Abstract
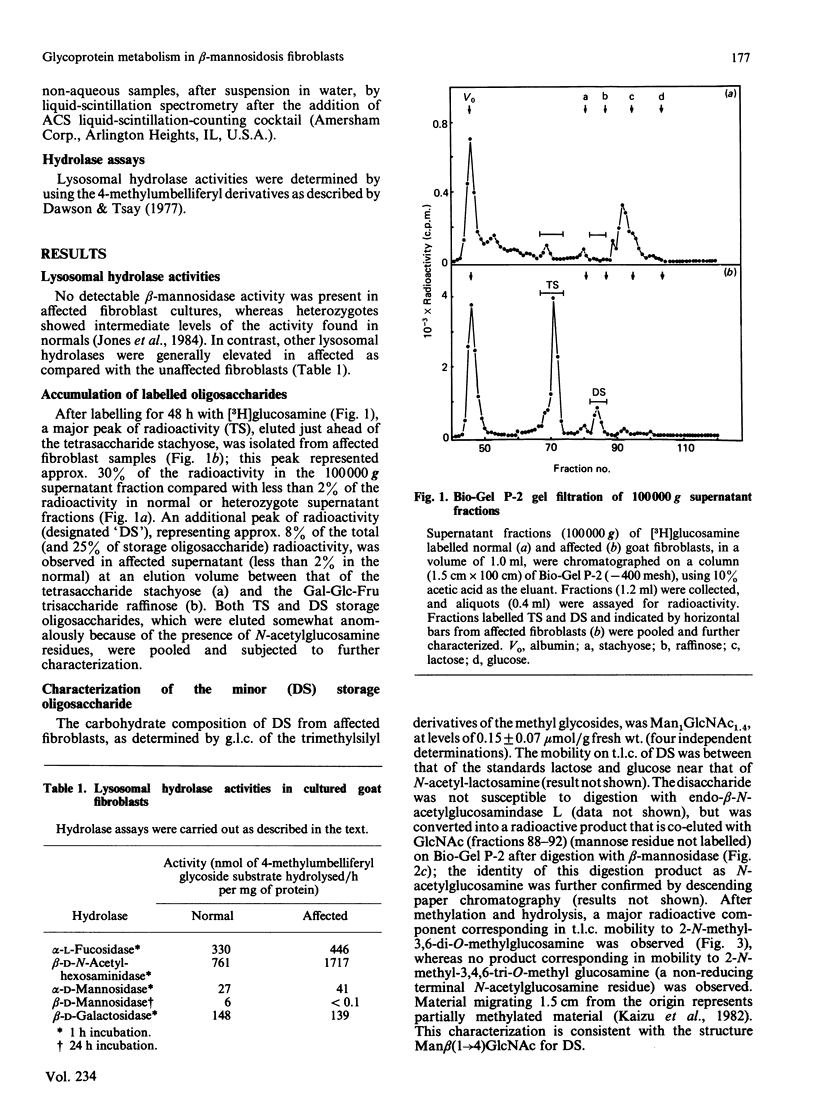
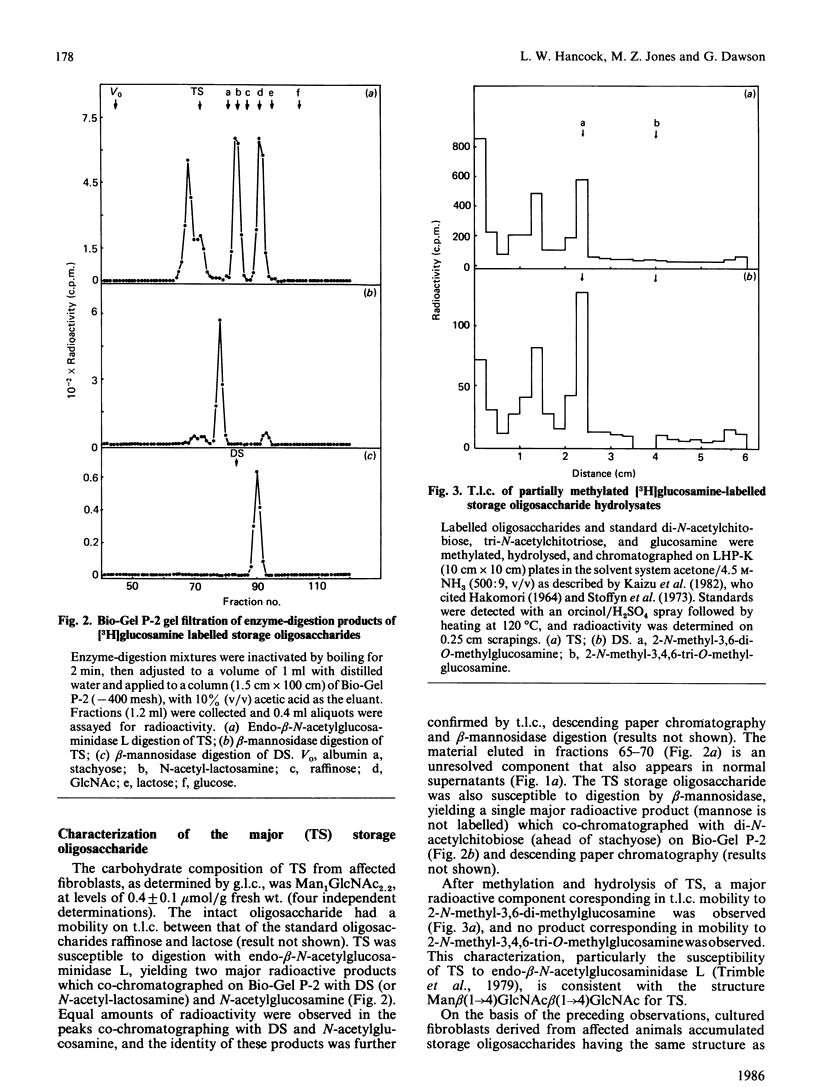
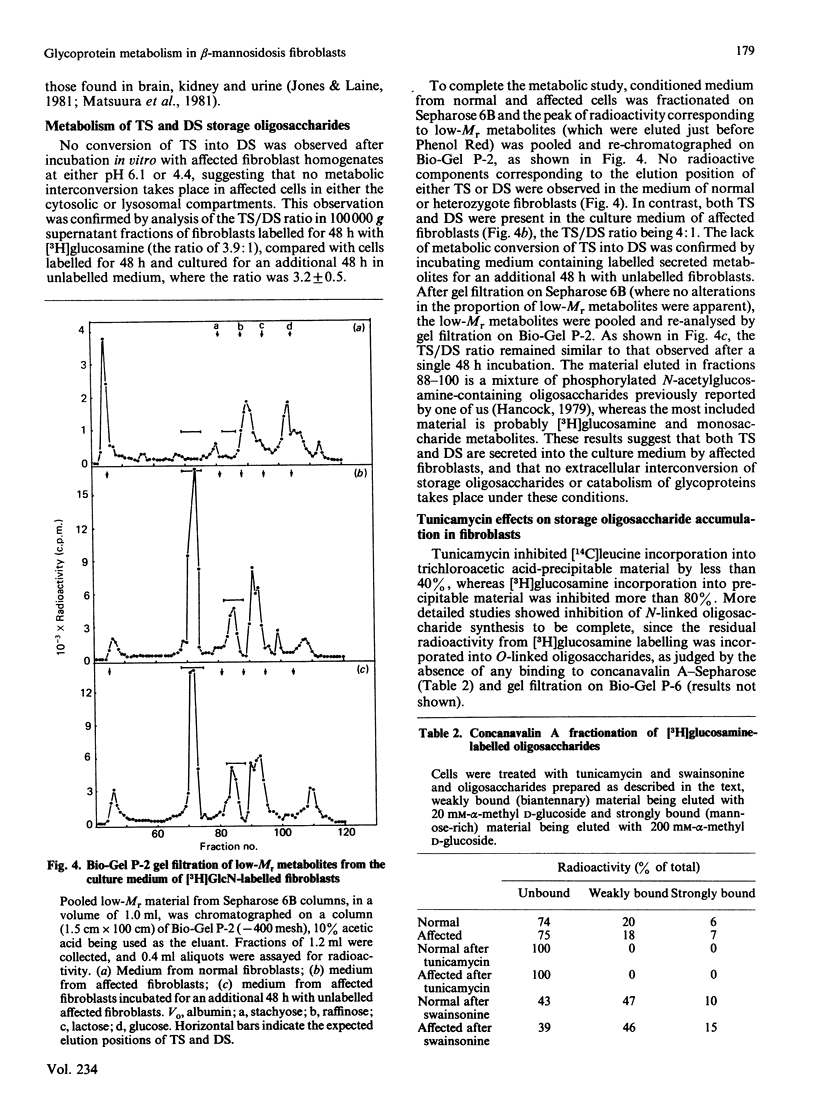
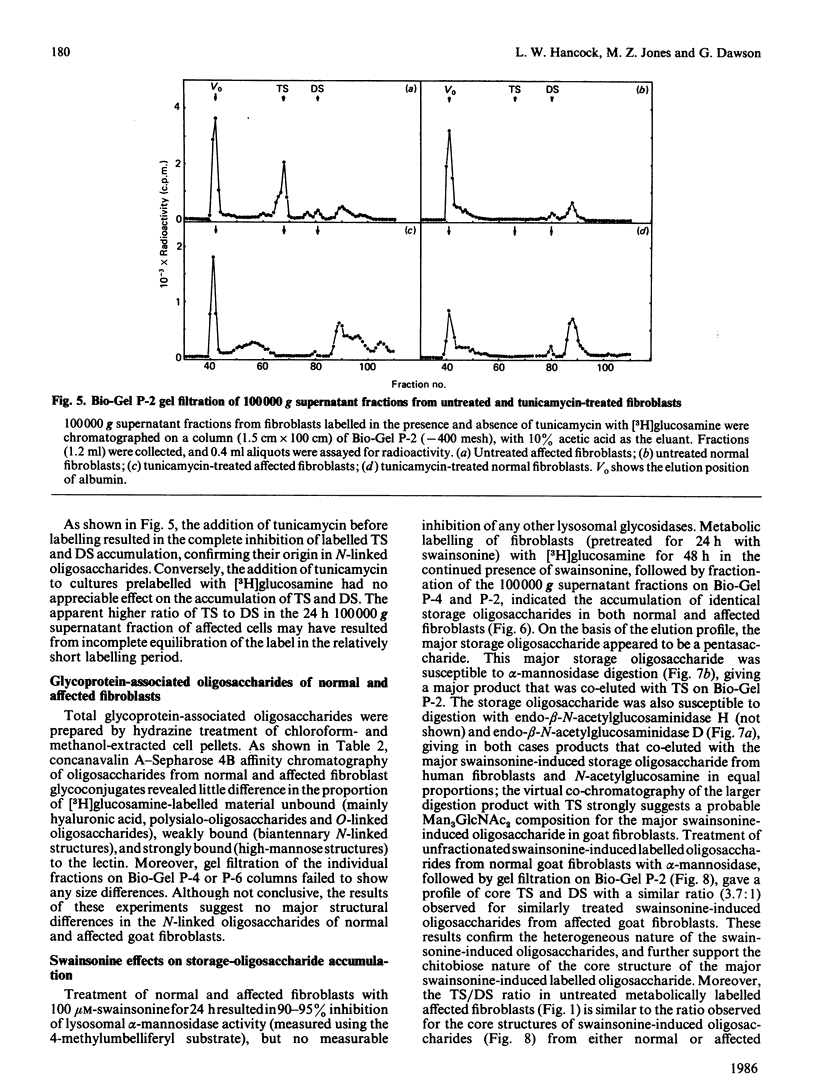
Cultured skin fibroblasts established from goats affected with beta-mannosidosis, an inherited neurovisceral storage disorder, showed an absence of lysosomal beta-mannosidase activity and the corresponding accumulation of a trisaccharide (TS) with the structure Man beta (1----4)GlcNAc beta (1----4)GlcNAc (0.4 mumol/g) and lesser amounts (0.15 mumol/g) of a Man beta (1----4)GlcNAc disaccharide (DS). By using purified storage TS isolated from fibroblasts metabolically labelled with [3H]GlcN, no conversion of TS into DS could be demonstrated in homogenates of affected cells at either lysosomal pH (4.4) or cytosolic pH (6.1), or in the culture medium (pH 7.0) of affected cells. Both TS and DS were secreted into the culture medium by affected fibroblasts. When affected fibroblasts were treated with tunicamycin before labelling with [3H]GlcN, the accumulation of both labelled TS and DS was completely inhibited. Treatment of both affected and normal goat fibroblasts with swainsonine resulted in the inhibition of lysosomal alpha-mannosidase activity and in the accumulation of the same labelled oligosaccharides in both. The major storage pentasaccharide from both normal and affected swainsonine-treated fibroblasts was sensitive to digestion with alpha-mannosidase and endo-beta-N-acetylhexosaminidase D, suggesting a branched mannose structure and a chitobiose core. In the absence of evidence for the existence of unusual N-linked glycoprotein-associated chitotriose oligosaccharide structures in affected goat fibroblasts, it must be concluded that degradative pathways for N-linked oligosaccharides are similar in both normal and affected goat fibroblasts, and that these pathways differ from catabolic pathways in human fibroblasts.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham D., Blakemore W. F., Jolly R. D., Sidebotham R., Winchester B. The catabolism of mammalian glycoproteins. Comparison of the storage products in bovine, feline and human mannosidosis. Biochem J. 1983 Dec 1;215(3):573–579. doi: 10.1042/bj2150573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burditt L. J., Chotai K., Hirani S., Nugent P. G., Winchester B. G., Blakemore W. F. Biochemical studies on a case of feline mannosidosis. Biochem J. 1980 Sep 1;189(3):467–473. doi: 10.1042/bj1890467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cenci di Bello I., Dorling P., Winchester B. The storage products in genetic and swainsonine-induced human mannosidosis. Biochem J. 1983 Dec 1;215(3):693–696. doi: 10.1042/bj2150693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson G. Disaccharide units from complex carbohydrates of animals. Methods Enzymol. 1978;50:272–284. doi: 10.1016/0076-6879(78)50028-1. [DOI] [PubMed] [Google Scholar]
- Dawson G., McLawhon R., Miller R. J. Opiates and enkephalins inhibit synthesis of gangliosides and membrane glycoproteins in mouse neuroblastoma cell line N4TG1. Proc Natl Acad Sci U S A. 1979 Feb;76(2):605–609. doi: 10.1073/pnas.76.2.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson G., Tsay G. Substrate specificity of human alpha-L-fucosidase. Arch Biochem Biophys. 1977 Nov;184(1):12–23. doi: 10.1016/0003-9861(77)90321-6. [DOI] [PubMed] [Google Scholar]
- HAKOMORI S. A RAPID PERMETHYLATION OF GLYCOLIPID, AND POLYSACCHARIDE CATALYZED BY METHYLSULFINYL CARBANION IN DIMETHYL SULFOXIDE. J Biochem. 1964 Feb;55:205–208. [PubMed] [Google Scholar]
- Jones M. Z., Dawson G. Caprine beta-mannosidosis. Inherited deficiency of beta-D-mannosidase. J Biol Chem. 1981 May 25;256(10):5185–5188. [PubMed] [Google Scholar]
- Jones M. Z., Laine R. A. Caprine oligosaccharide storage disease. Accumulation of beta-mannosyl (1 goes to 4) beta-N-acetylglucosaminyl (1 goes to 4) beta-N-acetylglucosamine in brain. J Biol Chem. 1981 May 25;256(10):5181–5184. [PubMed] [Google Scholar]
- Jones M. Z., Rathke E. J., Cavanagh K., Hancock L. W. Beta-mannosidosis: prenatal biochemical and morphological characteristics. J Inherit Metab Dis. 1984;7(2):80–85. doi: 10.1007/BF01805811. [DOI] [PubMed] [Google Scholar]
- Kaizu T., Turco S. J., Rush J. S., Laine R. A. Synthesis of the branched form of erythroglycan by Friend GM979 erythroleukemic cells. J Biol Chem. 1982 Jul 25;257(14):8272–8276. [PubMed] [Google Scholar]
- Krusius T., Finne J. Structural features of tissue glycoproteins. Fractionation and methylation analysis of glycopeptides derived from rat brain, kidney and liver. Eur J Biochem. 1977 Sep;78(2):369–379. doi: 10.1111/j.1432-1033.1977.tb11749.x. [DOI] [PubMed] [Google Scholar]
- Lundblad A., Nilsson B., Nordén N. E., Svensson S., Ockerman P. A., Jolly R. D. A urinary pentasaccharide in bovine mannosidosis. Eur J Biochem. 1975 Nov 15;59(2):601–605. doi: 10.1111/j.1432-1033.1975.tb02488.x. [DOI] [PubMed] [Google Scholar]
- Matalon R., Dorfman A. Hurler's syndrome: biosynthesis of acid mucopolysaccharides in tissue culture. Proc Natl Acad Sci U S A. 1966 Oct;56(4):1310–1316. doi: 10.1073/pnas.56.4.1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuura F., Jones M. Z., Frazier S. E. Structural analysis of the major caprine beta-mannosidosis urinary oligosaccharides. Biochim Biophys Acta. 1983 Aug 23;759(1-2):67–73. doi: 10.1016/0304-4165(83)90190-3. [DOI] [PubMed] [Google Scholar]
- Matsuura F., Laine R. A., Jones M. Z. Oligosaccharides accumulated in the kidney of a goat with beta-mannosidosis: mass spectrometry of intact permethylated derivatives. Arch Biochem Biophys. 1981 Oct 1;211(1):485–493. doi: 10.1016/0003-9861(81)90481-1. [DOI] [PubMed] [Google Scholar]
- Michalski J. C., Strecker G., Fournet B. Structures of sialyl-oligosaccharides excreted in the urine of a patient with mucolipidosis I. FEBS Lett. 1977 Jul 1;79(1):101–104. doi: 10.1016/0014-5793(77)80359-1. [DOI] [PubMed] [Google Scholar]
- Ng-Ying-Kin N. M., Wolfe L. S. Oligosaccharides accumulating in the liver from a patient with GM2-gangliosidosis variant O (Sandhoff-Jatzkewitz disease). Biochem Biophys Res Commun. 1974 Aug 5;59(3):837–844. doi: 10.1016/s0006-291x(74)80055-0. [DOI] [PubMed] [Google Scholar]
- Nishigaki M., Muramatsu T., Kobata A. Endoglycosidases acting on carbohydrate moieties of glycoproteins: demonstration in mammalian tissue. Biochem Biophys Res Commun. 1974 Jul 24;59(2):638–645. doi: 10.1016/s0006-291x(74)80027-6. [DOI] [PubMed] [Google Scholar]
- Nordén N. E., Lundblad A., Ockerman P. A., Jolly R. D. Mannosidosis in Angus cattle: partial characterization of two mannose containing oligosaccharides. FEBS Lett. 1973 Sep 15;35(2):209–212. doi: 10.1016/0014-5793(73)80286-8. [DOI] [PubMed] [Google Scholar]
- Nordén N. E., Lundblad A., Svensson S., Ockerman P. A., Autio S. A mannose-containing trisaccharide isolated from urines of three patients with mannosidosis. J Biol Chem. 1973 Sep 10;248(17):6210–6215. [PubMed] [Google Scholar]
- Overdijk B., van der Kroef W. M., Lisman J. J., Pierce R. J., Montreuil J., Spik G. Demonstration and partial characterization of endo-N-acetyl-beta-D-glucosaminidase in human tissues. FEBS Lett. 1981 Jun 15;128(2):364–366. doi: 10.1016/0014-5793(81)80118-4. [DOI] [PubMed] [Google Scholar]
- Rush J. S., Turco S. J., Laine R. A. Erythroglycan biosynthesis in K-562 cells. Inhibition of synthesis by tunicamycin and lack of attachment to the G-protein of vesicular-stomatitis virus. Biochem J. 1981 Jan 1;193(1):361–365. doi: 10.1042/bj1930361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadeh S., Warren C. D., Daniel P. F., Bugge B., James L. F., Jeanloz R. W. Characterization of oligosaccharides from the urine of loco-intoxicated sheep. FEBS Lett. 1983 Oct 31;163(1):104–109. doi: 10.1016/0014-5793(83)81173-9. [DOI] [PubMed] [Google Scholar]
- Stoffyn P., Stoffyn A., Hauser G. Structure of trihexosylceramide biosynthesized in vitro. J Biol Chem. 1973 Mar 25;248(6):1920–1923. [PubMed] [Google Scholar]
- Takasaki S., Ikehira H., Kobata A. Increase of asparagine-linked oligosaccharides with branched outer chains caused by cell transformation. Biochem Biophys Res Commun. 1980 Feb 12;92(3):735–742. doi: 10.1016/0006-291x(80)90765-2. [DOI] [PubMed] [Google Scholar]
- Trimble R. B., Tarentino A. L., Evans G., Maley F. Purification and properties of endo-beta-N-acetylglucosaminidase L from Streptomyces plicatus. J Biol Chem. 1979 Oct 10;254(19):9708–9713. [PubMed] [Google Scholar]
- Tsay G. C., Dawson G., Sung S. S. Structure of the accumulating oligosaccharide in fucosidosis. J Biol Chem. 1976 Oct 10;251(19):5852–5859. [PubMed] [Google Scholar]
- Tulsiani D. R., Touster O. Swainsonine causes the production of hybrid glycoproteins by human skin fibroblasts and rat liver Golgi preparations. J Biol Chem. 1983 Jun 25;258(12):7578–7585. [PubMed] [Google Scholar]
- Turco S. J., Rush J. S., Laine R. A. Presence of erythroglycan on human K-562 chronic myelogenous leukemia-derived cells. J Biol Chem. 1980 Apr 25;255(8):3266–3269. [PubMed] [Google Scholar]
- Warner T. G., O'Brien J. S. Structure analysis of the major oligosaccharides accumulating in canine GM1 gangliosidosis liver. J Biol Chem. 1982 Jan 10;257(1):224–232. [PubMed] [Google Scholar]
- Warren C. D., Sadeh S., Daniel P. F., Bugge B., James L. F., Jeanloz R. W. Induced mannosidosis-excretion of oligosaccharides by locoweed-intoxicated sheep. FEBS Lett. 1983 Oct 31;163(1):99–103. doi: 10.1016/0014-5793(83)81172-7. [DOI] [PubMed] [Google Scholar]
- Wolfe L. S., Senior R. G., Ng-Ying-Kin N. M. The structures of oligosaccharides accumulating in the liver of G-M1-gangliosidosis, type I. J Biol Chem. 1974 Mar 25;249(6):1828–1838. [PubMed] [Google Scholar]


