Abstract
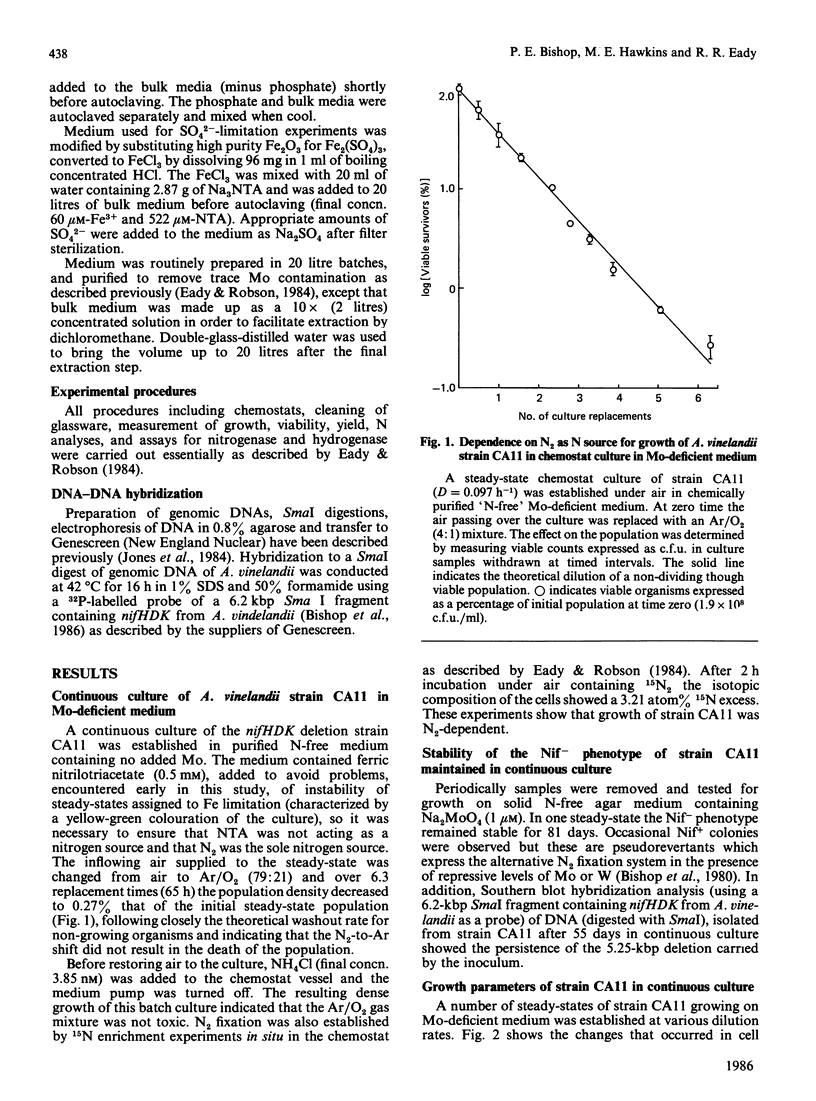
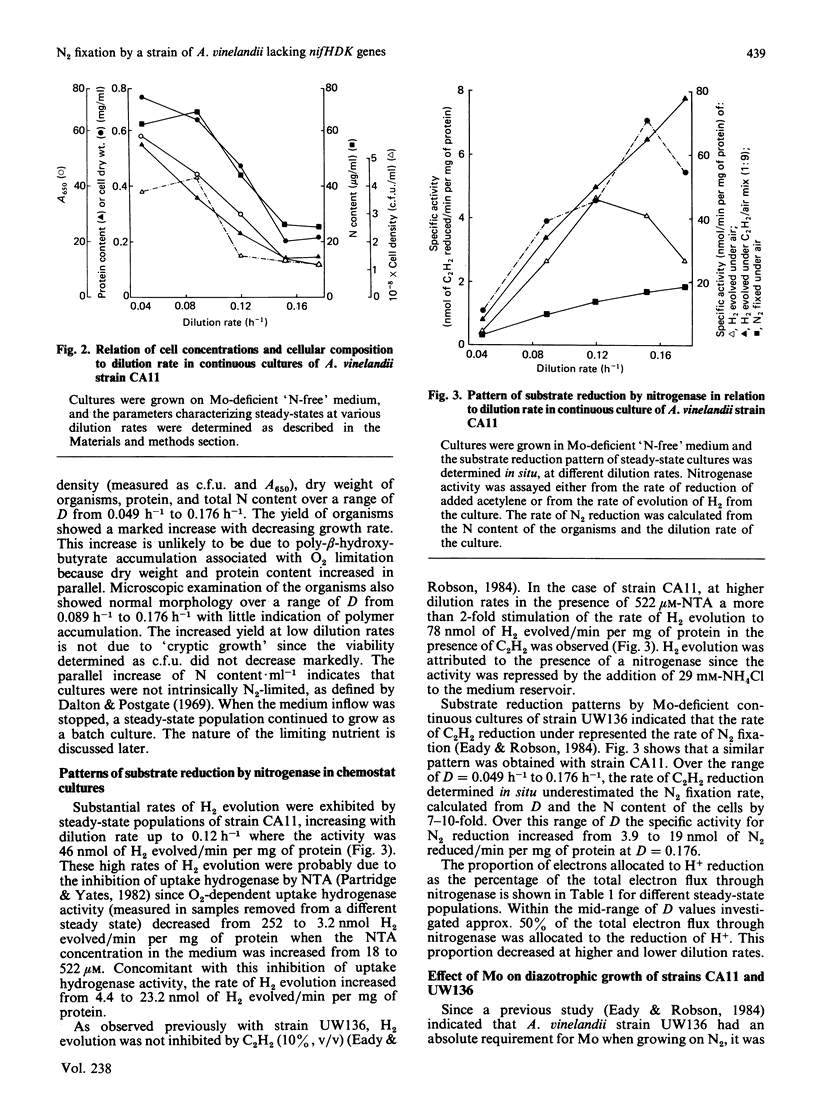
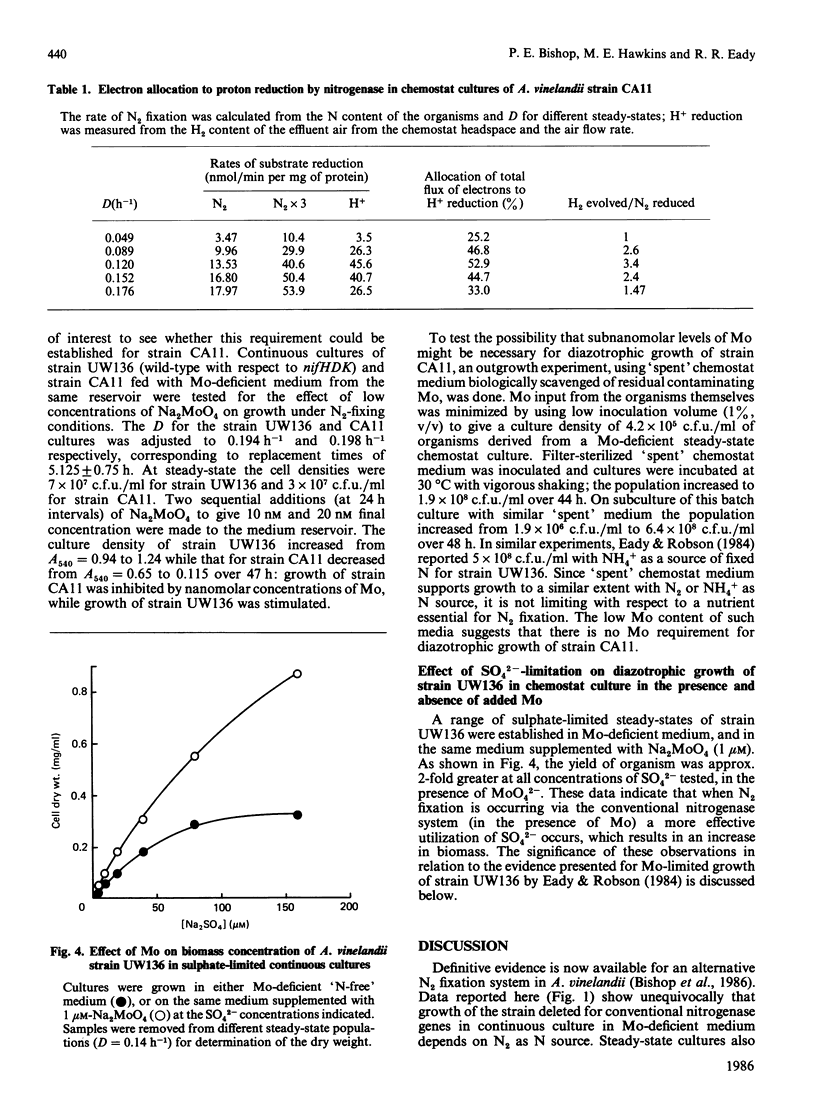
Steady-state chemostat cultures of Azotobacter vinelandii strain CA11, carrying a deletion of genes encoding the structural polypeptides of nitrogenase nifHDK, were established in a simple defined medium chemically purified to minimize contamination by Mo. The medium contained no utilizable N source. Growth was dependent on N2 (1.1 X 10(8) viable cells X ml-1 at D = 0.176 h-1), and was inhibited by Mo (20 nM). DNA hybridization showed the deletion to be stable during prolonged (55 days) growth in the chemostat (132 doublings). Since batch cultures, using unsupplemented 'spent' chemostat medium, showed good growth (1.9 X 10(8) cells X ml-1), no requirement for subnanomolar concentrations of Mo was found. The biomass yield, as the dilution rate (D) was varied, showed that the N content of the culture, protein and dry wt. increased as D was decreased, indicating that neither N2 nor O2 were limiting growth. The limiting nutrient was not identified. Substantial amounts of H2 were evolved by the chemostat cultures, probably as the result of inhibition of O2-dependent hydrogenase activity by nitrilotriacetic acid present in the medium. Over a range of D values approx. 50% of the electron flux through the alternative system was allocated to H+ reduction. C2H2 was a poor substrate, being reduced at 0.14-0.1 times the rate of N2 fixation, calculated from the N content of the cells. SO4(2-)-limited steady-state continuous cultures of strain UW136 (wild-type for nifHDK) had a 2-fold greater biomass in the presence of MoO4(2-) (1 microM). The significance of this finding for 'Mo-limited' continuous cultures [Eady & Robson (1984) Biochem. J. 224, 853-862] is discussed.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bishop P. E., Jarlenski D. M., Hetherington D. R. Evidence for an alternative nitrogen fixation system in Azotobacter vinelandii. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7342–7346. doi: 10.1073/pnas.77.12.7342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop P. E., Premakumar R., Dean D. R., Jacobson M. R., Chisnell J. R., Rizzo T. M., Kopczynski J. Nitrogen Fixation by Azotobacter vinelandii Strains Having Deletions in Structural Genes for Nitrogenase. Science. 1986 Apr 4;232(4746):92–94. doi: 10.1126/science.232.4746.92. [DOI] [PubMed] [Google Scholar]
- Dixon R. A. The genetic complexity of nitrogen fixation. The ninth Fleming lecture. J Gen Microbiol. 1984 Nov;130(11):2745–2755. doi: 10.1099/00221287-130-11-2745. [DOI] [PubMed] [Google Scholar]
- Eady R. R., Robson R. L. Characteristics of N2 fixation in Mo-limited batch and continuous cultures of Azotobacter vinelandii. Biochem J. 1984 Dec 15;224(3):853–862. doi: 10.1042/bj2240853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott B. B., Mortenson L. E. Transport of molybdate by Clostridium pasteurianum. J Bacteriol. 1975 Dec;124(3):1295–1301. doi: 10.1128/jb.124.3.1295-1301.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones R., Woodley P., Robson R. Cloning and organisation of some genes for nitrogen fixation from Azotobacter chroococcum and their expression in Klebsiella pneumoniae. Mol Gen Genet. 1984;197(2):318–327. doi: 10.1007/BF00330980. [DOI] [PubMed] [Google Scholar]
- Partridge C. D., Yates M. G. Effect of chelating agents on hydrogenase in Azotobacter chroococcum. Evidence that nickel is required for hydrogenase synthesis. Biochem J. 1982 Apr 15;204(1):339–344. doi: 10.1042/bj2040339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinto C., De La Vega H., Flores M., Leemans J., Cevallos M. A., Pardo M. A., Azpiroz R., De Lourdes Girard M., Calva E., Palacios R. Nitrogenase reductase: A functional multigene family in Rhizobium phaseoli. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1170–1174. doi: 10.1073/pnas.82.4.1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robson R., Woodley P., Jones R. Second gene (nifH*) coding for a nitrogenase iron protein in Azotobacter chroococcum is adjacent to a gene coding for a ferredoxin-like protein. EMBO J. 1986 Jun;5(6):1159–1163. doi: 10.1002/j.1460-2075.1986.tb04341.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadoff H. L., Shimel B., Ellis S. Characterization of Azotobacter vinelandii deoxyribonucleic acid and folded chromosomes. J Bacteriol. 1979 Jun;138(3):871–877. doi: 10.1128/jb.138.3.871-877.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scolnik P. A., Haselkorn R. Activation of extra copies of genes coding for nitrogenase in Rhodopseudomonas capsulata. Nature. 1984 Jan 19;307(5948):289–292. doi: 10.1038/307289a0. [DOI] [PubMed] [Google Scholar]
- Shah V. K., Ugalde R. A., Imperial J., Brill W. J. Molybdenum in nitrogenase. Annu Rev Biochem. 1984;53:231–257. doi: 10.1146/annurev.bi.53.070184.001311. [DOI] [PubMed] [Google Scholar]
- Strandberg G. W., Wilson P. W. Formation of the nitrogen-fixing enzyme system in Azotobacter vinelandii. Can J Microbiol. 1968 Jan;14(1):25–31. doi: 10.1139/m68-005. [DOI] [PubMed] [Google Scholar]


