Abstract
Introduction
DESTINY-PanTumor02 (NCT04482309) evaluated the efficacy and safety of trastuzumab deruxtecan (T-DXd) in pretreated patients with human epidermal growth factor receptor 2 (HER2)-expressing [immunohistochemistry (IHC) 3+/2+] solid tumors across seven cohorts: endometrial, cervical, ovarian, bladder, biliary tract, pancreatic, and other. Subgroup analyses by HER2 status were previously reported by central HER2 IHC testing, determined at enrollment or confirmed retrospectively. Reflecting the testing methods available in clinical practice, most patients (n = 202; 75.7%) were enrolled based on local HER2 IHC testing. Here, we report outcomes by HER2 IHC status as determined by the local or central test results used for study enrollment.
Methods
This phase 2, open-label study evaluated T-DXd (5.4 mg/kg once every 3 weeks) for HER2-expressing (IHC 3+/2+ by local or central testing) locally advanced or metastatic disease after ≥ 1 systemic treatment or without alternative treatments. The primary endpoint was investigator-assessed confirmed objective response rate (ORR). Secondary endpoints included safety, duration of response (DOR), progression-free survival (PFS), and overall survival.
Results
In total, 111 (41.6%) and 151 (56.6%) patients were enrolled with IHC 3+ and IHC 2+ tumors, respectively. In patients with IHC 3+ tumors, investigator-assessed confirmed ORR was 51.4% [95% confidence interval (CI) 41.7, 61.0], and median DOR was 14.2 months (95% CI 10.3, 23.6). In patients with IHC 2+ tumors, investigator-assessed ORR was 26.5% (95% CI 19.6, 34.3), and median DOR was 9.8 months (95% CI 4.5, 12.6). Safety was consistent with the known profile of T-DXd.
Conclusion
In line with previously reported results, T-DXd demonstrated clinically meaningful benefit in patients with HER2-expressing tumors, with the greatest benefit in patients with IHC 3+ tumors. These data support the antitumor activity of T-DXd in HER2-expressing solid tumors, irrespective of whether patients are identified by local or central HER2 IHC testing.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12325-024-02975-x.
Keywords: Advanced/metastatic solid tumors, HER2-expressing, HER2 testing, Trastuzumab deruxtecan
Key Summary Points
| In DESTINY-PanTumor02, trastuzumab deruxtecan (T-DXd) demonstrated durable responses across multiple solid tumor types, with the greatest benefit in those with human epidermal growth factor receptor 2 (HER2) immunohistochemistry (IHC) 3+ tumors (HER2 test result by central testing). |
| Reflecting the testing methods available in clinical practice, most patients (75.7%) were enrolled into DESTINY-PanTumor02 based on local HER2 IHC testing. |
| This post hoc analysis reports outcomes by HER2 IHC status used for study enrollment, as determined by the local or central test results. |
| T-DXd demonstrated clinically meaningful benefit in patients with HER2-expressing tumors when HER2 expression was determined by the local or central test results used for study enrollment; greatest benefit was observed in those with IHC 3+ tumors. |
| Patients with HER2-expressing (IHC 3+) solid tumors can be identified for potential T-DXd treatment using local HER2 IHC test results, which reflects HER2 testing practices. |
Introduction
Trastuzumab deruxtecan (T-DXd) is an antibody–drug conjugate comprising a humanized immunoglobulin G1 monoclonal antibody specifically targeting human epidermal growth factor receptor 2 (HER2), a tetrapeptide-based cleavable linker, and a potent topoisomerase I inhibitor payload [1]. T-DXd is approved in multiple countries worldwide for various indications, including HER2-positive and HER2-low breast cancer, HER2-positive gastric or gastroesophageal junction adenocarcinoma, and HER2-mutant non-small cell lung cancer (NSCLC) [2–4]. In April 2024, based in part on primary results from the DESTINY-PanTumor02 trial, T-DXd was granted accelerated approval in the USA for adult patients with unresectable or metastatic HER2-positive [immunohistochemistry (IHC) 3+] solid tumors that have progressed after prior treatment and have no satisfactory alternative therapy [2, 5].
In the open-label phase 2 DESTINY-PanTumor02 trial, T-DXd demonstrated clinically meaningful antitumor activity in pretreated patients with HER2-expressing solid tumors [6]. Subgroup analyses by HER2 status were previously reported by central HER2 IHC testing, with the greatest benefit reported in patients whose tumors had HER2 IHC 3+ expression [6]. HER2 expression for study enrollment was based on local or central IHC test result [6] and, reflective of HER2 testing methods used in clinical practice [7, 8], the majority of patients were enrolled based on results from local HER2 IHC testing (n = 202; 75.7%) [6].
Here, we report a post hoc efficacy analysis of T-DXd in DESTINY-PanTumor02 according to the local or central HER2 IHC test result used for enrollment.
Methods
Study Design and Participants
DESTINY-PanTumor02 (NCT04482309) was an open-label, phase 2 study evaluating T-DXd (5.4 mg/kg once every 3 weeks) for HER2-expressing locally advanced or metastatic disease after ≥ 1 systemic treatment or without alternative treatments. Study design details and outcome measures have been previously published [6]. Briefly, eligible patients were aged ≥ 18 years with histologically confirmed locally advanced, unresectable, or metastatic biliary tract, bladder, cervical, endometrial, ovarian, pancreatic, or other solid cancers (excluding NSCLC, breast, gastric, and colorectal cancers) that had progressed following prior treatment or with no satisfactory alternative treatment options.
HER2 expression for enrollment was based on a local IHC test result, where available; otherwise, enrollment was determined via a central IHC test result using the HER2 HercepTest™ (Dako). HER2 IHC scoring was based on current American Society of Clinical Oncology/College of American Pathologists guidelines for scoring HER2 for gastric cancer (in situ hybridization testing not required) [9]. Patients who were enrolled based on a local test result also had HER2 expression determined by retrospective central testing using the HER2 HercepTest™ (Dako) and scored according to gastric-specific criteria [9].
Procedures
T-DXd was administered intravenously once every 3 weeks at 5.4 mg/kg until documented disease progression [Response Evaluation Criteria in Solid Tumors version 1.1 (RECIST 1.1)], withdrawal of consent, or if any other discontinuation criteria were met.
Endpoints
The primary endpoint was confirmed objective response rate (ORR) by investigator assessment; secondary endpoints included safety, duration of response (DOR), progression-free survival (PFS), and overall survival (OS). An independent central review (ICR) per RECIST 1.1 was also conducted to support the investigator-assessed results for secondary outcomes. Exploratory endpoints included subgroup analysis by HER2 status. Secondary safety endpoints included occurrence of adverse events (AEs), including AEs of special interest [interstitial lung disease (ILD)/pneumonitis and left ventricular dysfunction].
Ethics
All patients provided written informed consent. The study was approved by independent institutional review boards of each participating site and was conducted in accordance with the ethics principles of the Declaration of Helsinki and with Good Clinical Practice guidelines defined by the International Conference on Harmonisation. A list of these individual review boards has been provided as a supplementary appendix.
Results
As reported previously, 268 patients with HER2-expressing solid tumors were enrolled between October 7, 2020, and July 7, 2022 [6]; 267 patients (99.6%) received ≥ 1 dose of T-DXd and were included in the full analysis set.
In total, 202 (75.7%) and 65 (24.3%) patients were enrolled based on local and central HER2 IHC test results, respectively. Per the local or central HER2 IHC test result used for study enrollment, 111 (41.6%) and 151 (56.6%) patients with IHC 3+ and IHC 2+ tumors were enrolled, respectively; 5 patients with IHC 1+ tumors were included following a protocol-specified interim analysis.
Baseline demographics and clinical characteristics in the full study population, and in patients with IHC 3+ and IHC 2+ tumors according to the local or central HER2 IHC test result used for study enrollment, are summarized in Table 1.
Table 1.
Baseline demographics and clinical characteristics
| All (n = 267) |
HER2 IHC 3+ by the local or central test result used for study enrollment (n = 111) |
HER2 IHC 2+ by the local or central test result used for study enrollment (n = 151) |
|
|---|---|---|---|
| Median age, years (range) | 62 (23–85) | 64 (23–85) | 61 (30–81) |
| Sex | |||
| Male | 89 (33.3) | 45 (40.5) | 44 (29.1) |
| Female | 178 (66.7) | 66 (59.5) | 107 (70.9) |
| Race | |||
| White | 163 (61.0) | 64 (57.7) | 94 (62.3) |
| Asian | 87 (32.6) | 38 (34.2) | 49 (32.5) |
| Black or African American | 6 (2.2) | 4 (3.6) | 2 (1.3) |
| Other | 6 (2.2) | 3 (2.7) | 3 (2.0) |
| Not reported | 5 (1.9) | 2 (1.8) | 3 (2.0) |
| ECOG performance statusa | |||
| 0 | 126 (47.2) | 54 (48.6) | 68 (45.0) |
| 1 | 140 (52.4) | 57 (51.4) | 82 (54.3) |
| HER2 IHC test used for study enrollment | |||
| Local | 202 (75.7) | 93 (83.8) | 107 (70.9) |
| Central | 65 (24.3) | 18 (16.2) | 44 (29.1) |
| HER2 IHC status at enrollment (by local or central test result) | |||
| IHC 3+ | 111 (41.6) | 111 (100) | – |
| IHC 2+ | 151 (56.6) | – | 151 (100) |
| IHC 1+ | 5 (1.9) | – | – |
| Centrally confirmed HER2 IHC status | |||
| IHC 3+ | 75 (28.1) | 69 (62.2) | 6 (4.0) |
| IHC 2+ | 125 (46.8) | 26 (23.4) | 98 (64.9) |
| IHC 1+ | 25 (9.4) | 4 (3.6) | 18 (11.9) |
| IHC 0 | 30 (11.2) | 6 (5.4) | 23 (15.2) |
| Unknown | 12 (4.5) | 6 (5.4) | 6 (4.0) |
| Prior therapy lines | |||
| Median (range) | 2.0 (0–12) | 2.0 (0–9) | 2.0 (0–12) |
| 0 | 3 (1.1) | 2 (1.8) | 1 (0.7) |
| 1 | 71 (26.6) | 35 (31.5) | 36 (23.8) |
| 2 | 84 (31.5) | 30 (27.0) | 52 (34.4) |
| 3 | 55 (20.6) | 21 (18.9) | 32 (21.2) |
| 4 | 21 (7.9) | 12 (10.8) | 8 (5.3) |
| ≥ 5 | 33 (12.4) | 11 (9.9) | 22 (14.6) |
| Tumor type | |||
| Biliary tract | 41 (15.4) | 22 (19.8) | 19 (12.6) |
| Bladder | 41 (15.4) | 27 (24.3) | 14 (9.3) |
| Cervical | 40 (15.0) | 10 (9.0) | 25 (16.6) |
| Endometrial | 40 (15.0) | 16 (14.4) | 24 (15.9) |
| Ovarian | 40 (15.0) | 15 (13.5) | 25 (16.6) |
| Pancreatic | 25 (9.4) | 5 (4.5) | 20 (13.2) |
| Other | 40 (15.0) | 16 (14.4) | 24 (15.9) |
| Prior HER2 therapy | |||
| Trastuzumab | 33 (12.4) | 22 (19.8) | 11 (7.3) |
| Pertuzumab | 5 (1.9) | 3 (2.7) | 2 (1.3) |
| Zanidatamab | 4 (1.5) | 2 (1.8) | 2 (1.3) |
| Trastuzumab emtansine | 3 (1.1) | 3 (2.7) | 0 |
| Trastuzumab duocarmazine | 1 (0.4) | 0 | 1 (0.7) |
| Tucatinib | 1 (0.4) | 1 (0.9) | 0 |
All values are n (%) unless stated otherwise. In the cervical cohort, 5 patients with IHC 1+ status were included after a protocol-specified interim analysis; for each cohort (except the “other” tumors cohort), up to 10 patients with IHC 1+ could have been included if ≥ 3 objective responses were observed in the first 15 patients with confirmed HER2 IHC 3+/2+ by central testing; for the “other” tumors cohort, only patients with HER2 IHC 3+/2+ were eligible for enrollment
ECOG Eastern Cooperative Oncology Group, HER2 human epidermal growth factor receptor 2, IHC immunohistochemistry
aOne patient with HER2 IHC 2+ by the test result used for study enrollment had an ECOG performance status of 2
The median (range) duration of follow-up was 16.0 months (0.4–31.6) and 11.7 months (0.7 to 31.1) in patients with IHC 3+ and IHC 2+ tumors, respectively.
Efficacy
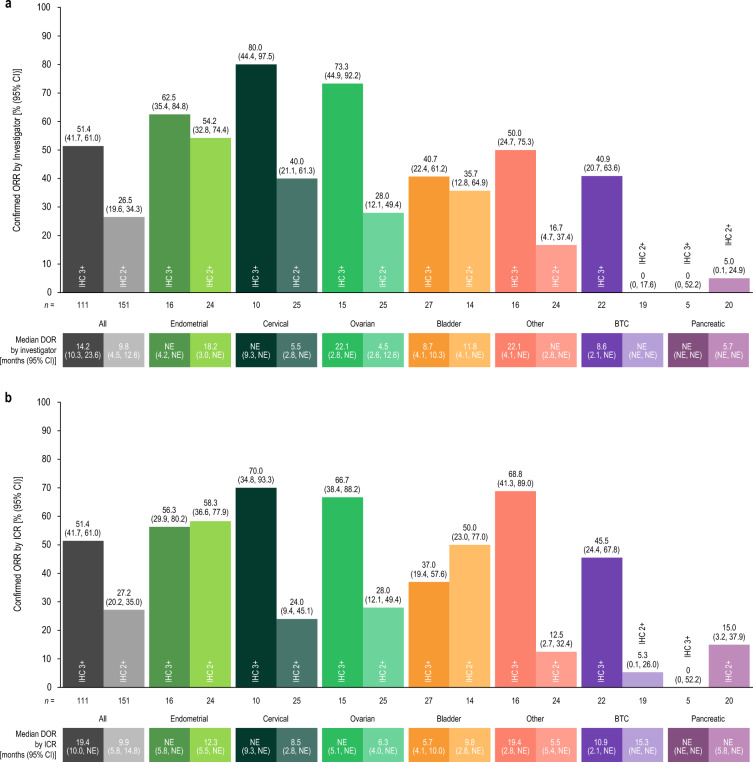
Investigator-assessed ORR and DOR by HER2 IHC status used to determine study enrollment and by tumor cohort are reported in Fig. 1. In patients with IHC 3+ tumors, investigator-assessed confirmed ORR was 51.4% [95% confidence interval (CI) 41.7, 61.0], and median DOR was 14.2 months (95% CI 10.3, 23.6). In patients with IHC 2+ tumors, investigator-assessed ORR was 26.5% (95% CI 19.6, 34.3), and median DOR was 9.8 months (95% CI 4.5, 12.6). ORR and DOR results by ICR are also presented in Fig. 1. Investigator-assessed disease control rate at 12 weeks was 78.4% (95% CI 69.6, 85.6) in patients with IHC 3+ tumors and 60.3% (95% CI 52.0, 68.1) in those with IHC 2+ tumors. PFS (by investigator assessment and ICR) and OS by tumor cohort and HER2 IHC status used to determine enrollment are reported in Table 2 and Supplementary Material Fig. S1.
Fig. 1.
ORR and DOR according to tumor type and HER2 IHC status by the local or central test result used for study enrollment by a investigator assessment and b ICR in patients with IHC 3+ and IHC 2+ tumors. BTC biliary tract cancer, CI confidence interval, DOR duration of response, HER2 human epidermal growth factor receptor 2, ICR independent central review, IHC immunohistochemistry, NE not estimable, ORR objective response rate
Table 2.
Survival outcomes according to tumor type and HER2 IHC status by the local or central test result used for study enrollment
| HER2 status by test for study enrollment | PFS | OS | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Investigator assessment | ICR | ||||||||||||
| Median, months (95% CI) | 6 months, % (95% CI) |
12 months, % (95% CI) | 18 months, % (95% CI) | Median, months (95% CI) | 6 months, % (95% CI) |
12 months, % (95% CI) | 18 months, % (95% CI) |
Median, months (95% CI) | 6 months, % (95% CI) |
12 months, % (95% CI) | 18 months, % (95% CI) | ||
| All patients |
IHC 3+ (n = 111) |
9.7 (7.0, 12.5) |
65.6 (55.8, 73.8) |
44.0 (34.4, 53.3) |
31.2 (22.4, 40.4) |
10.1 (7.0, 14.2) |
66.4 (56.6, 74.5) |
47.3 (37.2, 56.7) |
36.9 (26.9, 46.8) |
17.7 (12.8, 21.8) |
77.1 (68.1, 83.9) |
63.3 (53.5, 71.6) |
49.9 (40.1, 58.9) |
|
IHC 2+ (n = 151) |
5.1 (4.1, 6.0) |
43.2 (35.0, 51.2) |
19.2 (13.1, 26.3) |
13.0 (7.9, 19.6) |
5.6 (4.2, 7.0) |
47.4 (38.6, 55.7) |
23.7 (16.1, 32.1) |
18.4 (11.4, 26.7) |
12.0 (9.6, 13.5) |
71.9 (63.9, 78.4) |
50.6 (42.2, 58.3) |
31.0 (23.6, 38.7) |
|
| Endometrial cancer |
IHC 3+ (n = 16) |
NE (4.5, NE) |
74.5 (45.4, 89.6) |
60.9 (32.7, 80.3) |
54.2 (27.1, 75.0) |
NE (3.9, NE) |
74.5 (45.4, 89.6) |
67.0 (37.7, 84.9) |
59.6 (30.8, 79.6) |
26.0 (4.5, NE) |
75.0 (46.3, 89.8) |
75.0 (46.3, 89.8) |
68.8 (40.5, 85.6) |
|
IHC 2+ (n = 24) |
11.0 (6.0, 19.5) |
73.9 (50.7, 87.4) |
41.0 (20.7, 60.4) |
30.8 (13.0, 50.6) |
11.8 (6.0, 20.3) |
73.9 (50.7, 87.4) |
45.5 (22.5, 66.0) |
32.5 (12.6, 54.3) |
20.3 (8.1, NE) |
91.3 (69.5, 97.8) |
65.2 (42.3, 80.8) |
52.2 (30.5, 70.0) |
|
| Cervical cancer |
IHC 3+ (n = 10) |
NE (3.9, NE) |
90.0 (47.3, 98.5) |
68.6 (30.5, 88.7) |
51.4 (14.3, 79.6) |
NE (2.9, NE) |
90.0 (47.3, 98.5) |
77.1 (34.5, 93.9) |
57.9 (15.3, 85.2) |
NE (3.9, NE) |
90.0 (47.3, 98.5) |
90.0 (47.3, 98.5) |
90.0 (47.3, 98.5) |
|
IHC 2+ (n = 25) |
4.6 (1.4, 8.1) |
33.3 (15.9, 51.9) |
14.3 (2.9, 34.4) |
0.0 (NE, NE) |
4.6 (2.7, 8.3) |
36.1 (16.7, 56.0) |
9.6 (0.7, 33.1) |
NE (NE, NE) |
11.7 (8.0, NE) |
72.0 (50.1, 85.5) |
46.9 (26.6, 64.9) |
31.3 (13.4, 51.0) |
|
| Ovarian cancer |
IHC 3+ (n = 15) |
12.6 (4.1, NE) |
59.3 (30.7, 79.3) |
59.3 (30.7, 79.3) |
44.4 (18.9, 67.4) |
13.9 (4.4, NE) |
79.4 (48.8, 92.9) |
61.8 (30.4, 82.3) |
44.1 (16.7, 68.8) |
20.0 (7.2, NE) |
86.7 (56.4, 96.5) |
73.3 (43.6, 89.1) |
53.3 (26.3, 74.4) |
|
IHC 2+ (n = 25) |
4.4 (2.3, 7.1) |
42.1 (21.8, 61.2) |
14.0 (3.5, 31.6) |
4.7 (0.3, 19.4) |
5.6 (1.5, 8.2) |
48.9 (26.5, 68.0) |
18.3 (4.7, 39.0) |
12.2 (2.1, 31.9) |
10.7 (5.9, 14.8) |
71.6 (49.4, 85.3) |
46.3 (26.0, 64.4) |
21.1 (7.7, 38.8) |
|
| Bladder cancer |
IHC 3+ (n = 27) |
7.0 (3.9, 11.5) |
58.0 (37.0, 74.1) |
18.9 (6.2, 36.9) |
4.7 (0.3, 19.4) |
6.8 (2.6, 9.9) |
57.5 (36.4, 73.8) |
21.6 (7.4, 40.4) |
10.8 (1.9, 28.4) |
12.6 (6.7, 17.2) |
73.3 (52.0, 86.3) |
54.0 (33.4, 70.7) |
30.9 (14.7, 48.7) |
|
IHC 2+ (n = 14) |
7.0 (2.6, 13.0) |
57.1 (28.4, 78.0) |
28.6 (8.8, 52.4) |
14.3 (2.3, 36.6) |
7.0 (2.8, 11.1) |
57.9 (25.6, 80.2) |
19.3 (3.1, 46.0) |
19.3 (3.1, 46.0) |
13.5 (8.0, NE) |
85.7 (53.9, 96.2) |
78.6 (47.2, 92.5) |
35.7 (13.0, 59.4) |
|
| Other tumors |
IHC 3+ (n = 16) |
13.0 (6.3, NE) |
81.3 (52.5, 93.5) |
68.8 (40.5, 85.6) |
48.6 (22.9, 70.3) |
21.0 (5.6, 22.9) |
68.8 (40.5, 85.6) |
62.5 (34.9, 81.1) |
56.3 (29.5, 76.2) |
24.3 (11.1, NE) |
87.5 (58.6, 96.7) |
75.0 (46.3, 89.8) |
75.0 (46.3, 89.8) |
|
IHC 2+ (n = 24) |
6.6 (2.9, 8.8) |
51.2 (29.4, 69.4) |
18.6 (5.9, 37.0) |
18.6 (5.9, 37.0) |
8.4 (2.9, NE) |
75.1 (49.9, 88.9) |
35.0 (11.9, 59.6) |
23.4 (4.6, 50.3) |
15.5 (9.6, 22.4) |
95.8 (73.9, 99.4) |
68.5 (44.9, 83.6) |
41.1 (21.0, 60.2) |
|
| Biliary tract cancer |
IHC 3+ (n = 22) |
6.9 (3.0, 8.0) |
52.6 (29.9, 71.1) |
23.9 (8.7, 43.2) |
14.4 (3.6, 32.2) |
6.8 (2.8, 15.0) |
52.4 (29.7, 70.9) |
30.0 (11.4, 51.3) |
20.0 (4.4, 43.7) |
7.6 (4.6, NE) |
61.9 (38.1, 78.8) |
42.9 (21.9, 62.3) |
33.3 (14.9, 53.1) |
|
IHC 2+ (n = 19) |
3.7 (2.8, 5.1) |
15.8 (3.9, 34.9) |
5.3 (0.4, 21.4) |
5.3 (0.4, 21.4) |
3.0 (1.7, 4.2) |
10.5 (1.8, 28.4) |
5.3 (0.4, 21.4) |
5.3 (0.4, 21.4) |
5.3 (3.1, 10.2) |
42.1 (20.4, 62.5) |
15.8 (3.9, 34.9) |
15.8 (3.9, 34.9) |
|
| Pancreatic cancer |
IHC 3+ (n = 5) |
8.0 (1.2, NE) |
53.3 (6.8, 86.3) |
0.0 (NE, NE) |
0.0 (NE, NE) |
7.0 (1.4, NE) |
53.3 (6.8, 86.3) |
NE (NE, NE) |
NE (NE, NE) |
8.8 (2.4, NE) |
80.0 (20.4, 96.9) |
40.0 (5.2, 75.3) |
0.0 (NE, NE) |
|
IHC 2+ (n = 20) |
3.2 (1.4, 4.9) |
28.4 (10.4, 49.6) |
11.3 (1.9, 30.2) |
11.3 (1.9, 30.2) |
3.2 (1.2, 7.2) |
30.1 (11.4, 51.5) |
24.1 (7.7, 45.3) |
24.1 (7.7, 45.3) |
4.7 (3.2, 14.2) |
40.0 (19.3, 60.0) |
35.0 (15.7, 55.2) |
18.8 (5.2, 38.6) |
|
CI confidence interval, HER2 human epidermal growth factor receptor 2, ICR independent central review, IHC immunohistochemistry, NE not estimable, OS overall survival, PFS progression-free survival
All 5 patients enrolled with HER2 IHC 1+ tumors were in the cervical cancer cohort; 2 were enrolled based on local test results, and 3 were enrolled based on central test results. Two patients (40.0%; 95% CI 5.3, 85.3) had a confirmed partial response (by both investigator assessment and ICR).
Safety
Detailed safety outcomes have been reported previously [6]. Among the 267 treated patients (median follow-up of 12.75 months), 226 patients (84.6%) had ≥ 1 investigator-assessed drug-related AE; the most common drug-related AEs were nausea (55.1%), anemia (27.7%), diarrhea (25.8%), vomiting (24.7%), and fatigue (24.7%). Adjudicated drug-related events of ILD/pneumonitis occurred in 28 patients [10.5%; grade 1, n = 7 (2.6%); grade 2, n = 17 (6.4%); grade 3, n = 1 (0.4%)]; there were 3 (1.1%) fatal adjudicated drug-related cases of ILD/pneumonitis that occurred in the biliary tract, endometrial, and other tumor cohorts. Overall, no new safety signals were reported for T-DXd.
Discussion
DESTINY-PanTumor02 enrolled patients with HER2-expressing (IHC 3+/2+) solid tumors, as determined by local or central HER2 IHC test results, with 75.7% enrolled based on local HER2 IHC results. In real-world clinical practice, HER2 IHC testing for breast cancer, colorectal cancer, and endometrial cancers is frequently conducted via local laboratories [7, 8]. As such, demonstrating that T-DXd antitumor activity is observed, irrespective of whether HER2 expression is identified by local or central IHC testing, is important to clinicians who are considering T-DXd as a therapeutic option for their patients following HER2 testing.
In this post hoc analysis, T-DXd showed durable and clinically meaningful benefit in patients with HER2 IHC 3+ and IHC 2+ solid tumors per local or central HER2 IHC test results for study enrollment; efficacy results according to ICR were generally consistent with investigator-assessed outcomes. The highest response rate and longest DOR were seen in patients with IHC 3+ tumors. Overall, these IHC 3+ and IHC 2+ subgroup data by study enrollment HER2 IHC test results are comparable with the ORR and median DOR subgroup analyses previously reported according to HER2 IHC central test results using the HercepTest™ (Dako; investigator-assessed ORR of 61.3% and 27.2% and median DOR of 22.1 months and 9.8 months in patients with IHC 3+ and IHC 2+ tumors, respectively) [6, 10]. Favorable antitumor activity was also observed across a broad range of tumor types with IHC 3+ and IHC 2+ expression, similar to that previously shown by HER2 IHC central test results [6]. As the majority of patients were enrolled based on local HER2 IHC testing, this analysis supports use of local IHC test results to identify patients whose tumors have HER2 IHC 3+ expression and are likely to respond to T-DXd; the magnitude of T-DXd clinical benefit is irrespective of central IHC confirmation [6]. Considering the recent accelerated approval of T-DXd in the USA [2, 5], it is important that appropriately validated HER2 tests are used at local laboratories and that pathologists are appropriately trained to evaluate and score solid tumor samples.
Across studies of solid tumors, varying prevalence of HER2 IHC 3+/IHC 2+ expression has been observed, ranging from 16 to 33% of biliary tract cancers, 9–56% of urothelial carcinomas, 21–29% of cervical cancers, 18–56% of endometrial cancers, 4–28% of ovarian cancers, and 7–16% of pancreatic cancers [11–28]. Patients with HER2-expressing solid tumors typically have an inferior prognosis [29], and there remains a high unmet clinical need for efficacious treatment options. When considering outcomes associated with current standard of care for tumor types included in this study [30–33], the magnitude of clinical benefit observed in DESTINY-PanTumor02 supports T-DXd as a new therapeutic option for pretreated patients with a range of HER2 IHC 3+ solid tumors.
As previously reported, the safety findings in this trial are consistent with the known profile of T-DXd [6]. ILD/pneumonitis remains an important identified risk, and proactive monitoring, early detection, and active management are critical to prevent high-grade ILD/pneumonitis.
Limitations of the study include the single-arm design not enabling the inclusion of comparators owing to the range of tumor cohorts investigated and the small numbers of patients, reflecting the low prevalence of IHC 3+ and IHC 2+ expression in some tumor types.
Conclusion
This post hoc analysis affirms the tumor-agnostic activity of T-DXd in patients with HER2 IHC 3+ and IHC 2+ solid tumors when HER2 expression is determined primarily by locally available IHC assessment, which is reflective of HER2 testing practices in clinical practice; overall benefit is consistent whether HER2 testing is conducted by local or central testing.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We thank the patients who participated in the study, as well as their families and caregivers. We also thank the staff and investigators at all the study sites and the following AstraZeneca employees: Pippa Hadland for publication leadership, interpretation of the data, and scientific review of the manuscript; Nassim Morsli for clinical leadership of the study, interpretation of the data, and scientific review of the manuscript; Norah Shire for leadership of the study and data interpretation; Chiedozie Anoka, Fabiola Cecchi, Flavia Michelini, Jose David Hernandez Chagui, Abdennour Ferhat, Mark Gustavson, and Rob McEwen for interpretation and/or scientific review of the manuscript; Aleksandra Lorenc, Anna Dobrowolska, Jin Sakong, Magdalena Zakrzewska, Marta Czekaj, and Nicholas Holoweckyj for conduct of the study/data; Anubhavini Chaudhry, Chetan Shatapathy, Joao Simoes, and Nataliya Kuptsova-Clarkson for safety analyses and data interpretation; and Lindsey Jung and Galina Vinokurov for statistical analyses.
Medical Writing, Editorial, and Other Assistance
Medical writing support, under the direction of the authors, was provided by Jennifer Mitchell, PhD, of Helios Medical Communications, part of Helios Global Group, and was funded by AstraZeneca in accordance with Good Publication Practice (GPP) guidelines (http://www.ismpp.org/gpp-2022).
Author Contributions
Conception and design: Salvatore Siena, Soham Puvvada, Ann Smith, Jung-Yun Lee. Provision of study materials or patients: Ana Oaknin, Antonio González-Martín, Kyung Hae Jung, Luis Manso, Bohuslav Melichar, Salvatore Siena, Daniil Stroyakovskiy. Collection and assembly of data: Funda Meric-Bernstam, Do-Youn Oh, Antonio González-Martín, Aránzazu Manzano, Bohuslav Melichar, Salvatore Siena, Soham Puvvada, Jung-Yun Lee. Data analysis and interpretation: All authors. Manuscript writing: All authors. Final approval of manuscript: All authors. Accountable for all aspects of the work: All authors.
Funding
This study was sponsored by AstraZeneca in collaboration with Daiichi Sankyo. In March 2019, AstraZeneca entered into a global development and commercialization collaboration agreement with Daiichi Sankyo for trastuzumab deruxtecan (T-DXd; DS-8201). In collaboration with the authors, both AstraZeneca and Daiichi Sankyo assisted with data interpretation, the writing of the report, reviewing the manuscript, and the decision to submit the manuscript for publication. AstraZeneca funded the journal’s Rapid Service and Open Access fees.
Data Availability
Data underlying the findings described in this manuscript may be obtained in accordance with AstraZeneca’s data sharing policy, described at https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure. Data for studies directly listed on Vivli can be requested through Vivli at www.vivli.org. Data for studies not listed on Vivli can be requested through Vivli at https://vivli.org/members/enquiries-about-studies-not-listed-on-the-vivli-platform/. The AstraZeneca Vivli member page is also available, outlining further details: https://vivli.org/ourmember/astrazeneca/.
Declarations
Conflict of Interest
Ana Oaknin has received consulting fees from Agenus, AstraZeneca, Clovis Oncology, Corcept Therapeutics, Daiichi Sankyo, Debiopharm, Deciphera Pharmaceuticals, Eisai, Exelisis, Genmab, GSK, ImmunoGen, Itheos, MSD, Mersana Therapeutics, Myriad Genetics, Novocure, OncXerna Therapeutics, Pfizer, PharmaMar, Regeneron Pharmaceuticals, Roche, Shattuck Labs, Sutro Biopharma, and Zentalis Pharmaceuticals; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events from Asociación Colombiana de Ginecológos Oncólogos, AstraZeneca, European School of Oncology, GSK, Medscape, NSGO, PeerView, and PeerVoice; support for attending meetings and/or travel from AstraZeneca, PharmaMar, and Roche; participated on a Data Safety Monitoring Board or Advisory Board for Agenus, AstraZeneca, Clovis Oncology, Corcept Therapeutics, Daiichi Sankyo, Debiopharm, Deciphera Pharmaceuticals, Eisai, Exelisis, Genmab, GSK, ImmunoGen, Itheos, MSD, Mersana Therapeutics, Myriad Genetics, Novocure, OncXerna Therapeutics, Pfizer, PharmaMar, Regeneron Pharmaceuticals, Roche, Shattuck Labs, Sutro Biopharma, and Zentalis Pharmaceuticals; and held a leadership or fiduciary role in other board, society, committee, or advocacy group, paid or unpaid, for the European Society for Medical Oncology, as European Society for Medical Oncology Guidelines Gynecology Subject Editor, as European Society for Medical Oncology Gynecology Meeting Chair, as European Society for Medical Oncology Gynecology Faculty Member, for the Gynecologic Cancer Intergroup, and as Gynecologic Cancer Intergroup Cervical Cancer Committee Chair. Jung-Yun Lee has participated on a Data Safety Monitoring Board or Advisory Board for Eisai and GI Innovation Inc.; and has other financial or non-financial interests with Alkermes, AstraZeneca, BeiGene, BerGenBio, Cellid, Clovis Oncology, Eisai, GI Innovation, ImmunoGen, Janssen, Merck, Mersana Therapeutics, MSD, Novartis, OncoQuest Pharmaceuticals, ONO Pharmaceutical Co., Ltd., Roche, Seagen, Synthon, and Takeda Pharmaceuticals. Vicky Makker has received grants or contracts from AstraZeneca, Bristol-Myers Squibb, Clasi, Cullinan Therapeutics, Duality Biologics, Eisai, Faeth Therapeutics, Karyopharm Therapeutics, Merck, Takeda Pharmaceuticals, and Zymeworks; support for attending meetings and/or travel from Eisai, Karyopharm Therapeutics, and Merck; and has other financial or non-financial interests with Clovis, Cullinan Therapeutics, Duality Biologics, Eisai, Faeth Therapeutics, GSK, Immunocore, iTeos Therapeutics, Karyopharm Therapeutics, Lilly, Merck, Mereo BioPharma, MorphoSys, MSD, Novartis, Regeneron Pharmaceuticals, Sutro Biopharma, and Zymeworks. Do-Youn Oh has received grants or contracts from Array BioPharma, AstraZeneca, BeiGene, Eli Lilly and Company, HANDOK, MSD, Novartis, and Servier; and has participated on a Data Safety Monitoring Board or Advisory Board for Arcus Biosciences, ASLAN Pharmaceuticals, AstraZeneca, Basilea, Bayer, BeiGene, Bristol-Myers Squibb, Genentech/Roche, Halozyme, IQVIA, Merck, MSD, Novartis, Taiho Pharmaceutical Co., Ltd., Turning Point Therapeutics, Yuhan Corporation, and Zymeworks. Susanna Banerjee has received grants or contracts from AstraZeneca and GSK; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events from Amgen, AstraZeneca, Clovis Oncology, GSK, ImmunoGen, Medscape, Merck, Mersana Therapeutics, Novocure, Pfizer, Research to Practice, Roche, and Takeda Pharmaceuticals; support for attending meetings and/or travel from GSK and Verastem Oncology; participated on a Data Safety Monitoring Board or Advisory Board for AstraZeneca, Epsilogen, GSK, ImmunoGen, Merck, Mersana Therapeutics, Novartis, OncXerna Therapeutics, Seagen, Shattuck Labs, Regeneron Pharmaceuticals, and Verastem Oncology; held a leadership or fiduciary role in other board, society, committee or advocacy group, paid or unpaid, with the International Cancer Foundation; and has stock or stock options with Perci Health. Antonio González-Martín has received payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events from AstraZeneca, Clovis Oncology, GSK, Novocure, MSD, Roche, Takeda Pharmaceuticals, and Zai Lab; support for attending meetings and/or travel from AstraZeneca, GSK, and MSD; participated on a Data Safety Monitoring Board or Advisory Board for Alkermes, Amgen, AstraZeneca, BioNTech, Clovis Oncology, Daiichi Sankyo, Eisai, Genmab, GSK, Hedera Dx, Illumina, ImmunoGen, Incyte Corporation, Kartos Therapeutics, MacroGenics, Mersana Therapeutics, MSD, Novartis, Novocure, Oncoinvent, PharmaMar, Regeneron Pharmaceuticals, Roche, Sutro Biopharma, SOTIO Biotech, and Tubulis; and has other financial or non-financial interests with GSK and Roche. Kyung Hae Jung has received consulting fees from AstraZeneca, BIXINK, Celgene, Daiichi Sankyo, Eisai, Everest Medicines, Gilead Sciences, MSD, Novartis, Pfizer, Roche, and Takeda Pharmaceuticals. Iwona Ługowska has received grants or contracts from Agenus and Roche; and has other financial or non-financial interests with Agenus, AstraZeneca, Amgen, Bristol-Myers Squibb, Celon Pharma SA, CliniNote, the European Society for Medical Oncology, Incyte, Janssen, Menarini, MSCI, MSD, the Organisation of European Cancer Institutes, Pfizer, Rhizen, Roche, Ryvu Therapeutics, and Sairopa. Luis Manso declares no conflicts of interest. Aránzazu Manzano has received grants or contracts from AstraZeneca; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events from AstraZeneca, GSK, LEO Pharma, MSD, PharmaMar, and Sanofi; support for attending meetings and/or travel from AstraZeneca, GSK, and MSD; and participated on a Data Safety Monitoring Board or Advisory Board for Boehringer Ingelheim, GSK, and PharmaMar. Bohuslav Melichar has received consulting fees from AstraZeneca, Amgen, Bristol-Myers Squibb, Lilly, Merck, MSD, Novartis, Pfizer, Roche, and Servier; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events from AstraZeneca, Amgen, Bristol-Myers Squibb, EMD Serono, Lilly, Merck, MSD, Novartis, Pfizer, Roche, and Servier; support for attending meetings and/or travel from AstraZeneca, Bristol-Myers Squibb, Merck, and MSD; and has participated on a Data Safety Monitoring Board or Advisory Board for AstraZeneca, Bristol-Myers Squibb, Lilly, Merck, MSD, Novartis, Pfizer, and Roche. Salvatore Siena has participated on a Data Safety Monitoring Board or Advisory Board for Agenus, AstraZeneca, Bristol-Myers Squibb, Checkmab, Daiichi Sankyo, GSK, MSD, Novartis, Seagen, and T-ONE Therapeutics. Daniil Stroyakovskiy declares no conflicts of interest. Anitra Fielding holds stock or stock options with AstraZeneca and discloses other financial or non-financial interests with AstraZeneca (employment). Soham Puvvada holds stock or stock options with AstraZeneca and discloses other financial or non-financial interests with AstraZeneca (employment). Ann Smith holds stock or stock options with AstraZeneca and discloses other financial or non-financial interests with AstraZeneca (employment). Funda Meric-Bernstam has received grants or contracts from Aileron Therapeutics, AstraZeneca, Bayer, Calithera Biosciences, Curis, Inc., CytomX Therapeutics, Daiichi Sankyo, Debiopharm, eFFECTOR Therapeutics, Genentech Inc., Guardant Health, KLUS Pharma, Novartis, Puma Biotechnology, Takeda Pharmaceuticals, and Taiho Pharmaceutical Co., Ltd.; consulting fees from AbbVie, AstraZeneca, BD, Calibr-Skaggs at Scripps Research, Daiichi Sankyo, EcoR1 Capital, eFFECTOR Therapeutics, Exelixis, GT Apeiron, Incyte, Infinity Pharmaceuticals, Jazz Pharmaceuticals, LegoChem Biosciences, Lengo Therapeutics, Menarini, Molecular Templates, Protai, Roche, Tallac Therapeutics, and Zymeworks; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events from DAVA Oncology; received support for attending meetings and/or travel from the Cholangiocarcinoma Foundation, the European Organisation for Research and Treatment of Cancer, the European Society for Medical Oncology, and DAVA Oncology; and participated on a Data Safety Monitoring Board or Advisory Board for Black Diamond Therapeutics, Biovica International AB, Eisai, FogPharma, Harbinger Health, Karyopharm Therapeutics, Loxo Oncology, Mersana Therapeutics, OnCusp Therapeutics, Sanofi, Seagen, Theratechnologies, and Zentalis Pharmaceuticals.
Ethical Approval
All patients provided written informed consent. The study was approved by independent institutional review boards of each participating site and was conducted in accordance with the ethics principles of the Declaration of Helsinki and with Good Clinical Practice guidelines defined by the International Conference on Harmonisation. A list of these individual review boards has been provided as a supplementary appendix.
Footnotes
Prior Presentation. Primary analysis was published in Journal of Clinical Oncology, October 23rd, 2023; 10.1200/JCO.23.02005.
References
- 1.Ogitani Y, Aida T, Hagihara K, et al. DS-8201a, a novel HER2-targeting ADC with a novel DNA topoisomerase I inhibitor, demonstrates a promising antitumor efficacy with differentiation from T-DM1. Clin Cancer Res. 2016;22(20):5097–108. [DOI] [PubMed] [Google Scholar]
- 2.U.S. Food and Drug Administration (FDA). ENHERTU (fam-trastuzumab deruxtecan-nxki): highlights of prescribing information. 2024. www.accessdata.fda.gov/drugsatfda_docs/label/2024/761139s028lbl.pdf. Accessed Jun 21, 2024.
- 3.Daiichi Sankyo. Press release. ENHERTU® approved in Japan as first HER2 directed therapy for patients with HER2 mutant metastatic non-small cell lung cancer. 2023. Available from: https://www.daiichisankyo.com/files/news/pressrelease/pdf/202308/20230823_E.pdf. Accessed Jun 21, 2024.
- 4.European Medicines Agency (EMA). ENHERTU: summary of product characteristics. 2024. Available from: https://www.ema.europa.eu/en/documents/product-information/enhertu-epar-product-information_en.pdf. Accessed Jun 21, 2024.
- 5.AstraZeneca. Enhertu approved in the US as first tumour-agnostic HER2-directed therapy for previously treated patients with metastatic HER2-positive solid tumours. 2024. Available from: https://www.astrazeneca.com/media-centre/press-releases/2024/enhertu-approved-in-the-us-as-first-tumour-agnostic-her2-directed-therapy-for-previously-treated-patients-with-metastatic-her2-positive-solid-tumours.html. Accessed Jun 21, 2024.
- 6.Meric-Bernstam F, Makker V, Oaknin A, et al. Efficacy and safety of trastuzumab deruxtecan in patients with HER2-expressing solid tumors: primary results from the DESTINY-PanTumor02 Phase II trial. J Clin Oncol. 2024;42(1):47–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goddard KAB, Bowles EJA, Feigelson HS, et al. Utilization of HER2 genetic testing in a multi-institutional observational study. Am J Manag Care. 2012;18(11):704–12. [PMC free article] [PubMed] [Google Scholar]
- 8.Hagemann IS, Bridge JA, Tafe LJ, et al. Current laboratory testing practices for assessment of ERBB2/HER2 in endometrial serous carcinoma and colorectal carcinoma. Arch Pathol Lab Med. 2023;147(10):1148–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bartley AN, Washington MK, Ventura CB, et al. HER2 testing and clinical decision making in gastroesophageal adenocarcinoma: guideline from the College of American Pathologists, American Society for Clinical Pathology, and American Society of Clinical Oncology. Arch Pathol Lab Med. 2016;140(12):1345–63. [DOI] [PubMed] [Google Scholar]
- 10.Meric-Bernstam F, Makker V, Oaknin A, et al. Trastuzumab deruxtecan for pretreated patients with HER2-expressing solid tumors: primary analysis from the DESTINY-PanTumor02 study. Oral presentation at the 2023 European Society for Medical Oncology Annual Meeting, Madrid, Spain, Sep 13–17, 2023 (Abstract #1568).
- 11.Uzunparmak B, Haymaker C, Raso G, et al. HER2-low expression in patients with advanced or metastatic solid tumors. Ann Oncol. 2023;34(11):1035–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vivaldi C, Fornaro L, Ugolini C, et al. HER2 overexpression as a poor prognostic determinant in resected biliary tract cancer. Oncologist. 2020;25(10):886–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roa I, de Toro G, Schalper K, de Aretxabala X, Churi C, Javle M. Overexpression of the HER2/neu gene: a new therapeutic possibility for patients with advanced gallbladder cancer. Gastrointest Cancer Res. 2014;7(2):42–8. [PMC free article] [PubMed] [Google Scholar]
- 14.Fleischmann A, Rotzer D, Seiler R, Studer UE, Thalmann GN. HER2 amplification is significantly more frequent in lymph node metastases from urothelial bladder cancer than in the primary tumours. Eur Urol. 2011;60(2):350–7. [DOI] [PubMed] [Google Scholar]
- 15.Gårdmark T, Wester K, De La Torre M, Carlsson J, Malmström P-U. Analysis of HER2 expression in primary urinary bladder carcinoma and corresponding metastases. BJU Int. 2005;95(7):982–6. [DOI] [PubMed] [Google Scholar]
- 16.Moktefi A, Pouessel D, Liu J, et al. Reappraisal of HER2 status in the spectrum of advanced urothelial carcinoma: a need of guidelines for treatment eligibility. Mod Pathol. 2018;31(8):1270–81. [DOI] [PubMed] [Google Scholar]
- 17.Shi H, Shao Y, Lu W, Lu B. An analysis of HER2 amplification in cervical adenocarcinoma: correlation with clinical outcomes and the International Endocervical Adenocarcinoma Criteria and Classification. J Pathol Clin Res. 2021;7(1):86–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Halle MK, Tangen IL, Berg HF, et al. HER2 expression patterns in paired primary and metastatic endometrial cancer lesions. Br J Cancer. 2018;118(3):378–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tuefferd M, Couturier J, Penault-Llorca F, et al. HER2 status in ovarian carcinomas: a multicenter GINECO study of 320 patients. PLoS ONE. 2007;2(11):e1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chou A, Waddell N, Cowley MJ, et al. Clinical and molecular characterization of HER2 amplified-pancreatic cancer. Genome Med. 2013;5(8):78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Han S-H, Ryu KH, Kwon A-Y. The prognostic impact of HER2 genetic and protein expression in pancreatic carcinoma-HER2 protein and gene in pancreatic cancer. Diagnostics (Basel). 2021;11(4):653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Semiz H, Pala E, Can B, Atag E, Gungor H, Sanci M. cERBB-2/Her-2 Neu overexpression and prognostic significance in uterine carcinosarcoma. Turk Patoloji Derg. 2023;39(1):55–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vermij L, Singh N, Leon-Castillo A, et al. Performance of a HER2 testing algorithm specific for p53-abnormal endometrial cancer. Histopathology. 2021;79(4):533–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Buza N, English DP, Santin AD, Hui P. Toward standard HER2 testing of endometrial serous carcinoma: 4-year experience at a large academic center and recommendations for clinical practice. Mod Pathol. 2013;26(12):1605–12. [DOI] [PubMed] [Google Scholar]
- 25.Chung YW, Kim S, Hong JH, et al. Overexpression of HER2/HER3 and clinical feature of ovarian cancer. J Gynecol Oncol. 2019;30(5):e75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ersoy E, Cao QJ, Otis CN. HER2 protein overexpression and gene amplification in tubo-ovarian high-grade serous carcinomas. Int J Gynecol Pathol. 2022;41(4):313–9. [DOI] [PubMed] [Google Scholar]
- 27.Pils D, Pinter A, Reibenwein J, et al. In ovarian cancer the prognostic influence of HER2/neu is not dependent on the CXCR4/SDF-1 signalling pathway. Br J Cancer. 2007;96(3):485–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Panek G, Ligaj M. Prognostic significance of HER-2/neu expression in patients at early clinical stages of invasive cervical cancer. Gin Onkol. 2007;5(4):218–35. [Google Scholar]
- 29.Iqbal N, Iqbal N. Human epidermal growth factor receptor 2 (HER2) in cancers: overexpression and therapeutic implications. Mol Biol Int. 2014;2014: 852748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pujade-Lauraine E, Hilpert F, Weber B, et al. Bevacizumab combined with chemotherapy for platinum-resistant recurrent ovarian cancer: the AURELIA open-label randomized phase III trial. J Clin Oncol. 2014;32(13):1302–8. [DOI] [PubMed] [Google Scholar]
- 31.Lamarca A, Palmer DH, Wasan HS, et al. Second-line FOLFOX chemotherapy versus active symptom control for advanced biliary tract cancer (ABC-06): a phase 3, open-label, randomised, controlled trial. Lancet Oncol. 2021;22(5):690–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Makker V, Colombo N, Casado Herráez A, et al. Lenvatinib plus pembrolizumab for advanced endometrial cancer. N Engl J Med. 2022;386(5):437–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oaknin A, Moore KN, Meyer T, et al. Safety and efficacy of nivolumab (NIVO) ± ipilimumab (IPI) in patients (pts) with recurrent/metastatic cervical cancer (R/M Cx Ca) in CheckMate 358. Ann Oncol. 2022;33(Suppl):S782 (Abstract 520MO). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data underlying the findings described in this manuscript may be obtained in accordance with AstraZeneca’s data sharing policy, described at https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure. Data for studies directly listed on Vivli can be requested through Vivli at www.vivli.org. Data for studies not listed on Vivli can be requested through Vivli at https://vivli.org/members/enquiries-about-studies-not-listed-on-the-vivli-platform/. The AstraZeneca Vivli member page is also available, outlining further details: https://vivli.org/ourmember/astrazeneca/.