Abstract
A fundamental event in the pathogenesis of transmissible spongiform encephalopathies (TSE) is the conversion of a normal, proteinase K-sensitive, host-encoded protein, PrP-sen, into its protease-resistant isoform, PrP-res. During the formation of PrP-res, PrP-sen undergoes conformational changes that involve an increase of β-sheet secondary structure. While previous studies in which PrP-sen deletion mutants were expressed in transgenic mice or scrapie-infected cell cultures have identified regions in PrP-sen that are important in the formation of PrP-res, the exact role of PrP-sen secondary structures in the conformational transition of PrP-sen to PrP-res has not yet been defined. We constructed PrP-sen mutants with deletions of the first β-strand, the second β-strand, or the first α-helix and tested whether these mutants could be converted to PrP-res in both scrapie-infected neuroblastoma cells (Sc+-MNB cells) and a cell-free conversion assay. Removal of the second β-strand or the first α-helix significantly altered both processing and the cellular localization of PrP-sen, while deletion of the first β-strand had no effect on these events. However, all of the mutants significantly inhibited the formation of PrP-res in Sc+-MNB cells and had a greatly reduced ability to form protease-resistant PrP in a cell-free assay system. Thus, our results demonstrate that deletion of the β-strands and the first α-helix of PrP-sen can fundamentally affect PrP-res formation and/or PrP-sen processing.
Transmissible spongiform encephalopathies are progressive neuropathological diseases that include scrapie in sheep and goats, bovine spongiform encephalopathy, and Creutzfeldt- Jakob disease in humans. A hallmark of the transmissible spongiform encephalopathies is the accumulation of an abnormal isoform of a cellular protein, termed prion protein (PrP), in the central nervous system and sometimes in the lymphoid tissues of infected individuals (1, 4, 17, 30, 43). This pathologic form of the prion protein, PrP-res, is resistant to limited proteolysis and is closely associated with the infectious agent (35, 46). The precursor of PrP-res is a protease-sensitive protein, termed PrP-sen, which, following cleavage of a leader peptide and addition of a glycophosphatidylinositol anchor (GPI-anchor) (8, 58, 59, 63), consists of approximately 209 amino acid residues (38). PrP-sen can be variably glycosylated at two consensus sites for N-linked glycosylation (Asn180 and Asn196) (55, 61). While the cellular function of PrP-sen remains unclear, recent data suggest that it is involved in signal transduction in differentiated neurons (36).
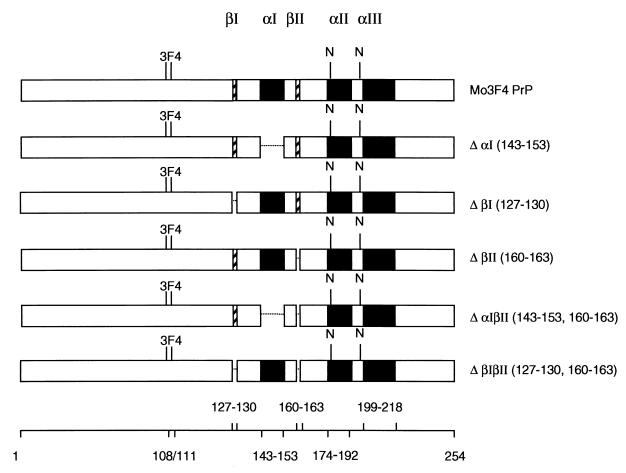
No characteristic sequence differences or chemical modifications between PrP-sen and PrP-res have been detected, indicating that they differ primarily in their conformational structure (44, 60). The nuclear magnetic resonance (NMR) structures of recombinant PrP-sen molecules from mice, Syrian hamsters, cattle, and humans have been determined (18, 33, 52, 66). All these PrP-sen molecules have a common architecture. In mouse PrP-sen, the N-terminal region remains flexibly disordered while residues 122 to 231 form a stable three-helix bundle with the α-helices located at amino acid positions 143 to 153, 174 to 192 and 199 to 218. This globular domain of PrP-sen also contains a short region of antiparallel β-sheet encompassing amino acid residues 127 to 130 and 160 to 163 (Fig. 1) (52, 53; R. Glockshuber, S. Hornemann, R. Riek, G. Wider, M. Billeter, and K. Wuthrich, Letter, Trends Biochem. Sci. 22:241–242, 1997). By contrast, Fourier transform infrared and circular dichroism studies have demonstrated that PrP-res consists largely of β-sheet secondary structure (12, 39, 56).
FIG. 1.
Structure of PrP-sen molecules lacking secondary-structure elements. Deletions of mouse PrP-sen secondary structures were based on NMR data that have been published previously (Glockshuber et al., Letter). Black boxes represent α-helical secondary structures, while hatched boxes symbolize β-strand. Deletions are shown as gaps. Clone names are given on the right, and the amino acid residues deleted are indicated in parentheses. The location of asparagine-linked glycosylation at positions 180 and 196 is indicated (N). To differentiate PrP deletion mutants from endogenous PrP molecules expressed in Sc+-MNB cells, all constructs contained the epitope for mouse monoclonal antibody 3F4. Amino acid positions of PrP-sen secondary-structure elements and the amino acids changed to generate the 3F4 epitope are shown at the bottom.
Conversion of PrP-sen to PrP-res is believed to involve direct interaction of the two PrP isoforms. Two different models have been proposed to explain the transition of PrP-sen to PrP-res. The template assistance model argues that PrP-res synthesis is dependent on the formation of PrP-sen/PrP-res heterodimers (47). In the seeded-polymerization model, the presence of a seed composed of aggregated PrP-res molecules is critical for the formation of new PrP-res (14).
Currently, the majority of available experimental evidence supports the latter hypothesis (6, 11, 19, 25, 45).
The secondary structures in PrP that are critical in the generation of PrP-res from PrP-sen are not fully defined. While deletion of the N-terminal residues 23 to 89 does not significantly affect the formation of PrP-res (21, 54), several studies indicate that the central part of the PrP molecule, encompassing amino acid residues 94 to 188, is critical for PrP-res synthesis (24, 31a, 42, 54, 57). The first α-helix and both β-strands are located in this region. The first α-helix has been proposed to be involved in the binding of PrP-sen to PrP-res (Glockshuber et al., Letter). Furthermore, it has been suggested that the short, antiparallel β-sheet in PrP-sen is involved in nucleating the conformational transition to PrP-res. In this instance, the β-sheet would serve as a nucleation site for the transition to β-strand secondary structure in regions of the molecule that are adjacent to the nucleation site (52; Glockshuber et al., Letter). By contrast, other studies suggested that neither the first β-strand nor the first α-helix were necessary for the formation of protease-resistant PrP. PrP-sen molecules with amino acid residues 23 to 88 and 141 to 176 (including the first α-helix and the second β-strand) (PrP106) deleted were converted to protease-resistant PrP in vivo (62). However, formation of protease-resistant PrP106 was variable in Sc+-MNB cells, indicating that the deleted regions may contain amino acid residues that at least facilitate the formation of PrP-res (37, 62).
The exact role of the first α-helix and the two β-strands in the conversion process has yet to be clearly elucidated. To more precisely study the potential role of individual PrP-sen secondary structures in the formation of PrP-res, we constructed PrP deletion molecules that lacked the β-strands and/or the first α-helix and determined whether these PrP-sen molecules could be converted to PrP-res. Here we show that deletion of either the first or the second β-strand or the first α-helix significantly reduced the ability of PrP-sen to be converted to PrP-res, demonstrating that these PrP-sen secondary structures are involved in PrP-res formation.
MATERIALS AND METHODS
Cell lines.
Scrapie-infected mouse neuroblastoma cells (Sc+-MNB cells) are persistently infected with the mouse-adapted scrapie strain RML and have been described previously (49, 50). These cells express mouse PrP-sen and accumulate mouse PrP-res. They were grown in OptiMEM (Life Technologies Inc.) supplemented with 10% fetal bovine serum (FBS). The retroviral packaging cell lines ψ2 and PA317 were maintained in RPMI containing 10% FBS and 300 U of penicillin per ml.
Antibodies.
The mouse monoclonal antibody 3F4 was raised against Syrian hamster PrP27-30 and recognizes an epitope in hamster PrP that can be inserted into mouse PrP by amino acid substitutions at codons 108 and 111 (2, 29). This antibody was used to distinguish mutant PrP molecules from the endogenous mouse PrP expressed in Sc+-MNB cells, which does not react with mouse monoclonal antibody 3F4. R30 is a rabbit polyclonal antiserum directed against a peptide spanning amino acids 89 to 103 of mouse PrP and reacts with both mutant and endogenous mouse PrP (51).
Site-directed mutagenesis.
Expression of recombinant PrP using the retroviral expression vector pSFF has been described elsewhere (13). All clones contained the 3F4 epitope and reacted with the 3F4 monoclonal antibody. Deletion of sequences coding for mouse PrP-sen secondary structures was performed by PCR mutagenesis. Several different oligonucleotides were used: For deletion of the first β-strand, 5′-GGCCTTGGTGGCAGCGCCATGAGCAGGCCC-3′ and 5′-GCTCATGGCGCTGCCACCAAGGCCCCCCAC-3′ (ΔβI); for deletion of the second β-strand, 5′-TACCGCTACCCTAACCAACCAGTGGATCAGTACAGC-3′ and 5′-CTGATCCACTGGTTGGTTAGGGTAGCGGTAC-3′ (ΔβII); for deletion of the first α-helix; 5′-CATTTTGGCAACTACCGCTACCCTAACC-3′ and 5′-TTGGTTAGGGTAGCGGTAGTTGCCAAAATGGATCATGG-3′ (ΔαI). Deletion of both β-strands as well as deletion of the first α-helix and the second β-strand was generated with these primers following deletion of the second β-strand. PCR fragments were cut with the restriction enzymes NaeI and BstEII and cloned into the open reading frame of 3F4 epitope-tagged mouse PrP (Mo3F4 PrP) as previously described (42). Recombinant PrP molecules (Fig. 1) were cloned into the retroviral expression vector pSFF (13) and verified by DNA sequence determination.
Transduction and analysis of Sc+-MNB cells expressing recombinant PrP.
Transfection of clones into retroviral packaging cells, production of infectious retroviral supernatant, and transduction of Sc+-MNB cells have been described elsewhere (13, 41). The cells were plated at a density of 3 × 105 cells/well in six-well plates. The next day, the cells were incubated with 4 ng of Polybrene per ml for 2 h and then exposed overnight to supernatant containing retroviral particles encoding the recombinant PrP of interest. Expression of recombinant PrP-sen in Sc+-MNB cells was confirmed by Western blot analysis of cell lysates and/or membrane immunofluorescence of live cells. For PrP-sen analysis, confluent cell culture monolayers were lysed in 32 mM sucrose containing 0.5% sodium deoxycholate and 0.5% NP-40. The lysate was cleared by centrifugation, and an equivalent amount of 2× sample buffer (4 M urea, 5% sodium dodecyl sulfate, 3 mM EDTA, 4% β-mercaptoethanol, 5% glycerol, 0.02% bromphenol blue, 63 mM Tris-HCl [pH 6.8]) was added. The samples were assayed on 14% Novex precast gels. For membrane immunofluorescence, live cells were exposed to 3F4 hybridoma cell culture supernatant for 30 min, rinsed three times with phosphate-buffered balanced salt solution (PBBS), and then incubated with fluorescein isothiocyanate-conjugated goat anti-mouse immunoglobulin G for 30 min. The cells were rinsed again in PBBS and analyzed for cell surface expression of recombinant PrP using a fluorescence microscope. Total PrP-res was assayed after proteinase K treatment of cell lysates (20 μg of Proteinase K per ml at 37°C for 1 h) by Western blot analysis using rabbit antiserum R30, while recombinant PrP-res was detected using monoclonal antibody 3F4.
Metabolic labeling and pulse-chase experiments.
Sc+-MNB cells that expressed different PrP constructs were labeled with 15 μCi of [35S]methionine-[35S]cysteine per ml for 10 min, and then 5 ml of OptiMEM–10% FBS was added. The cells were incubated for 0, 20, or 120 min or 8 h. The cells were lysed in lysis buffer (5 mM Tris-HCl [pH 7.4], 140 mM NaCl, 5 mM EDTA, 0.5% sodium deoxycholate, 0.5% Triton X-100). Recombinant PrP molecules were immunoprecipitated with monoclonal antibody 3F4 and then treated with endoglycosidase H (endo H) or N-glycosidase F (PNGase F) as specified by the manufacturer (New England BioLabs Inc., Beverly, Mass.).
For studying endogenous PrP-sen levels, cells were labeled with [35S]methionine-[35S]cysteine for 30 min, OptiMEM–10% FBS was added, and the mixture was incubated for 30 min. Cells were lysed with lysis buffer and centrifuged at 1,000 × g for 5 min. The lysates were precipitated with 4 volumes of methanol, and the pellets were sonicated into DLPC (4.2 mg of l-α-phosphatidylcholine per ml, 123 mM NaCl, 50 mM Tris-HCl [pH 7.5], 1% N-lauroylsarcosine). The resulting protein-lipid complexes were subject to consecutive cycles of immunoprecipitation with antibody 3F4 followed by a final immunoprecipitation with antiserum R30. To demonstrate cell surface expression, labeled cells were washed three times with PBBS and incubated with 0.4 U of phosphatidylinositol-specific phospholipase C (PI-PLC) for 30 min. Total protein in lysates and supernatants were precipitated with 4 volumes of methanol. After centrifugation, the pellets were sonicated into DLPC. Recombinant PrP-sen was first immunoprecipitated using monoclonal antibody 3F4, and the remaining PrP-sen was immunoprecipitated using polyclonal antiserum R30.
PI-PLC treatment.
For PI-PLC treatment of unlabeled cells, Sc+-MNB cells expressing recombinant PrP molecules were incubated with 0.4 U of PI-PLC in PBBS for 30 min. Supernatant was removed and precipitated with 4 volumes of methanol. The pellets were sonicated into sample buffer. Cell monolayers were lysed in lysis buffer, centrifuged to remove cell debris, and mixed with 2× sample buffer. Samples were heated to 95°C for 5 min and analyzed by Western blotting using monoclonal antibody 3F4.
Cell-free conversion assay.
PrP-res was isolated from the brains of VM/DK mice infected with scrapie strain 87V (51). The cell-free conversion assay was performed as described previously (31, 51). Briefly, purified PrP-res was partially denatured in 2.5 M guanidine hydrochloride for 1 h at 37°C. A 200-ng amount of PrP-res was incubated for 48 h at 37°C with 2 ng of radiolabeled PrP-sen isolated from ψ2/PA317 cells (5). One-tenth of the reaction mixture was removed and served as a PrP-sen control, while the remaining sample was treated with proteinase K (PK) for 1 h at 37°C. A proteinase inhibitor (Pefabloc; 2 mM) and carrier protein (thyroglobulin; 5 mg per ml) were added to both PK-treated and untreated samples, and proteins were precipitated with 4 volumes of methanol. Pellets were resuspended in 1× sample buffer and analyzed by SDS-polyacrylamide gel electrophoresis on 16% Novex precast gels.
RESULTS
PrP deletion mutants are not converted to PrP-res in Sc+-MNB cells.
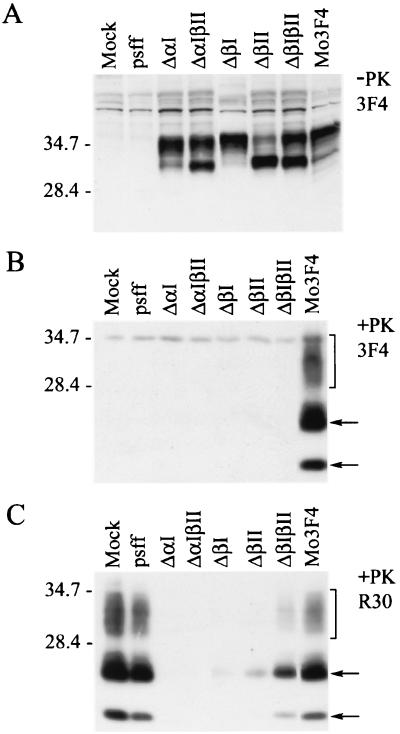
To determine if different PrP-sen secondary structures are involved in the conversion of PrP-sen to PrP-res, PrP mutants lacking the first α-helix and/or the β-strands were generated on the Mo3F4 PrP background (Fig. 1). Mo3F4 PrP-sen deletion mutants were expressed in Sc+-MNB cells using a retroviral expression system (13), and the formation of 3F4-positive PrP-res was assayed. Since the endogenous PrP-res accumulating in Sc+-MNB cells does not react with the 3F4 antibody, 3F4-reactive PrP-sen and PrP-res can be derived only from the recombinant PrP-sen molecules. Expression of recombinant PrP-sen by Sc+-MNB cells was confirmed by Western blotting of cell lysates and demonstrated that all constructs were expressed at similar levels. However, the glycosylation patterns of ΔβII, ΔβIβII, and ΔαIβII significantly differed from that of control Mo3F4 PrP-sen (Fig. 2A). Following protease treatment, substantial amounts of PK-resistant PrP were detected in the extract of cells expressing Mo3F4 PrP (Fig. 2B). By contrast, none of the Mo3F4 PrP-sen deletion mutants was converted to PrP-res. Thus, deletion of the first α-helix and/or either of the β-strands prevented the formation of PrP-res in Sc+-MNB cells.
FIG. 2.
PrP-sen deletion mutants are not converted to PrP-res and interfere with the accumulation of endogenous PrP-res. PrP deletion constructs ΔαI, ΔαIβII, ΔβI, ΔβII, and ΔβIβII were tested for their ability to form PrP-res. Retroviral supernatant coding for vector alone (pSFF), Mo3F4 PrP, or Mo3F4 PrP deletion constructs was used to transduce Sc+-MNB cells. (A) At 8 days posttransduction, cells were lysed and cell lysates were tested for the presence of 3F4-positive PrP-sen by Western blot analysis. All constructs were expressed at similar levels. The mock lane represents a cell lysate from untransduced cells. (B) Sc+-MNB cells were analyzed for recombinant PrP-res by immunoblotting following digestion of cell lysates with proteinase K. This blot was probed with monoclonal antibody 3F4, which detects the mutant PrP molecules but not the endogenous mouse PrP molecules already present in Sc+-MNB cells. The bottom arrow indicates unglycosylated PrP-res, the top arrow indicates partially glycosylated PrP-res, and the bracket indicates fully glycosylated PrP-res. While 3F4-positive PrP-res accumulated in cells transduced with Mo3F4 PrP, none of the deletion constructs was converted to PrP-res. (C) Western blot of PK-treated cell lysates using polyclonal antiserum R30, which reacts with both recombinant and endogenous mouse PrP. Expression of deletion mutant PrP-sen molecules either partially or totally abolished the formation of PrP-res.
Effect of PrP deletion mutants on the accumulation of endogenous mouse PrP-res.
Earlier data demonstrated that expression of hamster PrP molecules interfered with the accumulation of endogenous mouse PrP-res in Sc+-MNB cells (41). This interference was probably due to competition between endogenous and exogenous PrP-sen for either a PrP-res binding site or some as yet unidentified cellular factor that might be involved in the conversion process (26). To study the influence of PrP deletion mutant expression on endogenous PrP-res levels, total PrP-res levels were assayed by Western blotting using polyclonal antiserum R30, which reacts with both endogenous and recombinant PrP. Expression of Mo3F4 PrP-sen deletion mutants ΔαI, ΔβI, and ΔαIβII completely abolished the formation of PrP-res, while incomplete interference was achieved with deletion mutants ΔβII or ΔβIβII. This interference was not due to the retroviral expression system used in this study, since cells that were transduced with the vector alone (pSFF) accumulated similar amounts of PrP-res to those accumulated by nontransduced cells (Fig. 2C, lane Mock). The lower level of interference with ΔβII and ΔβIβII was probably due to a lower transduction efficiency, since complete interference could be detected with these mutants in some experiments where the transduction efficiency was higher (data not shown). These data demonstrate that PrP-sen molecules with deletions at the first α-helix or either of the two β-strands were capable of interfering with the formation of endogenous PrP-res. Furthermore, the results suggest that the inability of these deletion mutants to form PrP-res was not due to an inability to interact with PrP-res or other possible components of the conversion pathway.
Altered cell surface expression of PrP-sen deletion mutants.
The fact that all of the PrP deletion mutants interfered with PrP-res formation suggested that these mutants were located where events in the conversion process take place. Several studies indicate that the interaction of PrP-sen and PrP-res occurs after PrP-sen transits to the plasma membrane (3, 8). We therefore determined whether PrP deletion mutants were present on the cell surface of Sc+-MNB cells. Since experiments with Sc+-MNB cells showed that deletion of the first α-helix or either of the two β-strands was sufficient to prevent the formation of PrP-res, the following experiments were performed with the PrP deletion mutants lacking a single secondary-structure element (ΔαI, ΔβI, or ΔβII).
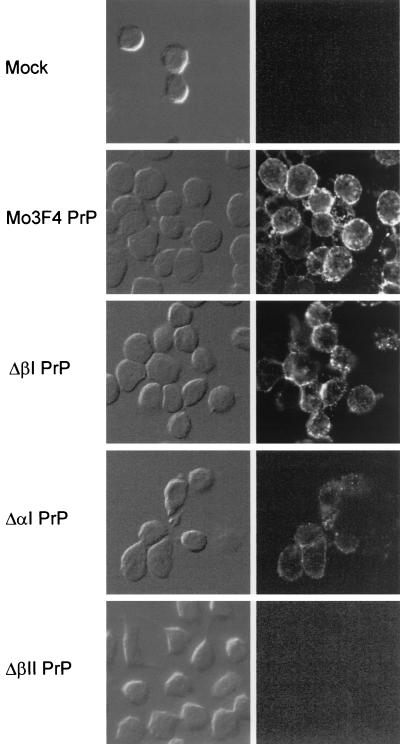
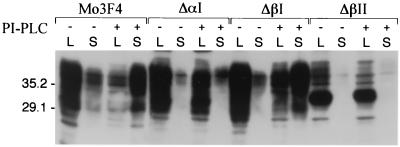
We first examined cell surface expression of the PrP deletion mutants by membrane immunofluorescence of live cells (Fig. 3). Although ΔαI and ΔβI were present on the cell surface, the level of cell surface ΔαI PrP-sen was markedly decreased compared to ΔβI or Mo3F4 PrP-sen. No 3F4-positive PrP could be detected on cells expressing ΔβII PrP. To determine if the weak membrane immunofluorescence observed with ΔαI and ΔβII was due to an inaccessibility of the 3F4 epitope to the antibody under the conditions used, we tested if mutant PrP-sen could be removed from the cell membrane by treatment with PI-PLC, an enzyme that releases wild-type PrP-sen from the cell surface (7, 59). Sc+-MNB cells expressing Mo3F4, ΔαI, ΔβI, or ΔβII PrP were incubated with or without PI-PLC, and supernatant and cell lysates were analyzed for the presence of recombinant PrP. While Mo3F4, ΔαI, and ΔβI PrP-sen could be removed from the cell surface by PI-PLC, only marginal amounts of glycosylated ΔβII PrP-sen were released by PI-PLC treatment or secreted into the cell culture supernatant (Fig. 4). Thus, at least some of the ΔαI molecules and the majority of ΔβII molecules remained inside the cell. These results suggest that interference of PrP-res formation by these mutants occurred inside the cell and not at the plasma membrane.
FIG. 3.
Cell surface expression of PrP deletion mutants. Mo3F4 PrP-sen and Mo3F4 PrP-sen molecules lacking the first α-helix (ΔαI PrP) or the β-strands (ΔβI PrP and ΔβII PrP) were expressed in Sc+-MNB cells. Cell surface expression was confirmed by membrane immunofluorescence of live cells using monoclonal antibody 3F4. Phase-contrast images are shown on the left, and immunofluorescence staining is shown on the right. While Mo3F4, ΔβI, and at least some ΔαI PrP-sen was present on the cell surface, no ΔβII PrP-sen could be detected by membrane immunofluorescence.
FIG. 4.
Sensitivity of Mo3F4 PrP-sen deletion mutants to treatment with PI-PLC. Sc+-MNB cells expressing Mo3F4, ΔαI, ΔβI, or ΔβII PrP were incubated with or without PI-PLC for 30 min. PI-PLC-releasable proteins in the supernatant were precipitated with methanol and sonicated into sample buffer. Cell monolayers were lysed in lysis buffer and mixed with equivalent amounts of 2× sample buffer. Blots were analyzed for the presence of recombinant PrP using the antibody 3F4. To detect PrP-sen in the supernatant, the whole sample was loaded and compared to 1/20 of the cell lysate. Molecular weight markers are indicated on the left in thousands. Lanes: S, supernatant; L, cell lysate.
Altered subcellular trafficking of PrP molecules lacking the first α-helix or the second β-strand.
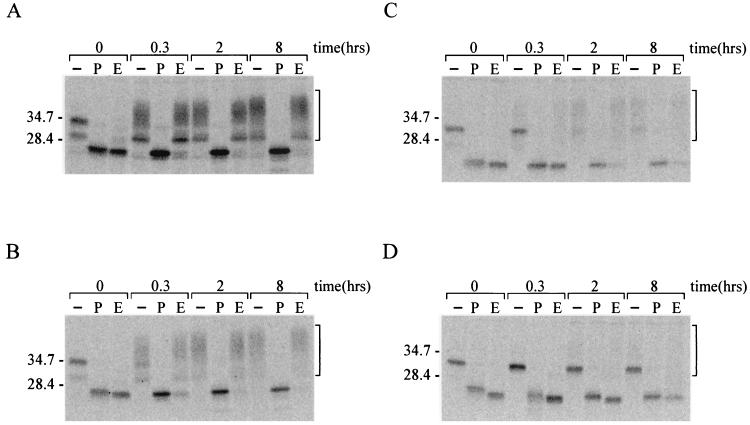
PrP-sen molecules on their way to the cell membrane are cotranslationally modified by the addition of carbohydrate moieties at their two N-linked glycosylation sites. These moieties become progressively altered as the protein traverses through the different compartments of the endoplasmic reticulum (ER) and Golgi (7, 63). To identify the cellular localization of the PrP deletion mutants, we analyzed N-linked glycosylation and the extent of PrP-sen carbohydrate modifications using pulse-chase analysis. Cells expressing Mo3F4 PrP-sen or Mo3F4 PrP-sen deletion mutants ΔαI, ΔβI, and ΔβII were labeled with [35S]methionine-[35S]cysteine and chased with complete medium for several different intervals. Immunoprecipitated, 3F4-positive PrP molecules were treated with PNGase F, an enzyme that removes N-linked oligosaccharides. All PrP molecules were sensitive to PNGase F, indicating that they had been glycosylated at their N-linked glycosylation sites within the lumen of the ER (Fig. 5).
FIG. 5.
Sensitivity of PrP-sen deletion mutants to endo H and PNGase F. To verify N-linked glycosylation and the processing of PrP-associated oligosaccharides to complex sugars, PrP deletion mutants were digested with PNGase F or endo H. Sc+-MNB cells expressing Mo3F4 PrP (A) or Mo3F4 PrP deletion mutants ΔβI (B), ΔαI (C), or ΔβII (D) were labeled with [35S]methionine-[35S]cysteine for 10 min and incubated in OptiMEM–10% FBS for the indicated intervals. PrP was immunoprecipitated from the cell lysate with monoclonal antibody 3F4 and treated with PNGase F or endo H. Samples were analyzed by SDS-PAGE. The brackets indicate glycosylated PrP molecules. Lanes: −, untreated sample; P, PNGase F-treated sample; E, endo H-treated sample. Molecular weight markers are indicated on the left in thousands.
To trace the movement of newly synthesized PrP-sen from the ER to the Golgi, we further examined the sensitivity of mutant PrP-sen to endo H. N-linked carbohydrates remain sensitive to endo H while they are in the ER and in early regions of the Golgi complex but become endo H resistant after they are processed by enzymes located in the medial Golgi. Thus, complex sugars present in terminally glycosylated proteins will not be cleaved by endo H. As expected, Mo3F4 PrP molecules were resistant to endo H treatment after less then 20 min, demonstrating that their oligosaccharide chains had been processed to complex sugars (Fig. 5A). Deletion of the first β-strand had only a slight influence on endo H sensitivity, and most ΔβI PrP molecules were resistant to endo H digestion after 20 min (Fig. 5B). However, deletion of the first α-helix or the second β-strand significantly altered PrP glycosylation (Fig. 5C and D). Although carbohydrate moieties on most ΔαI PrP molecules had been processed to complex sugars after 2 h, a small percentage of ΔαI PrP molecules remained endo H sensitive throughout the chase period of 8 h. By contrast, the vast majority of ΔβII PrP-sen molecules were sensitive to endo H treatment even after 8 h of chase. Thus, most carbohydrates on ΔβII and some carbohydrates on ΔαI were not processed to complex sugars, explaining the differences in glycosylation pattern for these PrP mutants and indicating that mutants that were not glycosylated properly were retained in a cellular compartment proximal to the medial Golgi. The retention of these PrP-sen molecules inside the cells provides an explanation for the significant drop in cell surface expression of ΔαI and the nearly complete absence of ΔβII from the plasma membrane.
Expression of endogenous mouse PrP-sen is unaltered in Sc+-MNB cells expressing PrP deletion mutants.
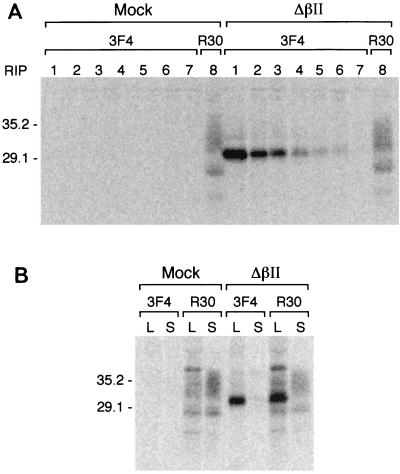
One possible explanation for the decrease of PrP-res accumulation in Sc+-MNB cells was that the expression of the deletion mutants led to a reduction of the endogenous PrP-sen level. To address this question, lysates from [35S]methionine-[35S]cysteine-labeled Sc+-MNB cells expressing ΔβII and untransduced control cells were immunoprecipitated with monoclonal antibody 3F4 for seven consecutive cycles to remove ΔβII. A subsequent immunoprecipitation step with the R30 antiserum demonstrated that the level of endogenous PrP-sen was not affected by the expression of ΔβII, since similar amounts of endogenous mouse PrP-sen were present in both control cells and cells expressing ΔβII (Fig. 6A). Similarly, the expression of the deletion mutants ΔαI and ΔβI had no effect on endogenous PrP-sen levels (data not shown).
FIG. 6.
Expression of ΔβII in Sc+-MNB cells does not impair expression of endogenous PrP-sen or its translocation to the cell surface. (A) Recombinant PrP-sen was removed from lysates of [35S]methionine-[35S]cysteine-labeled Sc+-MNB cells or Sc+-MNB cells expressing ΔβII by seven consecutive rounds of immunoprecipitation with antibody 3F4. The remaining PrP-sen was immunoprecipitated by R30 antiserum. (B) [35S]methionine-[35S]cysteine-labeled cells were chased for 30 min in OptiMEM–10% FBS and treated with PI-PLC to remove GPI-anchored PrP-sen from the cell surface. Since no detectable levels of 3F4-positive ΔβII were released into the supernatant by PI-PLC under these conditions, PrP-sen removed from the cell surface by PI-PLC represents endogenous mouse PrP-sen only. Molecular weights are shown on the left in thousands Lanes: S, supernatant; L, cell lysate.
We also assayed whether the apparent retention of ΔβII in a cellular compartment proximal to the medial Golgi had an effect on the translocation of endogenous PrP-sen to the cell surface. [35S]methionine-[35S]cysteine-labeled Sc+-MNB cells were chased for 30 min and incubated with PI-PLC to release GPI-anchored PrP molecules from the cell surface. Supernatants and cell lysates were first immunoprecipitated using the monoclonal antibody 3F4 and subsequently immunoprecipitated using the polyclonal antiserum R30 (Fig. 6B). Under these conditions, no detectable PrP-sen was released into the supernatant without PI-PLC treatment (data not shown). Similar amounts of endogenous mouse PrP-sen were released from the cell surface by PI-PLC, demonstrating that the transport of endogenous mouse PrP-sen to the cell surface was not affected by the expression of ΔβII. Thus, the reduced accumulation of PrP-res in Sc+-MNB cells expressing ΔβII was not due to either an altered endogenous PrP-sen level or to an impaired transport of endogenous PrP-sen to the cell surface.
Inefficient conversion of PrP deletion mutants in cell-free conversion reactions.
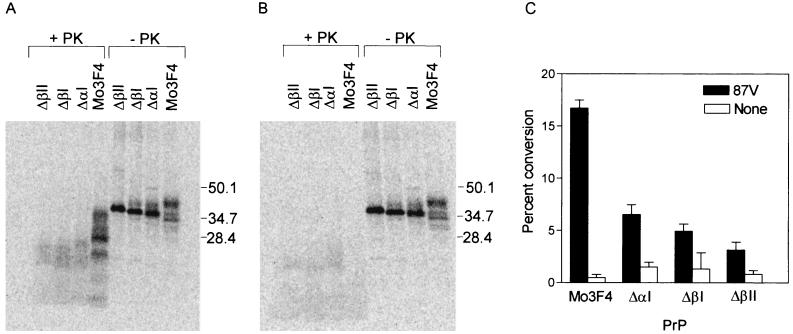
All of the PrP-sen deletion mutants tested inhibited PrP-res formation in Sc+-MNB cells, suggesting that they were interacting with PrP-res or other unknown components involved in PrP-res formation. However, none of these mutants were themselves converted to protease resistance. Thus, it was possible that the deletion mutants were interacting with PrP-res or other components of the conversion process in a cellular compartment where PrP-res formation could not occur. We therefore tested if PrP-sen deletion molecules could be converted to protease-resistant PrP in a cell-free conversion assay. In the presence of PrP-res, Mo3F4 PrP-sen was converted to the expected partially PK-resistant forms (Fig. 7A) while protease-resistant Mo3F4 PrP was not observed in the absence of PrP-res (Fig. 7B). Conversion of PrP-sen molecules lacking the first α-helix or the two β-strands was inefficient and led to indistinct PK-resistant products with lower molecular weights than protease-resistant Mo3F4 PrP (Fig. 7A). Faint PK-resistant bands were also detected when ΔαI, ΔβI, or ΔβII PrP-sen was incubated without PrP-res (Fig. 7B). The precise nature of these bands is unclear. While the conversion efficiency of Mo3F4 PrP-sen is consistent with earlier data (51), the conversion efficiency of the PrP deletion mutants was significantly decreased, by 60% (ΔαI), 76% (ΔβI) or 77% (ΔβII), compared to Mo3F4 PrP (Fig. 7C). Thus, the inability of PrP-sen molecules lacking either the β-strands or the first α-helix to form protease-resistant PrP is an intrinsic property of the PrP mutant and is not solely the consequence of localization in a cellular compartment where conversion cannot occur.
FIG. 7.
Cell-free conversion of PrP-sen molecules lacking the first or second β-strand or the first α-helix. Conversion efficiencies of the PrP-sen deletion mutants were determined using a cell-free conversion assay. (A and B) Metabolically labeled Mo3F4, ΔαI, ΔβI, or ΔβII PrP-sen immunoprecipitated from lysates of ψ2/PA317 cells were incubated for 48 h at 37°C with (A) or without (B) 87V PrP-res. One-tenth of the reaction mix was reserved as a control for total radiolabeled PrP (right four lanes), while the remaining sample was PK digested to determine the amount of protease-resistant PrP present (left four lanes). Molecular mass markers are indicated to the right of each panel. (C) Conversion percentages were determined by quantifying the integrated peak intensities of starting material (-PK lanes) with respect to PK-digested samples. Results were corrected for nonspecific background. Conversion efficiencies (with standard deviation indicated) are shown. All conversion reactions were independently repeated four to seven times for each PrP-sen variant.
DISCUSSION
Deletion of the first α-helix or the β-strands from PrP-sen drastically inhibited the formation of PrP-res. This effect is specific to the deletion of these secondary-structure elements since previous studies have shown that PrP-sen molecules deleted N- or C-terminally to the first α-helix or the β-strands were still converted to PrP-res (31a, 54, 62). The inability of the deletion mutants to efficiently form PrP-res could be due either to a misfolded PrP-sen molecule or to the absence of structural domains in PrP-sen that are important for the conformational transition to PrP-res. Interestingly, deletion of the first α-helix and the second β-strand, but not the first β-strand, significantly affected the processing and subcellular trafficking of PrP-sen. These results might be explained by the proposed role of the first α-helix and the second β-strand in the folding of the globular domain of PrP-sen. A recent NMR structure analysis indicates that PrP-sen polypeptide folding is stabilized by hydrophobic interactions within the globular domain of the molecule which includes amino acid side chains of the first α-helix (Tyr 149) and the second β-strand (Val 160) (52). Thus, deletion of these PrP-sen secondary structures is likely to destabilize PrP-sen, thereby leading to a misfolded polypeptide chain that is incapable of being converted efficiently to PrP-res.
By contrast, amino acid residues within the first β-strand do not contribute to the hydrophobic interactions in the central part of the molecule (52). Deletions of the first β-strand should therefore not destabilize PrP-sen. Our data are consistent with this prediction, demonstrating that the cellular processing of ΔβI is not significantly altered compared to Mo3F4 PrP-sen. However, the first β-strand could influence PrP-res formation and/or stability by contributing to the formation of a β-sheet-rich region from amino acids 90 to 144 (28). In fact, molecular dynamics analysis has predicted the formation of a third β-strand in PrP-res that would involve new hydrogen bonds between residues in the region spanning amino acids 123 to 125 and residues within the first β-strand. The first β-strand is therefore likely to stabilize a secondary structure required for the conformational transition of PrP-sen to its protease-resistant isoform (23).
Since transgenic mice expressing PrP-sen molecules that lack amino acid residues 23 to 88 and 141 to 176 (PrP106) were susceptible to scrapie and accumulated PK-resistant PrP106, it has been suggested that neither the first α-helix nor the second β-strand was necessary for PrP-res formation (62). Our data are not consistent with this proposal. However, it is possible that the deletion of the N-terminal amino acids 23 to 88 in addition to the region encompassing amino acids 141 to 176 rescued a phenotype similar to that of wild-type PrP-sen. Consistent with this hypothesis, deletion of either the first α-helix or the first β-strand in combination with the second β-strand was able to restore cell surface expression of PrP (data not shown).
The formation of PrP-res is believed to include two kinetically separable events: binding between the two PrP isoforms followed by conversion of PrP-sen to its protease-resistant state (26, 27). It is possible that the formation of protease-resistant PrP from the deletion mutants is influenced by inhibition of either the binding step or the step in which PK resistance is acquired. Previous publications reported that the initial binding of PrP-sen to PrP-res occurs through one or more surfaces adjacent to the C terminus in the three-dimensional structure of PrP-sen. In hamster PrP, the surface regions encompassing residues 119 to 138, 165 to 174, and/or 206 to 223 have been suggested to be potential sites for this intermolecular interaction (26). Based on this prediction, deletion of the first β-strand (residues 127 to 130) might strongly influence the binding of PrP-sen to PrP-res. The first α-helix, which is relatively isolated from the core of the structure, has also been proposed to be part of the PrP-res binding site in PrP-sen (52). However, two observations argue that binding of the deletion mutants to PrP-res still occurs. First, the conversion efficiency of PrP-sen ΔαI ΔβI, or ΔβII was drastically decreased but not completely abolished in a cell-free conversion system (Fig. 7). Second, expression of PrP-sen deletion mutants in Sc+-MNB cells substantially interfered with the formation of endogenous mouse PrP-res, suggesting a competition between wild-type and mutant PrP-sen molecules for PrP-res binding sites (26, 41). Further experiments are necessary to determine if the deletion of the first α-helix or the β-strands affects binding kinetics and/or binding stability.
While the formation of PrP-res has been studied in Sc+-MNB cells and cell-free conversion systems, little is known about the precise cellular compartment in which conversion and/or interference takes place. Several studies argue that PrP-res formation occurs either on the cell surface and/or along the endocytic pathway (3, 8, 10, 34, 63). Assuming that both conversion and interference take place in the same subcellular compartment, it was surprising to find that interference was observed even when the vast majority of ΔβII was retained inside the cell. If conversion and interference occur on the cell surface, a very low level of cell surface expression of ΔβII would have to be sufficient to inhibit PrP-res formation. However, interference is clearly dependent on the ratio of homologous and heterologous PrP-sen. The higher the ratio of heterologous to homologous PrP-sen, the higher the level of interference (27, 40, 47, 48). It is therefore unlikely that the nearly undetectable amount of ΔβII on the cell surface could account for its ability to strongly inhibit PrP-res formation. Conversion and interference could occur in the same cellular compartment but may not necessarily be restricted to the cell surface or the endocytic pathway. Some data suggest that a translocation of PrP-sen to the plasma membrane is not absolutely necessary for PrP-res formation (32, 64), providing a possible explanation for the interference of ΔβII molecules that are retained primarily in an intracellular compartment. It is, however, also possible that ΔβII molecules are directly transported from the Golgi to the endosomes or lysosomes, where they could interfere with the conversion of endogenous mouse PrP-sen, a trafficking route proposed for newly synthesized APP (16).
Interference and conversion, however, may not occur in the same cellular compartment. If the cellular compartments in which interference and conversion occur differ, interference could be the consequence of PrP-sen interacting with other cellular factors which are absolutely required for PrP-res formation, although no such factors have been isolated. However, it is possible that conversion-incompetent PrP-sen deletion mutants bind to cellular factors that influence the formation of PrP-res, such as chaperones or glycoaminoglycans (9, 15, 20, 22, 65). Thus, the deletion mutants presented here may provide a useful tool to more precisely determine the subcellular compartments and/or secondary factors involved in PrP-res formation.
ACKNOWLEDGMENTS
We thank Sonja Best, Byron Caughey, Victoria Lawson, and Rick Race for critically reading the manuscript and Gary Hettrick and Anita Mora for providing graphic assistance.
REFERENCES
- 1.Bolton D C, McKinley M P, Prusiner S B. Identification of a protein that purifies with the scrapie prion. Science. 1982;218:1309–1311. doi: 10.1126/science.6815801. [DOI] [PubMed] [Google Scholar]
- 2.Bolton D C, Seligman S J, Bablanian G, Windsor D, Scala L J, Kim K S, Chen C M J, Kascsak R J, Bendheim P E. Molecular location of a species specific epitope on the hamster scrapie agent protein. J Virol. 1991;65:3667–3675. doi: 10.1128/jvi.65.7.3667-3675.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Borchelt D R, Taraboulos A, Prusiner S B. Evidence for synthesis of scrapie prion protein in the endocytic pathway. J Biol Chem. 1992;267:16188–16199. [PubMed] [Google Scholar]
- 4.Brown K L, Stewart K, Ritchie D L, Mabbott N A, Williams A, Fraser H, Morrison W I, Bruce M E. Scrapie replication in lymphoid tissues depends on prion protein-expressing follicular dendritic cells. Nat Med. 1999;5:1308–1312. doi: 10.1038/15264. [DOI] [PubMed] [Google Scholar]
- 5.Caughey B. Formation of protease-resistant prion protein in cell-free systems. In: Harris D A, editor. Prions: molecular and cellular biology. Wymondham, United Kingdom: Horizon Scientific; 1999. pp. 27–44. [Google Scholar]
- 6.Caughey B, Kocisko D A, Raymond G J, Lansbury P T. Aggregates of scrapie associated prion protein induce the cell-free conversion of protease-sensitive prion protein to the protease-resistant state. Chem Biol. 1995;2:807–817. doi: 10.1016/1074-5521(95)90087-x. [DOI] [PubMed] [Google Scholar]
- 7.Caughey B, Race R E, Ernst D, Buchmeier M J, Chesebro B. Prion protein (PrP) biosynthesis in scrapie-infected and uninfected neuroblastoma cells. J Virol. 1989;63:175–181. doi: 10.1128/jvi.63.1.175-181.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Caughey B, Raymond G J. The scrapie-associated form of PrP is made from a cell surface precursor that is both protease- and phospholipase-sensitive. J Biol Chem. 1991;266:18217–18223. [PubMed] [Google Scholar]
- 9.Caughey B, Raymond G J. Sulfated polyanion inhibition of scrapie-associated PrP accumulation in cultured cells. J Virol. 1993;67:643–650. doi: 10.1128/jvi.67.2.643-650.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Caughey B, Raymond G J, Ernst D, Race R E. N-terminal truncation of the scrapie-associated form of PrP by lysosomal protease(s): implications regarding the site of conversion of PrP to the protease-resistant state. J Virol. 1991;65:6597–6603. doi: 10.1128/jvi.65.12.6597-6603.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Caughey B, Raymond G J, Kocisko D A, Lansbury P T., Jr Scrapie infectivity correlates with converting activity, protease resistance, and aggregation of scrapie-associated prion protein in guanidine denaturation studies. J Virol. 1997;71:4107–4110. doi: 10.1128/jvi.71.5.4107-4110.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Caughey B W, Dong A, Bhat K S, Ernst D, Hayes S F, Caughey W S. Secondary structure analysis of the scrapie-associated protein PrP 27–30 in water by infrared spectroscopy. Biochemistry. 1991;30:7672–7680. doi: 10.1021/bi00245a003. [DOI] [PubMed] [Google Scholar]
- 13.Chesebro B, Wehrly K, Caughey B, Nishio J, Ernst D, Race R. Foreign PrP expression and scrapie infection in tissue culture cell lines. Dev Biol Stand. 1993;80:131–140. [PubMed] [Google Scholar]
- 14.Come J H, Fraser P E, Lansbury P T., Jr A kinetic model for amyloid formation in the prion diseases: importance of seeding. Proc Natl Acad Sci USA. 1993;90:5959–5963. doi: 10.1073/pnas.90.13.5959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.DebBurman S K, Raymond G J, Caughey B, Lindquist S. Chaperone-supervised conversion of prion protein to its protease-resistant form. Proc Natl Acad Sci USA. 1997;94:13938–13943. doi: 10.1073/pnas.94.25.13938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Strooper B, Umans L, Van Leuven F, Van Den Berghe H. Study of the synthesis and secretion of normal and artificial mutants of murine amyloid precursor protein (APP): cleavage of APP occurs in a late compartment of the default secretion pathway. J Cell Biol. 1993;121:295–304. doi: 10.1083/jcb.121.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Diringer H, Gelderblom H, Hilmert H, Ozel M, Edelbluth C, Kimberlin R H. Scrapie infectivity, fibrils and low molecular weight protein. Nature. 1983;306:476–478. doi: 10.1038/306476a0. [DOI] [PubMed] [Google Scholar]
- 18.Donne D G, Viles J H, Groth D, Mehlhorn I, James T L, Cohen F E, Prusiner S B, Wright P E, Dyson H J. Structure of the recombinant full-length hamster prion protein PrP(29–231): the N terminus is highly flexible. Proc Natl Acad Sci USA. 1997;94:13452–13457. doi: 10.1073/pnas.94.25.13452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eigen M. Prionics or the kinetic basis of prion diseases. Biophys Chem. 1996;63:A1–A18. doi: 10.1016/s0301-4622(96)02250-8. [DOI] [PubMed] [Google Scholar]
- 20.Farquhar C F, Dickinson A G. Prolongation of scrapie incubation period by an injection of dextran sulphate 500 within the month before or after infection. J Gen Virol. 1986;67:463–473. doi: 10.1099/0022-1317-67-3-463. [DOI] [PubMed] [Google Scholar]
- 21.Fischer M, Rulicke T, Raeber A, Sailer A, Moser M, Oesch B, Brandner S, Aguzzi A, Weissmann C. Prion protein (PrP) with amino-proximal deletions restoring susceptibility of PrP knockout mice to scrapie. EMBO J. 1996;15:1255–1264. [PMC free article] [PubMed] [Google Scholar]
- 22.Gabizon R, Meiner Z, Halimi M, Bensasson S A. Heparin-like molecules bind differentially to prion proteins and change their intracellular metabolic-fate. J Cell Physiol. 1993;157:319–325. doi: 10.1002/jcp.1041570215. [DOI] [PubMed] [Google Scholar]
- 23.Guilbert C, Ricard F, Smith J C. Dynamic simulation of the mouse prion protein. Biopolymers. 2000;54:406–415. doi: 10.1002/1097-0282(200011)54:6<406::AID-BIP50>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 24.Holscher C, Delius J, Burkle A. Overexpression of nonconvertible PrPCdel114–121 in scrapie-infected mouse neuroblastoma cells leads to trans-dominant inhibition of wild-type PrPSc accumulation. J Virol. 1998;72:1153–1159. doi: 10.1128/jvi.72.2.1153-1159.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hope J. The nature of the scrapie agent: the evolution of the virino. Ann N Y Acad Sci. 1994;724:282–289. doi: 10.1111/j.1749-6632.1994.tb38917.x. [DOI] [PubMed] [Google Scholar]
- 26.Horiuchi M, Chabry J, Caughey B. Specific binding of normal prion protein to the scrapie form via a localized domain initiates its conversion to the protease- resistant state. EMBO J. 1999;18:3193–3203. doi: 10.1093/emboj/18.12.3193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Horiuchi M, Priola S A, Chabry J, Caughey B. Interactions between heterologous forms of prion protein: binding, inhibition of conversion, and species barriers. Proc Natl Acad Sci USA. 2000;97:5836–5841. doi: 10.1073/pnas.110523897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang Z, Prusiner S B, Cohen F E. Scrapie prions: a three-dimensional model of an infectious fragment. Fold Des. 1995;1:13–19. [PubMed] [Google Scholar]
- 29.Kascsak R J, Rubenstein R, Merz P A, Tonna-DeMasi M, Fersko R, Carp R I, Wisniewski H M, Diringer H. Mouse polyclonal and monoclonal antibody to scrapie-associated fibril proteins. J Virol. 1987;61:3688–3693. doi: 10.1128/jvi.61.12.3688-3693.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kitamoto T, Muramoto T, Mohri S, Doh-ura K, Tateishi J. Abnormal isoform of prion protein accumulates in follicular dendritic cells in mice with Creutzfeldt-Jakob disease. J Virol. 1991;65:6292–6295. doi: 10.1128/jvi.65.11.6292-6295.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kocisko D A, Come J H, Priola S A, Chesebro B, Raymond G J, Lansbury P T, Caughey B. Cell-free formation of protease-resistant prion protein. Nature. 1994;370:471–474. doi: 10.1038/370471a0. [DOI] [PubMed] [Google Scholar]
- 31a.Lawson, V. A., S. A. Priola, K. Wehrly, and B. Chesebro. N-terminal truncation of prion protein affects both formation and conformation of abnormal protease-resistant prion protein generated in vitro. J. Biol. Chem., in press. [DOI] [PubMed]
- 32.Lehmann S, Harris D A. Blockade of glycosylation promotes acquisition of scrapie-like properties by the prion protein in cultured cells. J Biol Chem. 1997;272:21479–21487. doi: 10.1074/jbc.272.34.21479. [DOI] [PubMed] [Google Scholar]
- 33.Lopez G F, Zahn R, Riek R, Wuthrich K. NMR structure of the bovine prion protein. Proc Natl Acad Sci USA. 2000;97:8334–8339. doi: 10.1073/pnas.97.15.8334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McKinley M P, Taraboulos A, Kenaga L, Serban D, Stieber A, DeArmond S J, Prusiner S B, Gonatas N. Ultrastructural localization of scrapie prion proteins in cytoplasmic vesicles of infected cultured cells. Lab Investig. 1991;65:622–630. [PubMed] [Google Scholar]
- 35.Meyer R K, McKinley M P, Bowman K A, Braunfeld M B, Barry R A, Prusiner S B. Separation and properties of cellular and scrapie prion protein. Proc Natl Acad Sci USA. 1986;83:2310–2314. doi: 10.1073/pnas.83.8.2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mouillet-Richard S, Ermonval M, Chebassier C, Laplanche J L, Lehmann S, Launay J M, Kellermann O. Signal transduction through prion protein. Science. 2000;289:1925–1928. doi: 10.1126/science.289.5486.1925. [DOI] [PubMed] [Google Scholar]
- 37.Muramoto T, Scott M, Cohen F E, Prusiner S B. Recombinant scrapie-like prion protein of 106 amino acids is soluble. Proc Natl Acad Sci USA. 1996;93:15457–15462. doi: 10.1073/pnas.93.26.15457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Oesch B, Westaway D, Walchli M, McKinley M P, Kent S B H, Aebersold R, Barry R A, Tempst P, Teplow D B, Hood L E, Prusiner S B, Weissmann C. A cellular gene encodes scrapie PrP 27–30 protein. Cell. 1985;40:735–746. doi: 10.1016/0092-8674(85)90333-2. [DOI] [PubMed] [Google Scholar]
- 39.Pan K-M, Baldwin M, Nguyen J, Gasset M, Serban A, Groth D, Mehlhorn I, Huang Z, Fletterick R J, Cohen F E, Prusiner S B. Conversion of alpha-helices into beta-sheets features in the formation of the scrapie prion protein. Proc Natl Acad Sci USA. 1993;90:10962–10966. doi: 10.1073/pnas.90.23.10962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Priola S A. Prion protein and species barriers in the transmissible spongiform encephalopathies. Biomed Pharmacother. 1999;53:27–33. doi: 10.1016/s0753-3322(99)80057-2. [DOI] [PubMed] [Google Scholar]
- 41.Priola S A, Caughey B, Race R E, Chesebro B. Heterologous PrP molecules interfere with accumulation of protease-resistant PrP in scrapie-infected murine neuroblastoma cells. J Virol. 1994;68:4873–4878. doi: 10.1128/jvi.68.8.4873-4878.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Priola S A, Chesebro B. A single hamster amino acid blocks conversion to protease-resistant PrP in scrapie-infected mouse neuroblastoma cells. J Virol. 1995;69:7754–7758. doi: 10.1128/jvi.69.12.7754-7758.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Prusiner S B. Novel proteinaceous infectious particles cause scrapie. Science. 1982;216:136–144. doi: 10.1126/science.6801762. [DOI] [PubMed] [Google Scholar]
- 44.Prusiner S B. Molecular biology of prion diseases. Science. 1991;252:1515–1522. doi: 10.1126/science.1675487. [DOI] [PubMed] [Google Scholar]
- 45.Prusiner S B, Groth D, Serban A, Stahl N, Gabizon R. Attempts to restore scrapie prion infectivity after exposure to protein denaturants. Proc Natl Acad Sci USA. 1993;90:2793–2797. doi: 10.1073/pnas.90.7.2793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Prusiner S B, Groth D F, Bolton D C, Kent S B, Hood L E. Purification and structural studies of a major scrapie prion protein. Cell. 1984;38:127–134. doi: 10.1016/0092-8674(84)90533-6. [DOI] [PubMed] [Google Scholar]
- 47.Prusiner S B, Scott M, Foster D, Pan K M, Groth D, Mirenda C, Torchia M, Yang S L, Serban D, Carlson G A, Hoppe P C, Westaway D, DeArmond S J. Transgenetic studies implicate interactions between homologous PrP isoforms in scrapie prion replication. Cell. 1990;63:673–686. doi: 10.1016/0092-8674(90)90134-z. [DOI] [PubMed] [Google Scholar]
- 48.Race R, Oldstone M, Chesebro B. Entry versus blockade of brain infection following oral or intraperitoneal scrapie administration: role of prion protein expression in peripheral nerves and spleen. J Virol. 2000;74:828–833. doi: 10.1128/jvi.74.2.828-833.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Race R E, Caughey B, Graham K, Ernst D, Chesebro B. Analyses of frequency of infection, specific infectivity, and prion protein biosynthesis in scrapie-infected neuroblastoma cell clones. J Virol. 1988;62:2845–2849. doi: 10.1128/jvi.62.8.2845-2849.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Race R E, Fadness L H, Chesebro B. Characterization of scrapie infection in mouse neuroblastoma cells. J Gen Virol. 1987;68:1391–1399. doi: 10.1099/0022-1317-68-5-1391. [DOI] [PubMed] [Google Scholar]
- 51.Raymond G J, Hope J, Kocisko D A, Priola S A, Raymond L D, Bossers A, Ironside J, Will R G, Chen S G, Petersen R B, Gambetti P, Rubenstein R, Smits M A, Lansbury P T, Jr, Caughey B. Molecular assessment of the transmissibilities of BSE and scrapie to humans. Nature. 1997;388:285–288. doi: 10.1038/40876. [DOI] [PubMed] [Google Scholar]
- 52.Riek R, Hornemann S, Wider G, Billeter M, Glockshuber R, Wuthrich K. NMR structure of the mouse prion protein domain PrP(121–231) Nature. 1996;382:180–182. doi: 10.1038/382180a0. [DOI] [PubMed] [Google Scholar]
- 53.Riek R, Hornemann S, Wider G, Glockshuber R, Wuthrich K. NMR characterization of the full-length recombinant murine prion protein, mPrP(23–21) FEBS Lett. 1997;413:282–288. doi: 10.1016/s0014-5793(97)00920-4. [DOI] [PubMed] [Google Scholar]
- 54.Rogers M, Yehiely F, Scott M, Prusiner S B. Conversion of truncated and elongated prion proteins into the scrapie isoform in cultured cells. Proc Natl Acad Sci USA. 1993;90:3182–3186. doi: 10.1073/pnas.90.8.3182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rudd P M, Endo T, Colominas C, Groth D, Wheeler S F, Harvey D J, Wormald M R, Serban H, Prusiner S B, Kobata A, Dwek R A. Glycosylation differences between the normal and pathogenic prion protein isoforms. Proc Natl Acad Sci USA. 1999;96:13044–13049. doi: 10.1073/pnas.96.23.13044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Safar J, Roller P P, Gajdusek D C, Gibbs C J., Jr Conformational transitions, dissociation, and unfolding of scrapie amyloid (prion) protein. J Biol Chem. 1993;268:20276–20284. [PubMed] [Google Scholar]
- 57.Scott M, Groth D, Foster D, Torchia M, Yang S L, DeArmond S J, Prusiner S B. Propagation of prions with artificial properties in transgenic mice expressing chimeric PrP genes. Cell. 1993;73:979–988. doi: 10.1016/0092-8674(93)90275-u. [DOI] [PubMed] [Google Scholar]
- 58.Stahl N, Baldwin M A, Teplow D B, Hood L, Gibson B W, Burlingame A L, Prusiner S B. Structural studies of the scrapie prion protein using mass spectrometry and amino acid sequencing. Biochemistry. 1993;32:1991–2002. doi: 10.1021/bi00059a016. [DOI] [PubMed] [Google Scholar]
- 59.Stahl N, Borchelt D R, Hsiao K, Prusiner S B. Scrapie prion protein contains a phosphatidylinositol glycolipid. Cell. 1987;51:229–240. doi: 10.1016/0092-8674(87)90150-4. [DOI] [PubMed] [Google Scholar]
- 60.Stahl N, Prusiner S B. Prions and prion proteins. FASEB J. 1991;5:2799–2807. doi: 10.1096/fasebj.5.13.1916104. [DOI] [PubMed] [Google Scholar]
- 61.Stimson E, Hope J, Chong A, Burlingame A L. Site-specific characterization of the N-linked glycans of murine prion protein by high-performance liquid chromatography/electrospray mass spectrometry and exoglycosidase digestions. Biochemistry. 1999;38:4885–4895. doi: 10.1021/bi982330q. [DOI] [PubMed] [Google Scholar]
- 62.Supattapone S, Bosque P, Muramoto T, Wille H, Aagaard C, Peretz D, Nguyen H O, Heinrich C, Torchia M, Safar J, Cohen F E, DeArmond S J, Prusiner S B, Scott M. Prion protein of 106 residues creates an artifical transmission barrier for prion replication in transgenic mice. Cell. 1999;96:869–878. doi: 10.1016/s0092-8674(00)80596-6. [DOI] [PubMed] [Google Scholar]
- 63.Taraboulos A, Raeber A J, Borchelt D R, Serban D, Prusiner S B. Synthesis and trafficking of prion proteins in cultured cells. Mol Biol Cell. 1992;3:851–863. doi: 10.1091/mbc.3.8.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Taraboulos A, Scott M, Semenov A, Avraham D, Laszlo L, Prusiner S B. Cholesterol depletion and modification of COOH-terminal targeting sequence of the prion protein inhibit formation of the scrapie isoform. J Cell Biol. 1995;129:121–132. doi: 10.1083/jcb.129.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Telling G C, Scott M, Hslao K K, Foster D, Yang S L, Torchia M, Sidle K C, Collinge J, DeArmond S J, Prusiner S B. Transmission of Creutzfeldt-Jakob disease from humans to transgenic mice expressing chimeric human-mouse prion protein. Proc Natl Acad Sci USA. 1994;91:9936–9940. doi: 10.1073/pnas.91.21.9936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zahn R, Liu A, Luhrs T, Riek R, von Schroetter C, Garcia F L, Billeter M, Calzolai L, Wider G, Wuthrich K. NMR solution structure of human prion protein. Proc Natl Acad Sci USA. 2000;97:145–150. doi: 10.1073/pnas.97.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]