Abstract
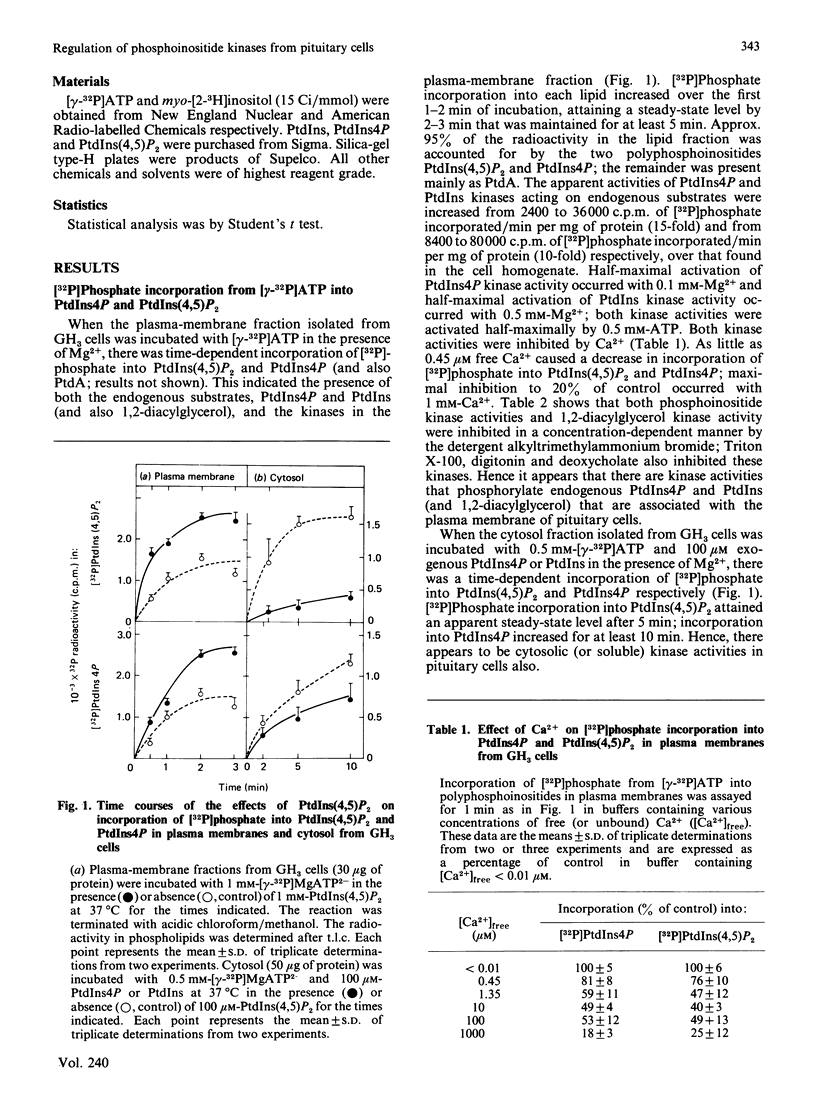
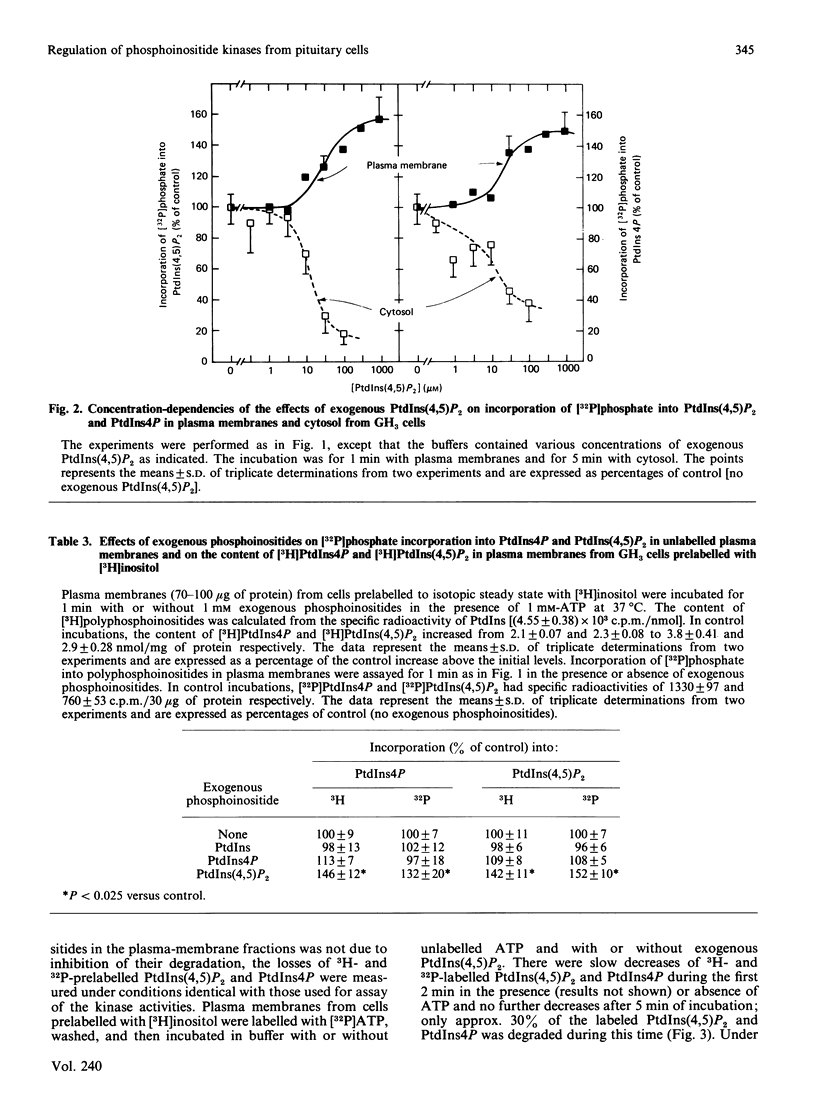
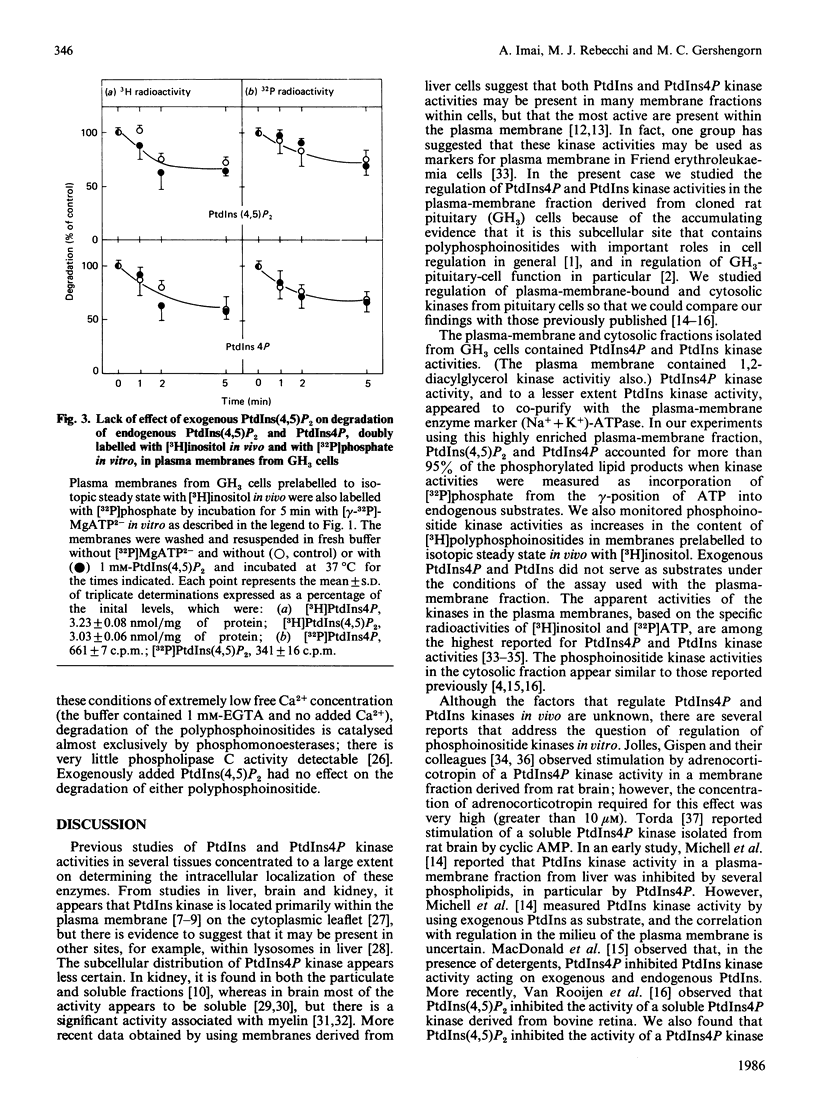
Regulation of phosphatidylinositol kinase (EC 2.7.1.67) and phosphatidylinositol 4-phosphate (PtdIns4P) kinase (EC 2.7.1.68) was investigated in highly enriched plasma-membrane and cytosolic fractions derived from cloned rat pituitary (GH3) cells. In plasma membranes, phosphatidylinositol 4,5-bisphosphate [PtdIns(4,5)P2] added exogenously enhanced incorporation of [32P]phosphate from [gamma-32P]MgATP2- into PtdIns(4,5)P2 and PtdIns4P to 150% of control; half-maximal effect occurred with 0.03 mM exogenous PtdIns(4,5)P2. Exogenous PtdIns4P and phosphatidylinositol (PtdIns) had no effect. When plasma membranes prepared from cells prelabelled to isotopic steady state with [3H]inositol were used, there was a MgATP2- dependent increase in the content of [3H]PtdIns(4,5)P2 and [3H]PtdIns4P that was enhanced specifically by exogenous PtdIns(4,5)P2 also. Degradation of 32P- and 3H-labelled PtdIns(4,5)P2 and PtdIns4P within the plasma-membrane fraction was not affected by exogenous PtdIns(4,5)P2. Phosphoinositide kinase activities in the cytosolic fraction were assayed by using exogenous substrates. Phosphoinositide kinase activities in cytosol were inhibited by exogenously added PtdIns(4,5)P2. These findings demonstrate that exogenously added PtdIns(4,5)P2 enhances phosphoinositide kinase activities (and formation of polyphosphoinositides) in plasma membranes, but decreases these kinase activities in cytosol derived from GH3 cells. These data suggest that flux of PtdIns to PtdIns4P to PtdIns(4,5)P2 in the plasma membrane cannot be increased simply by release of membrane-associated phosphoinositide kinases from product inhibition as PtdIns(4,5)P2 is hydrolysed.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berridge M. J. Inositol trisphosphate and diacylglycerol as second messengers. Biochem J. 1984 Jun 1;220(2):345–360. doi: 10.1042/bj2200345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burriss Garrett R. J., Redman C. M. Localization of enzymes involved in polyphosphoinositids metabolism on the cytoplasmic surface of the human erythrocyte membrane. Biochim Biophys Acta. 1975 Feb 28;382(1):58–64. doi: 10.1016/0005-2736(75)90372-7. [DOI] [PubMed] [Google Scholar]
- Collins C. A., Wells W. W. Identification of phosphatidylinositol kinase in rat liver lysosomal membranes. J Biol Chem. 1983 Feb 25;258(4):2130–2134. [PubMed] [Google Scholar]
- Colodzin M., Kennedy E. P. Biosynthesis of diphosphoinositide in brain. J Biol Chem. 1965 Oct;240(10):3771–3780. [PubMed] [Google Scholar]
- Cooper P. H., Hawthorne J. N. Phosphatidylinositol kinase and diphosphoinositide kinase of rat kidney cortex: properties and subcellular localization. Biochem J. 1976 Oct 15;160(1):97–105. doi: 10.1042/bj1600097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshmukh D. S., Bear W. D., Brockerhoff H. Polyphosphoinositide biosynthesis in three subfractions of rat brain myelin. J Neurochem. 1978 May;30(5):1191–1193. doi: 10.1111/j.1471-4159.1978.tb12417.x. [DOI] [PubMed] [Google Scholar]
- Galante Y. M., Hatefi Y. Resolution of complex I and isolation of NADH dehydrogenase and an iron--sulfur protein. Methods Enzymol. 1978;53:15–21. doi: 10.1016/s0076-6879(78)53007-3. [DOI] [PubMed] [Google Scholar]
- Gershengorn M. C., Geras E., Rebecchi M. J., Rubin B. G. Evidence that thyrotropin-releasing hormone transiently decreases membrane potential in mouse pituitary thyrotropic tumor cells in culture as monitored by triphenylmethylphosphonium ion. J Biol Chem. 1981 Dec 10;256(23):12445–12448. [PubMed] [Google Scholar]
- Gershengorn M. C., Hoffstein S. T., Rebecchi M. J., Geras E., Rubin B. G. Thyrotropin-releasing hormone stimulation of prolactin release from clonal rat pituitary cells: evidence for action independent of extracellular calcium. J Clin Invest. 1981 Jun;67(6):1769–1776. doi: 10.1172/JCI110216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershengorn M. C. Mechanism of thyrotropin releasing hormone stimulation of pituitary hormone secretion. Annu Rev Physiol. 1986;48:515–526. doi: 10.1146/annurev.ph.48.030186.002503. [DOI] [PubMed] [Google Scholar]
- Harwood J. L., Hawthorne J. N. Metabolism of the phosphoinositides in guinea-pig brain synaptosomes. J Neurochem. 1969 Sep;16(9):1377–1387. doi: 10.1111/j.1471-4159.1969.tb05989.x. [DOI] [PubMed] [Google Scholar]
- Harwood J. L., Hawthorne J. N. The properties and subcellular distribution of phosphatidylinositol kinase in mammalian tissues. Biochim Biophys Acta. 1969 Jan 7;171(1):75–88. doi: 10.1016/0005-2744(69)90107-7. [DOI] [PubMed] [Google Scholar]
- Iacobelli S. The biosynthesis of triiphosphoinositide by purified myelin of peripheral nerve. J Neurochem. 1969 Jun;16(3):909–911. doi: 10.1111/j.1471-4159.1969.tb08979.x. [DOI] [PubMed] [Google Scholar]
- Imai A., Ishizuka Y., Nakashima S., Nozawa Y. Differential activation of membrane phospholipid turnover by compound 48/80 and ionophore A23187 in rat mast cells. Arch Biochem Biophys. 1984 Jul;232(1):259–268. doi: 10.1016/0003-9861(84)90542-3. [DOI] [PubMed] [Google Scholar]
- Irvine R. F. The enzymology of stimulated inositol lipid turnover. Cell Calcium. 1982 Oct;3(4-5):295–309. doi: 10.1016/0143-4160(82)90018-5. [DOI] [PubMed] [Google Scholar]
- Jolles J., Zwiers H., Dekker A., Wirtz K. W., Gispen W. H. Corticotropin-(1--24)-tetracosapeptide affects protein phosphorylation and polyphosphoinositide metabolism in rat brain. Biochem J. 1981 Jan 15;194(1):283–291. doi: 10.1042/bj1940283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolles J., Zwiers H., van Dongen C. J., Schotman P., Wirtz K. W., Gispen W. H. Modulation of brain polyphosphoinositide metabolism by ACTH-sensitive protein phosphorylation. Nature. 1980 Aug 7;286(5773):623–625. doi: 10.1038/286623a0. [DOI] [PubMed] [Google Scholar]
- Kai M., Salway J. G., Hawthorne J. N. The diphosphoinositide kinase of rat brain. Biochem J. 1968 Feb;106(4):791–801. doi: 10.1042/bj1060791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kai M., White G. L., Hawthorne J. N. The phosphatidylinositol kinase of rat brain. Biochem J. 1966 Nov;101(2):328–337. doi: 10.1042/bj1010328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MacDonald M. L., Kuenzel E. A., Glomset J. A., Krebs E. G. Evidence from two transformed cell lines that the phosphorylations of peptide tyrosine and phosphatidylinositol are catalyzed by different proteins. Proc Natl Acad Sci U S A. 1985 Jun;82(12):3993–3997. doi: 10.1073/pnas.82.12.3993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marche P., Koutouzov S., Meyer P. Metabolism of phosphoinositides in the rat erythrocyte membrane. A reappraisal of the effect of magnesium on the 32P incorporation into polyphosphoinositides. Biochim Biophys Acta. 1982 Mar 12;710(3):332–340. doi: 10.1016/0005-2760(82)90116-3. [DOI] [PubMed] [Google Scholar]
- Michell R. H., Harwood J. L., Coleman R., Hawthorne J. N. Characteristics of rat liver phosphatidylinositol kinase and its presence in the plasma membrane. Biochim Biophys Acta. 1967 Dec 5;144(3):649–658. doi: 10.1016/0005-2760(67)90053-7. [DOI] [PubMed] [Google Scholar]
- Michell R. H., Kirk C. J., Jones L. M., Downes C. P., Creba J. A. The stimulation of inositol lipid metabolism that accompanies calcium mobilization in stimulated cells: defined characteristics and unanswered questions. Philos Trans R Soc Lond B Biol Sci. 1981 Dec 18;296(1080):123–138. doi: 10.1098/rstb.1981.0177. [DOI] [PubMed] [Google Scholar]
- Rawyler A. J., Roelofsen B., Wirtz K. W., Op den Kamp J. A. (poly) Phosphoinositide phosphorylation is a marker for plasma membrane in Friend erythroleukaemic cells. FEBS Lett. 1982 Nov 1;148(1):140–144. doi: 10.1016/0014-5793(82)81260-x. [DOI] [PubMed] [Google Scholar]
- Rebecchi M. J., Gershengorn M. C. Thyroliberin stimulates rapid hydrolysis of phosphatidylinositol 4,5-bisphosphate by a phosphodiesterase in rat mammotropic pituitary cells. Evidence for an early Ca2+-independent action. Biochem J. 1983 Nov 15;216(2):287–294. doi: 10.1042/bj2160287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebecchi M. J., Kolesnick R. N., Gershengorn M. C. Thyrotropin-releasing hormone stimulates rapid loss of phosphatidylinositol and its conversion to 1,2-diacylglycerol and phosphatidic acid in rat mammotropic pituitary cells. Association with calcium mobilization and prolactin secretion. J Biol Chem. 1983 Jan 10;258(1):227–234. [PubMed] [Google Scholar]
- Seyfred M. A., Wells W. W. Subcellular incorporation of 32P into phosphoinositides and other phospholipids in isolated hepatocytes. J Biol Chem. 1984 Jun 25;259(12):7659–7665. [PubMed] [Google Scholar]
- Smith C. D., Wells W. W. Phosphorylation of rat liver nuclear envelopes. II. Characterization of in vitro lipid phosphorylation. J Biol Chem. 1983 Aug 10;258(15):9368–9373. [PubMed] [Google Scholar]
- Straub R. E., Gershengorn M. C. Thyrotropin-releasing hormone and GTP activate inositol trisphosphate formation in membranes isolated from rat pituitary cells. J Biol Chem. 1986 Feb 25;261(6):2712–2717. [PubMed] [Google Scholar]
- Torda C. Cyclic AMP-dependent diphosphoinositide kinase. Biochim Biophys Acta. 1972 Dec 29;286(2):389–395. doi: 10.1016/0304-4165(72)90275-9. [DOI] [PubMed] [Google Scholar]
- Tou J. S., Hurst M. W., Huggins C. G., Foor W. E. Biosynthesis of triphosphoinositide in rat kidney cortex. Arch Biochem Biophys. 1970 Oct;140(2):492–502. doi: 10.1016/0003-9861(70)90093-7. [DOI] [PubMed] [Google Scholar]
- Tou J. S., Hurst M. W., Huggins C. G. Phosphatidylinositol kinase in rat kidney cortex. II. Subcellular distribution and kinetic properties. Arch Biochem Biophys. 1969 May;131(2):596–602. doi: 10.1016/0003-9861(69)90434-2. [DOI] [PubMed] [Google Scholar]
- Van Rooijen L. A., Rossowska M., Bazan N. G. Inhibition of phosphatidylinositol-4-phosphate kinase by its product phosphatidylinositol-4,5-bisphosphate. Biochem Biophys Res Commun. 1985 Jan 16;126(1):150–155. doi: 10.1016/0006-291x(85)90584-4. [DOI] [PubMed] [Google Scholar]
- Wilson D. B., Neufeld E. J., Majerus P. W. Phosphoinositide interconversion in thrombin-stimulated human platelets. J Biol Chem. 1985 Jan 25;260(2):1046–1051. [PubMed] [Google Scholar]


