Abstract
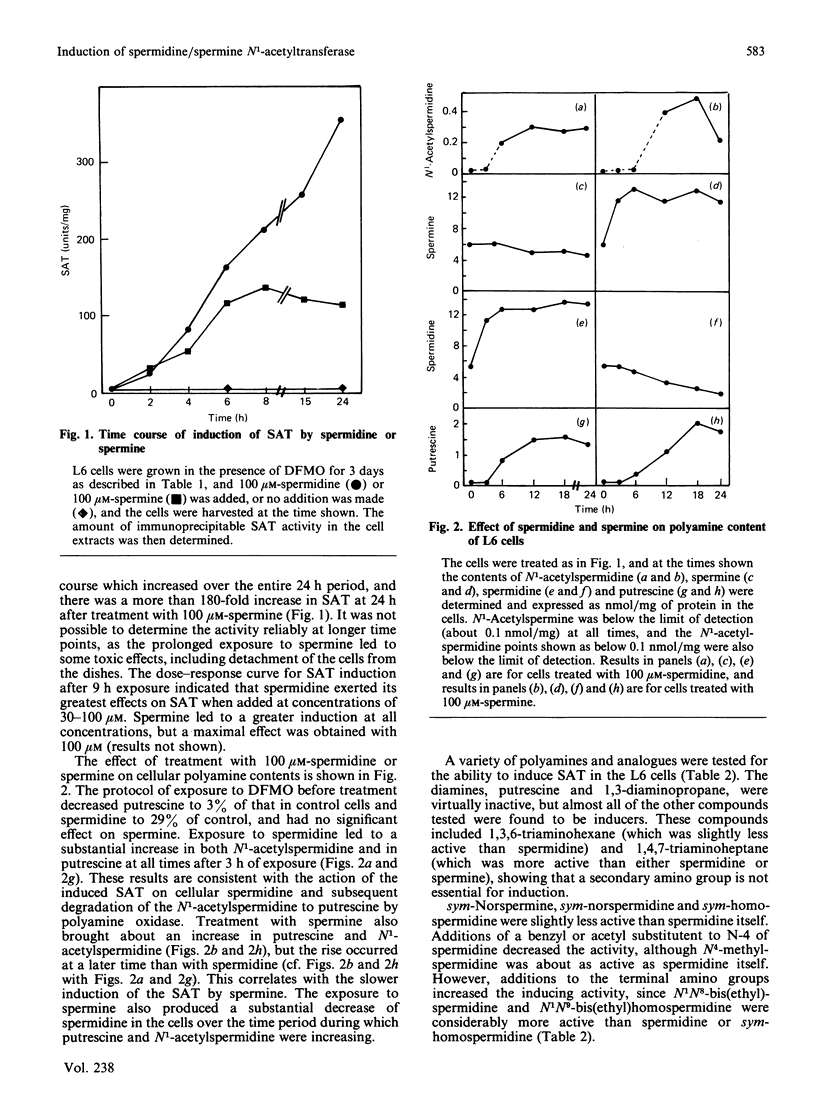
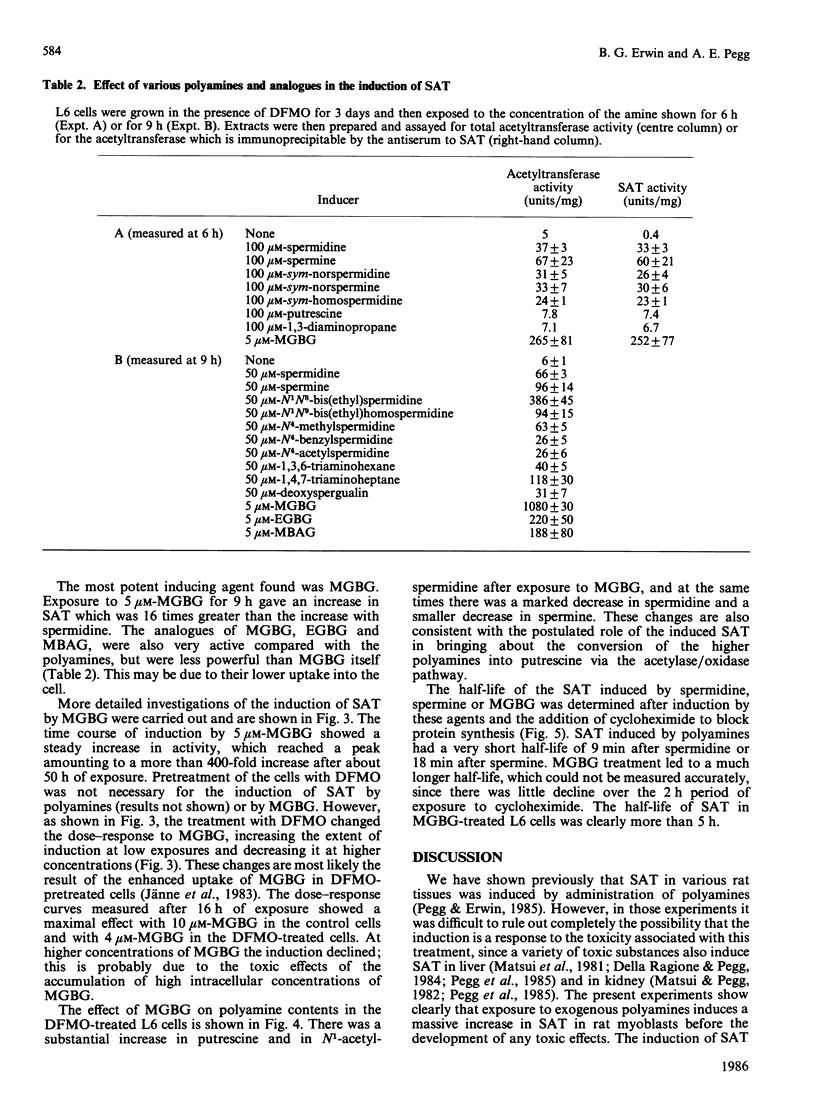
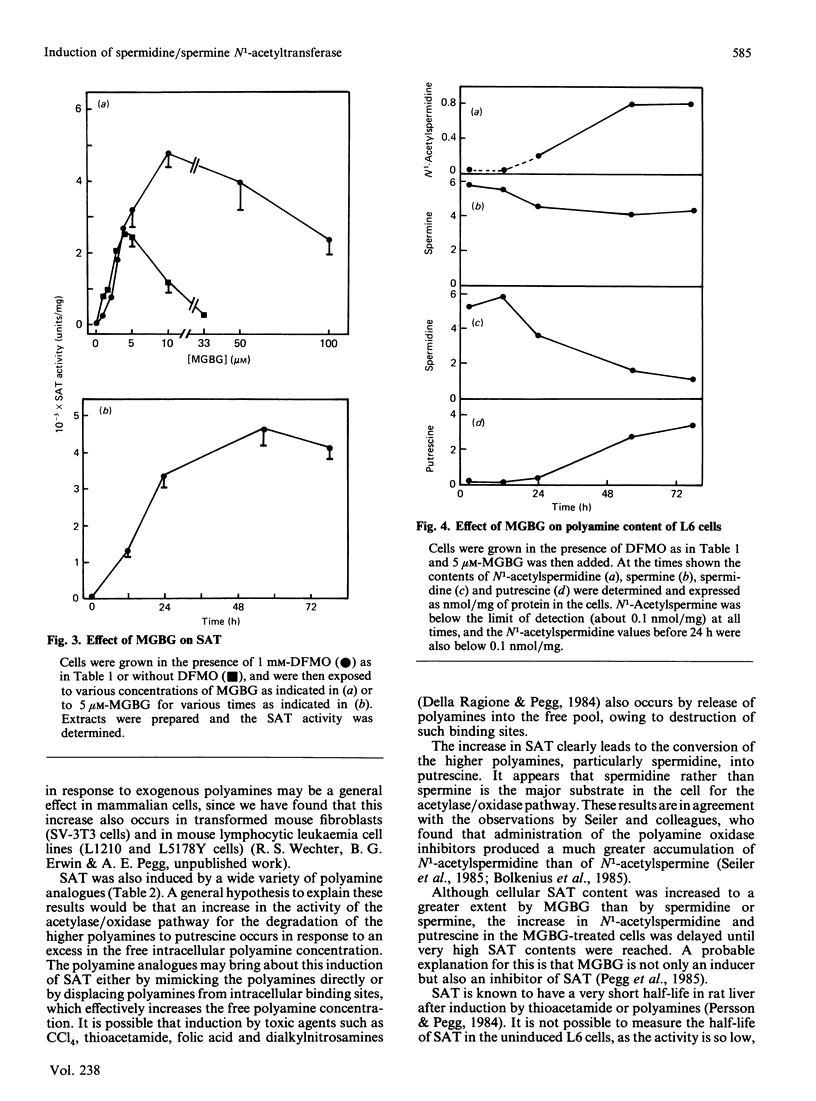
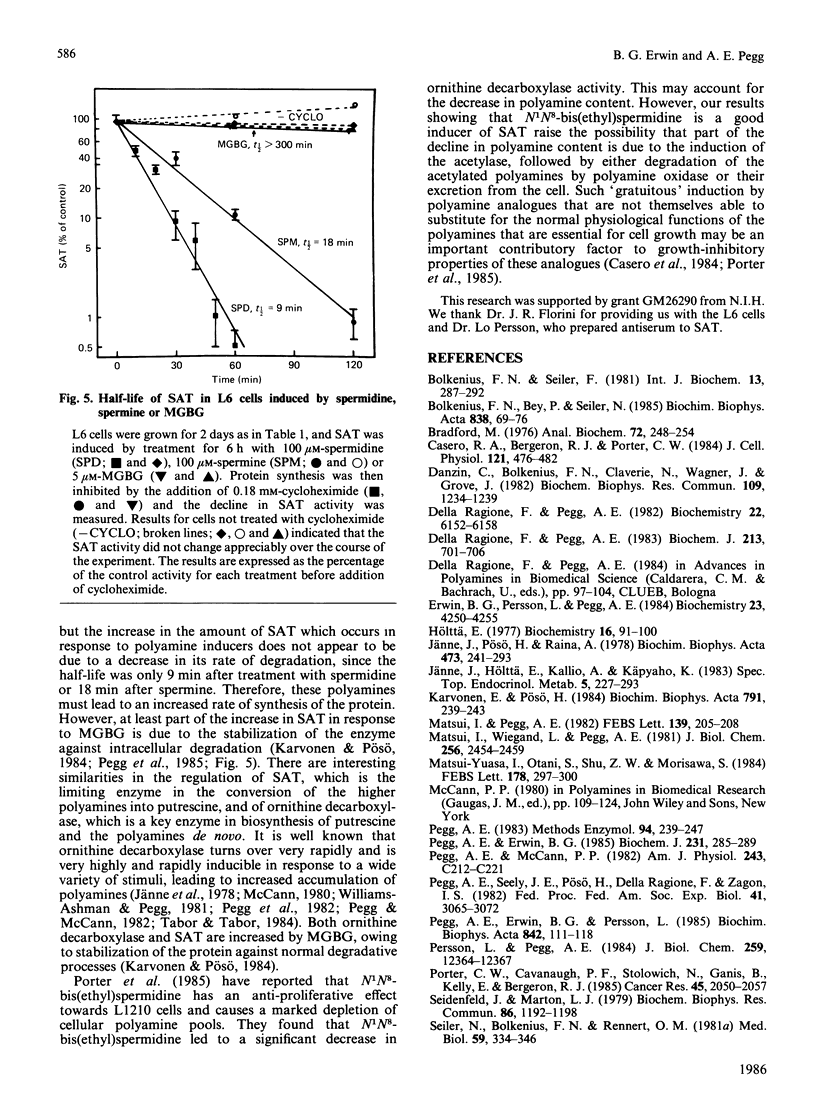
Exposure of rat L6 cells in culture to exogenous polyamines led to a very large increase in the activity of spermidine/spermine N1-acetyltransferase. Spermine was more potent than spermidine in bringing about this increase, but in both cases the elevated acetyltransferase activity increased the cellular conversion of spermidine into putrescine. The N1-acetyltransferase turned over very rapidly in the L6 cells, with a half-life of 9 min after spermidine and 18 min after spermine. A wide variety of synthetic polyamine analogues also brought about a substantial induction of spermidine/spermine N1-acetyltransferase activity. These included sym-norspermidine, sym-norspermine, sym-homospermidine, N4-substituted spermidine derivatives, 1,3,6-triaminohexane, 1,4,7-triaminoheptane and deoxyspergualin, which were comparable with spermidine in their potency, and N1N8-bis(ethyl)spermidine, N1N9-bis(ethyl)homospermidine, methylglyoxal bis(guanylhydrazone), ethylglyoxal bis(guanylhydrazone) and 1,1'-[(methylethanediylidene)dinitrilo]bis(3-amino-guanidine ), which were even more active than spermidine. It is suggested that these polyamine analogues may bring about a decrease in cellular polyamines not only by inhibiting biosynthesis but by stimulating the degradation of spermidine into putrescine.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bolkenius F. N., Bey P., Seiler N. Specific inhibition of polyamine oxidase in vivo is a method for the elucidation of its physiological role. Biochim Biophys Acta. 1985 Jan 28;838(1):69–76. doi: 10.1016/0304-4165(85)90251-x. [DOI] [PubMed] [Google Scholar]
- Bolkenius F. N., Seiler N. Acetylderivatives as intermediates in polyamine catabolism. Int J Biochem. 1981;13(3):287–292. doi: 10.1016/0020-711x(81)90080-x. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Casero R. A., Jr, Bergeron R. J., Porter C. W. Treatment with alpha-difluoromethylornithine plus a spermidine analog leads to spermine depletion and growth inhibition in cultured L1210 leukemia cells. J Cell Physiol. 1984 Dec;121(3):476–482. doi: 10.1002/jcp.1041210305. [DOI] [PubMed] [Google Scholar]
- Danzin C., Bolkenius F. N., Claverie N., Wagner J., Grove J. Secretin-induced accumulation of N1-acetylspermidine and putrescine in the rat pancreas. Biochem Biophys Res Commun. 1982 Dec 31;109(4):1234–1239. doi: 10.1016/0006-291x(82)91909-x. [DOI] [PubMed] [Google Scholar]
- Della Ragione F., Pegg A. E. Studies of the specificity and kinetics of rat liver spermidine/spermine N1-acetyltransferase. Biochem J. 1983 Sep 1;213(3):701–706. doi: 10.1042/bj2130701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erwin B. G., Persson L., Pegg A. E. Differential inhibition of histone and polyamine acetylases by multisubstrate analogues. Biochemistry. 1984 Aug 28;23(18):4250–4255. doi: 10.1021/bi00313a036. [DOI] [PubMed] [Google Scholar]
- Hölttä E. Oxidation of spermidine and spermine in rat liver: purification and properties of polyamine oxidase. Biochemistry. 1977 Jan 11;16(1):91–100. doi: 10.1021/bi00620a015. [DOI] [PubMed] [Google Scholar]
- Jänne J., Hölttä E., Kallio A., Käpyaho K. Role of polyamines and their antimetabolites in clinical medicine. Spec Top Endocrinol Metab. 1983;5:227–293. [PubMed] [Google Scholar]
- Jänne J., Pösö H., Raina A. Polyamines in rapid growth and cancer. Biochim Biophys Acta. 1978 Apr 6;473(3-4):241–293. doi: 10.1016/0304-419x(78)90015-x. [DOI] [PubMed] [Google Scholar]
- Karvonen E., Pösö H. Stabilization of ornithine decarboxylase and N1-spermidine acetyltransferase in rat liver by methylglyoxal bis(guanylhydrazone). Biochim Biophys Acta. 1984 Dec 7;791(2):239–243. doi: 10.1016/0167-4838(84)90014-1. [DOI] [PubMed] [Google Scholar]
- Matsui-Yuasa I., Otani S., Shu Z. W., Morisawa S. Phorbol esters stimulate spermidine/spermine N1-acetyltransferase activity in mitogen-stimulated bovine lymphocytes. FEBS Lett. 1984 Dec 10;178(2):297–300. doi: 10.1016/0014-5793(84)80620-1. [DOI] [PubMed] [Google Scholar]
- Matsui I., Pegg A. E. Induction of spermidine N1-acetyltransferase in rat kidney by treatment with folic acid. FEBS Lett. 1982 Mar 22;139(2):205–208. doi: 10.1016/0014-5793(82)80852-1. [DOI] [PubMed] [Google Scholar]
- Matsui I., Wiegand L., Pegg A. E. Properties of spermidine N-acetyltransferase from livers of rats treated with carbon tetrachloride and its role in the conversion of spermidine into putrescine. J Biol Chem. 1981 Mar 10;256(5):2454–2459. [PubMed] [Google Scholar]
- Pegg A. E., Erwin B. G. Induction of spermidine/spermine N1-acetyltransferase in rat tissues by polyamines. Biochem J. 1985 Oct 15;231(2):285–289. doi: 10.1042/bj2310285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pegg A. E., Erwin B. G., Persson L. Induction of spermidine/spermine N1-acetyltransferase by methylglyoxal bis(guanylhydrazone). Biochim Biophys Acta. 1985 Oct 17;842(2-3):111–118. doi: 10.1016/0304-4165(85)90192-8. [DOI] [PubMed] [Google Scholar]
- Pegg A. E. Inhibitors of S-adenosylmethionine decarboxylase. Methods Enzymol. 1983;94:239–247. doi: 10.1016/s0076-6879(83)94042-9. [DOI] [PubMed] [Google Scholar]
- Pegg A. E., McCann P. P. Polyamine metabolism and function. Am J Physiol. 1982 Nov;243(5):C212–C221. doi: 10.1152/ajpcell.1982.243.5.C212. [DOI] [PubMed] [Google Scholar]
- Pegg A. E., Seely J. E., Pösö H., della Ragione F., Zagon I. A. Polyamine biosynthesis and interconversion in rodent tissues. Fed Proc. 1982 Dec;41(14):3065–3072. [PubMed] [Google Scholar]
- Persson L., Pegg A. E. Studies of the induction of spermidine/spermine N1-acetyltransferase using a specific antiserum. J Biol Chem. 1984 Oct 25;259(20):12364–12367. [PubMed] [Google Scholar]
- Porter C. W., Cavanaugh P. F., Jr, Stolowich N., Ganis B., Kelly E., Bergeron R. J. Biological properties of N4- and N1,N8-spermidine derivatives in cultured L1210 leukemia cells. Cancer Res. 1985 May;45(5):2050–2057. [PubMed] [Google Scholar]
- Ragione F. D., Pegg A. E. Purification and characterization of spermidine/spermine N1-acetyltransferase from rat liver. Biochemistry. 1982 Nov 23;21(24):6152–6158. doi: 10.1021/bi00267a020. [DOI] [PubMed] [Google Scholar]
- Seidenfeld J., Marton L. J. Depletion of intracellular putrescine and spermidine by alpha-difluromethylornithine does not inhibit proliferation of 9L rat brain tumor cells. Biochem Biophys Res Commun. 1979 Feb 28;86(4):1192–1198. doi: 10.1016/0006-291x(79)90243-2. [DOI] [PubMed] [Google Scholar]
- Seiler N., Bolkenius F. N., Knödgen B. The influence of catabolic reactions on polyamine excretion. Biochem J. 1985 Jan 1;225(1):219–226. doi: 10.1042/bj2250219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiler N., Bolkenius F. N., Rennert O. M. Interconversion, catabolism and elimination of the polyamines. Med Biol. 1981 Dec;59(5-6):334–346. [PubMed] [Google Scholar]
- Shinki T., Takahashi N., Kadofuku T., Sato T., Suda T. Induction of spermidine N1-acetyltransferase by 1 alpha,25-dihydroxyvitamin D3 as an early common event in the target tissues of vitamin D. J Biol Chem. 1985 Feb 25;260(4):2185–2190. [PubMed] [Google Scholar]
- Stoscheck C. M., Erwin B. G., Florini J. R., Richman R. A., Pegg A. E. Effects of inhibitors of ornithine and S-adenosylmethionine decarboxylases on L6 myoblast proliferation. J Cell Physiol. 1982 Feb;110(2):161–168. doi: 10.1002/jcp.1041100209. [DOI] [PubMed] [Google Scholar]
- Tabor C. W., Tabor H. Polyamines. Annu Rev Biochem. 1984;53:749–790. doi: 10.1146/annurev.bi.53.070184.003533. [DOI] [PubMed] [Google Scholar]
- Wallace H. M., Macgowan S. H., Keir H. M. Polyamine metabolism in mammalian cells in culture. Biochem Soc Trans. 1985 Apr;13(2):329–330. doi: 10.1042/bst0130329. [DOI] [PubMed] [Google Scholar]
- Yaffe D. Retention of differentiation potentialities during prolonged cultivation of myogenic cells. Proc Natl Acad Sci U S A. 1968 Oct;61(2):477–483. doi: 10.1073/pnas.61.2.477. [DOI] [PMC free article] [PubMed] [Google Scholar]


