Abstract
The pyruvate: ferredoxin oxidoreductase from the anaerobic protozoon Trichomonas vaginalis is an extrinsic protein bound to the hydrogenosomal membrane. It has been solubilized and purified to homogeneity, principally by salting-out chromatography on Sepharose 4B. Low recoveries of active enzyme were caused by inactivation by O2 and the irreversible loss of thiamin pyrophosphate. It is a dimeric enzyme of overall Mr 240,000 and subunit Mr 120,000. The enzyme contains, per mol of dimer, 7.3 +/- 0.3 mol of iron and 5.9 +/- 0.9 mol of acid-labile sulphur, suggesting the presence of two [4Fe-4S] centres, and 0.47 mol of thiamin pyrophosphate. The absorption spectrum of the enzyme is characteristic of a non-haem iron protein. The pyruvate: ferredoxin oxidoreductase from T. vaginalis is therefore broadly similar to the 2-oxo acid: ferredoxin (flavodoxin) oxidoreductases purified from bacterial sources, except that it is membrane-bound.
Full text
PDF



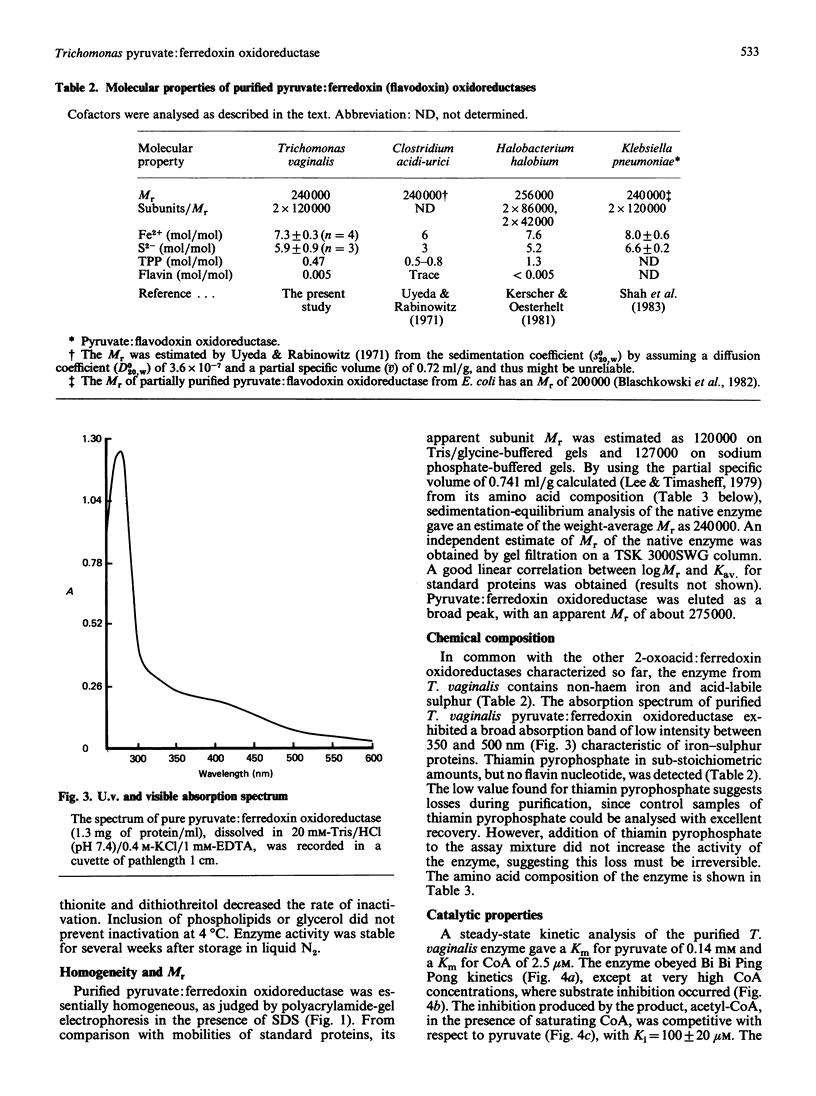



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson C. W., Baum P. R., Gesteland R. F. Processing of adenovirus 2-induced proteins. J Virol. 1973 Aug;12(2):241–252. doi: 10.1128/jvi.12.2.241-252.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arp D. J., Burris R. H. Purification and properties of the particulate hydrogenase from the bacteroids of soybean root nodules. Biochim Biophys Acta. 1979 Oct 11;570(2):221–230. doi: 10.1016/0005-2744(79)90142-6. [DOI] [PubMed] [Google Scholar]
- Blaschkowski H. P., Neuer G., Ludwig-Festl M., Knappe J. Routes of flavodoxin and ferredoxin reduction in Escherichia coli. CoA-acylating pyruvate: flavodoxin and NADPH: flavodoxin oxidoreductases participating in the activation of pyruvate formate-lyase. Eur J Biochem. 1982 Apr;123(3):563–569. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Carlson C. A., Ferguson L. P., Ingraham J. L. Properties of dissimilatory nitrate reductase purified from the denitrifier Pseudomonas aeruginosa. J Bacteriol. 1982 Jul;151(1):162–171. doi: 10.1128/jb.151.1.162-171.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coombs G. H., North M. J. An analysis of the proteinases of Trichomonas vaginalis by polyacrylamide gel electrophoresis. Parasitology. 1983 Feb;86(Pt 1):1–6. doi: 10.1017/s0031182000057103. [DOI] [PubMed] [Google Scholar]
- Edelhoch H. Spectroscopic determination of tryptophan and tyrosine in proteins. Biochemistry. 1967 Jul;6(7):1948–1954. doi: 10.1021/bi00859a010. [DOI] [PubMed] [Google Scholar]
- Gehring U., Arnon D. I. Purification and properties of -ketoglutarate synthase from a photosynthetic bacterium. J Biol Chem. 1972 Nov 10;247(21):6963–6969. [PubMed] [Google Scholar]
- Gorrell T. E. Effect of culture medium iron content on the biochemical composition and metabolism of Trichomonas vaginalis. J Bacteriol. 1985 Mar;161(3):1228–1230. doi: 10.1128/jb.161.3.1228-1230.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerscher L., Oesterhelt D. Purification and properties of two 2-oxoacid:ferredoxin oxidoreductases from Halobacterium halobium. Eur J Biochem. 1981 Jun 1;116(3):587–594. doi: 10.1111/j.1432-1033.1981.tb05376.x. [DOI] [PubMed] [Google Scholar]
- Kerscher L., Oesterhelt D. The catalytic mechanism of 2-oxoacid:ferredoxin oxidoreductases from Halobacterium halobium. One-electron transfer at two distinct steps of the catalytic cycle. Eur J Biochem. 1981 Jun 1;116(3):595–600. doi: 10.1111/j.1432-1033.1981.tb05377.x. [DOI] [PubMed] [Google Scholar]
- LOVENBERG W., BUCHANAN B. B., RABINOWITZ J. C. STUDIES ON THE CHEMICAL NATURE OF CLOSTRIDIAL FERREDOXIN. J Biol Chem. 1963 Dec;238:3899–3913. [PubMed] [Google Scholar]
- Lee J. C., Timasheff S. N. The calculation of partial specific volumes of proteins in 6 M guanidine hydrochloride. Methods Enzymol. 1979;61:49–57. doi: 10.1016/0076-6879(79)61006-6. [DOI] [PubMed] [Google Scholar]
- Lindmark D. G. Energy metabolism of the anaerobic protozoon Giardia lamblia. Mol Biochem Parasitol. 1980 Mar;1(1):1–12. doi: 10.1016/0166-6851(80)90037-7. [DOI] [PubMed] [Google Scholar]
- Lindmark D. G., Müller M. Hydrogenosome, a cytoplasmic organelle of the anaerobic flagellate Tritrichomonas foetus, and its role in pyruvate metabolism. J Biol Chem. 1973 Nov 25;248(22):7724–7728. [PubMed] [Google Scholar]
- Linstead D. New defined and semi-defined media for cultivation of the flagellate Trichomonas vaginalis. Parasitology. 1981 Aug;83(Pt 1):125–137. doi: 10.1017/s0031182000050101. [DOI] [PubMed] [Google Scholar]
- Lowe P. N., Rowe A. F. Aspartate: 2-oxoglutarate aminotransferase from trichomonas vaginalis. Identity of aspartate aminotransferase and aromatic amino acid aminotransferase. Biochem J. 1985 Dec 15;232(3):689–695. doi: 10.1042/bj2320689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mevarech M., Leicht W., Werber M. M. Hydrophobic chromatography and fractionation of enzymes from extremely halophilic bacteria using decreasing concentration gradients of ammonium sulfate. Biochemistry. 1976 Jun 1;15(11):2383–2387. doi: 10.1021/bi00656a021. [DOI] [PubMed] [Google Scholar]
- Müller M. Mode of action of metronidazole on anaerobic bacteria and protozoa. Surgery. 1983 Jan;93(1 Pt 2):165–171. [PubMed] [Google Scholar]
- Neuer G., Bothe H. The pyruvate: ferredoxin oxidoreductase in heterocysts of the cyanobacterium Anabaena cylindrica. Biochim Biophys Acta. 1982 Jun 16;716(3):358–365. doi: 10.1016/0304-4165(82)90028-9. [DOI] [PubMed] [Google Scholar]
- Nureddin A., Johnson P. Ultracentrifugal studies of human luteinizing hormone and its subunits: dependence on protein concentration and ionic strength. Biochemistry. 1977 Apr 19;16(8):1730–1737. doi: 10.1021/bi00627a033. [DOI] [PubMed] [Google Scholar]
- Penttinen H. K. Fluorometric determination of thiamine and its mono-, di-, and triphosphate esters. Methods Enzymol. 1979;62:58–59. doi: 10.1016/0076-6879(79)62190-0. [DOI] [PubMed] [Google Scholar]
- Rabinowitz J. C. Analysis of acid-labile sulfide and sulfhydryl groups. Methods Enzymol. 1978;53:275–277. doi: 10.1016/s0076-6879(78)53033-4. [DOI] [PubMed] [Google Scholar]
- Reeves R. E., Warren L. G., Susskind B., Lo H. S. An energy-conserving pyruvate-to-acetate pathway in Entamoeba histolytica. Pyruvate synthase and a new acetate thiokinase. J Biol Chem. 1977 Jan 25;252(2):726–731. [PubMed] [Google Scholar]
- Shah V. K., Stacey G., Brill W. J. Electron transport to nitrogenase. Purification and characterization of pyruvate:flavodoxin oxidoreductase. The nifJ gene product. J Biol Chem. 1983 Oct 10;258(19):12064–12068. [PubMed] [Google Scholar]
- Sim E., Vignais P. M. Hydrogenase activity in Paracoccus denitrificans. Partial purification and interaction with the electron transport chain. Biochimie. 1978;60(3):307–314. doi: 10.1016/s0300-9084(78)80827-x. [DOI] [PubMed] [Google Scholar]
- Steinbüchel A., Müller M. Anaerobic pyruvate metabolism of Tritrichomonas foetus and Trichomonas vaginalis hydrogenosomes. Mol Biochem Parasitol. 1986 Jul;20(1):57–65. doi: 10.1016/0166-6851(86)90142-8. [DOI] [PubMed] [Google Scholar]
- Thorneley R. N. A convenient electrochemical preparation of reduced methyl viologen and a kinetic study of the reaction with oxygen using an anaerobic stopped-flow apparatus. Biochim Biophys Acta. 1974 Mar 26;333(3):487–496. doi: 10.1016/0005-2728(74)90133-9. [DOI] [PubMed] [Google Scholar]
- Uyeda K., Rabinowitz J. C. Pyruvate-ferredoxin oxidoreductase. 3. Purification and properties of the enzyme. J Biol Chem. 1971 May 25;246(10):3111–3119. [PubMed] [Google Scholar]
- Wang Z. C., Burns A., Watt G. D. Complex formation and O2 sensitivity of Azotobacter vinelandii nitrogenase and its component proteins. Biochemistry. 1985 Jan 1;24(1):214–221. doi: 10.1021/bi00322a031. [DOI] [PubMed] [Google Scholar]
- Weinbach E. C., Claggett C. E., Keister D. B., Diamond L. S., Kon H. Respiratory metabolism of Giardia lamblia. J Parasitol. 1980 Apr;66(2):347–350. [PubMed] [Google Scholar]
- Yarlett N., Gorrell T. E., Marczak R., Müller M. Reduction of nitroimidazole derivatives by hydrogenosomal extracts of Trichomonas vaginalis. Mol Biochem Parasitol. 1985 Jan;14(1):29–40. doi: 10.1016/0166-6851(85)90103-3. [DOI] [PubMed] [Google Scholar]
- Yarlett N., Hann A. C., Lloyd D., Williams A. G. Hydrogenosomes in a mixed isolate of Isotricha prostoma and Isotricha intestinalis from ovine rumen contents. Comp Biochem Physiol B. 1983;74(2):357–364. doi: 10.1016/0305-0491(83)90025-1. [DOI] [PubMed] [Google Scholar]
- Yarlett N., Hann A. C., Lloyd D., Williams A. Hydrogenosomes in the rumen protozoon Dasytricha ruminantium Schuberg. Biochem J. 1981 Nov 15;200(2):365–372. doi: 10.1042/bj2000365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarlett N., Orpin C. G., Munn E. A., Yarlett N. C., Greenwood C. A. Hydrogenosomes in the rumen fungus Neocallimastix patriciarum. Biochem J. 1986 Jun 15;236(3):729–739. doi: 10.1042/bj2360729. [DOI] [PMC free article] [PubMed] [Google Scholar]




