Abstract
We are using avian leukosis-sarcoma virus (ALSV) vectors to generate mouse tumor models in transgenic mice expressing TVA, the receptor for subgroup A ALSV. Like other classical retroviruses, ALSV requires cell division to establish a provirus after infection of host cells. In contrast, lentiviral vectors are capable of integrating their viral DNA into the genomes of nondividing cells. With the intention of initiating tumorigenesis in resting, TVA-positive cells, we have developed a system for the preparation of a human immunodeficiency virus type 1 (HIV-1)-based lentiviral vector, pseudotyped with the envelope protein of ALSV subgroup A (EnvA). The HIV(ALSV-A) vector retains the requirement for TVA on the surface of target cells and can be produced at titers of 5 × 103 infectious units (IU)/ml. By inserting the central polypurine tract (cPPT) from the HIV-1 pol gene and removing the cytoplasmic tail of EnvA, the pseudotype can be produced at titers approaching 105 IU/ml and can be concentrated by ultracentrifugation to titers of 107 IU/ml. HIV(ALSV-A) also infects embryonic fibroblasts derived from transgenic mice in which TVA expression is driven by the β-actin promoter. In addition, this lentivirus pseudotype efficiently infects these fibroblasts after cell cycle arrest, when they are resistant to infection by ALSV vectors. This system may be useful for introducing genes into somatic cells in adult TVA transgenic animals and allows evaluation of the effects of altered gene expression in differentiated cell types in vivo.
Avian leukosis-sarcoma viruses (ALSV) have the ability to infect avian cells efficiently and replicate to high titer. Mammalian cells, however, are resistant to infection by these avian retroviruses and produce undetectable levels of infectious virus when rare infections occur (32). In 1993, Bates et al. cloned the gene that encodes the receptor for subgroup A ALSV (ALSV-A), termed TVA, and demonstrated that exogenous production of TVA on the surface of mammalian cells was both necessary and sufficient for the efficient infection of mammalian cells by ALSV-A (1, 34). Since susceptibility to infection is conferred by TVA, tissue- and cell type-specific infection in vivo can be achieved by expressing TVA from cell type- or tissue-specific promoters in transgenic animals. Federspiel et al. first demonstrated the utility of this system through specific infection of myocytes in which expression of TVA was directed by the α-actin promoter (6). Subsequent studies have demonstrated that this phenomenon is not restricted to a single cell type (8, 13, 21).
We are utilizing the TVA system to generate mouse tumor models for several types of human malignancy (8). In the mouse models generated to date, the target organs are readily accessible at birth, allowing the delivery of replication-competent ALSV vectors at a time when the target cells are still actively proliferating. However, in other organ systems, such as the pancreas, the target cells are not accessible at birth and proliferate very slowly in the adult animal (5). Like other classical retroviruses, ALSV vectors require cells to be actively dividing for the establishment of a provirus to occur (16, 19, 28). Therefore, for infection of nondividing cells in vivo, a retroviral vector that can generate a provirus in the absence of cell division is required.
Lentiviruses can integrate viral DNA into the genomes of nondividing cells (16, 19, 28). Naldini et al. have previously described the generation of a replication-deficient human immunodeficiency virus type 1 (HIV-1)-based vector pseudotyped with the vesicular stomatitis virus (VSV) envelope glycoprotein (VSVG) (22, 23). This vector can be generated at titers of 106 infectious units (IU)/ml and can infect many species and cell types. In addition, this vector was also shown to be more effective than a VSVG pseudotyped murine leukemia virus (MLV) vector at infecting several cell lineages in adult animals in vivo (15, 22, 23). Subsequently, other HIV-based pseudotypes have been described, as well as vectors based on other lentiviruses (25, 26, 27, 30).
To expand the utility of TVA technology, we sought to develop a replication-deficient, HIV-1-based lentiviral vector, pseudotyped with the envelope glycoprotein for ALSV subgroup A, named HIV(ALSV-A). We show here that this lentiviral vector can be produced at titers greater than 5 × 104 IU/ml and that it is stable during ultracentrifugation and can thus be concentrated to titers of 107 IU/ml. This vector retains the specificity of ALSV-based vectors, infecting only those mammalian cells engineered to express TVA. Further, this vector infects primary cells from multiple mouse tissues in culture and infects cell cycle-arrested mouse embryo fibroblasts (MEFs) that are resistant to infection by ALSV vectors. The development of this pseudotyped lentivirus vector will allow the expansion of TVA-directed gene delivery to include nondividing and terminally differentiated cells.
MATERIALS AND METHODS
Plasmids.
A self-inactivating lentiviral vector plasmid pCS-CG expressing green fluorescent protein (GFP) from an internal cytomegalovirus (CMV) promoter was used as a transfer vector (20). The packaging plasmids pCMVΔR 8.2 (encoding all accessory proteins), and pCMVΔR 8.91 (deleted for all accessory proteins) were used to express the HIV-1 gag, pol, rev, and tat gene products (22, 37). The ALSV-A envelope protein (EnvA) was expressed from plasmid pCB6WTA, and the VSVG envelope glycoprotein was expressed from plasmid pMD.G (10, 22). To generate the plasmid pCS-CG cPPT, a 118-bp fragment of the central polypurine tract was amplified from plasmid pCMVΔR 8.91 utilizing the primers cPPT 5′ Bam (5′-GCGGGGATCCTTTTAAAAGAAAAGGGGGG-3′) and cPPT 3′ (5′-GCGGAGATCTAAAATTTTGAATTTTTGTAATTTG-3′), digested with BamHI and BglII, and inserted into pCS-CG at the BamHI site upstream of the internal CMV promoter. The plasmid pCB6WTAΔ513 was generated by amplification of EnvA from the 5′ untranslated region to codon 513 with primers EnvA 5′ (5′-GCGGCAGGTACCCGTGCAGGGAGCCAACATACCC-3′) and EnvA 3′B (5′-GGCGCGGATCCGTCAGCATACGATTTGCAAAAGGCAAG-3′), digestion of the PCR product with Asp718 and BamHI, and insertion into pCB6 digested with the same restriction enzymes. pCB6WTA/VCT was generated by amplifying the same region of EnvA and fusing it in frame to the last 90 coding base pairs of the VSVG envelope glycoprotein gene, amplified with primers VSVG cyto (5′-GCGCGCTCGAGCGAGTTGGTATTTATCTTTA-3′) and VSVG 3′ (5′-GGCGCGGATCCGTTACCTTCCAAGTCGGTTCATCTC-3′), and inserted into the Asp718 and BamHI sites of pCB6. All PCRs were performed using Pfu Turbo DNA polymerase (Stratagene) in 10% dimethyl sulfoxide under the following amplification conditions: 94°C for 5 min, followed by 30 cycles at 94°C for 45 s, 50°C for 1 min, and 72°C for 2 min.
Vector production.
Replication-deficient lentiviral vectors were generated by transfection of three plasmids into 293T cells using calcium phosphate as previously described (22). Viral supernatant was collected 60 to 65 h after transfection. p24 levels were determined using the p24 enzyme-linked immunosorbent assay (ELISA) kit (Beckman-Coulter) according to the manufacturer's protocol. Serial dilution was performed by testing increasing dilutions of viral supernatant against 2 × 104 293-TVA cells. The percentage of GFP-positive cells was determined by flow cytometry. The infectious titer was determined for dilution ranges that showed a linear relationship. Vector was concentrated by ultracentrifugation of viral supernatant for 90 min at 50,000 × g. Particles were resuspended at 1/100 of the original volume.
Replication-competent ALSV-A GFP (RCAS-GFP) virus was collected from stable producing DF-1-GFP cells. The titer was determined by limiting dilution titration on DF-1 cells.
Cell lines.
The cell lines 293-TVA and Rat1a TVA were generated by stable transfection of 293 cells and Rat1a cells, respectively, with pCDNA6 TVA 950 and selection of clones with blasticidin (10 μg/ml). For 293-TVA cells, clone B3 was utilized for all experiments; for Rat1a TVA cells, clone 11 was used. MEFs were isolated from embryos of β-actin TVA transgenic mice (7). DF-1 cells have been previously described (12, 29).
Infection of target cells.
A total of 2 × 104 cells were placed in 12-well culture dishes and incubated with either HIV-GFP(ALSV-A), RCAS-GFP, or HIV-GFP(VSVG) overnight in the presence of 8 μg of Polybrene (Sigma)/ml. Culture medium was then replaced and cells grown for an additional 4 days. The percentage of GFP-positive cells was then determined by flow cytometry.
Cell cycle arrest.
For gamma-irradiation induced arrest, MEFs were treated with 7.5 Gy of gamma irradiation, centrifuged, resuspended, and plated at a density of 2 × 104 cells per well in six-well dishes. For colcemid-induced arrest, 2 × 104 cells were plated per well in six-well dishes, allowed to adhere for 12 h, and then treated with 20 ng of colcemid/ml. In both cases, viral vectors encoding GFP were added 30 h after initiation of arrest. For HIV-GFP(ALSV-A), 275 μl of virus (titer, 2.4 × 106) was added to each well; for RCAS-GFP, 160 μl of virus (titer, 1.3 × 106) was added to each well. Both amounts of virus were within the linear range as determined by serial dilution. Untreated cells were plated at the same density and infected simultaneously. Separate plates of cells treated identically and simultaneously were analyzed for cell cycle status by propidium iodide staining and flow cytometry 30 h after initiation of arrest.
RESULTS
Generation of an ALSV-A pseudotyped HIV vector.
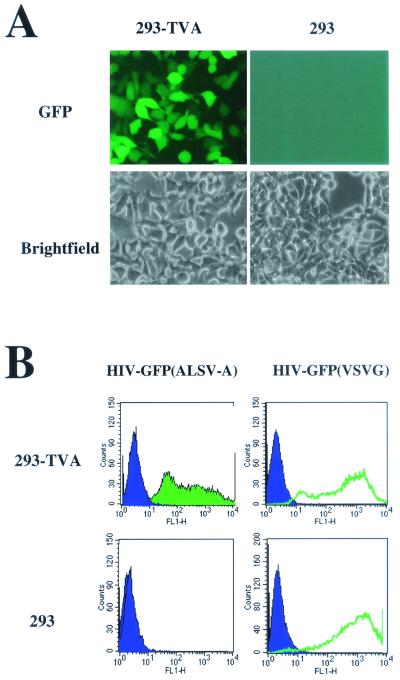
Mammalian cells are resistant to infection by ALSV-A vectors. Cells engineered to express the ALSV-A receptor TVA on their surface are rendered susceptible to infection by these vectors. ALSV-A vectors, like other classical retroviruses but unlike lentiviruses, require cells to be dividing for infection to occur. To expand the use of TVA-mediated gene delivery, we sought to develop a lentiviral vector whose entry is dependent on the presence of TVA on the surface of target cells. To generate pseudotyped HIV-based vectors, three plasmids—pCS-CG, which encodes GFP driven by the CMV promoter; pCMVΔR 8.2, which encodes the HIV structural and accessory proteins; and either pCB6WTA, encoding the ALSV-A envelope glycoprotein, or pMD.G, which encodes the VSVG envelope glycoprotein—were cotransfected into 293T cells, and the viral supernatant was harvested 60 to 65 h after transfection. The collected supernatants were placed on 293 cells engineered to express TVA (293-TVA cells) and parental 293 cells. More than 95% of 293-TVA cells were infected by the EnvA pseudotyped viral particles [HIV(ALSV-A)], whereas the parental 293 cells were not infected (Fig. 1). However, both cell lines were equally susceptible to infection by VSVG pseudotyped HIV particles [HIV(VSVG)] (Fig. 1B). These data demonstrate that the ALSV-A envelope can pseudotype HIV-1-based vectors and that these pseudotyped vectors require the presence of TVA on the surface of target cells for infection. 293-TVA cells infected with the HIV(ALSV-A) vector maintained expression of the GFP cassette for greater than 10 months (data not shown).
FIG. 1.
Infection of 293-TVA cells by the HIV-GFP(ALSV-A) vector. (A) Fluorescent and bright-field images of 293 and 293-TVA cells exposed to the HIV-GFP(ALSV-A) vector. (B) Flow cytometry plots of 293-TVA (upper panels) and 293 cells (lower panels) exposed to HIV-GFP(ALSV-A) (left) and HIV-GFP(VSVG) (right).
Having demonstrated that EnvA can pseudotype an HIV vector, we next sought to determine whether HIV accessory proteins, such as vpr, vpu, and nef, affect the infectious titer of the pseudotype. Vector particles were generated with plasmid pCMVΔR 8.2, which encodes all of the accessory proteins, or pCMVΔR 8.91, in which all accessory protein genes are deleted. Similar infectious titers were obtained with both constructs, suggesting that the HIV accessory proteins do not strongly influence the ability of the generated vector to infect 293-TVA cells (Table 1).
TABLE 1.
Role of HIV-1 accessory proteins in the infection of 293-TVA cells by HIV-GFP(ALSV-A)a
| Construct | Accessory genes present | Mean titer (103) ± SD |
|---|---|---|
| pCMVΔR8.2 | All | 4.6 ± 1.8 |
| pCMVΔR8.91 | None | 4.3 ± 2.0 |
The HIV accessory proteins are encoded by vif, vpr, vpu, and nef. The data are from two experiments done in duplicate. The error is standard deviation from the mean.
The HIV-1 central polypurine tract and altered ALSV-A envelopes increase the vector titer.
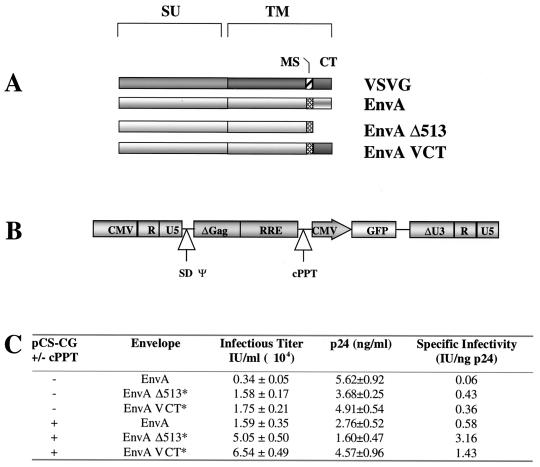
The work of Naldini et al., and subsequently of several other groups, demonstrated that the HIV(VSVG) vector can be generated at titers of ca. 106 to 107 particles per ml, whereas the titer of the HIV(ALSV-A) vector was consistently less than 104 IU/ml (15, 22, 36). Since the only difference between the two pseudotypes is the envelope glycoprotein and since the cytoplasmic tail of the transmembrane segment of the envelope is the only region that contacts the HIV core, we hypothesized that the cytoplasmic tail of the ALSV-A envelope might be inhibiting particle formation. We therefore generated a plasmid encoding EnvA truncated at residue 513, just beyond the membrane spanning region (pCB6 WTAΔ513), and a plasmid encoding a chimera in which the cytoplasmic tail of EnvA was replaced with that of VSVG (pCB6WTA VCT). These modified ALSV-A envelope proteins are shown in schematic form in Fig. 2A. Substitution of full-length EnvA with either of these proteins resulted in a fivefold increase in infectious titer to 1.7 × 104 IU/ml as determined by serial dilution (Fig. 2C).
FIG. 2.
(A) Schematic illustration of the wild-type ALSV-A (EnvA) and VSVG envelopes and altered ALSV-A envelopes. EnvA Δ513 is truncated at amino acid 513, four residues beyond the membrane spanning (MS) region of the envelope. In EnvA VCT the c-terminal 36 amino acids of EnvA, comprising the cytoplasmic tail (CT), are replaced with the 29 amino acids comprising the cytoplasmic tail of VSVG. SU, surface polypeptide; TM, transmembrane polypeptide. (B) Schematic illustration of the insertion point of the cPPT fragment into pCS-CG. The cPPT sequence is given in Follenzi et al. (9). SD, splice donor site; ψ, packaging signal. (C) Effect of altered ALSV-A envelope proteins and cPPT on the infectious and physical titers of generated HIV(ALSV-A) vectors. Data are from a single representative experiment done in triplicate. The error is the standard deviation from the mean. An asterisk indicates a P of <0.01.
Recent work has demonstrated the presence of a short purine-rich sequence (cPPT) within the HIV-1 pol gene that acts to generate a “plus-strand” flap in the double-stranded viral DNA prior to integration. This flap was shown to be important for efficient nuclear import of viral DNA (35). Furthermore, other recent work has demonstrated that insertion of this sequence into the expression plasmid increases the infectious titer of HIV(VSVG) fivefold (9). We therefore modified pCS-CG to include the cPPT sequence and measured its effect on the infectious titer of HIV(ALSV-A) (Fig. 2B). Consistent with the published work, we observed a fivefold increase in the infectious titer from 0.34 × 104 to 1.6 × 104 IU/ml after insertion of the cPPT sequence (Fig. 2C).
When both the cPPT sequence and the altered envelopes were included in the vector particles, a synergistic effect was observed, with the titers increased by ca. 20-fold to 6.5 × 104 IU/ml (Fig. 2C). In subsequent experiments, we have achieved virus preparations with titers of 105 IU/ml that can be concentrated by ultracentrifugation to titers of 107 IU/ml after a 100-fold reduction in volume. In our experiments we routinely recover 75 to 100% of the infectious particles.
When we determined the physical titers of the ALSV-A pseudotypes by using an HIV-1 p24 ELISA, we measured 5.62 ± 0.92 ng of p24/ml for the vector generated with wild-type EnvA and minus cPPT, where 1 ng of p24 is equivalent to ca. 1,000 to 5,000 viral particles (Fig. 2C) (36). This corresponds to a physical titer >15 times higher than the infectious titer determined by serial dilution on 293-TVA cells, suggesting that the majority of p24 was present in noninfectious viral particles. In contrast, we find that the titers determined by serial dilution and p24 ELISA for the HIV(VSVG) vector were similar (data not shown), implying a high specific infectious activity. When we measured the levels of p24 in vector particles with the cPPT sequence and modified envelope proteins, we observed that these changes did not increase the physical titer, although the infectious titers of these vectors were 5- to 20-fold higher (Fig. 2C). Therefore, the insertion of the cPPT sequence into the expression cassette and the removal of the cytoplasmic tail of EnvA act to increase the specific infectivity of the HIV(ALSV-A) vector (Fig. 2C).
Host range of HIV(ALSV-A).
Our main purpose in developing the HIV(ALSV-A) pseudotype was to generate a vector for in vivo gene delivery to stationary cells in TVA transgenic mice. We therefore tested the ability of HIV(ALSV-A) to infect primary mouse cells in culture, as well as cells from two additional nonprimate species, the rat and the chicken, to judge the host range of the pseudotype. The HIV(ALSV-A) vector was 5 to 10 times less efficient at infecting rodent cells than human 293-TVA cells and was a further 10-fold less efficient at infecting chicken cells (Table 2). In comparison, the ALSV-A vector, RCAS-GFP, infects MEFs ca. 30% as efficiently as 293-TVA cells (Table 2). In contrast to the HIV(ALSV-A) vector, the HIV-GFP(VSVG) vector was able to infect human, rat, and chicken cells with equal efficiency, although it was only half as efficient at infecting MEFs (Table 2). It is possible that the reduced infection of MEFs observed with all vectors reflects a difference between primary cells and immortalized cell lines.
TABLE 2.
Relative infection efficiency and host range of RCAS-GFP, HIV-GFP(ALSV-A), and HIV-GFP(VSVG) vectors
| Species | Cell line | Relative infection efficiencya
|
||
|---|---|---|---|---|
| RCAS-GFP | HIV-GFP(ALSV-A) | HIV-GFP(VSVG) | ||
| Human | 293-TVA | 1.00 | 1.00 | 1.00 |
| Mouse | β-Actin TVA MEF | 0.31 | 0.10 | 0.46 |
| Rat | Rat1a TVA | 0.76 | 0.22 | 1.04 |
| Chicken | DF-1 | ND | 0.01 | 1.00 |
For each vector, the infection efficiency for 293-TVA cells is set to 1. All other values are displayed relative to this infection efficiency. The data are from a single representative experiment done in triplicate. ND, not determined.
Infection of nondividing cells by HIV(ALSV-A).
The advantage of lentiviral vectors over classical retroviral vectors is their ability to infect nondividing cells (16). We therefore examined the ability of HIV(ALSV-A) to infect cells arrested during the cell cycle. MEFs from transgenic mice carrying the β-actin TVA transgene were arrested by either gamma irradiation or colcemid treatment. Cells were infected 30 h after the induction of arrest, and cell cycle arrest was verified through propidum iodide staining and flow cytometric analysis. The HIV(ALSV-A) pseudotype infected the arrested cells as efficiently as it did exponentially growing cells, whereas RCAS-GFP was only 10 to 16% as efficient at infecting arrested cells as exponentially growing cultures (Table 3). Next, we determined the ability of HIV(ALSV-A) to infect cells that had exited the cell cycle into G0. We allowed MEFs to exit the cell cycle into G0 by growing them to confluence and maintaining the cultures in 0.1% serum for 3 days after confluence. Cells were then infected with either RCAS-GFP or HIV-GFP(ALSV-A) vectors. Only HIV(ALSV-A) was able to infect the G0 cells (data not shown). Thus, like other HIV-based vectors, the ALSV pseudotype retains the ability to infect cells arrested during the cell cycle, as well as those which have exited the cell cycle into G0.
TABLE 3.
Infection of cell cycle-arrested β-actin TVA MEFs by HIV-GFP(ALSV-A) and RCAS-GFP vectors
| Treatment | Relative infection levela
|
|
|---|---|---|
| HIV-GFP(ALSV-A) | RCAS-GFP | |
| Growing | 1 | 1 |
| Gamma irradiation | 0.84 | 0.16 |
| Colcemid | 0.99 | 0.10 |
Data are from a single representative experiment done in duplicate.
DISCUSSION
ALSV cannot efficiently infect mammalian cells unless these cells are engineered to express the ALSV receptor on their surface (32). The TVA system provides a mechanism to deliver genes in a tissue-specific manner in vivo (6). The gene delivery vectors currently used with this system are all ALSV-based vectors and, as such, have an absolute requirement for cell division for the establishment of infection (16, 19, 28). Indeed, in their initial test of the TVA system in vivo, utilizing α-actin TVA transgenic mice, Federspiel et al. found that the ability of the ALSV vector to infect cells in the myocyte lineage dramatically decreased within a few days after birth (6). The requirement for cell division thus limits the systems to which the TVA technology can be applied.
We have described here the production of a replication-deficient, ALSV-A pseudotyped HIV-1 vector, HIV(ALSV-A). This virus, like the HIV(VSVG) pseudotype, is not produced in the target cells and therefore does not spread to infect neighboring cells. We have demonstrated that infection by HIV(ALSV-A) requires the presence of TVA on the surface of mammalian cells. Thus, using TVA transgenic mice, HIV(ALSV-A) infection can be cell type or tissue restricted in vivo.
HIV(ALSV-A) can be generated at infectious titers approaching 105 IU/ml and is stable during ultracentrifugation and can therefore be concentrated to titers of 107 IU/ml. This titer should be adequate for many in vivo and in vitro applications, particularly in circumstances in which ALSV vectors are ineffective because cells are in a resting state. Our data suggest, at least for 293 cells, that the absence of the HIV accessory proteins does not affect the ability of the pseudotype to infect these cells. However, an extensive study of multiple cell types was not conducted, and it is possible that there are cell types in which specific HIV-1 accessory proteins may affect infection efficiency. Indeed, there are conflicting reports in the literature concerning the requirement of the accessory factors in certain cell types (2, 15, 24).
The ability of EnvA to pseudotype the HIV vector is poor relative to the VSVG envelope glycoprotein, as demonstrated by its relatively low physical and infectious titers. While several heterologous envelopes, such as those of MLV, can pseudotype HIV-based vectors, others, such as that of gibbon ape leukemia virus (GALV), have failed to effectively pseudotype HIV-1-based vectors (27, 30; N. Chinnasamy and R. A. Morgan, unpublished data). We were able to increase the infectious titer of the ALSV pseudotype fivefold by using altered ALSV-A envelope proteins without increasing vector particle formation, suggesting that there are conformational constraints present in wild-type EnvA pseudotyped vectors that prevent proper interaction with receptors. Consistent with this idea, Stitz et al. recently reported that while wild-type GALV envelope cannot form an infectious pseudotype, chimeric GALV or MLV envelopes are capable of forming an infectious HIV-based pseudotype (30).
The HIV(ALSV-A) vector demonstrated the ability to infect cells of human and rodent origin, as did the ALSV-A vector RCAS-GFP. Although the titers for RCAS-GFP and HIV-GFP(ALSV-A) were determined by using different methods, an approximate comparison of the efficiencies of infection can be made. We found that, while both vectors infect 293-TVA cells with similar efficiency, RCAS-GFP is three times more efficient than HIV-GFP(ALSV-A) at infecting dividing MEFs. However, we found that this difference is not apparent for all cell types. Astrocytes from transgenic mice in which TVA expression is driven by the nestin promoter are infected at similar efficiency by both vectors. The HIV(ALSV-A) vector infects chicken cells poorly relative to its ability to infect cells of human and rodent origin. In contrast, we find that an HIV (VSVG) pseudotype is able to infect the chicken cell line DF-1 as efficiently as it does cells of human and rodent origin. One possible reason for the discrepancy between the two pseudotypes may lie in the different entry mechanisms of VSV and ALSV. ALSV utilizes glycoprotein receptors on the membrane surface, whereas VSV has been shown to fuse directly with membrane phospholipids (1, 17, 31, 33, 34). However, we have not formally investigated whether there are differences in other steps during the establishment of infection. Another possible explanation for the difference may be that endogenous TVA expression on DF-1 cells is very low in comparison to that generated in the 293 cell line and MEFs by the potent CMV and β-actin promoters.
We have further demonstrated in this work that HIV(ALSV-A) can readily infect primary murine cells that are arrested in the cell cycle or have exited the cell cycle. MEFs treated with either colcemid or gamma irradiation to induce cell cycle arrest were far less susceptible to infection by ALSV vectors but were equally susceptible to infection by HIV (ALSV-A) relative to logarithmically growing cells. Thus, the HIV(ALSV-A) pseudotype has the characteristic ability of other lentiviral vectors to infect nondividing cells.
Currently, the TVA system and ALSV vectors are used predominantly to generate mouse tumor models. Recently, however, Doetsch et al. and Murphy and Leavitt have used this system to monitor precursors of the glial and megakaryocyte cell lineages, respectively (4, 21). Previous studies have demonstrated effective gene delivery to liver, brain, and lung epithelia and to cells of the hematopoeitic lineage by HIV (VSVG), illustrating that lentivirus vectors can deliver genes efficiently to nondividing cells in vivo (3, 11, 14, 15, 18, 22, 23). These studies indicate that the HIV(ALSV-A) pseudotype should also provide an effective means of gene delivery to stationary cells in vivo. Thus, the development of the HIV (ALSV-A) pseudotype provides another valuable tool for groups interested in manipulating nondividing and terminally differentiated cells in vivo as a means to dissect molecular signaling and developmental pathways in these cells.
ACKNOWLEDGMENTS
B.C.L. and N.C. contributed equally to this study.
We thank Inder Verma (Salk Institute) and Didier Trono (University of Geneva) for the lentivirus constructs; Galen Fisher for pCDNA6 TVA; Judith White and Sue Delos (University of Virginia) for the pCB6WTA plasmid and sequence; Galen Fisher, Eric Collison, and Feng Cong for the MEFs; Doug Foster (University of Minnesota) for DF-1 cells; and Stacie Anderson (NHGRI) and Diane Domingo (MSKCC) for assistance with flow cytometry.
B.C.L. is a Helen Hay Whitney Foundation Fellow.
REFERENCES
- 1.Bates P, Young J A, Varmus H E. A receptor for subgroup A Rous sarcoma virus is related to the low density lipoprotein receptor. Cell. 1993;74:1043–1051. doi: 10.1016/0092-8674(93)90726-7. [DOI] [PubMed] [Google Scholar]
- 2.Chinnasamy D, Chinnasamy N, Enriquez M J, Otsu M, Morgan R A, Candotti F. Lentiviral-mediated gene transfer into human lymphocytes: role of HIV-1 accessory proteins. Blood. 2000;96:1309–1316. [PubMed] [Google Scholar]
- 3.Chinnasamy N, Chinnasamy D, Toso J F, Lapointe R, Candotti F, Morgan R A, Hwu P. Efficient gene transfer to human peripheral blood monocyte-derived dendritic cells using human immunodeficiency virus type 1-based lentiviral vectors. Hum Gene Ther. 2000;11:1901–1909. doi: 10.1089/10430340050129512. [DOI] [PubMed] [Google Scholar]
- 4.Doetsch F, Caille I, Lim D A, Garcia-Verdugo J M, Alvarez-Buylla A. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell. 1999;97:703–716. doi: 10.1016/s0092-8674(00)80783-7. [DOI] [PubMed] [Google Scholar]
- 5.Elsasser H P, Lutke H, Kern H F. Acinar and duct cell replication and regeneration. In: Go V L W, Dimagno E P, Gardner J D, Lebenthal E, Ceber H A, Scheele G A, editors. The exocrine pancreas: biology, pathobiology and diseases. New York, N.Y: Raven Press; 1986. pp. 45–53. [Google Scholar]
- 6.Federspiel M J, Bates P, Young J A, Varmus H E, Hughes S H. A system for tissue-specific gene targeting: transgenic mice susceptible to subgroup A avian leukosis virus-based retroviral vectors. Proc Natl Acad Sci USA. 1994;91:11241–11245. doi: 10.1073/pnas.91.23.11241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Federspiel M J, Swing D A, Eagleson B, Reid S W, Hughes S H. Expression of infected genes in mice generated by infecting blastocysts with avian leukosis virus-based retroviral vectors. Proc Natl Acad Sci USA. 1996;93:4931–4936. doi: 10.1073/pnas.93.10.4931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fisher G H, Orsulic S, Holland E, Hively W P, Li Y, Lewis B C, Williams B O, Varmus H E. Development of a flexible and specific gene delivery system for production of murine tumor models. Oncogene. 1999;18:5253–5260. doi: 10.1038/sj.onc.1203087. [DOI] [PubMed] [Google Scholar]
- 9.Follenzi A, Ailles L E, Bakovic S, Geuna M, Naldini L. Gene transfer by lentiviral vectors is limited by nuclear translocation and rescued by HIV-1 pol sequences. Nat Genet. 2000;25:217–222. doi: 10.1038/76095. [DOI] [PubMed] [Google Scholar]
- 10.Gilbert J M, Hernandez L D, Chernov-Rogan T, White J M. Generation of a water-soluble oligomeric ectodomain of the Rous sarcoma virus envelope glycoprotein. J Virol. 1993;67:6889–6892. doi: 10.1128/jvi.67.11.6889-6892.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guenechea G, Gan O I, Inamitsu T, Dorrell C, Pereira D S, Kelly M, Naldini L, Dick J E. Transduction of human CD34+ CD38− bone marrow and cord blood-derived SCID-repopulating cells with third-generation lentiviral vectors. Mol Ther. 2000;1:566–573. doi: 10.1006/mthe.2000.0077. [DOI] [PubMed] [Google Scholar]
- 12.Himly M, Foster D N, Bottoli I, Iacovoni J S, Vogt P K. The DF-1 chicken fibroblast cell line: transformation induced by diverse oncogenes and cell death resulting from infection by avian leukosis viruses. Virology. 1998;248:295–304. doi: 10.1006/viro.1998.9290. [DOI] [PubMed] [Google Scholar]
- 13.Holland E C, Varmus H E. Basic fibroblast growth factor induces cell migration and proliferation after glia-specific gene transfer in mice. Proc Natl Acad Sci USA. 1998;95:1218–1223. doi: 10.1073/pnas.95.3.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnson L G, Olsen J C, Naldini L, Boucher R C. Pseudotyped human lentiviral vector-mediated gene transfer to airway epithelia in vivo. Gene Ther. 2000;7:568–574. doi: 10.1038/sj.gt.3301138. [DOI] [PubMed] [Google Scholar]
- 15.Kafri T, Blomer U, Peterson D A, Gage F H, Verma I M. Sustained expression of genes delivered directly into liver and muscle by lentiviral vectors. Nat Genet. 1997;17:314–317. doi: 10.1038/ng1197-314. [DOI] [PubMed] [Google Scholar]
- 16.Lewis P F, Emerman M. Passage through mitosis is required for oncoretroviruses but not for the human immunodeficiency virus. J Virol. 1994;68:510–516. doi: 10.1128/jvi.68.1.510-516.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mastromarino P, Conti C, Goldoni P, Hauttecoeur B, Orsi N. Characterization of membrane components of the erythrocyte involved in vesicular stomatitis virus attachment and fusion at acidic pH. J Gen Virol. 1987;68:2359–2369. doi: 10.1099/0022-1317-68-9-2359. [DOI] [PubMed] [Google Scholar]
- 18.May C, Rivella S, Callegari J, Heller G, Gaensler K M, Luzzatto L, Sadelain M. Therapeutic haemoglobin synthesis in beta-thalassaemic mice expressing lentivirus-encoded human beta-globin. Nature. 2000;406:82–86. doi: 10.1038/35017565. [DOI] [PubMed] [Google Scholar]
- 19.Miller D G, Adam M A, Miller A D. Gene transfer by retrovirus vectors occurs only in cells that are actively replicating at the time of infection. Mol Cell Biol. 1990;10:4239–4242. doi: 10.1128/mcb.10.8.4239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miyoshi H, Blomer U, Takahashi M, Gage F H, Verma I M. Development of a self-inactivating lentivirus vector. J Virol. 1998;72:8150–8157. doi: 10.1128/jvi.72.10.8150-8157.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murphy G J, Leavitt A D. A model for studying megakaryocyte development and biology. Proc Natl Acad Sci USA. 1999;96:3065–3070. doi: 10.1073/pnas.96.6.3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Naldini L, Blomer U, Gage F H, Trono D, Verma I M. Efficient transfer, integration, and sustained long-term expression of the transgene in adult rat brains injected with a lentiviral vector. Proc Natl Acad Sci USA. 1996;93:11382–11388. doi: 10.1073/pnas.93.21.11382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Naldini L, Blomer U, Gallay P, Ory D, Mulligan R, Gage F H, Verma I M, Trono D. In vivo gene delivery and stable infection of nondividing cells by a lentiviral vector. Science. 1996;272:263–267. doi: 10.1126/science.272.5259.263. [DOI] [PubMed] [Google Scholar]
- 24.Park F, Ohashi K, Chiu W, Naldini L, Kay M A. Efficient lentiviral infection of liver requires cell cycling in vivo. Nat Genet. 2000;24:49–52. doi: 10.1038/71673. [DOI] [PubMed] [Google Scholar]
- 25.Poeschla E, Gilbert J, Li X, Huang S, Ho A, Wong-Staal F. Identification of a human immunodeficiency virus type 2 (HIV-2) encapsidation determinant and infection of nondividing human cells by HIV-2-based lentivirus vectors. J Virol. 1998;72:6527–6536. doi: 10.1128/jvi.72.8.6527-6536.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Poeschla E M, Wong-Staal F, Looney D J. Efficient infection of nondividing human cells by feline immunodeficiency virus lentiviral vectors. Nat Med. 1998;4:354–357. doi: 10.1038/nm0398-354. [DOI] [PubMed] [Google Scholar]
- 27.Reiser J, Harmison G, Kluepfel-Stahl S, Brady R O, Karlsson S, Schubert M. Transduction of nondividing cells using pseudotyped defective high-titer HIV type 1 particles. Proc Natl Acad Sci USA. 1996;93:15266–15271. doi: 10.1073/pnas.93.26.15266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roe T, Reynolds T C, Yu G, Brown P O. Integration of murine leukemia virus DNA depends on mitosis. EMBO J. 1993;12:2099–2108. doi: 10.1002/j.1460-2075.1993.tb05858.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schaefer-Klein J, Givol I, Barsov E V, Whitcomb J M, VanBrocklin M, Foster D N, Federspiel M J, Hughes S H. The EV-O-derived cell line DF-1 supports the efficient replication of avian leukosis-sarcoma viruses and vectors. Virology. 1998;248:305–311. doi: 10.1006/viro.1998.9291. [DOI] [PubMed] [Google Scholar]
- 30.Stitz J, Buchholz C J, Engelstadter M, Uckert W, Bloemer U, Schmitt I, Cichutek K. Lentiviral vectors pseudotyped with envelope glycoproteins derived from gibbon ape leukemia virus and murine leukemia virus 10A1. Virology. 2000;273:16–20. doi: 10.1006/viro.2000.0394. [DOI] [PubMed] [Google Scholar]
- 31.Superti F, Seganti L, Ruggeri F M, Tinari A, Donelli G, Orsi N. Entry pathway of vesicular stomatitis virus into different host cells. J Gen Virol. 1987;68:387–399. doi: 10.1099/0022-1317-68-2-387. [DOI] [PubMed] [Google Scholar]
- 32.Weiss R. Experimental biology and assay of RNA tumor viruses. In: Weiss R, Teich N, Varmus H, Coffin J, editors. RNA tumor viruses. Vol. 1. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1982. pp. 209–260. [Google Scholar]
- 33.Yamada S, Ohnishi S. Vesicular stomatitis virus binds and fuses with phospholipid domain in target cell membranes. Biochemistry. 1986;25:3703–3708. doi: 10.1021/bi00360a034. [DOI] [PubMed] [Google Scholar]
- 34.Young J A, Bates P, Varmus H E. Isolation of a chicken gene that confers susceptibility to infection by subgroup A avian leukosis and sarcoma viruses. J Virol. 1993;67:1811–1816. doi: 10.1128/jvi.67.4.1811-1816.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zennou V, Petit C, Guetard D, Nerhbass U, Montagnier L, Charneau P. HIV-1 genome nuclear import is mediated by a central DNA flap. Cell. 2000;101:173–185. doi: 10.1016/S0092-8674(00)80828-4. [DOI] [PubMed] [Google Scholar]
- 36.Zufferey R, Dull T, Mandel R J, Bukovsky A, Quiroz D, Naldini L, Trono D. Self-inactivating lentivirus vector for safe and efficient in vivo gene delivery. J Virol. 1998;72:9873–9880. doi: 10.1128/jvi.72.12.9873-9880.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zufferey R, Nagy D, Mandel R J, Naldini L, Trono D. Multiply attenuated lentiviral vector achieves efficient gene delivery in vivo. Nat Biotechnol. 1997;15:871–875. doi: 10.1038/nbt0997-871. [DOI] [PubMed] [Google Scholar]