Supplemental Digital Content is Available in the Text.
The holistic minimal clinical important difference provides a valid, personalized, and comprehensive measure of the impact of interventions for people with chronic pain.
Keywords: Chronic pain, Construct validity, Holistic composite outcome measure, Internal consistency, Minimal clinical important difference, Pain measurement
Abstract
Introduction:
Chronic pain is a personal experience influenced by multiple biopsychosocial factors. Using a pain intensity measure alone to assess the effectiveness of a chronic pain intervention fails to fully evaluate its impact on the multifaceted chronic pain experience. The holistic minimal clinically important difference (MCID) is a composite outcome developed to provide a comprehensive assessment of chronic pain in response to intervention, across 5 outcome domains: pain intensity, health-related quality of life, sleep quality, physical, and emotional function. To focus on domains where the individual need is greatest, the holistic MCID reflects the cumulative MCID averaged over only the domains where subjects were impaired preintervention.
Objectives:
To assess the internal and construct validity of the Holistic MCID score to inform its future use as an evidence-based tool.
Methods:
This validation study was undertaken using data from the EVOKE trial with 111 patients up to 24-month follow-up. Internal consistency of the holistic MCID was assessed using Cronbach alpha statistic and dimensional exploration using principal component analysis.
Results:
The holistic MCID measure demonstrated strong internal consistency with Cronbach alpha >0.7 at all follow-ups. Principal component analysis showed one overarching holistic dimension to be present in the composite. Construct validity was demonstrated by an increase in the holistic MCID score being associated with both increased Patients' Global Impression of Change, EuroQol visual analogue scale score, and each of the outcome domains in a “leave-one-out” analysis (all P < 0.001).
Conclusion:
The holistic MCID provides a valid measure for the comprehensive, personalized assessment of response after a chronic pain intervention. The validity of the holistic MCID requires further confirmation in other chronic pain populations and with different interventions.
1. Introduction
Chronic pain is a common, complex, and distressing condition that is difficult to quantify and experienced uniquely by each individual. The International Association for the Study of Pain (IASP) definition states that “pain is always a personal experience that is influenced to varying degrees by biological, psychological, and social factors.”47 Despite wide recognition of chronic pain as a complex biopsychosocial phenomenon, clinical trials of interventions for chronic pain etiologies focus primarily on pain intensity alone and regulatory agencies focus on pain intensity when deciding whether to approve therapies for use. The most commonly used methods for measuring pain intensity are visual analogue scale (VAS) and numeric rating scale (NRS), both of which are limited to subjective interpretations of pain that typically fail to adequately reflect the wider biopsychosocial chronic pain experience and how it affects an individual's overall health.13 This shortcoming has led scientists and practitioners, alike, to search for different means of evaluating pain that takes into account more aspects of well-being, thus allowing for a more accurate evaluation of the pain experience.
A composite outcome combines 2 or more outcomes into a single measure to evaluate the broader impact of health interventions.11 A holistic composite measure for chronic pain needs to capture the various outcome domains that reflect both the unmet health need of the condition and the response to intervention. These outcome domains should reflect their importance to patients with chronic pain,25,54 health care providers,24 and also consider current core outcome recommendations.15,32
An international expert panel developed the holistic minimal clinically important difference (MCID) outcome as a comprehensive composite outcome measure based on the Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials (IMMPACT) guidance for outcome assessment in chronic pain and US Food and Drug Administration (FDA) recommendations for composite outcomes.20 The holistic MCID uses recommended and validated patient-reported outcome measures (PROMs), assesses normative population values to determine unmet health needs, and evaluates treatment response based on changes that are meaningful to patients.35 The holistic MCID measure comprises the 5 domains of pain intensity, physical function, health-related quality of life (HRQoL), emotional function, and sleep quality. The holistic MCID score reflects the cumulative MCID score after intervention, averaged over only the domains where subjects were impaired preintervention. We have demonstrated the application of the holistic MCID score concept in the setting of a clinical trial.30
The validity of composite outcome measures for chronic pain has not been frequently assessed or reported. The objective of this study was to assess the internal and construct validity of the holistic MCID score to inform the future use of the holistic MCID score as an evidence-based tool.
2. Methods
2.1. Study population
The EVOKE [NCT02924129] trial was a participant, investigator, and outcome assessor-blinded, parallel-arm study. The study was conducted at 13 US centers and randomized 134 participants with chronic, intractable back and leg pain to evoked compound action potentials (ECAP)-controlled closed-loop spinal cord stimulation (SCS) or open-loop SCS. Details of the study design and outcomes are reported elsewhere.34,38–40 The study was conducted in compliance with ethical and regulatory guidelines and was approved by local ethics committees prior to subject enrollment. The current study utilizes individual patient EVOKE trial data for those 111 participants who provided complete outcome data at a follow-up to 24-month post-implant (n for participants at each time point is detailed in Table 1).
Table 1.
EVOKE trial results for 5 individual domains (mean number of MCIDs achieved), cumulative responder score, and holistic minimal clinically important difference score at 1- to 24-month follow up.
| Domains | 1-mo (n = 111) | 3-mo (n = 111) | 6-mo (n = 107) | 12-mo (n = 103) | 18-mo (n = 97) | 24-mo (n = 92) |
|---|---|---|---|---|---|---|
| Pain intensity (VAS overall ≥30%) | 2.3 (1.0) | 2.5 (0.9) | 2.4 (0.8) | 2.5 (0.9) | 2.5 (0.9) | 2.3 (1.0) |
| HRQoL (EQ-5D-5L index score ≥0.074) | 3.3 (2.1) | 3.5 (2.3) | 3.5 (2.1) | 3.2 (2.5) | 3.3 (2.2) | 3.1 (2.2) |
| Sleep quality (PSQI global score ≥3)* | 1.5 (1.4) | 1.7 (1.4) | 1.6 (1.4) | 1.8 (1.5) | 1.5 (1.5) | 1.5 (1.5) |
| Physical function (ODI score ≥10) | 2.5 (1.6) | 2.9 (1.6) | 2.7 (1.4) | 2.7 (1.5) | 2.6 (1.6) | 2.5 (1.4) |
| Emotional function (POMS TMD score ≥10)† | 2.3 (1.7) | 2.2 (2.0) | 2.3 (2.1) | 2.2 (2.1) | 2.0 (2.1) | 2.0 (2.0) |
| Cumulative responder score‡ | 11.1 (5.6) | 11.9 (6.2) | 11.7 (5.9) | 11.5 (6.4) | 11.1 (5.9) | 10.6 (5.6) |
| Holistic MCID§ | 2.4 (1.2) | 2.6 (1.3) | 2.5 (1.2) | 2.5 (1.3) | 2.4 (1.2) | 2.3 (1.2) |
Data presented as means and (standard deviations) of MCIDs achieved.
N of patients with baseline impairment for sleep quality that completed follow-up: 1 month (n = 108), 3 months (n = 108), 6 months (n = 104), 12 months (n = 100), 18 months (n = 94), 24 months (n = 89).
N of patients with baseline impairment for emotional function that completed follow-up: 1 month (n = 68), 3 months (n = 68), 6 months (n = 67), 12 months (n = 64), 18 months (n = 60), 24 months (n = 58).
Cumulative responder score: the total amount of MCIDs achieved after intervention across all 5 domains impaired at baseline for each individual patient.
Holistic MCID: cumulative responder score divided by the number of impaired domains at baseline for each individual patient.
EQ-5D-5L, EuroQol 5-dimension 5-level; HRQoL, health-related quality of life; MCID, minimal clinically important difference; ODI, Oswestry disability index; POMS, profile of mood states; PSQI, Pittsburgh sleep quality index; TMD, total mood disorder; VAS, visual analogue scale.
2.2. Basis of the holistic minimal clinically important difference score
The development of the holistic composite outcome and holistic treatment response has been previously described35 and was based on 5 key principles:
2.2.1. Components of the holistic composite outcome
The holistic MCID outcome comprises 5 domains of significance to a chronic pain population: pain intensity, HRQoL, sleep quality, physical, and emotional function. These domains have been judged as important in surveys of patients with chronic pain,25,54 and of health care providers,24 and are recommended by IMMPACT as core outcome domains.15,32
2.2.2. Validated patient-reported outcome measures
Assessment of each domain is based on the following validated PROMs:
(1) Pain intensity assessed with a 100-mm visual analogue scale (VAS) ranging from 0 (no pain) to 100 (worst possible pain).45
(2) HRQoL measured with the EuroQol 5-dimension 5-level (EQ-5D-5L) descriptive system that comprises 5 dimensions (mobility, self-care, usual activities, pain/discomfort, and depression/anxiety), where each dimension has 5 response levels: no problems, slight problems, moderate problems, severe problems, and unable to/extreme problems.28 Responses to the EQ-5D-5L were converted into single (utility) indices using the US value set for EQ-5D-5L crosswalk to EQ-5D-3L.55
(3) Sleep quality evaluated with the Pittsburgh Sleep Quality Index (PSQI) instrument that comprises 19 individual items that generate 7 component scores (subjective sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbances, use of sleeping medication, and daytime impairment); the sum of the component scores produces a single global score.6
(4) Physical function evaluated with the Oswestry Disability Index (ODI) that consists of 10 items/activities each with 6 scoring levels (range 0–5); the sum of the score for each item is divided by the total score possible for the items answered and multiplied by 100.18
(5) Emotional function estimated with the Profile of Mood States-Brief (POMS-B) tool that consists of 30 adjectives that describe feelings or moods that an individual may have experienced during the prior week; a total score (total mood disturbance) is derived from 6 mood states (tension, depression, anger, vigor, fatigue, and confusion).37
2.2.3. Assessment of unmet health need prior to treatment
Since chronic pain is a personal experience that affects people in different ways, it is important to assess which domains have been impaired on an individual patient basis. This is done by evaluation of baseline (preintervention) status of each patient relative to the normative value for each of the 5 outcome domains. We use published normative values for the 5 outcome domains. Pain intensity: VAS <60 mm (based on inclusion criterion required for study entry in the EVOKE trial of ≥60 mm [0–100 mm scale]); physical function: ODI < 10.1918; HRQoL: EQ-5D > 0.83052; sleep quality: PSQI <6.35; and emotional function: POMS < 17.7.37 Domains rated as being worse than normative values reflect areas where patients would place more value on improvements. The holistic MCID score is calculated for those baseline outcome domains demonstrated to score below (worse than) these normative cutoffs.
2.2.4. Intervention response (study outcome) assessed using minimal clinically important differences
Achievement of MCID thresholds are used to assess the intervention response (study outcome) for each of the domains and to calculate the cumulative responder score (sum of the total amount of MCIDs achieved after intervention across all 5 domains impaired at baseline for each individual patient). The following MCIDs were applied: pain intensity: ≥30% decrease in VAS16; physical function: ≥10-point decrease in ODI42; HRQoL: ≥0.074-point increase in EQ-5D index score56; sleep quality: ≥3-point decrease in PSQI4; and emotional function: ≥10-point decrease in POMS total mood disorder (TMD).16
2.2.5. Adjustment of holistic minimal clinically important difference score to baseline impairments
To avoid a ceiling effect and to focus on domains where the individual clinical need is greatest, the holistic MCID is calculated by summing each of the MCID domain scores, averaged over each patients' number of impaired domains at baseline (Fig. 1 for additional explanation on how to calculate the holistic MCID for each individual patient). Worsening in the different domains would have a negative contribution to the cumulative score. The holistic MCID enables standardization of the score irrespective of number of impaired domains at baseline or number of domains considered in a holistic composite outcome (Fig. 1). A holistic MCID of 1.0 indicates that a clinically meaningful change was obtained on average across all domains impaired at baseline.
Figure 1.
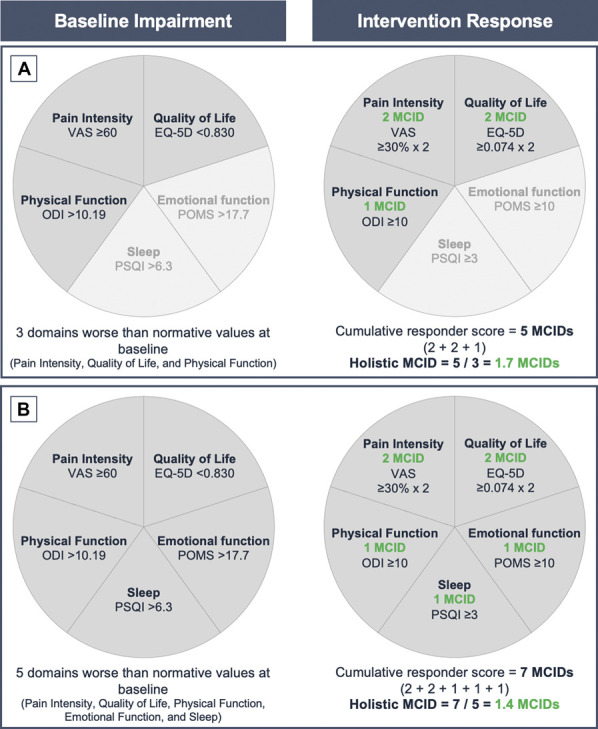
Theoretical representation of the holistic MCID. Five core domains of pain intensity, physical function, health-related quality of life, sleep quality, and emotional function were evaluated. (A) In this example, a hypothetical “Patient A” had baseline impairment for pain intensity, health-related quality of life, and physical function. Patient A obtained 2 MCIDs for each of pain intensity and health-related quality of life domains, and 1 MCID for physical function domain, which corresponds to a cumulative responder score of 5 MCIDs. Adjusting the cumulative responder score of 5 MCIDs by 3 impaired domains at baseline results in a holistic MCID score of 1.7. (B) A hypothetical “Patient B” had baseline impairment in the 5 domains and obtained 2 MCIDs for each of pain intensity and health-related quality of life domains, and 1 MCID for each of sleep quality, physical, and emotional function domains, corresponding to a cumulative responder score of 7 MCIDs. Adjusting the cumulative responder score of 7 MCIDs by 5 impaired domains at baseline results in a holistic MCID score of 1.4.
2.3. Data analysis
Internal consistency of holistic MCID was assessed using exploratory dimensional analysis of all domains as a singular “holistic” measure followed by well-established consistency analysis utilizing Cronbach α at each follow-up.53 Internal consistency dimensional exploration was performed using principal component analysis (PCA), utilizing standard eigenvalue and variance explained analysis visualized with factoextra.31 Cronbach α statistic and results were computed via the psych package.48 As a minimum sample size, it is recommended that we would require between 5 and 10 observations for each variable (ie, a sample size of ∼50 patients are necessary for the statistical models used in the current study).12
To assess the construct validity of the holistic MCID score, 3 approaches were taken. First, comparison of intervention response on the holistic MCID score vs the Patients' Global Impression of Change (PGIC) ordinal 7-category scale (Very Much Worse, Much Worse, Minimally Worse, No Change, Minimally Improved, Much Improved, and Very Much Improved) using ordinal mixed effect longitudinal regression.10,26 Second, comparison of intervention response on the holistic MCID score vs EuroQol visual analogue scale (EQ-VAS) self-rating scale from 0% (worst imaginable health) to 100% (best imaginable health) using Generalized Linear Mixed Model (GLMM) longitudinal beta-regression.3,19 Third, use of the take-one-out method removing one dimension of the holistic MCID score and comparing that dimension to the 4-item holistic MCID score using Gaussian Linear Mixed Model (LMM) via lme4.2 Data cleaning and visualization were performed within the tidyverse.57 All statistical analysis were performed using R version 4.3.1.46
Additional sensitivity analyses were undertaken with the results standardized cross-sectionally by visit to obtain a balanced or equally weighted holistic MCID score (Supplementary material 1, Figs. S1-3 and Tables S1-3, http://links.lww.com/PR9/A256), and each holistic MCID approach was compared to VAS MCID alone (Supplementary material 2, Figs. S4-6 and Tables S4-5, http://links.lww.com/PR9/A256).
3. Results
3.1. Participant characteristics and domain scores before intervention
Detailed characteristics of the patients with chronic pain recruited to the EVOKE trial, number of patients randomized to each treatment group, and results for the randomized controlled trial through 24 months are presented elsewhere.38,39 A total of 111 patients received a SCS system after a successful trial period, completed the 1-month follow-up assessment, and contributed data to the current analysis. The patients included in the analysis had a mean age of 56 years (SD = 10), relatively equal representation by sex (female n = 54 [48.6%]), and a mean duration of pain of 12.7 years (SD = 10.3) prior to treatment with SCS (Table 2).
Table 2.
Baseline characteristics and domain scores.
| Baseline characteristics | Included patients (n = 111) |
|---|---|
| Age (y) | 56.0 (10.0) |
| Sex, female (%), male (%) | 54 (48.6%), 57 (51.4%) |
| BMI (kg/m2) | 32.5 (6.2) |
| Duration of pain (y) | 12.7 (10.3) |
| Race | |
| American Indian or Alaska Native, n (%) | 2 (1.8%) |
| Black or African American, n (%) | 8 (7.2%) |
| White, n (%) | 99 (89.2%) |
| Other, n (%) | 2 (1.8%) |
| Ethnicity, Hispanic/Latino (%), Non-Hispanic/Latino (%) | 6 (5.4%), 105 (94.6%) |
| Baseline domain scores | |
| Pain intensity (VAS overall) | 82.2 (9.7) |
| HRQoL (EQ-5D-5L index score) | 0.501 (0.137) |
| Sleep quality (PSQI global score) | 13.6 (3.9) |
| Physical function (ODI score) | 55.3 (9.1) |
| Emotional function (POMS TMD score) | 24.8 (18.8) |
Data presented as means and (standard deviations) or n (%).
BMI, body mass index; EQ-5D-5L, EuroQol 5-dimension 5-level; HRQoL, health-related quality of life; ODI, Oswestry disability index; POMS, profile of mood states; PSQI, Pittsburgh sleep quality index; TMD, total mood disorder; VAS, visual analogue scale.
At baseline, 100% of patients (111/111) presented scores worse than normative population values for pain intensity (VAS), physical function (ODI), and HRQoL (EQ-5D-5L); 97% (108/111) were also impaired for sleep quality (PSQI) and 60% (67/111) for emotional function (POMS). Ninety-eight percent (109/111) of patients presented impaired scores for 4 domains and 60% (67/111) for all 5 outcome domains.
3.2. Domain and holistic minimal clinically important difference scores observed in the EVOKE trial
Treatment response assessed using the MCIDs obtained for each of the individual components of the holistic composite outcome, cumulative responder score, and holistic MCID are presented in Table 1. Meaningful changes to patients, characterized by ≥ 1 MCID after treatment with SCS, were obtained at all time points for each of the individual domains. The cumulative responder score ranged from a mean of 10.6 MCIDs at 24-month follow-up to mean of 11.9 MCIDs at 3-month follow-up. A holistic MCID of 1.0 indicates that a clinically meaningful change was obtained on average across all domains impaired at baseline. In the current cohort, we observed a holistic MCID >2 at all time points.
3.3. Internal consistency: multivariate dimensional analysis of holistic minimal clinically important difference
A Cronbach α > 0.7 was observed at each follow-up demonstrating strong internal consistency of the holistic MCID outcome (Fig. 2). Removing any one of the 5 outcome domains at each follow-up time point did not significantly improve this statistic.
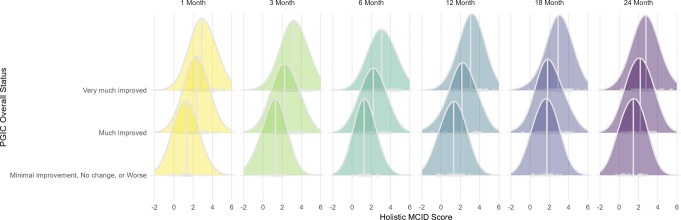
Figure 2.
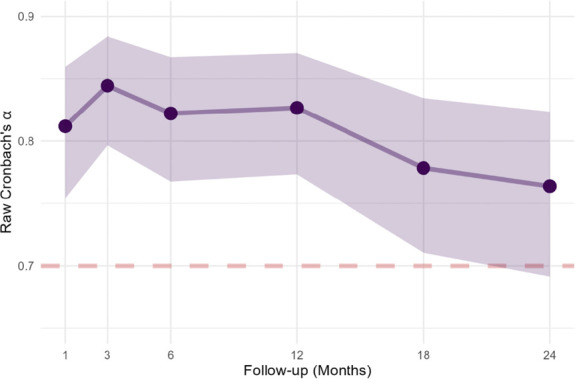
Internal consistency of holistic MCID at each follow-up. MCID, minimal clinically important difference.
Factor analysis of MCID scores for the 5 individual outcome domains showed one predominant dimension (#1) that included all 5 outcome domains with an eigenvalue of 2.95 (see Supplementary material 3, Table S6, Figs. S7-8, http://links.lww.com/PR9/A256). The eigenvalues of the other 4 identified dimensions were all less than 1 (ie, lower than the average). Dimension #1 explained most of the variance (58.98%). Each of other 4 dimensions explain less than 14% of the variance. There was an equally balanced contribution from the 5 outcome domains to the first component dimension. The analysis supports the holistic 5 outcome basis of the holistic MCID measure.
3.4. Construct validity: holistic minimal clinically important difference association with patients' global impression of change
Due to heavily skewed response rates for PGIC favoring the top 2 categories of improvement “much improved” to “very much improved,” and nearly zero observations in the lower categories, responses were collapsed into 3 categories: “minimal improvement, no change, or worse”; “much improved”; and “very much improved.” Significant positive associations were observed between increasing holistic MCID score and increased levels of the ordinal PGIC response at all EVOKE trial follow-ups (Fig. 3). While similar positive associations were observed between PGIC response and VAS MCID, the distribution of VAS MCID alone shows uneven variability and less symmetry than the holistic MCID (see Supplementary material 2, Fig. S4, http://links.lww.com/PR9/A256). Therefore, the holistic MCID provides a more balanced or well-calibrated score than VAS MCID alone.
Figure 3.
Association between holistic MCID score and PGIC. MCID, minimal clinically important difference; PGIC, patients' global impression of change.
The association between PGIC and holistic MCID is supported by longitudinal ordinal GLMM model, which showed that a one unit increase in the holistic MCID score was associated with an odds ratio of 6.69 (95% CI: 4.62–9.69, P < 0.001), ie, relative increase in the expected odds of increasing PGIC category. Full model results can be found in Supplementary material 3, Table S7, http://links.lww.com/PR9/A256.
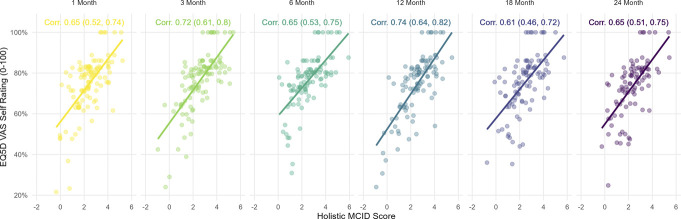
3.5. Construct validity: holistic minimal clinically important difference association with EQ-VAS
There were strong positive correlations (≥0.61) at all EVOKE follow-up time points between holistic MCID and EQ-VAS (see Fig. 4). The holistic MCID scores appeared to be more evenly distributed horizontally, indicating that they are less prone to ceiling and floor effects than seen with VAS MCID scores (Supplementary material 2, Fig. S5, http://links.lww.com/PR9/A256).
Figure 4.
Association between holistic MCID score and EQ-VAS. EQ-VAS, EuroQol visual analogue scale; MCID, minimal clinically important difference.
Results from the 100% response-inflated GLMM longitudinal beta-regression show for each additional unit increase in the holistic MCID score, an expected 39.1% increase (P < 0.001) in the odds of higher EQ-VAS per patient was observed, adjusted for longitudinal correlation and 100% response inflation (Supplementary material 3, Table S8, http://links.lww.com/PR9/A256). Further, this association was approximately 47% stronger than the positive association observed with VAS MCID alone (Supplementary material 2, Table S5, http://links.lww.com/PR9/A256).
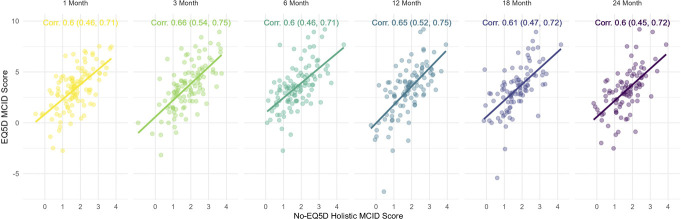
3.6. Construct validity: “leave-one-out” 4-item holistic minimal clinically important difference validation
Strong associations (Pearson correlation ≥0.6) were seen across all EVOKE trial follow-ups between the 4-item holistic MCID (minus EQ-5D) vs EQ-5D MCID score (see Fig. 5).
Figure 5.
Association between 4-item holistic MCID and EQ-5D MCID. EQ-5D, EuroQol 5-dimension; MCID, minimal clinically important difference.
These correlations were supported by significant Gaussian LMM results showing a 1.0 unit increase in composite 4-item holistic MCID was associated with an expected 1.44 (95% CI: 1.27–1.62, P < 0.001) increase in EQ-5D MCID score (Supplementary material 4, Table S9, http://links.lww.com/PR9/A256). Similarly, significant positive 4-item associations were seen during leave-one-out analyses with omission of ODI, POMS, and PSQI MCID items, respectively, and were likewise observed to be stronger than VAS MCID alone (Supplementary material 4, Figs. S9-S11, http://links.lww.com/PR9/A256).
4. Discussion
The holistic MCID is a composite measure for the personalized and comprehensive assessment of the impact of interventions for people with chronic pain. The 5 outcome domains contributing to the holistic MCID were informed by IMMPACT core outcome recommendations and judged as important by patients with chronic pain and health care providers.15,24,32,54 Formal assessment of the internal and construct validity is a key requirement for the implementation of a new composite outcome in clinical practice, research, and regulatory evaluations. Our study showed consistently high Cronbach alpha (>0.7) at all follow-up time points indicating good internal validity. Factor analysis confirmed that the holistic MCID composite measure is based on an overarching holistic dimension based on all 5 outcome components. Construct validity of the holistic MCID was demonstrated with its strong association with PGIC, EQ-5D VAS, and leave-one-out analysis.
It is now widely accepted that the biopsychosocial model is the most heuristic approach to chronic pain,21 and that treatment goals and successes encompass more than just pain scores. Yet, there has not been consensus on a methodology that can be used widely and adapted to various chronic pain conditions. Consequentially, different methods have been described for the evaluation of a holistic response,30,35 such as defining a holistic treatment responder as a patient who obtains a response (≥1 MCID) for each of the domains that were impaired at baseline. This concept of holistic treatment responder is valuable on an individual level and to ascertain the proportion of patients that obtain such a response; however, it does not allow quantification of the magnitude of the holistic treatment effect. Alternatively described is a cumulative responder score, computed as the sum of MCIDs for each domain impaired at baseline. Such a cumulative responder score can, however, be greater or smaller solely on the basis of the number of domains that were impaired at baseline and contribute to the score, or when additional domains are added as components of a holistic composite outcome. Our previous report showed that both the cumulative responder score and holistic MCID can be used to demonstrate benefit of an intervention over an alternative.30
Although a number of composite outcomes have been used in chronic pain intervention trials,1,22,23,44,49 their validity has often not been assessed or reported, and there is a clear lack of consensus. The ACTTION analysis of the validity of 10 different composite outcomes found that 2 composites (≥30% reduction in pain intensity or ≥30% improvement in physical function; ≥50% reduction in pain intensity, or ≥20% reduction in pain intensity and ≥30% improvement in physical function) were more strongly associated with ratings of “much improved” or “very much improved” in the PGIC.43 The study used a data-driven approach to identify a composite responder score based on level of improvement in pain intensity and physical function (ie, 2 different domains). A limitation of a data-driven approach to development of a composite is that the findings may only be applicable to that specific study population. A recent review observed that composite outcomes of benefits and harms are underutilized in chronic pain trials.41 Two composite outcomes of benefits and harms were identified, both dichotomous “responder” outcomes that categorized participants into those with or without a favorable outcome.41 The composite outcomes used a combination of response to pain and absence of stimulation-related neurological deficits14 or response to pain with no change in baseline pain medications, no discontinuation of the study drug due to lack of efficacy or tolerability, and no moderate or severe adverse drug reactions.27 It is important to note that these composite outcomes did not consider the breadth of health domains included in the holistic MCID. Nevertheless, separate inclusion of harms in composite outcomes should be further evaluated.
The holistic composite outcome and methods to evaluate treatment response proposed in the current study were informed by previous literature and its framework elaborated by an expert panel.35 The outcome domains that contribute to the holistic MCID reflect the recommendations of IMMPACT for the collection of PROMs for patients with chronic pain, ie, pain intensity, physical function, HRQoL, emotional function, and sleep quality.15,32,54 These domains have also been identified as core outcomes for clinical trials in specific pain conditions such as nonspecific low back pain and whiplash associated disorders.7,9 The selected PROMs have been recommended in core outcome sets for other conditions, including the VAS, ODI, and EQ-5D-5L.8,51 However, it is important to recognize that other domains or PROMs could contribute to a holistic composite outcome and core outcome sets and PROMs vary widely across different areas of health including chronic pain.29 A systematic review of PROMs used in chronic neuropathic pain trials found that some 200 different PROMs were used across 251 included studies, and only 27 PROMSs had been recommended by IMMPACT or NeuPSIG guidelines.50
The MCIDs used to assess intervention response should be clearly reported and justified. Where appropriate or required, alternative MCID thresholds could be evaluated in sensitivity analysis. The results of the holistic composite outcome should be reported for the composite itself (eg, cumulative responder score or holistic MCID) and for each individual domain to demonstrate the intervention effects on each of the components of the holistic composite outcome. Both the European Medicines Agency17 and the US Food and Drug Administration (FDA)20 recommend that the results for each of the individual domains of the composite should also be examined.
To our knowledge, this is the first study to formally assess the validity of a composite outcome to assess the impact of interventions for people with chronic pain. Furthermore, our demonstration of the validity of the holistic MCID was robust to sensitivity analysis.
We recognize that there are some potential limitations to the current study. The data were derived from a single study in a population of patients with significant, treatment refractory chronic pain. It is possible that the results would differ in a population with less severe chronic pain or with different phenotypes of pain. The assumption that participants would place more value on improvements for domains worse than normative values was not directly tested. Our finding of the validity of the holistic MCID outcome should, therefore, be evaluated in other chronic pain populations and interventions. In addition, the treatment response to ECAP-guided SCS showed skewed results in the improvement categories of the PGIC and nearly zero observations in the lower categories, which would reflect a poor treatment response. The wider spread or variability of the holistic MCID score suggests that this metric may be more representative of patients' pain experience when compared with PGIC responses. To overcome the limitations observed with the use of the PGIC, the validity of the holistic MCID was also assessed against the EQ-VAS self-rating scale. The EQ-VAS has been found to have poorer responsiveness but better predictive validity than the EQ-5D-5L index and allows patients to consider more quality-of-life constructs in their subjective rating of health.36 Comparisons against alternative outcomes, eg, PROMIS-29 or SF-36, would allow further investigation of the construct validity of the holistic MCID.
We observed that the cumulative responder score, without adjustment for the number of impaired domains, is also a valid measure. However, this score may vary considerably solely due to the number of domains that are included in the holistic composite. As such, until there is a consensus on the core outcome domains required to be included in a holistic composite outcome, the holistic MCID score may be more appropriate to compare relative treatment effects. Since the holistic MCID score only incorporates domains for which an individual subject presents worse than normative values, it provides a clinically focused and potentially statistically powerful summary measure. In addition, we also standardized results cross sectionally by visit to obtain a balanced or equally weighted holistic MCID score. Overall, the holistic approach has been shown to be a valuable tool for quantitatively representing a broader spectrum of the pain experience, treatment goals, and treatment successes by combining validated pain intensity, sleep quality, HRQoL, emotional, and physical function measurement, indicating an improved measure to assess the efficacy of interventions for chronic pain. Further studies are warranted on the use of the holistic MCID in other chronic pain conditions where more variability across domains may be present.
5. Conclusion
Pain is subjective and can be difficult to measure objectively—asking a patient to simply rate their pain on a scale of 0 to 10 may fail to consider other factors (eg, function, sleep, emotional well-being, HRQoL) without clarification of the individual's personal meaning of pain.33 The current study demonstrates that the holistic MCID is a valid composite outcome measure for patients after a chronic pain intervention. By capturing treatment responses across 5 different outcome domains in a single measure, the holistic MCID provides a personalized and more comprehensive measure of the impact of interventions for people with chronic pain than pain response alone.
Disclosures
R.S.T. reports consultancy fees from Medtronic, Nevro and Saluda Medical outside the submitted work. C.M.M. is employed by NAMSA, a company that provides consulting and testing services to medical device manufacturers. N.A.M. reports receiving grants from Neuros, Mesoblast, and Vivex Biologics, as well as consulting as a medical monitor for Saluda Medical, Nevro, Vivex Biologics, Mainstay, Sollis Therapeutics, and Vertos outside the submitted work. J.W.K. is an advisory board member for Boston Scientific, Medtronic, Abbott, and Saluda Medical. J.E.P. reports research and consulting fees from Saluda Medical during the conduct of the study; consultancy for Abbott, Medtronic, Saluda Medical, Flowonix, SpineThera, Vertos, Vertiflex, SPR Therapeutics, Tersera, Aurora, Spark, Ethos, Biotronik, Mainstay, WISE, Boston Scientific, and Thermaquil outside the submitted work; has received grant and research support from: Abbott, Flowonix, Aurora, Painteq, Ethos, Muse, Boston Scientific, SPR Therapeutics, Mainstay, Vertos, AIS, and Thermaquil outside the submitted work; and is a shareholder of Vertos, SPR Therapeutics, Painteq, Aurora, Spark, Celeri Health, Neural Integrative Solutions, Pacific Research Institute, Thermaquil, and Anesthetic Gas Reclamation. C.W.H. has received consultancy fees from Saluda Medical and Genecentrix outside the submitted work. S.J.C. reports receiving grants from Saluda Medical, Vertos, Mainstay, and Vivex outside the submitted work. L.K. reports receiving grants from Nevro, Neuros, Avanos, Medtronic, Neuralace, and Xalud Therapeutics and financial support from Nevro, Avanos, and Saluda Medical outside the submitted work. C.A.G. reports personal fees and other from SPR, and personal fees from Nevro, Nalu, Biotronik, Boston Scientific and Saluda Medical outside the submitted work. E.A.P. has received research support from Mainstay, Medtronic, Neuros Medical, Nevro Corp, ReNeuron, SPR, and Saluda Medical outside the submitted work, as well as personal fees from Abbott Neuromodulation, Biotronik, Medtronic Neuromodulation, Nalu, Neuros Medical, Nevro, Presidio Medical, Saluda Medical, and Vertos outside the submitted work. She holds stock options from SynerFuse and neuro42. K.V.P. is a consultant and speaker for Abbott. S.E. reports consultancy fees from Medtronic, and Mainstay Medical outside the submitted work. He has received department research funding from the National Institute of Health Research, Saluda Medical and Medtronic. R.M.L. is an uncompensated consultant for Biotronik, Abbott, Nalu, Saluda Medical, and Mainstay Medical and has stock options from Nalu and Saluda Medical. C.G. reports payment to his institution (for part of his salary) and stock options received from Mainstay, personal fees from Mainstay, Saluda Medical, Persica, and Iliad outside the submitted work, research funded by Sollis, expert witness testimony fees, and serves as Editor-in-Chief of Pain Practice. S.D. has received consulting payments from Averitas Pharma and Biotronik outside the submitted work. A.A. reports consultancy for Medtronic, Avanos, and StimWave outside the submitted work. P.B. reports consulting fees from Abbott and PainTEQ outside the submitted work. E.H., A.L., N.S. and R.V.D. are employees of Saluda Medical. R.V.D. has previously received consultancy fees from Mainstay Medical, Medtronic, and Saluda Medical. D.J.C. has consulted for AbbVie, Heron Therapeutics, Aptinyx, Neumentum, Regeneron Pharmaceuticals, Swing Therapeutics, Virios Therapeutics, Allergan Sales, Eli Lilly and Company, H. Lundback A/S, Pfizer, Samumed, Tonix Pharmaceuticals. T.J.N. has received consultancy fees from Saluda Medical outside the submitted work. The remaining authors declare no conflict of interest.
Appendix A. Supplemental digital content
Supplemental digital content associated with this article can be found online at http://links.lww.com/PR9/A256.
Supplementary Material
Acknowledgements
Funding statement: This study was funded by Saluda Medical.
Data availability statement: Saluda Medical is committed to responsible data sharing regarding the clinical trials we sponsor. This includes access to anonymized, individual, and trial-level data (analysis data sets), as well as other information (eg, protocols and Clinical Study Reports), provided the trials are not part of an ongoing or planned regulatory submission. This includes requests for clinical trial data for unlicensed products and indications. These clinical trial data can be requested by any qualified researchers who engage in rigorous, independent scientific research and will be provided after review and approval of a research proposal and statistical analysis plan and execution of a data sharing agreement. Data requests can be submitted at any time, and the data will be accessible for 12 months, with possible extensions considered. For more information on the process or to submit a request, visit https://www.saludamedical.com/us/contact-us/.
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.painrpts.com).
Contributor Information
Rod S. Taylor, Email: Rod.Taylor@glasgow.ac.uk.
Quinton Neville, Email: nevil066@umn.edu.
Christopher M. Mullin, Email: cmullin@namsa.com.
Nagy A. Mekhail, Email: MEKHAIN@ccf.org.
Jan W. Kallewaard, Email: jkallewaard@rijnstate.nl.
Salim Hayek, Email: salim.hayek@uhhospitals.org.
Jason E. Pope, Email: popeje@me.com.
Corey W. Hunter, Email: corey.hunter@me.com.
Shrif J. Costandi, Email: COSTANS2@ccf.org.
Leonardo Kapural, Email: lkapuralmd@gmail.com.
Christopher A. Gilmore, Email: CGilmore@ccrpain.com.
Erika A. Petersen, Email: eapetersen@uams.edu.
Kiran V. Patel, Email: kiranpatelmd@yahoo.com.
Sam Eldabe, Email: seldabe@mac.com.
Robert M. Levy, Email: rml199@northwestern.edu.
Christopher Gilligan, Email: chrisgilliganmd@gmail.com.
Shravani Durbhakula, Email: Shravani87@gmail.com.
Alaa Abd-Elsayed, Email: abdelsayed@wisc.edu.
Marshall Bedder, Email: marshall.bedder@gmail.com.
Patrick Buchanan, Email: pbuchana@gmail.com.
Erin Hanson, Email: Erin.Hanson@saludamedical.com.
Angela Leitner, Email: Angela.Leitner@saludamedical.com.
Nicole Soliday, Email: Nicole.Soliday@saludamedical.com.
Daniel J. Clauw, Email: dclauw@med.umich.edu.
Turo J. Nurmikko, Email: turo.nurmikko@gmail.com.
References
- [1].Arnold LM, Williams DA, Hudson JI, Martin SA, Clauw DJ, Crofford LJ, Wang F, Emir B, Lai C, Zablocki R, Mease PJ. Development of responder definitions for fibromyalgia clinical trials. Arthritis Rheum 2012;64:885–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Bates D, Mächler M, Bolker B, Walker S. Fitting linear mixed-effects models using lme4. J Stat Softw 2015;67:1–48. [Google Scholar]
- [3].Brooks ME, Kristensen K, Benthem K, Magnusson A, Berg CW, Nielsen A, Skaug HJ, Machler M, Bolker BM. glmmTMB balances speed and flexibility among packages for zero-inflated generalized linear mixed modeling. R J 2017;9:378–400. [Google Scholar]
- [4].Buysse DJ, Germain A, Moul DE, Franzen PL, Brar LK, Fletcher ME, Begley A, Houck PR, Mazumdar S, Reynolds CF, III, Monk TH. Efficacy of brief behavioral treatment for chronic insomnia in older adults. Arch Intern Med 2011;171:887–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Buysse DJ, Hall ML, Strollo PJ, Kamarck TW, Owens J, Lee L, Reis SE, Matthews KA. Relationships between the Pittsburgh sleep quality index (PSQI), epworth sleepiness scale (ESS), and clinical/polysomnographic measures in a community sample. J Clin Sleep Med 2008;4:563–71. [PMC free article] [PubMed] [Google Scholar]
- [6].Buysse DJ, Reynolds CF, III, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res 1989;28:193–213. [DOI] [PubMed] [Google Scholar]
- [7].Chen K, Andersen T, Carroll L, Connelly L, Côté P, Curatolo M, Elliott J, Grant G, Jull G, Kasch H, MacDermid J, Malmström EM, Maujean A, McLean SA, Nielsen M, Rebbeck T, Söderlund A, Sterling J, Treleaven J, Walton DM, Westergren H, Sterling M. Recommendations for core outcome domain set for whiplash-associated disorders (CATWAD). Clin J Pain 2019;35:727–36. [DOI] [PubMed] [Google Scholar]
- [8].Chiarotto A, Boers M, Deyo RA, Buchbinder R, Corbin TP, Costa LOP, Foster NE, Grotle M, Koes BW, Kovacs FM, Lin CWC, Maher CG, Pearson AM, Peul WC, Schoene ML, Turk DC, van Tulder MW, Terwee CB, Ostelo RW. Core outcome measurement instruments for clinical trials in nonspecific low back pain. PAIN 2018;159:481–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Chiarotto A, Deyo RA, Terwee CB, Boers M, Buchbinder R, Corbin TP, Costa LO, Foster NE, Grotle M, Koes BW, Kovacs FM, Lin CW, Maher CG, Pearson AM, Peul WC, Schoene ML, Turk DC, van Tulder MW, Ostelo RW. Core outcome domains for clinical trials in non-specific low back pain. Eur Spine J 2015;24:1127–42. [DOI] [PubMed] [Google Scholar]
- [10].Christensen RHB. Regression models for ordinal data. 2022. Available at: https://cran.r-project.org/web/packages/ordinal/ordinal.pdf. Accessed September 6, 2023. [Google Scholar]
- [11].Cordoba G, Schwartz L, Woloshin S, Bae H, Gøtzsche PC. Definition, reporting, and interpretation of composite outcomes in clinical trials: systematic review. BMJ 2010;341:c3920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Costello A, Osborne JW. Best practices in exploratory factor analysis: four recommendations for getting the most from your analysis. Pract Assess Res Eval 2005;10:1–9. [Google Scholar]
- [13].de Williams AC, Davies HTO, Chadury Y. Simple pain rating scales hide complex idiosyncratic meanings. PAIN 2000;85:457–63. [DOI] [PubMed] [Google Scholar]
- [14].Deer TR, Levy RM, Kramer J, Poree L, Amirdelfan K, Grigsby E, Staats P, Burton AW, Burgher AH, Obray J, Scowcroft J, Golovac S, Kapural L, Paicius R, Kim C, Pope J, Yearwood T, Samuel S, McRoberts WP, Cassim H, Netherton M, Miller N, Schaufele M, Tavel E, Davis T, Davis K, Johnson L, Mekhail N. Dorsal root ganglion stimulation yielded higher treatment success rate for complex regional pain syndrome and causalgia at 3 and 12 months: a randomized comparative trial. PAIN 2017;158:669–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Dworkin RH, Turk DC, Farrar JT, Haythornthwaite JA, Jensen MP, Katz NP, Kerns RD, Stucki G, Allen RR, Bellamy N, Carr DB, Chandler J, Cowan P, Dionne R, Galer BS, Hertz S, Jadad AR, Kramer LD, Manning DC, Martin S, McCormick CG, McDermott MP, McGrath P, Quessy S, Rappaport BA, Robbins W, Robinson JP, Rothman M, Royal MA, Simon L, Stauffer JW, Stein W, Tollett J, Wernicke J, Witter J, IMMPACT. Core outcome measures for chronic pain clinical trials: IMMPACT recommendations. PAIN 2005;113:9–19. [DOI] [PubMed] [Google Scholar]
- [16].Dworkin RH, Turk DC, Wyrwich KW, Beaton D, Cleeland CS, Farrar JT, Haythornthwaite JA, Jensen MP, Kerns RD, Ader DN, Brandenburg N, Burke LB, Cella D, Chandler J, Cowan P, Dimitrova R, Dionne R, Hertz S, Jadad AR, Katz NP, Kehlet H, Kramer LD, Manning DC, McCormick C, McDermott MP, McQuay HJ, Patel S, Porter L, Quessy S, Rappaport BA, Rauschkolb C, Revicki DA, Rothman M, Schmader KE, Stacey BR, Stauffer JW, von Stein T, White RE, Witter J, Zavisic S. Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. J Pain 2008;9:105–21. [DOI] [PubMed] [Google Scholar]
- [17].EMA. Guideline on multiplicity issues in clinical trials. 2016. Available at: https://www.ema.europa.eu/en/documents/scientific-guideline/draft-guideline-multiplicity-issues-clinical-trials_en.pdf. Accessed May 9, 2023. [Google Scholar]
- [18].Fairbank JC, Pynsent PB. The Oswestry disability index. Spine (Phila PA 1976) 2000;25:2940–52; discussion 2952. [DOI] [PubMed] [Google Scholar]
- [19].Feng Y, Parkin D, Devlin NJ. Assessing the performance of the EQ-VAS in the NHS PROMs programme. Qual Life Res 2014;23:977–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Food and Drug Administration. Multiple endpoints in clinical trials: guidance for industry. 2022. Available at: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/multiple-endpoints-clinical-trials-guidance-industry. Accessed May 9, 2023. [Google Scholar]
- [21].Gatchel RJ, Peng YB, Peters ML, Fuchs PN, Turk DC. The biopsychosocial approach to chronic pain: scientific advances and future directions. Psychol Bull 2007;133:581–624. [DOI] [PubMed] [Google Scholar]
- [22].Gewandter JS, McDermott MP, Evans S, Katz NP, Markman JD, Simon LS, Turk DC, Dworkin RH. Composite outcomes for pain clinical trials: considerations for design and interpretation. PAIN 2021;162:1899–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Goudman L, Billot M, Duarte RV, Eldabe S, Rigoard P, Moens M. Gradation of clinical holistic response as new composite outcome to evaluate success in spinal cord stimulation studies for pain. Neuromodulation 2023;26:139–46. [DOI] [PubMed] [Google Scholar]
- [24].Goudman L, De Smedt A, Billot M, Roulaud M, Rigoard P, Moens M. Opinions of health care providers about neuromodulation for pain: results of an online survey at the 2nd joint congress of the international neuromodulation society European chapters. Neuromodulation Technol Neural Interf 2023;26:1887–92. [DOI] [PubMed] [Google Scholar]
- [25].Goudman L, De Smedt A, Linderoth B, Eldabe S, Witkam R, Henssen D, Moens M. Identifying goals in patients with chronic pain: a European survey. Eur J Pain 2021;25:1959–70. [DOI] [PubMed] [Google Scholar]
- [26].Guy W. ECDEU assessment manual for psychopharmacology. Rockville, MD: U.S. Dept. of Health, Education, and Welfare, Public Health Service, Alcohol, Drug Abuse, and Mental Health Administration, National Institute of Mental Health, Psychopharmacology Research Branch, Division of Extramural Research Programs, 1976. [Google Scholar]
- [27].Haanpää M, Cruccu G, Nurmikko TJ, McBride WT, Docu Axelarad A, Bosilkov A, Chambers C, Ernault E, Abdulahad AK. Capsaicin 8% patch versus oral pregabalin in patients with peripheral neuropathic pain. Eur J Pain 2016;20:316–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Herdman M, Gudex C, Lloyd A, Janssen M, Kind P, Parkin D, Bonsel G, Badia X. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res 2011;20:1727–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Hughes KL, Clarke M, Williamson PR. A systematic review finds Core Outcome Set uptake varies widely across different areas of health. J Clin Epidemiol 2021;129:114–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Kapural L, Mekhail NA, Costandi S, Gilmore C, Pope JE, Li S, Hunter CW, Poree L, Staats PS, Taylor RS, Eldabe S, Kallewaard JW, Thomson S, Petersen EA, Sayed D, Deer TR, Antony A, Budwany R, Leitner A, Soliday N, Duarte RV, Levy RM. Durable multimodal and holistic response for physiologic closed-loop spinal cord stimulation supported by objective evidence from the EVOKE double-blind randomized controlled trial. Reg Anesth Pain Med 2024;49:233–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Kassambara A, Mundt F. Factoextra: extract and visualize the results of multivariate data analyses. 2020. Available at: https://CRAN.R-project.org/package=factoextra. Accessed September 4, 2023. [Google Scholar]
- [32].Katz N, Dworkin RH, North R, Thomson S, Eldabe S, Hayek SM, Kopell BH, Markman J, Rezai A, Taylor RS, Turk DC, Buchser E, Fields H, Fiore G, Ferguson M, Gewandter J, Hilker C, Jain R, Leitner A, Loeser J, McNicol E, Nurmikko T, Shipley J, Singh R, Trescot A, van Dongen R, Venkatesan L. Research design considerations for randomized controlled trials of spinal cord stimulation for pain: initiative on methods, measurement, and pain assessment in clinical trials/institute of neuromodulation/international neuromodulation society recommendations. PAIN 2021;162:1935–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Langford DJ, Gewandter JS, Amtmann D, Reeve BB, Hertz S, Loeser JD, Veasley C, Turk DC, Dworkin RH. Patient-reported chronic pain intensity: more than meets the eye. Patient 2022;15:383–7. [DOI] [PubMed] [Google Scholar]
- [34].Levy R, Deer TR, Poree L, Rosen SM, Kapural L, Amirdelfan K, Soliday N, Leitner A, Mekhail N. Multicenter, randomized, double-blind study protocol using human spinal cord recording comparing safety, efficacy, and neurophysiological responses between patients being treated with evoked compound action potential-controlled closed-loop spinal cord stimulation or open-loop spinal cord stimulation (the EVOKE study). Neuromodulation 2019;22:317–26. [DOI] [PubMed] [Google Scholar]
- [35].Levy R, Mekhail N, Abd-Elsayed A, Abejón D, Anitescu M, Deer T, Eldabe S, Goudman L, Kallewaard J, Moens M, Petersen E, Pilitsis J, Pope J, Poree L, Raslan A, Russo M, Sayed D, Staats P, Taylor R, Thomson S, Verrills P, Duarte R. Holistic treatment response: an international expert panel definition and criteria for a new paradigm in the assessment of clinical outcomes of spinal cord stimulation. Neuromodulation 2023;26:1015–22. [DOI] [PubMed] [Google Scholar]
- [36].Lin DY, Cheok TS, Kaambwa B, Samson AJ, Morrison C, Chan T, Kroon HM, Jaarsma RL. Evaluation of the EQ-5D-5L, EQ-VAS stand-alone component and Oxford knee score in the Australian knee arthroplasty population utilising minimally important difference, concurrent validity, predictive validity and responsiveness. Health Qual Life Outcomes 2023;21:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].McNair D, Heuchert J. Profile of mood states—technical update. North Tonawanda, NY: Multi-Health Systems, 2003. [Google Scholar]
- [38].Mekhail N, Levy RM, Deer TR, Kapural L, Li S, Amirdelfan K, Hunter CW, Rosen SM, Costandi SJ, Falowski SM, Burgher AH, Pope JE, Gilmore CA, Qureshi FA, Staats PS, Scowcroft J, Carlson J, Kim CK, Yang MI, Stauss T, Poree L, EVOKE Study Group, Gorman R, Gmel GE, Hanson E, Karantonis DM, Khurram A, Kiefer D, Leitner A, Mugan D, Obradovic M, Parker J, Single P, Soliday N. Long-term safety and efficacy of closed-loop spinal cord stimulation to treat chronic back and leg pain (EVOKE): a double-blind, randomised, controlled trial. Lancet Neurol 2020;19:123–34. [DOI] [PubMed] [Google Scholar]
- [39].Mekhail N, Levy RM, Deer TR, Kapural L, Li S, Amirdelfan K, Hunter CW, Rosen SM, Costandi SJ, Falowski SM, Burgher AH, Pope JE, Gilmore CA, Qureshi FA, Staats PS, Scowcroft J, McJunkin T, Carlson J, Kim CK, Yang MI, Stauss T, Pilitsis J, Poree L, EVOKE Study Group, Brounstein D, Gilbert S, Gmel GE, Gorman R, Gould I, Hanson E, Karantonis DM, Khurram A, Leitner A, Mugan D, Obradovic M, Ouyang Z, Parker J, Single P, Soliday N. Durability of clinical and quality-of-life outcomes of closed-loop spinal cord stimulation for chronic back and leg pain: a secondary analysis of the evoke randomized clinical trial. JAMA Neurol 2022;79:251–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Mekhail NA, Levy RM, Deer TR, Kapural L, Li S, Amirdelfan K, Pope JE, Hunter CW, Rosen SM, Costandi SJ, Falowski SM, Burgher AH, Gilmore CA, Qureshi FA, Staats PS, Scowcroft J, McJunkin T, Carlson J, Kim CK, Yang MI, Stauss T, Petersen EA, Hagedorn JM, Rauck R, Kallewaard JW, Baranidharan G, Taylor RS, Poree L, Brounstein D, Duarte RV, Gmel GE, Gorman R, Gould I, Hanson E, Karantonis DM, Khurram A, Leitner A, Mugan D, Obradovic M, Ouyang Z, Parker J, Single P, Soliday N, EVOKE Study Group. ECAP-controlled closed-loop versus open-loop SCS for the treatment of chronic pain: 36-month results of the EVOKE blinded randomized clinical trial. Reg Anesth Pain Med 2024;49:346–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Nishtar M, Mark R, Langford DJ, McDermott MP, Markman JD, Evans SR, France FO, Park M, Sharma S, Turk DC, Dworkin RH, Gewandter JS. Evaluating the balance of benefits and harms in chronic pain clinical trials: prioritizing individual participants over individual outcomes. Reg Anesth Pain Med 2024;49:363–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Ostelo RW, de Vet HC. Clinically important outcomes in low back pain. Best Pract Res Clin Rheumatol 2005;19:593–607. [DOI] [PubMed] [Google Scholar]
- [43].Patel KV, Allen R, Burke L, Farrar JT, Gewandter JS, Gilron I, Katz NP, Markman JD, Marshall SF, Resnick M, Rice ASC, Rowbotham MC, Smith SM, Vanhove GF, Wasan AD, Zhang S, Dworkin RH, Turk DC. Evaluation of composite responder outcomes of pain intensity and physical function in neuropathic pain clinical trials: an ACTTION individual patient data analysis. PAIN 2018;159:2245–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Pilitsis JG, Fahey M, Custozzo A, Chakravarthy K, Capobianco R. Composite score is a better reflection of patient response to chronic pain therapy compared with pain intensity alone. Neuromodulation 2021;24:68–75. [DOI] [PubMed] [Google Scholar]
- [45].Price DD, McGrath PA, Rafii A, Buckingham B. The validation of visual analogue scales as ratio scale measures for chronic and experimental pain. PAIN 1983;17:45–56. [DOI] [PubMed] [Google Scholar]
- [46].R Core Team. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing, 2022. [Google Scholar]
- [47].Raja SN, Carr DB, Cohen M, Finnerup NB, Flor H, Gibson S, Keefe FJ, Mogil JS, Ringkamp M, Sluka KA, Song XJ, Stevens B, Sullivan MD, Tutelman PR, Ushida T, Vader K. The revised International Association for the Study of Pain definition of pain: concepts, challenges, and compromises. PAIN 2020;161:1976–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Revelle WR. Psych: procedures for psychological, psychometric, and personality research. 2023. Available at: https://cran.r-project.org/web/packages/psych/index.html. Accessed September 6, 2023. [Google Scholar]
- [49].Russo M, Verrills P, Santarelli D, Gupta S, Martin J, Hershey B. A novel composite metric for predicting patient satisfaction with spinal cord stimulation. Neuromodulation 2020;23:687–97. [DOI] [PubMed] [Google Scholar]
- [50].Sachau J, Sendel M, Péchard M, Schnabel K, Schmieg I, Medkour T, Ecochard L, Woischnik M, Liedgens H, Pogatzki-Zahn E, Baron R, Bouhassira D. Patient reported outcome measures in chronic neuropathic pain clinical trials—a systematic literature review. J Pain 2023;24:38–54. [DOI] [PubMed] [Google Scholar]
- [51].Sterling M, Andersen T, Carroll L, Connelly L, Côté P, Curatolo M, Grant G, Jull G, Kasch H, Ravn SL, MacDermid J, Malmström EM, Rebbeck T, Söderlund A, Treleaven J, Walton DM, Westergren H. Recommendations for a core outcome measurement set for clinical trials in whiplash associated disorders. PAIN 2023;164:2265–72. [DOI] [PubMed] [Google Scholar]
- [52].Szende A, Janssen B, Cabases J. Self-reported population health: An international perspective based on EQ-5D. Dordrecht, NL: Springer, 2014. [PubMed] [Google Scholar]
- [53].Taber KS. The use of Cronbach's alpha when developing and reporting research instruments in science education. Res Sci Educ 2018;48:1273–96. [Google Scholar]
- [54].Turk DC, Dworkin RH, Revicki D, Harding G, Burke LB, Cella D, Cleeland CS, Cowan P, Farrar JT, Hertz S, Max MB, Rappaport BA. Identifying important outcome domains for chronic pain clinical trials: an IMMPACT survey of people with pain. PAIN 2008;137:276–85. [DOI] [PubMed] [Google Scholar]
- [55].van Hout B, Janssen MF, Feng YS, Kohlmann T, Busschbach J, Golicki D, Lloyd A, Scalone L, Kind P, Pickard AS. Interim scoring for the EQ-5D-5L: mapping the EQ-5D-5L to EQ-5D-3L value sets. Value Health 2012;15:708–15. [DOI] [PubMed] [Google Scholar]
- [56].Walters SJ, Brazier JE. Comparison of the minimally important difference for two health state utility measures: EQ-5D and SF-6D. Qual Life Res 2005;14:1523–32. [DOI] [PubMed] [Google Scholar]
- [57].Wickham H, Averick M, Bryan J, Chang W, McGowan LD, François R, Grolemund G, Hayes A, Henry L, Hester J, Kuhn M, Pedersen TL, Miller E, Bache SM, Müller K, Ooms J, Robinson D, Seidel DP, Spinu V, Takahashi K, Vaughan D, Wilke C, Woo K, Yutani H. Welcome to the tidyverse. J Open Source Softw 2019;4:1686. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental digital content associated with this article can be found online at http://links.lww.com/PR9/A256.