Abstract
We have shown that changes in fluorescence intensity for the Ca2+ + Mg2+-activated ATPase of sarcoplasmic reticulum labelled with fluorescein isothiocyanate following the addition of Ca2+ can give the ratio of the two conformations (E1 and E2) of the ATPase. We show that the fluorescence response to Ca2+ is unaffected by Mg2+, phosphate or K+, implying that these ions bind equally well to the E1 and E2 conformations. A model is presented for phosphorylation of the ATPase by phosphate as a function of pH, Mg2+, K+ and Ca2+.
Full text
PDF
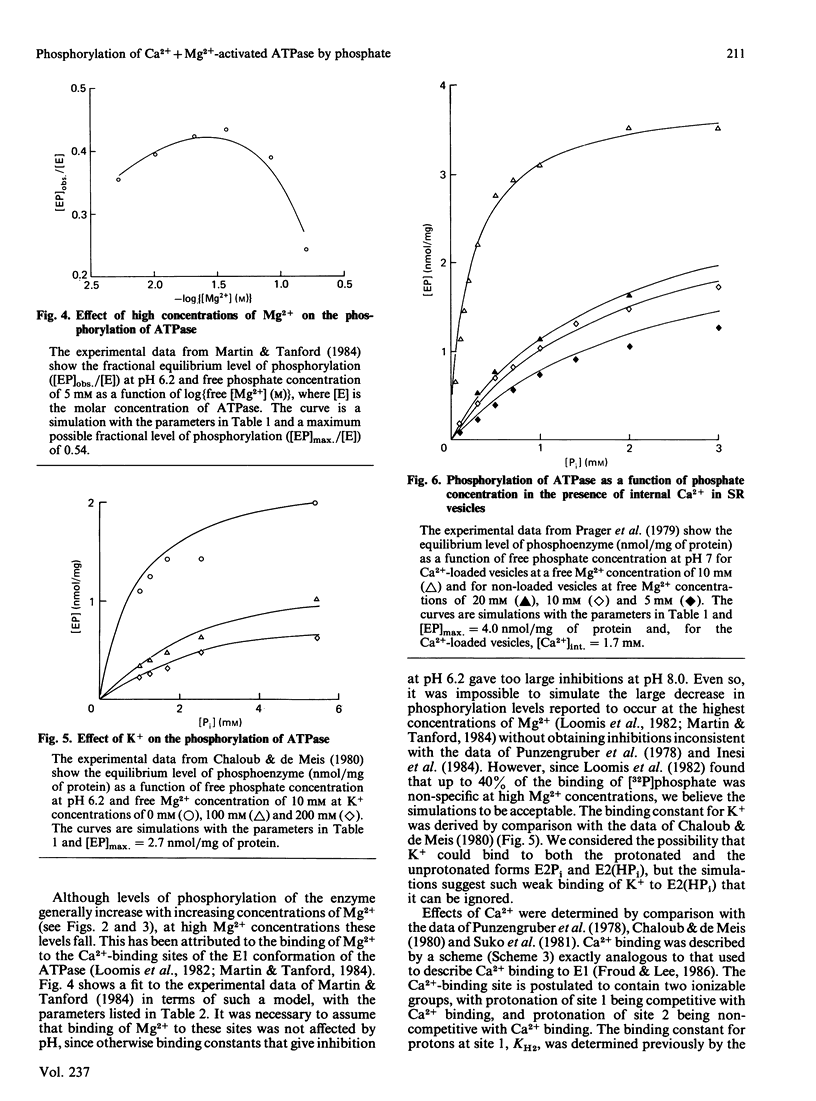
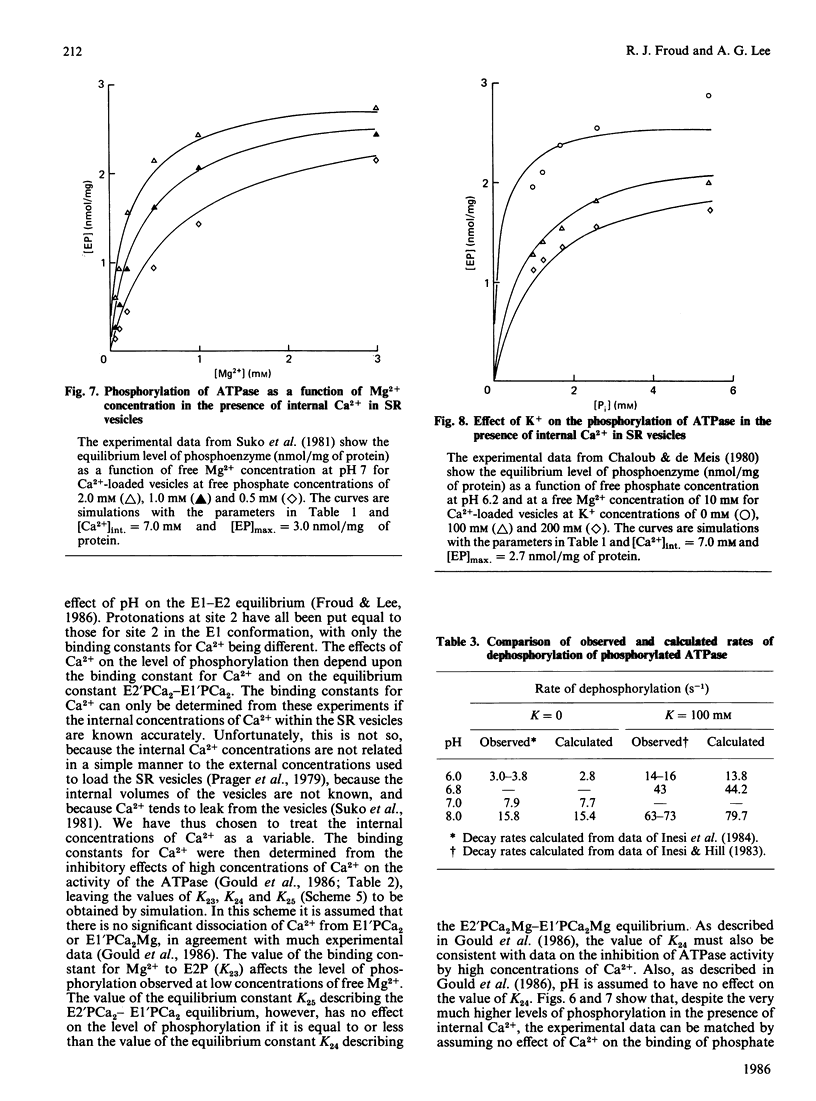
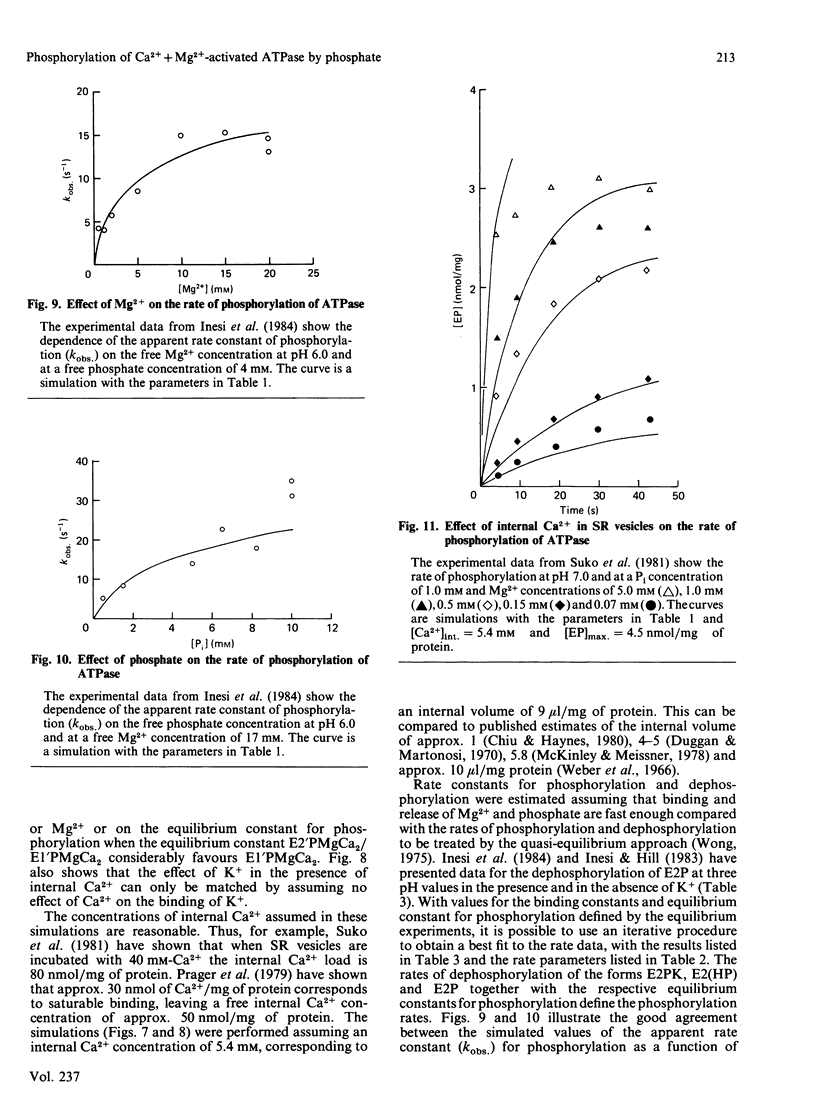


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chaloub R. M., de Meis L. Effect of K+ on phosphorylation of the sarcoplasmic reticulum ATPase by either Pi or ATP. J Biol Chem. 1980 Jul 10;255(13):6168–6172. [PubMed] [Google Scholar]
- Champeil P., Guillain F., Vénien C., Gingold M. P. Interaction of magnesium and inorganic phosphate with calcium-deprived sarcoplasmic reticulum adenosinetriphosphatase as reflected by organic solvent induced perturbation. Biochemistry. 1985 Jan 1;24(1):69–81. doi: 10.1021/bi00322a012. [DOI] [PubMed] [Google Scholar]
- Chiu V. C., Haynes D. H. Rapid kinetic study of the passive permeability of a Ca2+-ATPase rich fraction of the sarcoplasmic reticulum. J Membr Biol. 1980 Oct 31;56(3):203–218. doi: 10.1007/BF01869477. [DOI] [PubMed] [Google Scholar]
- Coll R. J., Murphy A. J. Purification of the CaATPase of sarcoplasmic reticulum by affinity chromatography. J Biol Chem. 1984 Nov 25;259(22):14249–14254. [PubMed] [Google Scholar]
- Degani C., Boyer P. D. A borohydride reduction method for characterization of the acyl phosphate linkage in proteins and its application to sarcoplasmic reticulum adenosine triphosphatase. J Biol Chem. 1973 Dec 10;248(23):8222–8226. [PubMed] [Google Scholar]
- Duggan P. F., Martonosi A. Sarcoplasmic reticulum. IX. The permeability of sarcoplasmic reticulum membranes. J Gen Physiol. 1970 Aug;56(2):147–167. doi: 10.1085/jgp.56.2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froud R. J., Lee A. G. Conformational transitions in the Ca2+ + Mg2+-activated ATPase and the binding of Ca2+ ions. Biochem J. 1986 Jul 1;237(1):197–206. doi: 10.1042/bj2370197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froud R. J., Lee A. G. Conformational transitions in the Ca2+ + Mg2+-activated ATPase and the binding of Ca2+ ions. Biochem J. 1986 Jul 1;237(1):197–206. doi: 10.1042/bj2370197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gafni A., Boyer P. D. Characterization of sarcoplasmic reticulum adenosinetriphosphatase purified by selective column adsorption. Biochemistry. 1984 Sep 11;23(19):4362–4367. doi: 10.1021/bi00314a018. [DOI] [PubMed] [Google Scholar]
- Gould G. W., East J. M., Froud R. J., McWhirter J. M., Stefanova H. I., Lee A. G. A kinetic model for the Ca2+ + Mg2+-activated ATPase of sarcoplasmic reticulum. Biochem J. 1986 Jul 1;237(1):217–227. doi: 10.1042/bj2370217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillain F., Champeil P., Boyer P. D. Sarcoplasmic reticulum adenosinetriphosphatase phosphorylation from inorganic phosphate. Theoretical and experimental reinvestigation. Biochemistry. 1984 Sep 25;23(20):4754–4761. doi: 10.1021/bi00315a034. [DOI] [PubMed] [Google Scholar]
- Inesi G., Hill T. L. Calcium and proton dependence of sarcoplasmic reticulum ATPase. Biophys J. 1983 Nov;44(2):271–280. doi: 10.1016/S0006-3495(83)84299-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inesi G., Lewis D., Murphy A. J. Interdependence of H+, Ca2+, and Pi (or vanadate) sites in sarcoplasmic reticulum ATPase. J Biol Chem. 1984 Jan 25;259(2):996–1003. [PubMed] [Google Scholar]
- Loomis C. R., Martin D. W., McCaslin D. R., Tanford C. Phosphorylation of calcium adenosinetriphosphatase by inorganic phosphate: reversible inhibition at high magnesium ion concentrations. Biochemistry. 1982 Jan 5;21(1):151–156. doi: 10.1021/bi00530a026. [DOI] [PubMed] [Google Scholar]
- Martin D. W., Tanford C. Phosphorylation of calcium adenosinetriphosphatase by inorganic phosphate: van't Hoff analysis of enthalpy changes. Biochemistry. 1981 Aug 4;20(16):4597–4602. doi: 10.1021/bi00519a013. [DOI] [PubMed] [Google Scholar]
- Martin D. W., Tanford C. Solubilized monomeric sarcoplasmic reticulum Ca pump protein. Phosphorylation by inorganic phosphate. FEBS Lett. 1984 Nov 5;177(1):146–150. doi: 10.1016/0014-5793(84)81000-5. [DOI] [PubMed] [Google Scholar]
- McIntosh D. B., Boyer P. D. Adenosine 5'-triphosphate modulation of catalytic intermediates of calcium ion activated adenosinetriphosphatase of sarcoplasmic reticulum subsequent to enzyme phosphorylation. Biochemistry. 1983 Jun 7;22(12):2867–2875. doi: 10.1021/bi00281a015. [DOI] [PubMed] [Google Scholar]
- McKinley D., Meissner G. Evidence for a K+, Na+ permeable channel in sarcoplasmic reticulum. J Membr Biol. 1978 Dec 15;44(2):159–186. doi: 10.1007/BF01976037. [DOI] [PubMed] [Google Scholar]
- Pick U., Karlish S. J. Regulation of the conformation transition in the Ca-ATPase from sarcoplasmic reticulum by pH, temperature, and calcium ions. J Biol Chem. 1982 Jun 10;257(11):6120–6126. [PubMed] [Google Scholar]
- Pickart C. M., Jencks W. P. Energetics of the calcium-transporting ATPase. J Biol Chem. 1984 Feb 10;259(3):1629–1643. [PubMed] [Google Scholar]
- Prager R., Punzengruber C., Kolassa N., Winkler F., Suko J. Ionized and bound calcium inside isolated sarcoplasmic reticulum of skeletal muscle and its significance in phosphorylation of adenosine triphosphatase by orthophosphate. Eur J Biochem. 1979 Jun;97(1):239–250. doi: 10.1111/j.1432-1033.1979.tb13108.x. [DOI] [PubMed] [Google Scholar]
- Punzengruber C., Prager R., Kolassa N., Winkler F., Suko J. Calcium gradient-dependent and calcium gradient-independent phosphorylation of sarcoplasmic reticulum by orthophosphate. The role of magnesium. Eur J Biochem. 1978 Dec;92(2):349–359. doi: 10.1111/j.1432-1033.1978.tb12754.x. [DOI] [PubMed] [Google Scholar]
- Suko J., Plank B., Preis P., Kolassa N., Hellmann G., Conca W. Formation of magnesium-phosphoenzyme and magnesium-calcium-phosphoenzyme in the phosphorylation of adenosine triphosphatase by orthophosphate in sarcoplasmic reticulum. Models of a reaction sequence. Eur J Biochem. 1981 Oct;119(2):225–236. doi: 10.1111/j.1432-1033.1981.tb05598.x. [DOI] [PubMed] [Google Scholar]
- Tanford C., Martin D. W. Equilibrium constants for some steps of the reaction cycle of the sarcoplasmic reticulum calcium pump. Z Naturforsch C. 1982 May-Jun;37(5-6):522–526. doi: 10.1515/znc-1982-5-626. [DOI] [PubMed] [Google Scholar]
- de Meis L., Vianna A. L. Energy interconversion by the Ca2+-dependent ATPase of the sarcoplasmic reticulum. Annu Rev Biochem. 1979;48:275–292. doi: 10.1146/annurev.bi.48.070179.001423. [DOI] [PubMed] [Google Scholar]
- de Meis L., de Souza Otero A., Martins O. B., Alves E. W., Inesi G., Nakamoto R. Phosphorylation of sarcoplasmic reticulum ATPase by orthophosphate in the absence of Ca2+ gradient. Contribution of water activity to the enthalpy and the entropy changes. J Biol Chem. 1982 May 10;257(9):4993–4998. [PubMed] [Google Scholar]


