Abstract
Reduction of human 5-aminolaevulinate dehydratase with NaBH4 in the presence of 14C-labelled substrate led to complete loss of catalytic activity and to incorporation of label into the enzyme protein. By comparison with authentic lysyl-aminolaevulinic acid, prepared chemically, the modified active-site amino acid obtained by acid hydrolysis was shown to be lysine. Sequencing of a CNBr-cleavage peptide isolated from the inactivated 14C-labelled enzyme revealed that the lysine was present within the sequence M-V-K-P-G-M.
Full text
PDF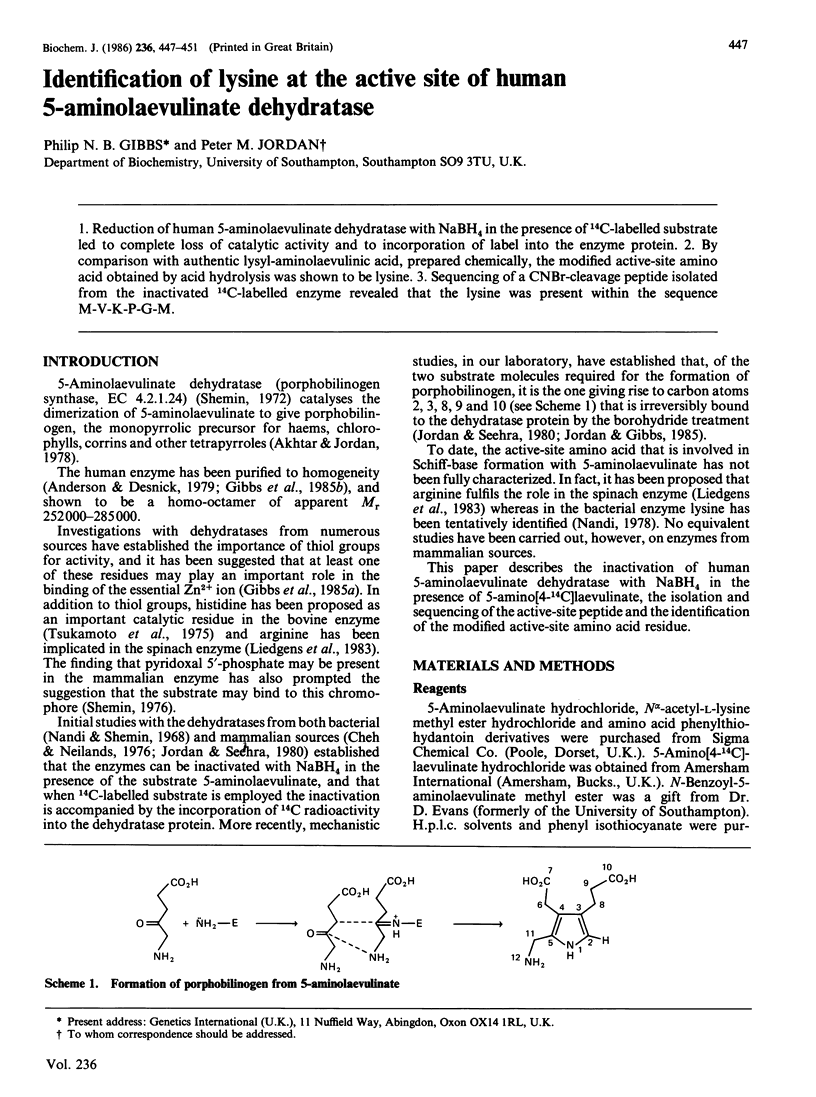


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson P. M., Desnick R. J. Purification and properties of delta-aminolevulinate dehydrase from human erythrocytes. J Biol Chem. 1979 Aug 10;254(15):6924–6930. [PubMed] [Google Scholar]
- Bloxham D. P., Parmelee D. C., Kumar S., Walsh K. A., Titani K. Complete amino acid sequence of porcine heart citrate synthase. Biochemistry. 1982 Apr 27;21(9):2028–2036. doi: 10.1021/bi00538a009. [DOI] [PubMed] [Google Scholar]
- Gibbs P. N., Chaudhry A. G., Jordan P. M. Purification and properties of 5-aminolaevulinate dehydratase from human erythrocytes. Biochem J. 1985 Aug 15;230(1):25–34. doi: 10.1042/bj2300025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs P. N., Gore M. G., Jordan P. M. Investigation of the effect of metal ions on the reactivity of thiol groups in human 5-aminolaevulinate dehydratase. Biochem J. 1985 Feb 1;225(3):573–580. doi: 10.1042/bj2250573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan P. M., Gibbs P. N. Mechanism of action of 5-aminolaevulinate dehydratase from human erythrocytes. Biochem J. 1985 May 1;227(3):1015–1020. doi: 10.1042/bj2271015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan P. M., Seehra J. S. 13C NMR as a probe for the study of enzyme-catalysed reactions: mechanism of action of 5-aminolevulinic acid dehydratase. FEBS Lett. 1980 Jun 2;114(2):283–286. doi: 10.1016/0014-5793(80)81134-3. [DOI] [PubMed] [Google Scholar]
- Liedgens W., Lütz C., Schneider H. A. Molecular properties of 5-aminolevulinic acid dehydratase from Spinacia oleracea. Eur J Biochem. 1983 Sep 1;135(1):75–79. doi: 10.1111/j.1432-1033.1983.tb07619.x. [DOI] [PubMed] [Google Scholar]
- Nandi D. L. Lysine as the substrate binding site of porphobilinogen synthase of Rhodopseudomonas spheroides. Z Naturforsch C. 1978 Sep-Oct;33(9-10):799–802. doi: 10.1515/znc-1978-9-1036. [DOI] [PubMed] [Google Scholar]
- Nandi D. L., Shemin D. Delta-aminolevulinic acid dehydratase of Rhodopseudomonas spheroides. 3. Mechanism of porphobilinogen synthesis. J Biol Chem. 1968 Mar 25;243(6):1236–1242. [PubMed] [Google Scholar]
- Shemin D. 5-Aminolaevulinic acid dehydratase: structure, function, and mechanism. Philos Trans R Soc Lond B Biol Sci. 1976 Feb 5;273(924):109–115. doi: 10.1098/rstb.1976.0004. [DOI] [PubMed] [Google Scholar]
- Tsukamoto I., Yoshinaga T., Sano S. Evidence for histidine as another functional group of delta-aminolevulinic acid dehydratase from beef liver. Biochem Biophys Res Commun. 1975 Nov 3;67(1):294–300. doi: 10.1016/0006-291x(75)90315-0. [DOI] [PubMed] [Google Scholar]


