ABSTRACT
The unique cell biology presented by members of the phylum Planctomycetota has puzzled researchers ever since their discovery. Initially thought to have eukaryotic-like features, their traits are now recognized as exceptional but distinctly bacterial. However, recently discovered strains again added novel and stunning aspects to the planctomycetal cell biology—shapeshifting by members of the “Saltatorellus” clade to an extent that is unprecedented in any other bacterial phylum, and phagocytosis-like cell engulfment in the bacterium “Candidatus Uabimicrobium amorphum.” These recent additions to the phylum Planctomycetota indicate hitherto unexplored members with unique cell biology, which we aimed to make accessible for further investigations. Targeting bacteria with features like “Ca. U. amorphum”, we first studied both the morphology and behavior of this microorganism in more detail. While similar to eukaryotic amoeboid organisms at first sight, we found “Ca. U. amorphum” to be rather distinct in many regards. Presenting a detailed description of “Ca. U. amorphum,” we furthermore found this organism to divide in a fashion that has never been described in any other organism. Employing the obtained knowledge, we isolated a second “bacterium of prey” from the harbor of Heligoland Island (North Sea, Germany). Our isolate shares key features with “Ca. U. amorphum”: phagocytosis-like cell engulfment, surface-dependent motility, and the same novel mode of cell division. Being related to “Ca. U. amorphum” within genus thresholds, we propose the name “Ca. Uabimicrobium helgolandensis” for this strain.
IMPORTANCE
“Candidatus Uabimicrobium helgolandensis” HlEnr_7 adds to the explored bacterial biodiversity with its phagocytosis-like uptake of prey bacteria. Enrichment of this strain indicates that there might be “impossible” microbes out there, missed by metagenomic analyses. Such organisms have the potential to challenge our understanding of nature. For example, the origin of eukaryotes remains enigmatic, with a contentious debate surrounding both the mitochondrial host entity and the moment of uptake. Currently, favored models involve a proteobacterium as the mitochondrial progenitor and an Asgard archaeon as the fusion partner. Models in which a eukaryotic ancestor engulfed the mitochondrial ancestor via phagocytosis had been largely rejected due to bioenergetic constraints. Thus, the phagocytosis-like abilities of planctomycetal bacteria might influence the debate, demonstrating that prey engulfment is possible in a prokaryotic cellular framework.
KEYWORDS: evolutionary biology, endocytosis, planctomycetes
OBSERVATION
The bacterial phylum Planctomycetota has intrigued microbiologists for years, with some suggesting that these bacteria represent a “missing link” between prokaryotic and eukaryotic cells (1–3). Initially, this theory was supported for example by a protein uptake mechanism in Gemmata obscuriglobus, resembling to some extent eukaryotic endocytosis (4). However, extensive research over the past decade has largely doubted these hypotheses (5–8). Even though planctomycetes still present an unconventional cell biology, they seemed to generally align with a Gram-negative cell plan (9). However, in 2019, we first described the phylogenetically distinct “Saltatorellus” clade (10, 11). A few months later, Shiratori et al., published “Candidatus Uab amorphum” (12) later renamed to “Ca. Uabimicrobium amorphum” (UA) acknowledging taxonomic rules (13). Showing unprecedented cell biological features, these new strains seem to challenge not only aspects of the planctomycetotal cell plan but also that of diderm bacteria in general. Especially UA’s unique capability of feeding on other bacteria via phagocytosis-like prey uptake contradicts previous predictions based on theoretical calculations as well as features of the Gram-negative cell plan (14, 15). Although other studies have suggested that the proposed energetic barrier preventing bacteria from developing complex traits such as phagocytosis does not exist, living evidence has not been found until now (16, 17). Thus, the discovery of organisms such as UA and the “Saltatorellus” clade is of major importance for several fields from biodiversity to evolution.
Aiming to isolate bacteria possessing similar phagocytosis-like capabilities as “Ca. U. amorphum” from the environment, we first needed to assess possibilities for their identification from both morphological and genomic perspectives. Therefore, we obtained a UA culture from the Japan Collection of Microorganisms (JCM 39082). Observing UA under the microscope, its striking resemblance to eukaryotic amoeboid organisms became evident (Fig. 1; Movie S1 and S2). Since this posed a high danger of mistaking amoebae for such bacteria, we screened literature and culture collections for size-wise comparable eukaryotic amoeboid organisms, to study similarities and differences. Based on such morphological criteria, we found Hartmannella sp. CCAP 1534/15 and Squamamoeba japonica CCAP 1493/1 to be suitable for the comparison.
Fig 1.
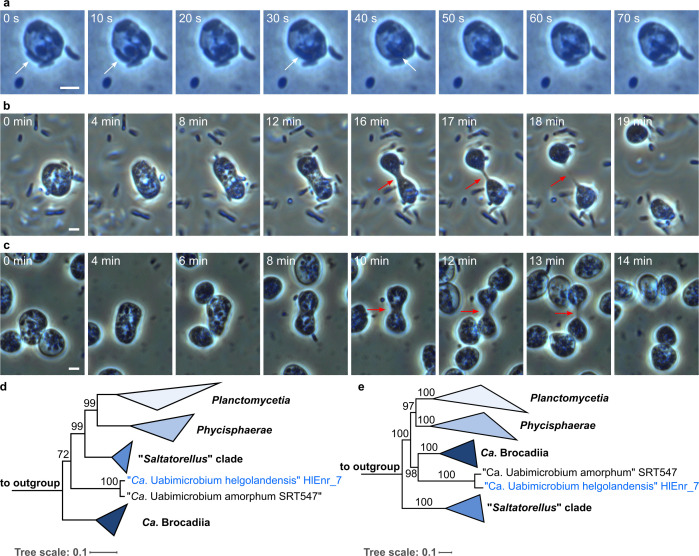
Overview on the cell biology and phylogeny of “Ca. U. amorphum” SRT547 as well as the novel isolate “Ca. U. helgolandensis” HIEnr_7. Phagocytosis-like uptake of surrounding prey bacteria by “Ca. U. amorphum” (large cell) (a); white arrows indicate the prey bacterium being internalized. Cell division of “Ca. U. amorphum” (b), and “Ca. U. helgolandensis” HlEnr_7 (c). Two opposite cell poles move apart until only a thin, thread-like connection remains (red arrows) that finally disrupts. 16S rRNA gene sequence- (d) and multi-locus sequence analysis (MLSA)- (e) based phylogenies showing the deep branching of the “Ca. Uabimicrobium” clade within the phylum Planctomycetota.
Despite certain similarities, we noticed clear differences: both, Hartmannella sp. and S. japonica have a rather uniform cell size (about 20 µm and 6 µm, respectively) and intracellular granulation. In contrast, UA cells differ significantly in size (4–20 µm) and granularity. Furthermore, the locomotion of UA contrasts that of the tested amoebae: while amoebae form pseudopodia to crawl along surfaces, UA cells show no such arm-like protrusions and maintain a mostly round cell shape during crawling. Additional distinctions can be found in their cellular division process: UA cells require a surface to generate the force needed for dividing into two daughter cells (Fig. 1b; Fig. S1; Movie S2). Opposite cell poles move apart until they are only connected by a thin, thread-like structure. Its length is up to three times the cell diameter prior to division initiation and both cell poles continue crawling apart until the structure disrupts. In comparison, division of the two amoebae seemed not to require pulling by the emerging daughter cells, and neither of the tested amoebae formed such long tubules (Fig. S1; Movie S3). Despite the process of prey engulfment itself appearing quite similar (Fig. S2; Movie S1 and S4), further differences can be found in the organisms’ feeding behavior: while UA seemed to engulf every bacterium encountered on the surface, both amoebae frequently let some bacteria escape. Additionally, the amoebae tend to move around a lot, frequently crossing areas where they already fed on bacteria before. In contrast, UA cells move less and instead internalize every bacterium in their proximity. This leads to the formation of “feeding circles”—accumulations of UA cells grazing in a circular line around areas with no prey bacteria left (Fig. S3). While both amoebae seem to sense accumulations of prey and move toward them over long distances, UA cells just feed in their proximity and ignore more distant prey accumulations.
Although morphological distinctions seemed to suffice for identifying further bacteria of prey, we wondered, whether an amoeba contamination could unquestionably be excluded by our sequencing approaches. Therefore, we sequenced the UA cell culture including Alteromonas macleodii prey bacteria, a UA DNA sample (JCM), and the two amoebae cultures. For UA samples, only the two expected bacterial genomes were found [NCBI accession number JAZFBE000000000 (A. macleodii, prey bacterium) and JAZFBD000000000 (UA)]. The amoebae cultures yielded genome fragments of the two amoebae, multiple prey bacteria, and mitochondria. Thus, our sequencing method is suitable to distinguish amoeba and bacteria and we can confirm that Shiratori et al.’s UA culture is that of a planctomycetotal bacterium.
Using the information obtained from studying UA, we repeated the original enrichment strategy and obtained a close relative from water sampled in Heligoland (North Sea, Germany) (18). Both morphology and behavior of the obtained isolate appear very similar to UA (12): the majority of its cells are 4–6 µm in size, it obligately feeds on other bacteria (Supplementary results), and cells divide like UA (Fig. 1c). Sequencing an enrichment culture, we obtained three bacterial bins, among them the genome of A. macleodii (added prey bacterium). A second, 9.3 Mb bacterial genome (CP165719) relates to UA within genus thresholds (Table S1 and S2), for which we propose the name “Candidatus Uabimicrobium helgolandensis” strain HlEnr_7 (UH). Pangenome analysis revealed 4,319 shared genes while 2,442 and 2,398 genes were unique for UH and UA, respectively (Fig. S4). Both multi-locus sequence analysis- (MLSA) and 16S rRNA gene-based tree reconstructions demonstrate deep phylogenetic branching of both “Ca. Uabimicrobium spp.” within the phylum Planctomycetota (Fig. 1). However, this branching pattern might be compromised by DNA G+C content differences between 70% (“Saltatorellus” clade), 43% (Ca. Brocadiales), and 39% (UA) (19). Such differences affect sequence similarity and thus alignment accuracy, as sequences with similar DNA G+C content have fewer mismatches. This can influence phylogenetic tree construction and gene prediction: a KEGG KOfam analysis of cell division and peptidoglycan synthesis genes revealed the lack of most such genes in UA and UH, indicating that “Ca. Uabimicrobium spp.” do not employ a canonical bacterial cell division mechanism (Fig. S5).
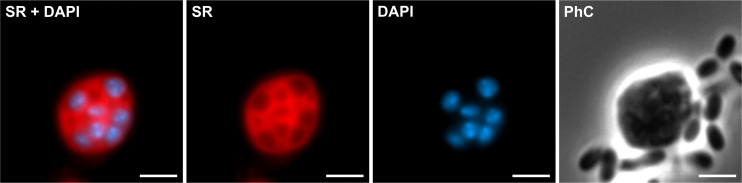
While confirming the findings of Shiratori et al., UH adds further evidence for undiscovered planctomycetotal biodiversity. Besides phagocytosis-like cell engulfment as their hallmark trait, both strains present a novel mode of cell division as well as other unique features that require further investigation. Especially their cell envelope and the process of prey engulfment need careful examination considering the previous critique regarding “true endocytotic invaginations” in Gram-negative bacteria (14). However, membrane coat-like proteins might play a role, as such a protein was recently identified in UA (20). Furthermore, bioenergetics in these cells need to be addressed, since controversial opinions exist on the complexity achievable by prokaryotic cells (15–17). Although further analyses are required, a first stain of membranes and DNA revealed extensive membrane signals inside the cell (Fig. 2), which might serve a similar purpose as mitochondrial cristae increasing the metabolically active surface (15).
Fig 2.
Fluorescent staining of “Ca. U. amorphum” with SynaptoRed C2 (SR, membranes) and DAPI (DNA). Prey bacteria do not show fluorescence signals in this figure, since they are in a slightly different focal plane and have much lower fluorescence intensity making them disappear upon brightness and contrast adjustment. Scale bars are 2 µm.
Taken together, the study of “Ca. Uabimicrobium spp.” indicates the conservation of a phagocytosis-like bacterial uptake mechanism, a predatory lifestyle, and unconventional cell biology. Such traits point toward yet unexplored evolutionary complexities and bioenergetic principles of Planctomycetota bacteria.
ACKNOWLEDGMENTS
We thank Muriel C. F. van Teeseling for fruitful discussions. We further thank the Biological Institute Helgoland, Alfred Wegener Institute, and Helmholtz Centre for Polar and Marine Research for providing a seawater sample. The sample was sent to us as part of the material dispatch service (Grant number: AWI_BAH_o4).
The study was funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) under Germany’s Excellence Strategy—EXC 2051—Project-ID 390713860. This study is part of the Collaborative Research Centre AquaDiva of the Friedrich Schiller University Jena, funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation)—SFB 1076—Project Number 218627073.
We acknowledge support by the German Research Foundation Projekt-Nr. 512648189 and the Open Access Publication Fund of the Thueringer Universitaets- und Landesbibliothek Jena. N.K. was funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) under Project ID 239748522—CRC 1127 ChemBioSys (project A07). C.E.W. is funded by the Studienstiftung des deutschen Volkes. J.H. is funded by the Carl Zeiss Stiftung. T.H. is funded by the Landesgraduiertenstipendium of the Free State of Thuringia awarded by the Friedrich Schiller University Jena. J.H. and T.H. are supported by the Jena School for Microbial Communications.
C.E.W. performed cultivation, microscopy, and whole genome amplification for all organisms as well as sampling (Baltic Sea), enrichment, and isolation of the novel strains. J.H. performed Oxford Nanopore sequencing as well as genome assembly and analyses with the DNA amplified from the enrichment. T.H. performed microscopy and cultivation. S.W. performed initial computational analyses. N.K. performed the phylogenetic analyses on the novel isolate and helped supervise the study. C.J. supervised the study, arranged sampling (Heligoland), performed microscopy, and wrote the manuscript together with C.E.W. and N.K. All authors read and approved the final version of the manuscript.
Contributor Information
Christian Jogler, Email: Christian.jogler@uni-jena.de.
Nicole Dubilier, Max Planck Institute for Marine Microbiology, Bremen, Germany.
ETHICS APPROVAL
This article does not contain any studies with animals performed by any of the authors.
DATA AVAILABILITY
The re-sequenced genome of “Ca. U. amorphum” SRT547 is available from NCBI under accession number JAZFBD000000000. The genome of “Ca. U. helgolandensis” HlEnr_7 was deposited under accession number CP165719. The 16S rRNA gene sequences were deposited in GenBank under the accession numbers OR886618 (“Ca. U. amorphum” SRT547) and OR832863 (“Ca. U. helgolandensis” HlEnr_7).
SUPPLEMENTAL MATERIAL
The following material is available online at https://doi.org/10.1128/mbio.02044-24.
Supplemental text, tables, figures, and movie legends.
Endocytosis-like prey uptake of “Ca. U. amorphum” (large cells).
Cell division of “Ca. U. amorphum.”
Cell division of Hartmannella sp. CCAP 1534/15.
Phagocytosis of Squamamoeba japonica CCAP 1493/1.
ASM does not own the copyrights to Supplemental Material that may be linked to, or accessed through, an article. The authors have granted ASM a non-exclusive, world-wide license to publish the Supplemental Material files. Please contact the corresponding author directly for reuse.
REFERENCES
- 1. Devos DP. 2021. Reconciling asgardarchaeota phylogenetic proximity to eukaryotes and planctomycetes cellular features in the evolution of life. Mol Biol Evol 38:3531–3542. doi: 10.1093/molbev/msab186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Devos DP, Reynaud EG. 2010. Evolution. Intermediate steps. Science 330:1187–1188. doi: 10.1126/science.1196720 [DOI] [PubMed] [Google Scholar]
- 3. Forterre P. 2011. A new fusion hypothesis for the origin of Eukarya: better than previous ones, but probably also wrong. Res Microbiol 162:77–91. doi: 10.1016/j.resmic.2010.10.005 [DOI] [PubMed] [Google Scholar]
- 4. Lonhienne TGA, Sagulenko E, Webb RI, Lee K-C, Franke J, Devos DP, Nouwens A, Carroll BJ, Fuerst JA. 2010. Endocytosis-like protein uptake in the bacterium Gemmata obscuriglobus. Proc Natl Acad Sci U S A 107:12883–12888. doi: 10.1073/pnas.1001085107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Boedeker C, Schüler M, Reintjes G, Jeske O, van Teeseling MCF, Jogler M, Rast P, Borchert D, Devos DP, Kucklick M, Schaffer M, Kolter R, van Niftrik L, Engelmann S, Amann R, Rohde M, Engelhardt H, Jogler C. 2017. Determining the bacterial cell biology of planctomycetes. Nat Commun 8:14853. doi: 10.1038/ncomms14853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Santarella-Mellwig R, Pruggnaller S, Roos N, Mattaj IW, Devos DP. 2013. Three-dimensional reconstruction of bacteria with a complex endomembrane system. PLoS Biol 11:e1001565. doi: 10.1371/journal.pbio.1001565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Devos DP. 2014. PVC bacteria: variation of, but not exception to, the Gram-negative cell plan. Trends Microbiol 22:14–20. doi: 10.1016/j.tim.2013.10.008 [DOI] [PubMed] [Google Scholar]
- 8. Devos DP. 2014. Re-interpretation of the evidence for the PVC cell plan supports a Gram-negative origin. Antonie van Leeuwenhoek 105:271–274. doi: 10.1007/s10482-013-0087-y [DOI] [PubMed] [Google Scholar]
- 9. Wiegand S, Jogler M, Jogler C. 2018. On the maverick planctomycetes. FEMS Microbiol Rev 42:739–760. doi: 10.1093/femsre/fuy029 [DOI] [PubMed] [Google Scholar]
- 10. Wiegand S, Jogler M, Kohn T, Awal RP, Oberbeckmann S, Kesy K, Jeske O, Schumann P, Peeters SH, Kallscheuer N, Strauss M, Heuer A, Jetten MSM, Labrenz M, Rohde M, Boedeker C, Engelhardt H, Schüler D, Jogler C. 2019. The novel shapeshifting bacterial phylum Saltatorellota. bioRxiv. doi: 10.1101/817700:817700 [DOI] [Google Scholar]
- 11. Wiegand S, Jogler M, Boedeker C, Pinto D, Vollmers J, Rivas-Marín E, Kohn T, Peeters SH, Heuer A, Rast P, et al. 2020. Cultivation and functional characterization of 79 planctomycetes uncovers their unique biology. Nat Microbiol 5:126–140. doi: 10.1038/s41564-019-0588-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shiratori T, Suzuki S, Kakizawa Y, Ishida KI. 2019. Phagocytosis-like cell engulfment by a planctomycete bacterium. Nat Commun 10:5529. doi: 10.1038/s41467-019-13499-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lodha T, Narvekar S, Karodi P. 2021. Classification of uncultivated anammox bacteria and Candidatus Uabimicrobium into new classes and provisional nomenclature as Candidatus Brocadiia classis nov. and Candidatus Uabimicrobiia classis nov. of the phylum Planctomycetes and novel family Candidatus Scalinduaceae fam. nov to accommodate the genus Candidatus Scalindua. Syst Appl Microbiol 44:126272. doi: 10.1016/j.syapm.2021.126272 [DOI] [PubMed] [Google Scholar]
- 14. Mills DB. 2020. The origin of phagocytosis in Earth history. Interface Focus 10:20200019. doi: 10.1098/rsfs.2020.0019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lane N, Martin W. 2010. The energetics of genome complexity. Nature 467:929–934. doi: 10.1038/nature09486 [DOI] [PubMed] [Google Scholar]
- 16. Lynch M, Marinov GK. 2015. The bioenergetic costs of a gene. Proc Natl Acad Sci U S A 112:15690–15695. doi: 10.1073/pnas.1514974112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chiyomaru K, Takemoto K. 2020. Revisiting the hypothesis of an energetic barrier to genome complexity between eukaryotes and prokaryotes. R Soc Open Sci 7:191859. doi: 10.1098/rsos.191859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dummermuth A, Wiltshire KH, Kirstein I, Brodte E-M, Wichels A, Shama L, Bergmann A, Hofmann C, Fischer P, Mölter K, Strasser M. 2023. Marine stations helgoland and sylt operated by the Alfred Wegener Institute Helmholtz Centre for Polar and Marine research. JLSRF 8:A184. doi: 10.17815/jlsrf-8-184 [DOI] [Google Scholar]
- 19. Odelgard A, Hägglund E, Guy L, Andersson SGE. 2024. Phylogeny and expansion of serine/threonine kinases in phagocytotic bacteria in the phylum Planctomycetota. Genome Biol Evol 16:evae068. doi: 10.1093/gbe/evae068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ferrelli ML, Pidre ML, García-Domínguez R, Alberca LN, Del Saz-Navarro D, Santana-Molina C, Devos DP. 2023. Prokaryotic membrane coat - like proteins: an update. J Struct Biol 215:107987. doi: 10.1016/j.jsb.2023.107987 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental text, tables, figures, and movie legends.
Endocytosis-like prey uptake of “Ca. U. amorphum” (large cells).
Cell division of “Ca. U. amorphum.”
Cell division of Hartmannella sp. CCAP 1534/15.
Phagocytosis of Squamamoeba japonica CCAP 1493/1.
Data Availability Statement
The re-sequenced genome of “Ca. U. amorphum” SRT547 is available from NCBI under accession number JAZFBD000000000. The genome of “Ca. U. helgolandensis” HlEnr_7 was deposited under accession number CP165719. The 16S rRNA gene sequences were deposited in GenBank under the accession numbers OR886618 (“Ca. U. amorphum” SRT547) and OR832863 (“Ca. U. helgolandensis” HlEnr_7).