol Cell Biol. 1998;18:7556–7564. doi: 10.1128/mcb.18.12.7556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mungre S, Enderle K, Turk B, Porras A, Wu Y Q, Mumby M C, Rundell K. Mutations which affect the inhibition of protein phosphatase 2A by simian virus 40 small-t antigen in vitro decrease viral transformation. J Virol. 1994;68:1675–1681. doi: 10.1128/jvi.68.3.1675-1681.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nuydens R, de Jong M, Van Den Kieboom G, Heers C, Dispersyn G, Cornelissen F, Nuyens R, Borgers M, Geerts H. Okadaic acid-induced apoptosis in neuronal cells: evidence for an abortive mitotic attempt. J Neurochem. 1998;70:1124–1133. doi: 10.1046/j.1471-4159.1998.70031124.x. [DOI] [PubMed] [Google Scholar]
- 50.Ogryzko V V, Wong P, Howard B H. WAF1 retards S-phase progression primarily by inhibition of cyclin-dependent kinases. Mol Cell Biol. 1997;17:4877–4882. doi: 10.1128/mcb.17.8.4877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ojala P M, Yamamoto K, Castanos-Velez E, Biberfeld P, Korsmeyer S J, Makela T P. The apoptotic v-cyclin-CDK6 complex phosphorylates and inactivates Bcl-2. Nat Cell Biol. 2000;2:819–825. doi: 10.1038/35041064. [DOI] [PubMed] [Google Scholar]
- 52.Pallas D C, Shahrik L K, Martin B L, Jaspers S, Miller T B, Brautigan D L, Roberts T M. Polyoma small and middle T antigens and SV40 small t antigen form stable complexes with protein phosphatase 2A. Cell. 1990;60:167–176. doi: 10.1016/0092-8674(90)90726-u. [DOI] [PubMed] [Google Scholar]
- 53.Pan H, Griep A E. Altered cell cycle regulation in the lens of HPV-16 E6 or E7 transgenic mice: implications for tumor suppressor gene function in development. Genes Dev. 1994;8:1285–1299. doi: 10.1101/gad.8.11.1285. [DOI] [PubMed] [Google Scholar]
- 54.Phillips A C, Ernst M K, Bates S, Rice N R, Vousden K H. E2F-1 potentiates cell death by blocking antiapoptotic signaling pathways. Mol Cell. 1999;4:771–781. doi: 10.1016/s1097-2765(00)80387-1. [DOI] [PubMed] [Google Scholar]
- 55.Porras A, Bennett J, Howe A, Tokos K, Bouck N, Henglein B, Sathyamangalam S, Thimmapaya B, Rundell K. A novel simian virus 40 early region domain mediates transactivation of the cyclin A promoter by small-t antigen and is required for transformation in small-t antigen-dependent assays. J Virol. 1996;70:6902–6908. doi: 10.1128/jvi.70.10.6902-6908.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Porras A, Gaillard S, Rundell K. The simian virus 40 small-t and large-T antigens jointly regulate cell cycle reentry in human fibroblasts. J Virol. 1999;73:3102–3107. doi: 10.1128/jvi.73.4.3102-3107.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Querido E, Marcellus R C, Lai A, Charbonneau R, Teodoro J G, Ketner G, Branton P E. Regulation of p53 levels by the E1B 55-kilodalton protein and E4orf6 in adenovirus-infected cells. J Virol. 1997;71:3788–3798. doi: 10.1128/jvi.71.5.3788-3798.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rajesh D, Schell K, Verma A K. Ras mutation, irrespective of cell type and p53 status, determines a cell's destiny to undergo apoptosis by okadaic acid, an inhibitor of protein phosphatase 1 and 2A. Mol Pharmacol. 1999;56:515–525. doi: 10.1124/mol.56.3.515. [DOI] [PubMed] [Google Scholar]
- 59.Roulston A, Marcellus R C, Branton P E. Viruses and apoptosis. Annu Rev Microbiol. 1999;53:577–628. doi: 10.1146/annurev.micro.53.1.577. [DOI] [PubMed] [Google Scholar]
- 60.Rubin H, Figge J, Bladon M T, Chen L B, Ellman M, Bikel I, Farrell M, Livingston D M. Role of small t antigen in the acute transforming activity of SV40. Cell. 1982;30:469–480. doi: 10.1016/0092-8674(82)90244-6. [DOI] [PubMed] [Google Scholar]
- 61.Rundell K, Gaillard S, Porras A. Small-t and large-T antigens cooperate to drive cell proliferation. Dev Biol Stand. 1998;94:289–295. [PubMed] [Google Scholar]
- 62.Rundell K, Parakati R. The role of the SV40 ST antigen in cell growth promotion and transformation. Semin Cancer Biol. 2001;11:5–13. doi: 10.1006/scbi.2000.0341. [DOI] [PubMed] [Google Scholar]
- 63.Ryan K M, Ernst M K, Rice N R, Vousden K H. Role of NF-κB in p53-mediated programmed cell death. Nature. 2000;404:892–897. doi: 10.1038/35009130. [DOI] [PubMed] [Google Scholar]
- 64.Samuelson A V, Lowe S W. Selective induction of p53 and chemosensitivity in RB-deficient cells by E1A mutants unable to bind the RB-related proteins. Proc Natl Acad Sci USA. 1997;94:12094–12099. doi: 10.1073/pnas.94.22.12094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schlegel R, Benjamin T L. Cellular alterations dependent upon the polyoma virus Hr-t function: separation of mitogenic from transforming capacities. Cell. 1978;14:587–599. doi: 10.1016/0092-8674(78)90244-1. [DOI] [PubMed] [Google Scholar]
- 66.Schuchner S, Wintersberger E. Binding of polyomavirus small T antigen to protein phosphatase 2A is required for elimination of p27 and support of S-phase induction in concert with large T antigen. J Virol. 1999;73:9266–9273. doi: 10.1128/jvi.73.11.9266-9273.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sheng Q, Love T M, Schaffhausen B. J domain-independent regulation of the Rb family by polyomavirus large T antigen. J Virol. 2000;74:5280–5290. doi: 10.1128/jvi.74.11.5280-5290.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shtrichman R, Kleinberger T. Adenovirus type 5 E4 open reading frame 4 protein induces apoptosis in transformed cells. J Virol. 1998;72:2975–2982. doi: 10.1128/jvi.72.4.2975-2982.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shtrichman R, Sharf R, Barr H, Dobner T, Kleinberger T. Induction of apoptosis by adenovirus E4orf4 protein is specific to transformed cells and requires an interaction with protein phosphatase 2A. Proc Natl Acad Sci USA. 1999;96:10080–10085. doi: 10.1073/pnas.96.18.10080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sleigh M J, Topp W C, Hanich R, Sambrook J F. Mutants of SV40 with an altered small t protein are reduced in their ability to transform cells. Cell. 1978;14:79–88. doi: 10.1016/0092-8674(78)90303-3. [DOI] [PubMed] [Google Scholar]
- 71.Slinskey A, Barnes D, Pipas J M. Simian virus 40 large T antigen J domain and Rb-binding motif are sufficient to block apoptosis induced by growth factor withdrawal in a neural stem cell line. J Virol. 1999;73:6791–6799. doi: 10.1128/jvi.73.8.6791-6799.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sontag E, Fedorov S, Kamibayashi C, Robbins D, Cobb M, Mumby M. The interaction of SV40 small tumor antigen with protein phosphatase 2A stimulates the map kinase pathway and induces cell proliferation. Cell. 1993;75:887–897. doi: 10.1016/0092-8674(93)90533-v. [DOI] [PubMed] [Google Scholar]
- 73.Sontag E, Sontag J M, Garcia A. Protein phosphatase 2A is a critical regulator of protein kinase C zeta signaling targeted by SV40 small t to promote cell growth and NF-κB activation. EMBO J. 1997;16:5662–5671. doi: 10.1093/emboj/16.18.5662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Srinivasan A, McClellan A J, Vartikar J, Marks I, Cantalupo P, Li Y, Whyte P, Rundell K, Brodsky J L, Pipas J M. The amino-terminal transforming region of simian virus 40 large T and small t antigens functions as a J domain. Mol Cell Biol. 1997;17:4761–4773. doi: 10.1128/mcb.17.8.4761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Stubdal H, Zalvide J, Campbell K S, Schweitzer C, Roberts T M, DeCaprio J A. Inactivation of pRB-related proteins p130 and p107 mediated by the J domain of simian virus 40 large T antigen. Mol Cell Biol. 1997;17:4979–4990. doi: 10.1128/mcb.17.9.4979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sugano S, Yamaguchi N, Shimojo H. Small t protein of simian virus 40 is required for dense focus formation in a rat cell line. J Virol. 1982;41:1073–1075. doi: 10.1128/jvi.41.3.1073-1075.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Symonds H, Krall L, Remington L, Saenz-Robles M, Lowe S, Jacks T, Van Dyke T. p53-dependent apoptosis suppresses tumor growth and progression in vivo. Cell. 1994;78:703–711. doi: 10.1016/0092-8674(94)90534-7. [DOI] [PubMed] [Google Scholar]
- 78.Tooze J E. DNA tumor viruses. The molecular biology of tumor viruses. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1980. [Google Scholar]
- 79.Tsai S C, Pasumarthi K B, Pajak L, Franklin M, Patton B, Wang H, Henzel W J, Stults J T, Field L J. Simian virus 40 large T antigen binds a novel Bcl-2 homology domain 3-containing proapoptosis protein in the cytoplasm. J Biol Chem. 2000;275:3239–3246. doi: 10.1074/jbc.275.5.3239. [DOI] [PubMed] [Google Scholar]
- 80.Virshup D M. Protein phosphatase 2A: a panoply of enzymes. Curr Opin Cell Biol. 2000;12:180–185. doi: 10.1016/s0955-0674(99)00074-5. [DOI] [PubMed] [Google Scholar]
- 81.Wang W B, Bikel I, Marsilio E, Newsome D, Livingston D M. Transrepression of RNA polymerase II promoters by the simian virus 40 small t antigen. J Virol. 1994;68:6180–6187. doi: 10.1128/jvi.68.10.6180-6187.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wang X, Martindale J L, Holbrook N J. Requirement for ERK activation in cisplatin-induced apoptosis. J Biol Chem. 2000;275:39435–39443. doi: 10.1074/jbc.M004583200. [DOI] [PubMed] [Google Scholar]
- 83.Watanabe G, Howe A, Lee R J, Albanese C, Shu I W, Karnezis A N, Zon L, Kyriakis J, Rundell K, Pestell R G. Induction of cyclin D1 by simian virus 40 small tumor antigen. Proc Natl Acad Sci USA. 1996;93:12861–12866. doi: 10.1073/pnas.93.23.12861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Whalen B, Laffin J, Friedrich T D, Lehman J M. SV40 small T antigen enhances progression to >G2 during lytic infection. Exp Cell Res. 1999;251:121–127. doi: 10.1006/excr.1999.4572. [DOI] [PubMed] [Google Scholar]
- 85.Yan Y, Shay J W, Wright W E, Mumby M C. Inhibition of protein phosphatase activity induces p53-dependent apoptosis in the absence of p53 transactivation. J Biol Chem. 1997;272:15220–15226. doi: 10.1074/jbc.272.24.15220. [DOI] [PubMed] [Google Scholar]
- 86.Yang S I, Lickteig R L, Estes R, Rundell K, Walter G, Mumby M C. Control of protein phosphatase 2A by simian virus 40 small-t antigen. Mol Cell Biol. 1991;11:1988–1995. doi: 10.1128/mcb.11.4.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yen A, Placanica L, Bloom S, Varvayanis S. Polyomavirus small t antigen prevents retinoic acid-induced retinoblastoma protein hypophosphorylation and redirects retinoic acid-induced G0 arrest and differentiation to apoptosis. J Virol. 2001;75:5302–5314. doi: 10.1128/JVI.75.11.5302-5314.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zalvide J, Stubdal H, DeCaprio J A. The J domain of simian virus 40 large T antigen is required to functionally inactivate RB family proteins. Mol Cell Biol. 1998;18:1408–1415. doi: 10.1128/mcb.18.3.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]