Abstract
Over the past several years, there has been a surge in blood biomarker studies examining the value of plasma or serum neurofilament light (NfL) as a biomarker of neurodegeneration for Alzheimer's disease. However, there have been limited efforts to combine existing findings to assess the utility of blood NfL as a biomarker of neurodegeneration for Alzheimer's disease. In addition, we still need better insight into the specific aspects of neurodegeneration that are reflected by the elevated plasma or serum concentration of NfL.
In this review, we survey the literature on the cross-sectional and longitudinal relationships between blood-based NfL levels and other, neuroimaging-based, indices of neurodegeneration in individuals on the Alzheimer's continuum. Then, based on the biomarker classification established by the FDA-NIH Biomarker Working group, we determine the utility of blood-based NfL as a marker for monitoring the disease status (i.e. monitoring biomarker) and predicting the severity of neurodegeneration in older adults with and without cognitive decline (i.e. a prognostic or a risk/susceptibility biomarker). The current findings suggest that blood NfL exhibits great promise as a monitoring biomarker because an increased NfL level in plasma or serum appears to reflect the current severity of atrophy, hypometabolism and the decline of white matter integrity, particularly in the brain regions typically affected by Alzheimer's disease. Longitudinal evidence indicates that blood NfL can be useful not only as a prognostic biomarker for predicting the progression of neurodegeneration in patients with Alzheimer's disease but also as a susceptibility/risk biomarker predicting the likelihood of abnormal alterations in brain structure and function in cognitively unimpaired individuals with a higher risk of developing Alzheimer's disease (e.g. those with a higher amyloid-β).
There are still limitations to current research, as discussed in this review. Nevertheless, the extant literature strongly suggests that blood NfL can serve as a valuable prognostic and susceptibility biomarker for Alzheimer's disease-related neurodegeneration in clinical settings, as well as in research settings.
Keywords: dementia, atrophy, glucose metabolism, white matter microstructure
Based on a review of the literature, Jung and Damoiseaux conclude that blood levels of neurofilament light hold great promise for predicting progression of neurodegeneration in individuals with cognitive decline, and subsequent rate of neurodegeneration in cognitively unimpaired individuals at risk of Alzheimer’s disease.
Introduction
In Alzheimer's disease (AD) research, the development of techniques allowing a reliable measurement of blood-based neurofilament light chain (NfL) proteins has inspired many researchers to investigate the potential of blood NfL as a marker of neurodegeneration for AD. However, relatively little effort has been made to synthesize the accumulated findings to evaluate the utility of blood NfL as a neurodegenerative marker for AD. In addition, in the extant literature, little attention is paid to what aspects of neurodegeneration the observed increase of blood NfL is indicative of. In this paper, we will review existing knowledge on the relationship between blood NfL and other indices of neurodegeneration in AD research to determine the utility of NfL as a neurodegenerative marker for AD. Moreover, we will discuss the aspects of AD-related neurodegeneration and pathology likely reflected by the change in the blood level of NfL and suggest possible (clinical) applications of blood NfL. This review consists of four sections. In the first section, we will provide some background on NfL as a general marker of neuroaxonal damage and a neurodegenerative marker for AD. In the second section, we will summarize existing findings on the relationship between blood NfL and other measures of neurodegeneration derived from MRI and PET imaging, which have been the most commonly used methods for in vivo assessment of neurodegeneration in the human brain. Furthermore, we will discuss the potential of NfL as a monitoring and a susceptibility or prognostic biomarker for AD. The third section will discuss current challenges and future directions of research on blood-based NfL as a neurodegenerative marker for AD. Finally, the last section concludes by providing potential practical applications of blood NfL in clinical and research settings.
Background: NfL as a potential biomarker for Alzheimer’s disease
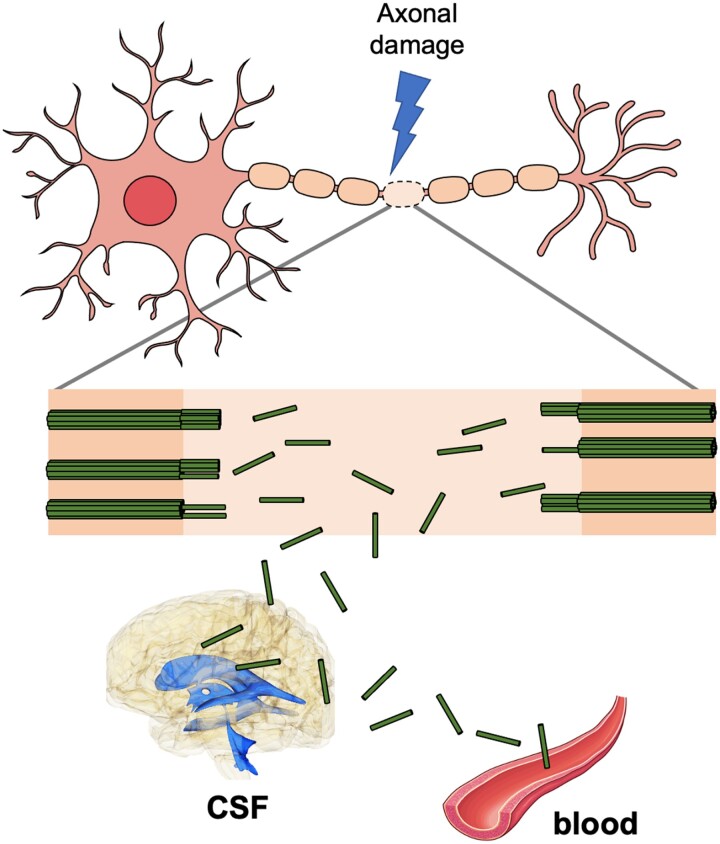
Neurofilaments are major components of the axonal cytoskeleton and are particularly abundant in myelinated large-calibre axons.1 Neurofilaments, although scarce, also exist in soma and dendrites.2 They provide stability to the cytoskeletal structure of neurons and affect conduction speed by controlling the calibre of axons.1 There are five subunits of neurofilaments, neurofilament heavy chain, neurofilament medium chain, NfL, α-internexin and peripherin, which differ by the structure of the head and tail domains of the chains and functional roles.2 Neurofilaments, particularly NfL, have been used as a marker of neuroaxonal degeneration because they are released into the CSF and blood as a result of axonal injury or neuronal death (Fig. 1).2
Figure 1.
Influx of NfL into the CSF and the bloodstream following axonal damage. Following damage to axons, the fragments of neurofilament chains (green sticks) are released into the CSF and the bloodstream. The mechanisms of the influx of neurofilament light chain (NfL) into CSF and blood are not yet clearly known. See Gafson et al.3 and Yuan and Nixon4 for discussions on potential CSF drainage pathways responsible for the entry of NfL into CSF. A possible route for the influx of NfL fragments into the blood is the drainage of NfL-containing CSF to dural venous sinuses through arachnoid villi.5 The figure contains images adapted from an icon, ‘Motor neuron’ (https://reactome.org/content/detail/R-ICO-014093) by reactome.org, used under CC BY 4.0, and an image, ‘Arteries’ (https://smart.servier.com/smart_image/artery-20/) by Servier Medical Art, used under CC BY 3.0.
The CSF or blood level of NfL is not specific to a disease. The CSF or blood NfL level increases in various neurological and neurodegenerative diseases such as traumatic brain injury,6 multiple sclerosis,7,8 frontotemporal lobar degeneration9,10 and AD11,12 (see Kahlil et al.13 for a review). Not only that, but normal ageing also leads to an increase in CSF and blood NfL. NfL has been detected in CSF and blood in middle-aged to older adults without neurodegenerative diseases, but they showed a lower level of NfL or a lower rate of increase in NfL over 2 years compared to people in similar age groups with neurodegenerative disorders.14,15 Moreover, within-age group variability of NfL level increases as age increases in middle-aged to older adults, possibly due to subclinical or inconspicuous diseases or injuries.16 Thus, ageing may further increase the overlap between pathological and non-pathological groups in their CSF/blood NfL level.
Recently, many studies have tested the value of CSF or blood NfL as a measure of neurodegeneration or as a diagnostic marker for AD. Meta-analyses on the diagnostic performance of NfL consistently showed that patients with AD dementia displayed significantly higher levels of NfL compared to control participants17–19 and studies show that the level of NfL differentiates between AD and control groups with a reasonably high area under the receiver operating characteristic curve (e.g. 0.87 in Mattsson et al.12 and 0.798 in Barker et al.20). However, studies show mixed results on the difference in the level of NfL between control and mild cognitive impairment (MCI) groups or MCI and AD groups.21–24 Moreover, NfL showed moderate or poor performance in discriminating AD from other dementias or other neurological diseases sharing symptoms with AD.19,25 As these findings show, NfL may not be that useful as a diagnostic biomarker, particularly when it is used independently. However, previous findings suggest that NfL can be more useful as a biomarker for monitoring and predicting the severity of AD. A higher blood/CSF level of NfL has been associated with lower cognitive function and more advanced neurodegeneration, as well as more accelerated cognitive decline or neurodegeneration in AD.21,22,26,27
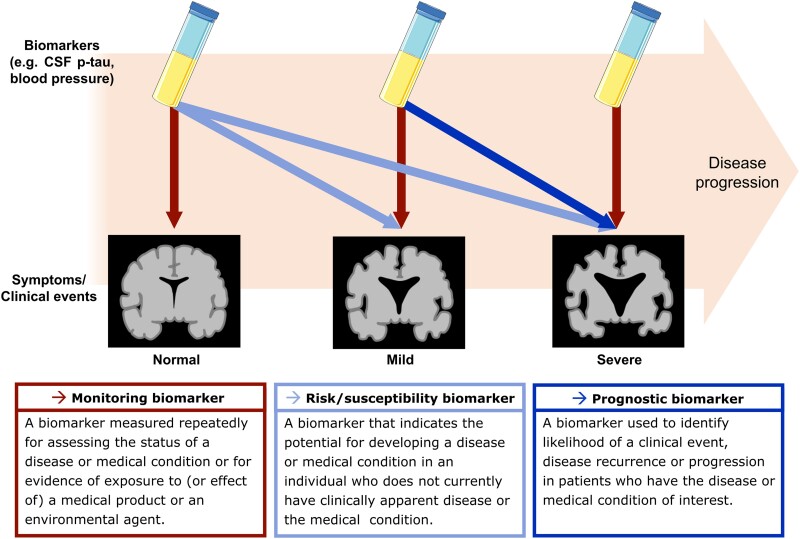
Indicating and predicting the severity of a disease are the roles of monitoring, prognostic and susceptibility/risk biomarkers defined by the FDA-NIH Biomarker Working Group in 2016 (Fig. 2).28 The working group defined the monitoring biomarker as ‘A biomarker measured repeatedly for assessing the status of a disease or medical condition or for evidence of exposure to (or effect of) a medical product or an environmental agent’. Thus, the role of a monitoring biomarker is to indicate the current status or severity of a disease. Both prognostic and susceptibility/risk biomarkers indicate future risk for a disease-related event. However, the difference is that a prognostic biomarker is used for patients, whereas a susceptibility/risk biomarker is used for those who do not currently have a disease. The working group defined the prognostic biomarker as ‘A biomarker used to identify likelihood of a clinical event, disease recurrence or progression in patients who have the disease or medical condition of interest’. The susceptibility/risk biomarker was defined as ‘A biomarker that indicates the potential for developing a disease or medical condition in an individual who does not currently have clinically apparent disease or the medical condition’.
Figure 2.
The definitions of a monitoring biomarker, a risk/susceptibility biomarker, and a prognostic biomarker proposed by the FDA-NIH Biomarker Working Group. The red box on the bottom presents the definition of a monitoring biomarker, and the red arrows show the role of monitoring biomarkers, which is to indicate the status or severity of disease at the time of the collection of a biomarker. The light blue box presents the definition of a risk/susceptibility biomarker, and the light blue arrows show the role of risk/susceptibility biomarkers. A risk/susceptibility biomarker measured in individuals who do not have a disease (e.g. normal cognitive function, no disease-related neurodegeneration) indicates the risk of developing a disease-related event (e.g. cognitive decline, disease-related neurodegeneration) in the future. The dark blue box presents the definition of a prognostic biomarker, and the dark blue arrow shows the role of prognostic biomarkers. A prognostic biomarker measured in individuals already having a disease or medical condition (e.g. cognitive decline, neurodegeneration) predicts the future disease progression. This figure contains images adapted from ‘Tube’ (https://smart.servier.com/smart_image/tube/) and ‘Alzheimer disease’ (https://smart.servier.com/smart_image/alzheimer-3/) by Servier Medical Art, used under CC BY 3.0.
As mentioned, blood/CSF NfL has the potential to be useful as a monitoring biomarker for AD. Particularly, more recent studies have shown the potential utility of blood-based NfL,29–31 which is minimally invasive and more accessible compared to other markers requiring MRI, PET imaging or lumbar puncture. Despite such advantages of blood-based NfL, there are concerns that plasma/serum concentration of NfL may not properly reflect the severity of neurodegeneration occurring in the CNS. However, a previous histopathological study demonstrated a significant correlation between higher plasma concentration of NfL measured in vivo and later lower density of NfL staining in post-mortem medial temporal lobe (MTL) tissue samples with a moderate magnitude of correlation (ρ = −0.47 after adjusting for age at post-mortem, interval between plasma sampling and death and the burden of co-pathology, including vascular lesions, TDP-43 and Lewy body pathology) in older adults predominately with AD.32 Also, the blood concentration of NfL has consistently shown a linear relationship with the CSF concentration of NfL in AD,23,33–35 as well as in other conditions, such as multiple sclerosis,36,37 traumatic brain injury38 and HIV.39 However, to determine whether blood NfL can serve as a useful monitoring or prognostic/susceptibility biomarker in AD, it is imperative to have a comprehensive review of findings on the association between blood NfL and the severity of AD. Previous review or meta-analysis papers discussed the associations of CSF/blood NfL with cognitive function,40 other fluid markers40 and neuroimaging measures.41,42 Since structural/functional brain measures from MRI and PET imaging are commonly used as markers of neurodegeneration in AD research with human subjects, an in-depth review of the association between blood NfL and structural/functional brain imaging measures will provide a meaningful contribution to the current literature. Moreover, to determine whether blood NfL can be useful as prognostic or susceptibility biomarkers, we need to comprehensively review the findings on the relationship between blood NfL and subsequent changes in other indicators of neurodegeneration in individuals with or without cognitive decline.
In the later sections of this review, we will first summarize and discuss research findings on the cross-sectional association between baseline blood NfL and structural and functional brain measures in individuals on the AD continuum and cognitively unimpaired individuals to determine the potential of blood NfL as a monitoring biomarker for AD. Next, we will review longitudinal findings revealing the relationship between blood NfL and the future progression of neurodegeneration to determine the potential of blood NfL as a prognostic and susceptibility marker for AD. We will focus on the studies examining the association between serum or plasma NfL and other measures of neurodegeneration, including cortical atrophy, glucose metabolism measured by 18F-fluorodeoxyglucose-PET (FDG-PET), diffusion tensor imaging (DTI) indices and functional MRI (fMRI) measures. We will not include amyloid and tau measures here but focus on the neuroimaging markers of neurodegeneration.43
We conducted a literature search using PubMed, Web of Science and Scopus databases using the search terms ‘(neurofilament light OR NfL) AND (volume OR thickness OR atrophy OR cortical OR subcortical OR "T1-weighted" OR MRI OR fMRI OR "functional MRI" OR "glucose metabolism" OR FDG-PET)’. Studies were included if their samples included people on the spectrum of late-onset AD, MCI or subjective cognitive decline (SCD), cognitively unimpaired or autosomal dominant familial Alzheimer's disease; measured NfL level from either plasma or serum; examined the relationship between the NfL level and structural or functional brain MRI/PET measures, including cortical thickness/volume, grey matter density, diffusion measures, glucose metabolism (FDG-PET) and fMRI. We excluded conference proceedings, letters, abstracts, review articles and meta-analyses; and studies that only included participants diagnosed with non-AD dementia.
The potential of blood neurofilament light chain as a monitoring biomarker for Alzheimer’s disease
Cross-sectional association between cortical atrophy and blood neurofilament light chain
The brain measures that have been associated most frequently with blood NfL are cortical structural measures, including cortical volume, cortical thickness and grey matter density. Supplementary Table 1 provides an overview of cross-sectional studies on the relationship between plasma/serum NfL and neuroimaging measures of neurodegeneration, with the statistics representing the strength of the effects and the variables statistically controlled for. Many studies focused on examining the association between blood NfL and cortical regions restricted to the MTL and other temporal regions,12,20,21,27,44–52 such as the hippocampus, parahippocampal gyrus, entorhinal cortex, inferior and middle temporal lobe, which are the focus of many existing AD studies. The most consistent finding across these studies is that higher baseline plasma NfL level was associated with more severe baseline atrophy of the MTL and other temporal regions determined by either volumetric measures or visual ratings in patients with AD dementia or in older adults with MCI or SCD.20,52 Two Alzheimer Disease Neuroimaging Initiative (ADNI) studies conducted by the same research group found a significant association between higher plasma NfL level and lower hippocampal volume, as well as the cortical thickness of AD-vulnerable temporal regions in their ADNI subsamples regardless of the diagnosis.12,21 A study on autosomal dominant AD also found a significant association between lower hippocampal volume and higher serum NfL in all carriers (both symptomatic and presymptomatic) of familial AD mutations (i.e. mutations in the APP, PSEN1 or PSEN2 genes) but not in presymptomatic carriers.27 In addition to the previously described studies solely focused on temporal brain regions, whole brain studies also confirmed baseline associations between higher plasma or serum NfL and more atrophy of the hippocampus and temporal cortical regions in patients with MCI or AD dementia but not in cognitively unimpaired participants.53–55 These findings are in line with previous results that the MTL shows earlier and more pronounced cortical atrophy in AD.56,57 Also, the findings suggest that the blood level of NfL indicates the severity of MTL atrophy from the early stages of AD. Whole brain studies also revealed correlations between plasma NfL level and cortical atrophy in areas overlapping with the brain regions comprising the default mode network (DMN), such as posterior cingulate cortex, precuneus, medial frontal and superior frontal gyrus, in patients with MCI or AD.55,58 These findings suggest the level of NfL is likely indicative of neurodegeneration happening not only in the MTL but also in the DMN regions, which show pronounced cortical atrophy, change in metabolism and amyloid deposition in AD.59
Interestingly, the association between blood NfL and cortical atrophy seemed to differ by the disease stage and amyloid-β (Aβ) load. The associations between blood NfL and atrophy have been consistently found in patients with MCI and AD but not in cognitively unimpaired participants with or without significant Aβ burden.20,47–50 Moreover, the cross-sectional association between the level of blood NfL and cortical atrophy in regions vulnerable to AD was more pronounced in MCI patients with high Aβ burden than those with low Aβ burden.55 In another study, only cognitively unimpaired individuals having APOE ε4 alleles, a primary genetic risk factor for late-onset AD,60 showed an association between higher plasma NfL level and lower volumes of the posterior cingulate, frontal and temporal cortices, whereas cognitively unimpaired APOE ε4 non-carriers did not show a significant association.61 The pronounced association between blood NfL and the atrophy of cortical regions typically more vulnerable to AD pathologies in people with higher Aβ burden or APOE ε4 allele(s) suggests that the blood NfL level in people with a higher risk of AD or those at the preclinical stages of AD reflects the severity of patterns of neurodegeneration often seen in AD rather than the patterns of neurodegeneration shown in typical ageing.
Overall, findings showed regional specificity centring on the temporal and DMN regions in the association between blood NfL and cortical atrophy. Nevertheless, an association was also found in less AD-specific regions, such as the paracentral lobule61 and cerebellum.55 The association in less AD-specific regions may be the result of a wide array of pathological effects on cells across the cortex, considering that neither blood NfL nor cortical atrophy is an AD-specific measure of neuronal damage. It may be reasonable to interpret the association between NfL and cortical atrophy as the outcome of the influence of third variables affecting both the blood NfL level and cortical atrophy rather than as a cause-and-effect relationship between blood NfL and atrophy. Since both NfL and cortical atrophy are non-specific markers, it is possible that potential third variables would include many non-AD-specific pathological factors, such as oxidative stress and inflammation. A previous study involving YKL-40, an inflammatory marker,62 suggests that the strength of inflammatory response in the ageing brain could explain the severity of atrophy happening in cortical areas that have not been indicated as ‘typical’ AD-specific regions, such as the paracentral lobule,63 cerebellum and inferior frontal gyrus.64
Cross-sectional association between cortical glucose metabolism and blood neurofilament light chain
Although cortical atrophy has been the focus of most investigations on the relationship between blood NfL and functional or structural brain imaging measures, several studies examined how plasma or serum NfL is associated with brain glucose metabolism measured by FDG-PET (see Supplementary Table 1 for the overview of the studies). Glucose hypometabolism has been associated with early synaptic dysfunction, mitochondrial dysfunction and cell loss,65–67 but the association with Aβ has been mixed.68–70 Four of six studies that examined the cross-sectional association between blood NfL and cortical glucose metabolism either used the mean FDG uptake level measured in a priori regions in which FDG-PET uptake level has been most affected by AD in previous studies, such as angular gyrus, temporal cortex and posterior cingulate cortex,21,48 or measured in almost the entire cortex.12,50 A study by Mattsson and colleagues21 found that higher baseline plasma NfL level predicted lower baseline mean FDG uptake level in the bilateral angular, temporal and posterior cingulate cortices in their sample from ADNI that included both cognitively impaired and unimpaired older adults. However, another ADNI study conducted several years earlier by the same research group found no baseline association between plasma NfL and the mean FDG uptake in broader regions of interest, including lateral and medial frontal, anterior cingulate, posterior cingulate, lateral parietal and lateral temporal regions in their sample that consisted of cognitively impaired and unimpaired older adults.12 These results may indicate that there is a regional specificity focused on the posterior cingulate, temporal and angular gyrus, which largely overlap with posterior DMN regions. However, more evidence is needed to substantiate this conclusion. Several studies examined how the blood level of NfL relates to FDG uptake level in individual voxels across the entire cortex or in several individual regions. An ADNI study by Mayeli and colleagues71 found that the MCI group showed cross-sectional associations between the plasma NfL level and FDG uptake level in parietal, temporal and some frontal regions. Another ADNI study by Benedet and colleagues72 showed significant voxel-wise negative associations between plasma NfL and the FDG uptake level in the hippocampus and insula only in the cognitively impaired group, which included MCI and AD patients, but not in the cognitively unimpaired group. Similar to the results regarding cortical atrophy, studies in which all or most of the participants were cognitively unimpaired did not find a significant association between plasma NfL and the level of FDG uptake.48,50,72 It is worth noting that the studies that reported a significant relationship between FDG uptake and blood NfL are not independent of one another in that they all used the ADNI database. Still, the results from the latter two studies, which did not use a priori regions of interest,71,72 are consistent with former two studies focusing on the a priori regions,12,21 as well as with previous literature showing reduced FDG uptake mostly in temporoparietal cortices in AD patients.68,73 Compared to the NfL–atrophy associations, which also showed significance in brain regions that are not highly vulnerable to AD, the associations between NfL and FDG uptake appear to be more focused on the brain regions found to be vulnerable to AD-related hypometabolism. It seems that blood NfL is more strongly associated with hypometabolism localized in areas vulnerable to AD pathologies rather than with atrophy in the same regions. This slight difference in regional specificity may be because FDG-PET is a more specific marker of neurodegeneration, reflecting a specific aspect of neurodegeneration, i.e. glucose hypometabolism. Thus, the above results suggest that the level of blood NfL in individuals on the AD continuum can be an indicator of the severity of hypometabolism happening in cortical areas whose glucose metabolism is commonly affected by AD.
Cross-sectional association between white matter microstructural characteristics and blood neurofilament light chain
There are only a few studies on the relationship between blood NfL and white matter microstructural characteristics, all of which used DTI as indicators of white matter structural integrity (Supplementary Table 1). A study showed that higher blood NfL was cross-sectionally associated with lower fractional anisotropy (FA), a DTI metric representing the degree of anisotropy of water molecules and often used as an indicator of axonal integrity, in widespread white matter areas in MCI patients but not in cognitively unimpaired older adults.24 A study with early-onset AD patients also showed similar results, showing the association between the serum NfL level and multiple DTI indices in nearly all white matter tracts in autosomal dominant AD mutation carriers regardless of cognitive decline but not in non-carriers.74 In two publications from the Mayo Clinic Study of Aging focusing on the FA of the corpus callosum, baseline plasma NfL was not associated with the baseline FA of the corpus callosum but did predict the rate of change in the same measure in their samples, which included MCI and cognitively unimpaired older adults.48,75 The correlation between blood NfL level and DTI measures suggests that the level of blood NfL possibly reflects a wide array of white matter damage happening in AD, such as destabilization of the cytoskeleton due to tau phosphorylation,76 axonal dystrophy77 and demyelination.78 White matter hyperintensities (WMH) are also a manifestation of white matter damage, particularly due to abnormalities in cerebral small vessels. However, WMH seems less likely to be a major cause of NfL increase in AD, considering that the association between WMH burden and NfL level has been more inconsistent compared to the relationship of NfL with cortical volume or glucose metabolism.12,16,47,75,79,80 Schultz and colleagues74 found that the association between WMH and plasma NfL was explained by a DTI measure, while the associations between multiple tract-specific DTI metrics and plasma NfL were not explained by global WMH burden. In addition, another study found that age largely explained the relationship between WMH and plasma NfL.80 These findings suggest that the association between NfL and WMH may be largely driven by either white matter damage of non-vascular origin or biological processes that worsen with ageing, such as a capacity for axon repair81 and inflammation.82,83 It is hard to generalize the results due to the limited number of studies, but the current findings on the association between blood-based NfL and DTI measures are in line with the findings of previous DTI studies conducted with patients in the AD continuum showing a decrease in FA and an increase in mean diffusivity (MD) in widespread regions.84–86 MD is a DTI metric representing the overall diffusivity of water molecules and has often been associated with the degree of myelination87–89 and shown to be elevated in multiple neurological conditions, such as multiple sclerosis (see Inglese and Bester90 and Rovaris et al.91 for reviews), Alzheimer's disease (see Sexton et al.84 for a meta-analysis) and brain areas with WMHs in healthy older adults.92–94 However, there is also evidence suggesting the vulnerability of specific localized white matter areas to AD, particularly in the early stages. In individuals with MCI, compared to cognitively unimpaired controls, white matter tracts connected to the parietal or occipital lobe, such as the parahippocampal part of the cingulum and posterior thalamic radiation, exhibited lower FA, higher radial diffusivity (RD) or higher neurite orientation dispersion index (ODI).95 Also, a study observed lower fibre density and thinner fibre bundle cross-section in the posterior cingulum and uncinate fasciculus in those with MCI compared to the control group.96 Further investigations using indices that are capable of measuring more specific characteristics of white matter microstructure than DTI indices, such as neurite orientation dispersion and density imaging (NODDI) indices,97 which can quantify the characteristics of different tissue compartments or myelin water imaging, which can measure myelin content,98 are needed to elucidate the aspects of white matter degeneration reflected by increased plasma/serum concentration of NfL in AD.
Cross-sectional association between functional brain measures and blood neurofilament light chain
We did not find any studies that investigated direct associations between the level of either plasma or serum NfL with functional brain measures using fMRI in AD. However, there is a study by Pereira and colleagues99 that examined the association between the level of CSF NfL and resting state functional connectivity of the DMN. In that study, they found no significant association between CSF NfL and functional connectivity of the anterior and posterior DMN in the sample of cognitively unimpaired and cognitively impaired (i.e. MCI or dementia) participants. These null results may have been due to the high proportion of cognitively unimpaired participants in the sample. Another fMRI-based study examined blood oxygen level-dependent (BOLD) variability, which has been associated with ageing and cognitive decline,100,101 and showed that lower BOLD variability was related to higher CSF NfL, as well as lower CSF Aβ-42, in cognitively unimpaired adults.102 This result suggests there may be a link between BOLD variability and change in CSF or blood NfL due to amyloidosis. Further investigations are needed in samples at different stages of AD to elucidate how neuronal damage, indexed by blood/CSF NfL, is associated with various aspects of AD-related changes in the activity or functional organization of the brain.
Conclusion on the potential of blood neurofilament light chain as a monitoring biomarker for Alzheimer’s disease
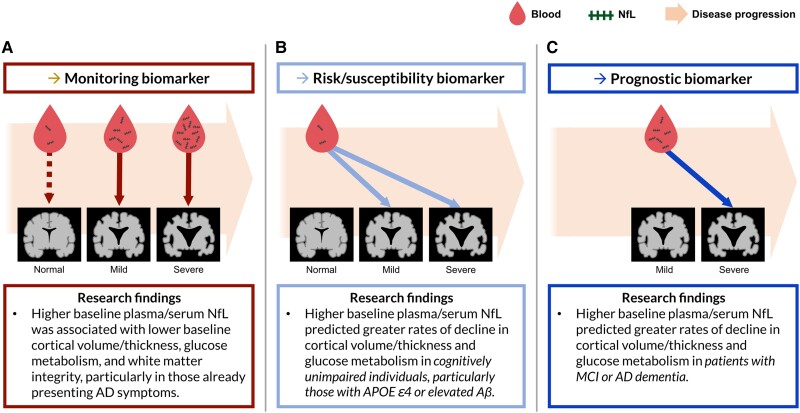
In sum, the existing cross-sectional literature indicates that blood NfL shows great promise as a monitoring biomarker indicating the current severity of neurodegeneration in AD (Fig. 3A). In particular, it seems that blood NfL as a monitoring marker would be useful for cognitively unimpaired individuals who already display AD pathology or are highly likely to develop AD (i.e. Aβ-positive or APOE ε4-positive individuals), as well as patients who already display cognitive decline. Specifically, increased blood NfL seems to reflect the severity of atrophy, hypometabolism and the decline of white matter integrity, while more strongly representing neurodegenerative changes happening in distinct areas of the brain—MTL and DMN areas for cortical atrophy and temporoparietal areas for FDG-PET—in people on the Alzheimer's continuum. Furthermore, although blood NfL is a non-specific marker of neuronal damage that also increases during typical ageing, the level of blood NfL in individuals who are likely to progress to Alzheimer's dementia [i.e. those without dementia with a high Aβ burden or APOE ε4 allele(s)] appears to reflect the severity of neuronal damage in brain regions commonly affected by AD-related neurodegeneration.55,61
Figure 3.
The potential roles of blood-based NfL as different types of biomarkers and supporting research findings. (A) Plasma/serum neurofilament light chain (NfL) has been correlated with neurodegeneration at the time of the blood draw in patients showing cognitive impairment. This supports the potential utility of plasma/serum NfL as a monitoring biomarker indicating the severity of neurodegeneration in Alzheimer's disease (AD). The dashed red line represents weak correlations between NfL and neurodegeneration in those without cognitive decline. (B) Plasma/serum NfL in cognitively unimpaired individuals has predicted the subsequent rate of neurodegeneration in brain areas susceptible to AD, supporting the potential utility of plasma/serum NfL as a risk/susceptibility biomarker for AD. (C) Plasma/serum NfL in individuals showing the symptoms of AD has predicted the subsequent rate of neurodegeneration, suggesting that plasma/serum NfL is a promising prognostic biomarker for neurodegeneration in patients with AD. Aβ = amyloid-β; MCI = mild cognitive impairment. The figure contains images adapted from ‘Alzheimer disease’ (https://smart.servier.com/smart_image/alzheimer-3/) by Servier Medical Art, used under CC BY 3.0.
The potential of blood neurofilament light chain as a prognostic and susceptibility marker for Alzheimer’s disease
Progression of cortical atrophy predicted from baseline blood neurofilament light chain
The existing literature on the longitudinal relationship between blood NfL and cortical atrophy supports the role of blood NfL as a prognostic or susceptibility marker predicting the rate of cortical atrophy (Supplementary Table 2). Similar to cross-sectional research, longitudinal studies also consistently found significant relationships between blood NfL and atrophy in brain regions vulnerable to AD pathology. More specifically, there is evidence that higher baseline blood NfL predicts a greater rate of atrophy in the hippocampal and temporal cortical regions,12,26,47,48,50,51,61,75 as well as DMN regions26,30,35,61 in late-onset AD patients and presymptomatic and symptomatic carriers of autosomal dominant AD. However, one notable difference between longitudinal and cross-sectional findings is that longitudinal studies reported significant associations between blood NfL and brain atrophy in cognitively unimpaired participants more often, particularly in those with higher Aβ burden, as opposed to the cross-sectional findings showing weak or no significant associations in cognitively unimpaired participants. An ADNI study by Hu and colleagues,50 which only included cognitively unimpaired older adults as participants, showed that greater baseline plasma NfL predicted a steeper decline in the hippocampal volume over the follow-up period ranging from 1 to 10 years in cognitively unimpaired participants with higher Aβ burden, while the same association was not significant cross-sectionally. Moscoso and colleagues26 found an association between baseline plasma NfL and cortical atrophy over the median follow-up period of 5 years in multiple large areas across parietal, temporal and frontal cortices in cognitively unimpaired participants with high Aβ load but in confined small cortical areas across paracentral lobule and superior frontal gyrus in those with low Aβ load. These results suggest that an increase in blood NfL may precede AD-related change in cortical atrophy and may likely already be elevated in the preclinical stages of AD. The differential predictive power of NfL for the neurodegeneration depending on the binary Aβ status (i.e. Aβ positive or negative) may seem contradictory to the weak association between fluid NfL and amyloid burden measured using CSF/blood-based immunoassays,53,103,104 PET imaging24,35,48 or histological staining method.105 These weak associations suggest that Aβ may not have a strong direct effect on neuroaxonal damage reflected by the increase of CSF/blood NfL. Compared to Aβ as a continuous variable, which specifically indicates the severity of amyloid pathology, the binary Aβ status provides less specific information. The binary status seems to indicate whether AD-related pathological processes have already started. Thus, the greater predictive power of NfL for neurodegeneration in Aβ-positive individuals with mild or no cognitive decline compared to Aβ-negative individuals could be due to the more pronounced progression of neurodegeneration over time in Aβ-positive individuals, as supported by previous findings.106–108 This is further supported by the study by Hu and colleagues,50 which demonstrated that baseline NfL, as well as the rate of change, was already higher in cognitively unimpaired Aβ-positive individuals compared to Aβ-negative individuals (P = 0.0044 for the difference in the baseline NfL; P < 0.0001 for the difference in the rate of increase in NfL). Although Aβ showed a weak correlation with CSF/plasma NfL, there are other markers that exhibited stronger associations with CSF/plasma NfL, such as YKL-40,104,109,110 which is a marker of inflammation,62 as well as t-tau (see Jin et al.40 for a meta-analysis on the association between CSF/plasma NfL and other AD markers). There are also findings on pathological processes that seem to affect axonal damage, such as dysfunctional trafficking of organelles in axons due to lysosomal dysfunction,77,111 increased oxidative stress,112,113 which can be caused by factors that are not directly related to Aβ,114 and tau phosphorylation and accumulation.115 These findings suggest that some of these pathological processes may have more proximal influence on neuroaxonal damage than Aβ has.
Despite the consistent findings regarding the relationship between baseline plasma or serum NfL and the longitudinal rate of atrophy, there were also several studies that did not support the relationship between blood NfL and longitudinal atrophy. Weston and colleagues27 did not find a significant association between baseline serum NfL and the rate of change in hippocampal volume in autosomal dominant AD mutation carriers, and Steinacker and colleagues103 found no significant association between baseline serum NfL and regional cortical volume at follow-up in either the AD or MCI group. These negative results may be attributed to the relatively shorter mean follow-up intervals (Weston et al.27: the mean of 1.3 years; Steinacker et al.103: 1 year ± 3 months) and small sample sizes (Weston et al.27: 33 participants with one follow-up MRI scan; Steinacker et al.103: 15 controls, 17 MCI and 26 AD patients). Cavedo and colleagues116 performed a multiple regression analysis in which both plasma NfL and plasma tau were included as predictors and found that baseline p-tau predicted change in basal forebrain cholinergic system (BFCS) volume over 12 months in older adults with subjective memory complaints while baseline plasma NfL did not in a linear model with NfL, t-tau and the interaction between NfL and t-tau as predictors and an adjustment for age, sex, education, amyloid status and APOE status. This result may be attributable to the central role of tauopathy in the degeneration of BFCS in AD.117,118 The null finding may also be attributed to either the short follow-up interval or the mild symptoms. Another explanation could be that atrophy of the BFCS is more closely associated with the damage of unmyelinated axons, as the authors suggest in their discussion, whereas NfL is abundant in myelinated large-calibre axons.1 Lastly, such results could have been obtained because the degeneration of the BFCS occurs prior to the degeneration of the other cortical and subcortical areas.119,120 If this is the case, the level of NfL, which reflects the overall cortical and subcortical neurodegeneration, may be predicted by atrophy of the BFCS rather than predicting the change in BFCS atrophy.
Progression of glucose hypometabolism predicted from baseline blood neurofilament light chain
Longitudinal FDG-PET studies also show strong evidence supporting the role of blood NfL as a prognostic or susceptibility marker predicting the rate of decline in glucose metabolism. Consistent with the longitudinal findings on atrophy above, not only those who exhibit cognitive symptoms,12,21,26,48,71,72 but individuals who are still at preclinical stages of AD and cognitively unimpaired individuals also demonstrated a longitudinal relationship between baseline blood NfL and change in glucose metabolism.26 In a study by Mielke and colleagues,48 the participants with either MCI or no cognitive decline showed that higher baseline plasma NfL predicted a higher rate of decline in the mean FDG uptake over 15 to 30 months from the baseline in the brain regions whose glucose metabolism is highly impacted by AD pathologies, while the cross-sectional relationship examined in the same sample was not significant. Also, the association seemed to be more pronounced in those with high Aβ load compared to those with low Aβ load. Two studies using the ADNI dataset revealed an association between higher baseline plasma NfL and a higher rate of decline in FDG uptake in the parietal and temporal cortices over 2 years in cognitively unimpaired individuals with high Aβ load but not in cognitively unimpaired individuals with low Aβ load,26 as well as in cognitively impaired individuals with high Aβ load but not in cognitively impaired individuals with low Aβ load.72 As these studies show, longitudinal studies exhibit that baseline NfL predicts change in glucose metabolism in focal areas that are vulnerable to AD-related metabolic change, including parietal and temporal regions, consistent with cross-sectional associations.26,71,72
Conclusion on the potential of neurofilament light chain as a prognostic or risk/susceptibility biomarker for Alzheimer’s disease
In sum, existing studies provide evidence that the level of blood NfL consistently predicts a future rate of changes in atrophy and glucose metabolism, mainly in brain areas commonly affected by AD pathology, similar to the regional specificity shown in cross-sectional investigations. Among cognitively unimpaired individuals, the relationship between baseline blood NfL and subsequent rate of neurodegeneration has been more pronounced in those with a higher likelihood of developing AD (i.e. those with high Aβ load) than those without significant amyloid deposition. Furthermore, in cognitively unimpaired individuals, significant prediction of the progression of neurodegeneration by baseline level of blood NfL has been more consistently observed than the prediction of the current state of neurodegeneration by blood NfL, providing strong support for the usefulness of blood NfL as a susceptibility/risk biomarker for AD. Therefore, the evidence strongly indicates that blood NfL would be a useful prognostic marker for predicting the progression of neurodegeneration in patients, as well as a useful susceptibility/risk marker for assessing the likelihood that a cognitively unimpaired individual with a higher risk for AD will show abnormal changes in brain structure and function (Fig. 3B and C).
How does neurofilament light chain predict neurodegeneration as an early marker of Alzheimer’s disease?
A recurring finding was that baseline blood NfL does not correlate with baseline neuroimaging markers of neurodegeneration in early AD but does predict the subsequent rate of change in these neuroimaging markers. This finding suggests that the elevation of blood NfL likely precedes changes in the neuroimaging markers. This empirical evidence highlights the potential of blood NfL as an early neurodegenerative marker of AD. Nevertheless, a careful interpretation of the lead-lag relationship between baseline NfL and subsequent changes in the neuroimaging markers is warranted because those findings do not guarantee that the damage of axons, measured by blood NfL, actually precedes the pathological processes underlying the neuroimaging markers in the progression of AD. Therefore, it would be worthwhile to speculate on why such a lead-lag relationship between NfL and neuroimaging indices has been observed in light of current knowledge of the order of neurodegenerative processes in AD and characteristics of different neuroimaging modalities measuring neurodegeneration.
Plasma/serum concentration of NfL, which presumably reflects axonal integrity, has demonstrated predictive capabilities for the baseline and the rate of change in cortical atrophy. These findings raise the question of whether they indicate that white matter integrity is affected earlier in the AD process than grey matter volume. There is a finding that the change in white matter integrity but not in the change in grey matter volume, are observed in the late preclinical (i.e. SCD) and prodromal (i.e. early amnestic MCI) stages of AD,121,122 suggesting white matter damage may precede cortical damage. Moreover, molecular or cellular level evidence suggests that axonal damage (i.e. white matter damage) may happen earlier than damage to the cell body (i.e. grey matter).77,123 However, it is also possible that the lead-lag relationship between baseline NfL and subsequent change in cortical volume/thickness commonly observed in previous studies was due to the technical limitations of cortical structural MRI, such that cortical structural MRI measures may not be sensitive enough to detect early subtle damage of the cortex because of its large minimum spatial unit, voxel, which consists of several hundred thousand neurons. There is research supporting this idea, which suggests that white matter damage happens as a result of cortical damage.124,125 Investigating the microstructural changes (e.g. NODDI measures) in the cortex in AD may help elucidate the temporal order between cortical damage and axonal damage.
The level of FDG uptake measured by FDG-PET, an index of glucose metabolic rate, is thought to be affected by multiple pathological processes, such as mitochondrial and synaptic dysfunction, which start to occur at the very early stages of AD.65,67 Therefore, the pathological processes reflected by FDG-PET are likely to happen earlier than axonal damage. However, this view is contradictory to the empirical findings that baseline NfL predicts subsequent change in glucose metabolism but not baseline glucose metabolism in the early stages of AD. This apparent contradiction may be due to the limited sensitivity of PET imaging. FDG-PET measures, particularly standardized uptake value, may not be sensitive enough to capture subtle changes in early pathological events causing the decline in glucose metabolic rate in AD, such as mitochondrial and synaptic dysfunction.
Both DTI measures and the NfL level reflect the degree of axonal damage. However, DTI measures are likely less sensitive to axonal damage than NfL because DTI measures are less specific to neuronal damage. DTI indices measured in one voxel represent averaged water diffusion signals from different brain tissue compartments (e.g. intra-axonal, intra-glial and extracellular compartments).126 Thus, some of the change in water diffusion due to axonal degeneration might be cancelled out at the same time by the water diffusion in different directions stemming from other factors in the same voxel. Therefore, it is likely that the change in DTI measures is observed after the level of blood NfL starts to rise. As will be further discussed below, more advanced diffusion imaging analysis approaches, such as NODDI, may help clarify the relationship between NfL and white matter tissue damage.
Limitations of current research and future directions
The extant literature has unveiled many aspects of blood NfL and yielded evidence supporting the potential of NfL as a neurodegenerative biomarker for AD. However, there are important limitations to the current research that should be considered.
First, most studies examining the association between blood NfL and neuroimaging measures of neurodegeneration used either cortical volume/thickness or FDG uptake level as their neuroimaging measures of interest. There is a relative lack of studies examining the relationship between NfL and other brain imaging modalities. Therefore, we still have a limited understanding of the extent to which the level of blood or CSF NfL reflects different aspects of neuronal damage, such as the damage to the myelin, cytoskeletal structure and dendrites. We need more research on how blood or CSF NfL is related to various brain imaging modalities to have a more detailed knowledge of the aspects of neurodegeneration that blood or CSF NfL reflects. Considering that NfL is mainly located in the axons, it is likely that the increased level of blood or CSF NfL is most intimately related to the damage of axons and myelin sheath. Only recently have researchers begun to investigate the relationship between NfL and diffusion-weighted imaging, an imaging technique mainly used to measure the integrity of white matter tracts. However, DTI measures, such as FA and MD, do not provide precise information on microstructural properties. Thus, more studies on the relationship between blood NfL and measures indicating more specific characteristics of white matter, such as NODDI, which can quantify the density and distribution of neurites,97 or myelin water imaging, which can measure myelin content,98 should be conducted on individuals in the AD continuum. Furthermore, investigating how blood or CSF NfL is related to more diverse measures from functional neuroimaging may also provide valuable information on how neurodegeneration causes cognitive decline in AD. We still do not have a clear understanding of how neuronal damage leads to cognitive change. Since functional brain measures are closely associated with cognitive function, investigating how blood or CSF NfL is related to functional brain measures would enable a more accurate prediction of cognitive decline in AD.
Second, it is still not clear which AD pathologies have the most substantial influence on the increase of blood/CSF NfL. Blood and CSF NfL have shown weak associations with Aβ,24,35 which has often been considered the primary upstream pathology of AD,127,128 Moreover, blood/CSF NfL has inconsistently been associated with p-tau but more consistently with t-tau with larger effect sizes.12,23,40,50,53 As discussed earlier, this weak or inconsistent relationship with Aβ, as well as p-tau, may be attributed to the existence of other pathological processes having a substantial influence on axonal damage, which needs further investigation. Another possible cause of the weak relationship between NfL and Aβ, as well as p-tau, maybe the different trajectories of these markers in the progression of AD. A data-driven modelling study demonstrated that blood NfL gradually increases without showing a strong peak of change rate as AD progresses,129 while the trajectory of Aβ is known to reach its peak during the early stages of AD.130,131 There is also a finding that the trajectories of plasma NfL and p-tau181 increase are different from each other.132 Indeed, the association between plasma NfL and Aβ measurements from the CSF, as well as PET imaging, differed by disease stage, showing significant association in the preclinical or prodromal stages of AD and no significant association in later stages.21,24,61 The association between tau and plasma NfL also varied across different disease stages, with CSF p-tau showing a more robust relationship with plasma NfL in preclinical or prodromal stages,21,133 while tau PET exhibited a significant relationship with plasma NfL in cognitively impaired individuals but not in cognitively unimpaired individuals.61 In an effort to examine which of the known AD pathologies have the most direct influence on the blood/CSF NfL level, it is essential to conduct longitudinal studies tracking the changes in blood/CSF NfL and other markers of AD pathology, such as Aβ, tau, synaptic function, neuroinflammation and mitochondrial function over a long period starting at presymptomatic stages.
Lastly, it should be noted that blood NfL is a peripheral marker of neuronal damage. It is still not clear how the change in the permeability of the blood–brain barrier (BBB) due to diseases or ageing affects the blood concentration of NfL, given the inconsistent results on the impact of BBB permeability on serum NfL levels from a few number of studies.134,135 Also, we lack knowledge on the degree to which the NfL released from the peripheral nervous system136,137 contributes to the plasma/serum level of NfL. Studies showed that peripheral neuropathies,138,139 as well as cardiovascular disease,140 diabetes140,141 and kidney functions140–142 are associated with blood NfL concentration. In vivo measurement of α-internexin, a neurofilament subunit mainly expressed in the CNS, and peripherin, a subunit predominantly expressed in the peripheral nervous system, may aid in a more accurate assessment of the neuronal damage in the CNS based on plasma or serum neurofilament. However, only a few attempts have been made to measure the fluid level of α-internexin or peripherin.143,144 Further research is needed to assess the reliability and sensitivity of α-internexin or peripherin as fluid markers. Additionally, the extent to which plasma proteases degrade the NfL in the blood remains unclear. Further research is warranted to enhance the understanding of the degradation of NfL in the blood, as well as the pathways of the drainage of NfL into the bloodstream. While there are still several unanswered questions, as outlined above, they do not seem to undermine the utility of blood NfL as a biomarker. Studies have consistently demonstrated significant linear relationships between plasma/serum NfL and CSF NfL in different neurological conditions.12,23,33,35,36,39 Also, plasma/serum NfL has shown comparable performance to CSF NfL in predicting the likelihood of developing AD.145 However, the field should seek to answer these unresolved questions to learn more about potential factors influencing the NfL concentration in the blood.
Finally, we need to be cautious when interpreting the results of the studies introduced in this review for several reasons. First, the diagnostic criteria for AD dementia and MCI used in many studies were based solely on clinical symptoms, not considering the A (Aβ), T (pathologic tau), or N (neurodegeneration) biomarkers suggested by the NIA-AA research framework.43 Thus, we cannot rule out the possibility that the cognitive impairment of some participants was not due to AD but another disease. Thus, researchers should seek to include biomarker levels, as well as clinical symptoms, in their diagnostic criteria for AD. Second, many studies in the scope of this review were conducted using ADNI data with considerably overlapping samples (see ‘Sample source’ columns in Supplementary Tables 1 and 2). In addition, the ADNI dataset is biased toward highly educated white participants. Thus, it needs to be verified whether the results of the ADNI studies are replicated in other large cohorts and in more diverse samples. Finally, not all studies accounted for potential confounders in their statistical model. While most studies adjusted for age and sex, there were several studies that performed a simple correlation analysis relating NfL and another variable without adjusting for possible confounding variables. While sex has been independent of NfL in many previous studies, it is important to consider the influence of age due to its strong association with NfL concentration.16,50,146 When controlling for age, there are several things to be considered. First, age and NfL concentration are in a non-linear relationship. Specifically, CSF/blood NfL tends to increase more rapidly as age increases across adulthood.16,146,147 Log-transforming NfL may help address this complexity because log-transformed NfL exhibit a relatively linear relationship with age.16,146,147 However, researchers would need to carefully interpret the results when using log-transformed NfL values. Second, controlling for age would significantly diminish the shared variability between NfL and a neuroimaging measure because both NfL and neuroimaging measures are heavily influenced by age, which might result in NfL being an insignificant or marginal predictor of neuroimaging measures. Thus, when studying the relationship between NfL and neuroimaging measures while adjusting for age, researchers should assess the amount of shared variance between age and a neuroimaging measure, as well as NfL, which will provide a clearer understanding of the findings. Also, studies performed using the data collected from multiple sites should consider adjusting for the site differences because variations in study protocols, such as MRI scanners and imaging sequences, across different sites could impact the statistical association between NfL and another variable of interest. Only two of the studies within our scope accounted for scanner field strength (1.5 T or 3 T) in their models,26,61 and unaccounted effects of site differences in the other studies might indeed explain some of the diverging results observed in the literature. Therefore, future studies should seek to include appropriate confounding variables in their statistical model.
Conclusions
The literature shows that the level of blood NfL indicates the severity of multiple aspects of neurodegeneration in the continuum of AD, from preclinical stages to dementia, demonstrating that blood NfL has a high potential to be used as an early monitoring biomarker for assessing the severity of neurodegeneration. Thus, blood NfL will be useful, for example, for screening for neurodegeneration or tracking the status of neurodegeneration in individuals with an increased risk for AD. Furthermore, blood NfL may be useful as an indicator assessing the effect of treatment on neurodegeneration in clinical trials. Studies also showed that blood NfL predicts the future progression of structural and functional neurodegeneration in AD patients and cognitively unimpaired individuals at risk for AD. This suggests that blood NfL would be useful as a prognostic marker predicting the progression of neurodegeneration for patients showing AD symptoms, as well as a risk/susceptibility marker assessing how likely a cognitively unimpaired individual will develop pathological neurodegeneration. Hence, blood NfL may aid clinicians in predicting the symptom progression in patients and determining the initiation of treatment for AD. Establishing a consensus on the criteria for an abnormal level of blood NfL may facilitate the use of blood NfL in clinical settings. Lastly, blood NfL may also be used as a proxy measure of neuronal damage in research investigating the mechanisms of how pathological processes, including neuronal damage, lead to cognitive decline in AD. Future studies should investigate the association between blood/CSF NfL and various brain measures and perform long-term investigations tracking dynamic changes of blood/CSF NfL in parallel with different AD markers. Compared to markers requiring a spinal tap, MRI or PET imaging, blood NfL is a more cost-efficient, more accessible and less invasive biomarker. These advantages would facilitate more frequent measurement of blood NfL in more diverse populations, including the populations that have been under-represented in previous studies. Therefore, using blood NfL as a biomarker of neuronal damage will advance our understanding of the pathological mechanisms of AD and improve our prediction of disease progression.
Supplementary Material
Acknowledgements
We would like to thank Dr Ana M. Daugherty and Dr John L. Woodard for providing constructive feedback on the earlier versions of the manuscript.
Contributor Information
Youjin Jung, Department of Psychology, Wayne State University, Detroit, MI 48202, USA; Institute of Gerontology, Wayne State University, Detroit, MI 48202, USA.
Jessica S Damoiseaux, Department of Psychology, Wayne State University, Detroit, MI 48202, USA; Institute of Gerontology, Wayne State University, Detroit, MI 48202, USA.
Funding
The authors’ time was supported in part by funding from the National Institutes of Health (P30AG072931 to J.D.) and the Blue Cross Blue Shield of Michigan Foundation Student Award Program grant (2021010116.SAP to Y.J.).
Competing interests
The authors report no competing interests.
Supplementary material
Supplementary material is available at Brain online.
References
- 1. Lee MK, Cleveland DW. Neuronal intermediate filaments. Annu Rev Neurosci. 1996;19:187–217. [DOI] [PubMed] [Google Scholar]
- 2. Yuan A, Rao MV, Veeranna, Nixon RA. Neurofilaments and neurofilament proteins in health and disease. Cold Spring Harb Perspect Biol. 2017;9:a018309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gafson AR, Barthélemy NR, Bomont P, et al. Neurofilaments: Neurobiological foundations for biomarker applications. Brain. 2020;143:1975–1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yuan A, Nixon RA. Neurofilament proteins as biomarkers to monitor neurological diseases and the efficacy of therapies. Front Neurosci. 2021;15:689938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Welch K, Friedman V. The cerebrospinal fluid valves. Brain. 1960;83:454–469. [DOI] [PubMed] [Google Scholar]
- 6. Karantali E, Kazis D, McKenna J, Chatzikonstantinou S, Petridis F, Mavroudis I. Neurofilament light chain in patients with a concussion or head impacts: A systematic review and meta-analysis. Eur J Trauma Emerg Surg. 2022;48:1555–1567. [DOI] [PubMed] [Google Scholar]
- 7. Lycke JN, Karlsson JE, Andersen O, Rosengren LE. Neurofilament protein in cerebrospinal fluid: A potential marker of activity in multiple sclerosis. J Neurol Neurosurg Psychiatry. 1998;64:402–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Benkert P, Meier S, Schaedelin S, et al. Serum neurofilament light chain for individual prognostication of disease activity in people with multiple sclerosis: A retrospective modelling and validation study. Lancet Neurol. 2022;21:246–257. [DOI] [PubMed] [Google Scholar]
- 9. van der Ende E, Bron EE, Poos JM, et al. A data-driven disease progression model of fluid biomarkers in genetic FTD. Alzheimer’s Dement. 2022;145:1805–1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Benussi A, Karikari TK, Ashton N, et al. Diagnostic and prognostic value of serum NfL and p-Tau 181 in frontotemporal lobar degeneration. J Naeurology, Neurosurg Psychiatry. 2020;91:960–967. [DOI] [PubMed] [Google Scholar]
- 11. Mattsson N, Insel PS, Palmqvist S, et al. Cerebrospinal fluid tau, neurogranin, and neurofilament light in Alzheimer’s disease. EMBO Mol Med. 2016;8:1184–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mattsson N, Andreasson U, Zetterberg H, et al. Association of plasma neurofilament light with neurodegeneration in patients with Alzheimer disease. JAMA Neurol. 2017;74:557–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Khalil M, Teunissen CE, Otto M, et al. Neurofilaments as biomarkers in neurological disorders. Nat Rev Neurol. 2018;14:577–589. [DOI] [PubMed] [Google Scholar]
- 14. Milà-Alomà M, Salvadó G, Gispert JD, et al. Amyloid beta, tau, synaptic, neurodegeneration, and glial biomarkers in the preclinical stage of the Alzheimer’s continuum. Alzheimer’s Dement. 2020;16:1358–1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Naude JP, Gill S, Hu S, et al. Plasma neurofilament light: A marker of neurodegeneration in mild behavioral impairment. J Alzheimer’s Dis. 2020;76:1017–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Khalil M, Pirpamer L, Hofer E, et al. Serum neurofilament light levels in normal aging and their association with morphologic brain changes. Nat Commun. 2020;11:812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Olsson B, Lautner R, Andreasson U, et al. CSF And blood biomarkers for the diagnosis of Alzheimer’s disease: A systematic review and meta-analysis. Lancet Neurol. 2016;15:673–684. [DOI] [PubMed] [Google Scholar]
- 18. Zhao Y, Xin Y, Meng S, He Z, Hu W. Neurofilament light chain protein in neurodegenerative dementia: A systematic review and network meta-analysis. Neurosci Biobehav Rev. 2019;102:123–138. [DOI] [PubMed] [Google Scholar]
- 19. Forgrave LM, Ma M, Best JR, DeMarco ML. The diagnostic performance of neurofilament light chain in CSF and blood for Alzheimer’s disease, frontotemporal dementia, and amyotrophic lateral sclerosis: A systematic review and meta-analysis. Alzheimer’s Dement Diagnosis. Assess Dis Monit. 2019;11:730–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Barker W, Quinonez C, Greig MT, et al. Utility of plasma neurofilament light in the 1Florida Alzheimer’s Disease Research Center (ADRC). J Alzheimer’s Dis. 2021;79:59–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mattsson N, Cullen NC, Andreasson U, Zetterberg H, Blennow K. Association between longitudinal plasma neurofilament light and neurodegeneration in patients with Alzheimer disease. JAMA Neurol. 2019;76:791–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sugarman MA, Zetterberg H, Blennow K, et al. A longitudinal examination of plasma neurofilament light and total tau for the clinical detection and monitoring of Alzheimer’s disease. Neurobiol Aging. 2020;94:60–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lewczuk P, Ermann N, Andreasson U, et al. Plasma neurofilament light as a potential biomarker of neurodegeneration in Alzheimer’s disease. Alzheimers Res Ther. 2018;10:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Andersson E, Janelidze S, Lampinen B, et al. Blood and cerebrospinal fluid neurofilament light differentially detect neurodegeneration in early Alzheimer’s disease. Neurobiol Aging. 2020;95:143–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bridel C, Van Wieringen WN, Zetterberg H, et al. Diagnostic value of cerebrospinal fluid neurofilament light protein in neurology: A systematic review and meta-analysis. JAMA Neurol. 2019;76:1035–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Moscoso A, Grothe MJ, Ashton NJ, et al. Longitudinal associations of blood phosphorylated Tau181 and neurofilament light chain with neurodegeneration in Alzheimer disease. JAMA Neurol. 2021;78:396–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Weston PSJ, Poole T, Ryan NS, et al. Serum neurofilament light in familial Alzheimer disease A marker of early neurodegeneration. Neurology. 2017;89:2167–2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. FDA-NIH Biomarker Working Group . BEST (Biomarkers, EndpointS, and other tools) resource. Food and Drug Administration (US); 2016. [PubMed] [Google Scholar]
- 29. Zetterberg H, Burnham SC. Blood-based molecular biomarkers for Alzheimer’s disease. Mol Brain. 2019;12:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Simrén J, Leuzy A, Karikari TK, et al. The diagnostic and prognostic capabilities of plasma biomarkers in Alzheimer’s disease. Alzheimer’s Dement. 2021;17:1145–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Illán-Gala I, Lleo A, Karydas A, et al. Plasma tau and neurofilament light in frontotemporal lobar degeneration and Alzheimer disease. Neurology. 2021;96:e671–e683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ashton NJ, Leuzy A, Lim YM, et al. Increased plasma neurofilament light chain concentration correlates with severity of post-mortem neurofibrillary tangle pathology and neurodegeneration. ACTA Neuropathol Commun. 2019;7:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gaiottino J, Norgren N, Dobson R, et al. Increased neurofilament light chain blood levels in neurodegenerative neurological diseases. PLoS One. 2013;8:e75091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Janelidze S, Berron D, Smith R, et al. Associations of plasma phospho-Tau217 levels with tau positron emission tomography in early Alzheimer disease. JAMA Neurol. 2021;78:149–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Preische O, Schultz SA, Apel A, et al. Serum neurofilament dynamics predicts neurodegeneration and clinical progression in presymptomatic Alzheimer’s disease. Nat Med. 2019;25:277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Disanto G, Barro C, Benkert P, et al. Serum neurofilament light: A biomarker of neuronal damage in multiple sclerosis. Ann Neurol. 2017;81:857–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kuhle J, Barro C, Disanto G, et al. Serum neurofilament light chain in early relapsing remitting MS is increased and correlates with CSF levels and with MRI measures of disease severity. Mult Scler. 2016;22:1550–1559. [DOI] [PubMed] [Google Scholar]
- 38. Shahim P, Politis A, van der Merwe A, et al. Neurofilament light as a biomarker in traumatic brain injury. Neurology. 2020;95:e610–e622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gisslén M, Price RW, Andreasson U, et al. Plasma concentration of the neurofilament light protein (NFL) is a biomarker of CNS injury in HIV infection: A cross-sectional study. EBioMedicine. 2016;3:135–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Jin M, Cao L, Dai YP. Role of neurofilament light chain as a potential biomarker for Alzheimer’s disease: A correlative meta-analysis. Front Aging Neurosci. 2019;11:254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Alirezaei Z, Pourhanifeh MH, Borran S, Nejati M, Mirzaei H, Hamblin MR. Neurofilament light chain as a biomarker, and correlation with magnetic resonance imaging in diagnosis of CNS-related disorders. Mol Neurobiol. 2020;57:469–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Xiong YL, Meng T, Luo J, Zhang H. The potential of neurofilament light as a biomarker in Alzheimer’s disease. Eur Neurol. 2021;84:6–15. [DOI] [PubMed] [Google Scholar]
- 43. Jack CR, Bennett DA, Blennow K, et al. NIA-AA Research framework: Toward a biological definition of Alzheimer’s disease. Alzheimer’s Dement. 2018;14:535–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Shahid SS, Wen Q, Risacher SL, et al. Hippocampal-subfield microstructures and their relation to plasma biomarkers in Alzheimer’s disease. Brain. 2022;145:2149–2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Chen Y, Therriault J, Luo J, Ba M, Zhang H. Neurofilament light as a biomarker of axonal degeneration in patients with mild cognitive impairment and Alzheimer’s disease. J Integr Neurosci. 2021;20:861–870. [DOI] [PubMed] [Google Scholar]
- 46. Chatterjee P, Pedrini S, Ashton NJ, et al. Diagnostic and prognostic plasma biomarkers for preclinical Alzheimer’s disease. Alzheimer’s Dement. 2022;18:1141–1154. [DOI] [PubMed] [Google Scholar]
- 47. Rajan KB, Aggarwal NT, McAninch EA, et al. Remote blood biomarkers of longitudinal cognitive outcomes in a population study. Ann Neurol. 2020;88:1065–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Mielke MM, Syrjanen JA, Blennow K, et al. Plasma and CSF neurofilament light: Relation to longitudinal neuroimaging and cognitive measures. Neurology. 2019;93:E252–E260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Chatterjee P, Goozee K, Sohrabi HR, et al. Association of plasma neurofilament light chain with neocortical amyloid-β load and cognitive performance in cognitively normal elderly participants. J Alzheimer’s Dis. 2018;63:479–487. [DOI] [PubMed] [Google Scholar]
- 50. Hu H, Chen KL, Ou YN, et al. Neurofilament light chain plasma concentration predicts neurodegeneration and clinical progression in nondemented elderly adults. Aging (Albany NY). 2019;11:6904–6914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Pereira JB, Janelidze S, Stomrud E, et al. Plasma markers predict changes in amyloid, tau, atrophy and cognition in non-demented subjects. Brain. 2021;144:2826–2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Verberk IMW, Thijssen E, Koelewijn J, et al. Combination of plasma amyloid beta(1–42/1–40) and glial fibrillary acidic protein strongly associates with cerebral amyloid pathology. Alzheimers Res Ther. 2020;12:118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Pereira JB, Westman E, Hansson O. Association between cerebrospinal fluid and plasma neurodegeneration biomarkers with brain atrophy in Alzheimer’s disease. Neurobiol Aging. 2017;58:14–29. [DOI] [PubMed] [Google Scholar]
- 54. Shi Y, Lu X, Zhang L, et al. Potential value of plasma amyloid-β, total tau, and neurofilament light for identification of early Alzheimer’s disease. ACS Chem Neurosci. 2019;10:3479–3485. [DOI] [PubMed] [Google Scholar]
- 55. Kang MS, Aliaga AA, Shin M, et al. Amyloid-beta modulates the association between neurofilament light chain and brain atrophy in Alzheimer’s disease. Mol Psychiatry. 2020;26:5989–6001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Jack CR, Petersen RC, Xu YC, et al. Medial temporal atrophy on MRI in normal aging and very mild Alzheimer’s disease. Neurology. 1997;49:786–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Whitwell JL, Przybelski SA, Weigand SD, et al. 3D Maps from multiple MRI illustrate changing atrophy patterns as subjects progress from mild cognitive impairment to Alzheimer’s disease. Brain. 2007;130:1777–1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Parbo P, Madsen LS, Ismail R, et al. Low plasma neurofilament light levels associated with raised cortical microglial activation suggest inflammation acts to protect prodromal Alzheimer’s disease. Alzheimers Res Ther. 2020;12:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Buckner RL, Snyder AZ, Shannon BJ, et al. Molecular, structural, and functional characterization of Alzheimer’s disease: Evidence for a relationship between default activity, amyloid, and memory. J Neurosci. 2005;25:7709–7717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Strittmatter WJ, Roses AD. Apolipoprotein E and Alzheimer disease. Proc Natl Acad Sci U S A. 1995;92:4725–4727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Benedet AL, Leuzy A, Pascoal TA, et al. Stage-specific links between plasma neurofilament light and imaging biomarkers of Alzheimer’s disease. Brain. 2020;143:3793–3804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Connolly K, Lehoux M, O’Rourke R, et al. Potential role of chitinase-3-like protein 1 (CHI3L1/YKL-40) in neurodegeneration and Alzheimer’s disease. Alzheimer’s Dement. 2023;19:9–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Schaeverbeke J, Gille B, Adamczuk K, et al. Cerebrospinal fluid levels of synaptic and neuronal integrity correlate with gray matter volume and amyloid load in the precuneus of cognitively intact older adults. J Neurochem. 2019;149:139–157. [DOI] [PubMed] [Google Scholar]
- 64. Gispert JD, Monté GC, Falcon C, et al. CSF YKL-40 and pTau181 are related to different cerebral morphometric patterns in early AD. Neurobiol Aging. 2016;38:47–55. [DOI] [PubMed] [Google Scholar]
- 65. Perez Ortiz JM, Swerdlow RH. Mitochondrial dysfunction in Alzheimer’s disease: Role in pathogenesis and novel therapeutic opportunities. Br J Pharmacol. 2019;176:3489–3507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Mosconi L. Brain glucose metabolism in the early and specific diagnosis of Alzheimer’s disease: FDG-PET studies in MCI and AD. Eur J Nucl Med Mol Imaging. 2005;32:486–510. [DOI] [PubMed] [Google Scholar]
- 67. Herholz K. PET Studies in dementia. Ann Nucl Med. 2003;17:79–89. [DOI] [PubMed] [Google Scholar]
- 68. La Joie R, Perrotin A, Barré L, et al. Region-specific hierarchy between atrophy, hypometabolism, and 2-amyloid (Aβ) load in Alzheimer’s disease dementia. J Neurosci. 2012;32:16265–16273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Cohen AD, Price JC, Weissfeld LA, et al. Basal cerebral metabolism may modulate the cognitive effects of aβ in mild cognitive impairment: An example of brain reserve. J Neurosci. 2009;29:14770–14778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Li Y, Rinne JO, Mosconi L, et al. Regional analysis of FDG and PIB-PET images in normal aging, mild cognitive impairment, and Alzheimer’s disease. Eur J Nucl Med Mol Imaging. 2008;35:2169–2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Mayeli M, Mirshahvalad SM, Aghamollaii V, Tafakhori A, Abdolalizadeh A, Rahmani F. Plasma neurofilament light chain levels are associated with cortical hypometabolism in Alzheimer disease signature regions. J Neuropathol Exp Neurol. 2019;78:709–716. [DOI] [PubMed] [Google Scholar]
- 72. Benedet AL, Ashton NJ, Pascoal TA, et al. Plasma neurofilament light associates with Alzheimer’s disease metabolic decline in amyloid-positive individuals. Alzheimers Dement (Amst). 2019;11:679–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Herholz K, Salmon E, Perani D, et al. Discrimination between Alzheimer dementia and controls by automated analysis of multicenter FDG PET. Neuroimage. 2002;17:302–316. [DOI] [PubMed] [Google Scholar]
- 74. Schultz SA, Strain JF, Adedokun A, et al. Serum neurofilament light chain levels are associated with white matter integrity in autosomal dominant Alzheimer’s disease. Neurobiol Dis. 2020;142:104960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Marks JD, Syrjanen JA, Graff-Radford J, et al. Comparison of plasma neurofilament light and total tau as neurodegeneration markers: Associations with cognitive and neuroimaging outcomes. Alzheimers Res Ther. 2021;13:199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Billingsley ML, Kincaid RL. Regulated phosphorylation and dephosphorylation of tau protein: Effects on microtubule interaction, intracellular trafficking and neurodegeneration. Biochem J. 1997;323:577–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Kanaan NM, Pigino GF, Brady ST, Lazarov O, Binder LI, Morfini GA. Axonal degeneration in Alzheimer’s disease: When signaling abnormalities meet the axonal transport system. Exp Neurol. 2013;246:44–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Nasrabady SE, Rizvi B, Goldman JE, Brickman AM. White matter changes in Alzheimer’s disease: A focus on myelin and oligodendrocytes. Acta Neuropathol Commun. 2018;6:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Sun Y, Tan L, Xu W, et al. Plasma neurofilament light and longitudinal progression of white matter hyperintensity in elderly persons without dementia. J Alzheimer’s Dis. 2020;75:729–737. [DOI] [PubMed] [Google Scholar]
- 80. Walsh P, Sudre CH, Fiford CM, et al. The age-dependent associations of white matter hyperintensities and neurofilament light in early- and late-stage Alzheimer’s disease. Neurobiol Aging. 2021;97:10–17. [DOI] [PubMed] [Google Scholar]
- 81. Boulanger JJ, Messier C. From precursors to myelinating oligodendrocytes: Contribution of intrinsic and extrinsic factors to white matter plasticity in the adult brain. Neuroscience. 2014;269:343–366. [DOI] [PubMed] [Google Scholar]
- 82. Raj D, Yin Z, Breur M, et al. Increased white matter inflammation in aging- and Alzheimer’s disease brain. Front Mol Neurosci. 2017;10:206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Satizabal CL, Zhu YC, Mazoyer B, Dufouil C, Tzourio C. Circulating IL-6 and CRP are associated with MRI findings in the elderly: the 3C-Dijon study. Neurology. 2012;78:720–727. [DOI] [PubMed] [Google Scholar]
- 84. Sexton CE, Kalu UG, Filippini N, Mackay CE, Ebmeier KP. A meta-analysis of diffusion tensor imaging in mild cognitive impairment and Alzheimer’s disease. Neurobiol Aging. 2011;32:2322.e5–2322.e18. [DOI] [PubMed] [Google Scholar]
- 85. Mayo CD, Mazerolle EL, Ritchie L, Fisk JD, Gawryluk JR. Neuroimage : Clinical longitudinal changes in microstructural white matter metrics in Alzheimer ’ s disease. NeuroImage Clin. 2017;13:330–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Ohlhauser L, Parker AF, Smart CM, Gawryluk JR. White matter and its relationship with cognition in subjective cognitive decline. Alzheimer’s Dement Diagnosis, Assess Dis Monit. 2019;11:28–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Back SA, Kroenke CD, Sherman LS, et al. White matter lesions defined by diffusion tensor imaging in older adults. Ann Neurol. 2011;70:465–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Kolasinski J, Stagg CJ, Chance SA, et al. A combined post-mortem magnetic resonance imaging and quantitative histological study of multiple sclerosis pathology. Brain. 2012;135:2938–2951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Peters JM, Struyven RR, Prohl AK, et al. White matter mean diffusivity correlates with myelination in tuberous sclerosis complex. Ann Clin Transl Neurol. 2019;6:1178–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Inglese M, Bester M. Diffusion imaging in multiple sclerosis: research and clinical implications. NMR Biomed. 2010;23:865–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Rovaris M, Gass A, Bammer R, et al. Diffusion MRI in multiple sclerosis. Neurology. 2005;65:1526–1532. [DOI] [PubMed] [Google Scholar]
- 92. van Leijsen EMC, Bergkamp MI, van Uden IWM, et al. Progression of white matter hyperintensities preceded by heterogeneous decline of microstructural integrity. Stroke. 2018;49:1386–1393. [DOI] [PubMed] [Google Scholar]
- 93. Reginold W, Sam K, Poublanc J, Fisher J, Crawley A, Mikulis DJ. Impact of white matter hyperintensities on surrounding white matter tracts. Neuroradiology. 2018;60:933–944. [DOI] [PubMed] [Google Scholar]
- 94. Muñoz Maniega S, Chappell FM, Valdés Hernández MC, et al. Integrity of normal-appearing white matter: Influence of age, visible lesion burden and hypertension in patients with small-vessel disease. J Cereb Blood Flow Metab. 2017;37:644–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Wen Q, Mustafi SM, Li J, et al. White matter alterations in early-stage Alzheimer’s disease: A tract-specific study. Alzheimer’s Dement Diagnosis. Assess Dis Monit. 2019;11:576–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Mito R, Raffelt D, Dhollander T, et al. Fibre-specific white matter reductions in Alzheimer’s disease and mild cognitive impairment. Brain. 2018;141:888–902. [DOI] [PubMed] [Google Scholar]
- 97. Zhang H, Schneider T, Wheeler-Kingshott CA, Alexander DC. NODDI: practical in vivo neurite orientation dispersion and density imaging of the human brain. Neuroimage. 2012;61:1000–1016. [DOI] [PubMed] [Google Scholar]
- 98. Mackay A, Whittall K, Adler J, Li D, Paty D, Graeb D. In vivo visualization of myelin water in brain by magnetic resonance. Magn Reson Med. 1994;31:673–677. [DOI] [PubMed] [Google Scholar]
- 99. Pereira JB, Janelidze S, Ossenkoppele R, et al. Untangling the association of amyloid-beta and tau with synaptic and axonal loss in Alzheimer’s disease. BRAIN. 2021;144:310–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Nomi JS, Bolt TS, Ezie CEC, Uddin LQ, Heller AS. Moment-to-Moment BOLD signal variability reflects regional changes in neural flexibility across the lifespan. J Neurosci. 2017;37:5539–5548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Garrett DD, Kovacevic N, McIntosh AR, Grady CL. The importance of being variable. J Neurosci. 2011;31:4496–4503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Millar PR, Ances BM, Gordon BA, et al. Evaluating resting-state BOLD variability in relation to biomarkers of preclinical Alzheimer’s disease. Neurobiol Aging. 2020;96:233–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Steinacker P, Anderl-Straub S, Diehl-Schmid J, et al. Serum neurofilament light chain in behavioral variant frontotemporal dementia. Neurology. 2018;91:E1390–E1401. [DOI] [PubMed] [Google Scholar]
- 104. Hampel H, Toschi N, Baldacci F, et al. Alzheimer’s disease biomarker-guided diagnostic workflow using the added value of six combined cerebrospinal fluid candidates: Aβ 1–42, total-tau, phosphorylated-tau, NFL, neurogranin, and YKL-40. Alzheimer’s Dement. 2018;14:492–501. [DOI] [PubMed] [Google Scholar]
- 105. Salvadó G, Ossenkoppele R, Ashton NJ, et al. Specific associations between plasma biomarkers and postmortem amyloid plaque and tau tangle loads. EMBO Mol Med. 2023;15:e17123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Huijbers W, Mormino EC, Schultz AP, et al. Amyloid-β deposition in mild cognitive impairment is associated with increased hippocampal activity, atrophy and clinical progression. Brain. 2015;138:1023–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Petersen RC, Wiste HJ, Weigand SD, et al. Association of elevated amyloid levels with cognition and biomarkers in cognitively normal people from the community. JAMA Neurol. 2016;73:85–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Armstrong NM, Huang CW, Williams OA, et al. Sex differences in the association between amyloid and longitudinal brain volume change in cognitively normal older adults. NeuroImage Clin. 2019;22:101769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Bos I, Vos S, Verhey F, et al. Cerebrospinal fluid biomarkers of neurodegeneration, synaptic integrity, and astroglial activation across the clinical Alzheimer’s disease spectrum. Alzheimer’s Dement. 2019;15:644–654. [DOI] [PubMed] [Google Scholar]
- 110. Melah KE, Lu SYF, Hoscheidt SM, et al. Cerebrospinal fluid markers of Alzheimer’s disease pathology and microglial activation are associated with altered white matter microstructure in asymptomatic adults at risk for Alzheimer’s disease. J Alzheimer’s Dis. 2016;50:873–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Lee S, Sato Y, Nixon RA. Lysosomal proteolysis inhibition selectively disrupts axonal transport of degradative organelles and causes an Alzheimer’s-like axonal dystrophy. J Neurosci. 2011;31:7817–7830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Back SA, Gan X, Li Y, Rosenberg PA, Volpe JJ. Maturation-dependent vulnerability of oligodendrocytes to oxidative stress-induced death caused by glutathione depletion. J Neurosci. 1998;18:6241–6253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. French HM, Reid M, Mamontov P, Simmons RA, Grinspan JB. Oxidative stress disrupts oligodendrocyte maturation. J Neurosci Res. 2009;87:3076–3087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Tönnies E, Trushina E. Oxidative stress, synaptic dysfunction, and Alzheimer’s disease. J Alzheimer’s Dis. 2017;57:1105–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Hoover BR, Reed MN, Su J, et al. Tau mislocalization to dendritic spines mediates synaptic dysfunction independently of neurodegeneration. Neuron. 2010;68:1067–1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Cavedo E, Lista S, Houot M, et al. Plasma tau correlates with basal forebrain atrophy rates in people at risk for Alzheimer disease. Neurology. 2020;94(1 PG-30-41):e30–e41. [DOI] [PubMed] [Google Scholar]
- 117. Cantero JL, Atienza M, Lage C, et al. Atrophy of basal forebrain initiates with tau pathology in individuals at risk for Alzheimer’s disease. Cereb Cortex. 2020;30:2083–2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Tiernan CT, Mufson EJ, Kanaan NM, Counts SE. Tau oligomer pathology in nucleus basalis neurons during the progression of Alzheimer disease. J Neuropathol Exp Neurol. 2018;77:246–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Fernández-Cabello S, Kronbichler M, van Dijk KRA, Goodman JA, Spreng RN, Schmitz TW. Basal forebrain volume reliably predicts the cortical spread of Alzheimer’s degeneration. Brain. 2020;143:993–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Schmitz TW, Spreng RN. Basal forebrain degeneration precedes and predicts the cortical spread of Alzheimer’s pathology. Nat Commun. 2016;7:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Zhuang L, Sachdev PS, Trollor JN, et al. Microstructural white matter changes, not hippocampal atrophy, detect early amnestic mild cognitive impairment. PLoS One. 2013;8:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Selnes P, Fjell AM, Gjerstad L, et al. White matter imaging changes in subjective and mild cognitive impairment. Alzheimer’s Dement. 2012;8(SUPPL. 5):S112–S121. [DOI] [PubMed] [Google Scholar]
- 123. Coleman M. Axon degeneration mechanisms: Commonality amid diversity. Nat Rev Neurosci. 2005;6:889–898. [DOI] [PubMed] [Google Scholar]
- 124. Chung SJ, Jeon S, Yoo HS, et al. Neural correlates of cognitive performance in Alzheimer’s disease- and Lewy bodies-related cognitive impairment. J Alzheimers Dis. 2020;73:873–885. [DOI] [PubMed] [Google Scholar]
- 125. Leys D, Pruvo JP, Parent M, et al. Could wallerian degeneration contribute to “leuko-araiosis” in subjects free of any vascular disorder? J Neurol Neurosurg Psychiatry. 1991;54:46–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Beaulieu C. The biological basis of diffusion anisotropy. In: Johansen-Berg H, Behrens TEJ, eds. Diffusion MRI. 2nd ed. Academic Press; 2014:155–183. [Google Scholar]
- 127. Hardy JA, Higgins GA. Alzheimer’s disease: The amyloid cascade hypothesis. Science (80-). 1992;256:184–185. [DOI] [PubMed] [Google Scholar]
- 128. Selkoe DJ, Hardy J. The amyloid hypothesis of Alzheimer’s disease at 25 years. EMBO Mol Med. 2016;8:595–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Hadjichrysanthou C, Evans S, Bajaj S, et al. The dynamics of biomarkers across the clinical spectrum of Alzheimer’s disease. Alzheimers Res Ther. 2020;12:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Stomrud E, Minthon L, Zetterberg H, Blennow K, Hansson O. Longitudinal cerebrospinal fluid biomarker measurements in preclinical sporadic Alzheimer’s disease: A prospective 9-year study. Alzheimers Dement (Amst). 2015;1:403–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Buchhave P, Minthon L, Zetterberg H, Wallin ÅK, Blennow K, Hansson O. Cerebrospinal fluid levels of β-amyloid 1–42, but not of tau, are fully changed already 5 to 10 years before the onset of Alzheimer dementia. Arch Gen Psychiatry. 2012;69:98–106. [DOI] [PubMed] [Google Scholar]
- 132. Rauchmann BS, Schneider-Axmann T, Perneczky R. Associations of longitudinal plasma p-tau181 and NfL with tau-PET, Aβ-PET and cognition. J Neurol Neurosurg Psychiatry. 2021;92:1289–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Giacomucci G, Mazzeo S, Bagnoli S, et al. Plasma neurofilament light chain as a biomarker of Alzheimer’s disease in subjective cognitive decline and mild cognitive impairment. J Neurol. 2022;269:4270–4280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Kalm M, Boström M, Sandelius Å, et al. Serum concentrations of the axonal injury marker neurofilament light protein are not influenced by blood-brain barrier permeability. Brain Res. 2017;1668:12–19. [DOI] [PubMed] [Google Scholar]
- 135. Uher T, McComb M, Galkin S, et al. Neurofilament levels are associated with blood–brain barrier integrity, lymphocyte extravasation, and risk factors following the first demyelinating event in multiple sclerosis. Mult Scler J. 2021;27:220–231. [DOI] [PubMed] [Google Scholar]
- 136. Trojanowski JQ, Walkenstein N, Lee VMY. Expression of neurofilament subunits in neurons of the central and peripheral nervous system: An immunohistochemical study with monoclonal antibodies. J Neurosci. 1986;6:650–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Schlaepfer WW, Lynch RG. Immunofluorescence studies of neurofilaments in the rat and human peripheral and central nervous system. J Cell Biol. 1977;74:241–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Mariotto S, Farinazzo A, Magliozzi R, Alberti D, Monaco S, Ferrari S. Serum and cerebrospinal neurofilament light chain levels in patients with acquired peripheral neuropathies. J Peripher Nerv Syst. 2018;23:174–177. [DOI] [PubMed] [Google Scholar]
- 139. Sandelius Å, Zetterberg H, Blennow K, et al. Plasma neurofilament light chain concentration in the inherited peripheral neuropathies. Neurology. 2018;90:e518–e524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Polymeris AA, Coslovksy M, Aeschbacher S, et al. Serum neurofilament light in atrial fibrillation: Clinical, neuroimaging and cognitive correlates. Brain Commun. 2020;2:fcaa166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Ciardullo S, Muraca E, Bianconi E, et al. Diabetes Mellitus is associated with higher Serum neurofilament light chain levels in the general US population. J Clin Endocrinol Metab. 2023;108:361–367. [DOI] [PubMed] [Google Scholar]
- 142. Akamine S, Marutani N, Kanayama D, et al. Renal function is associated with blood neurofilament light chain level in older adults. Sci Rep. 2020;10:20350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Martínez-Morillo E, García Hernández P, Begcevic I, et al. Identification of novel biomarkers of brain damage in patients with hemorrhagic stroke by integrating bioinformatics and mass spectrometry-based proteomics. J Proteome Res. 2014;13:969–981. [DOI] [PubMed] [Google Scholar]
- 144. Sabbatini D, Raggi F, Ruggero S, et al. Evaluation of peripherin in biofluids of patients with motor neuron diseases. Ann Clin Transl Neurol. 2021;8:1750–1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Palmqvist S, Tideman P, Cullen N, et al. Prediction of future Alzheimer’s disease dementia using plasma phospho-tau combined with other accessible measures. Nat Med. 2021;27:1034–1042. [DOI] [PubMed] [Google Scholar]
- 146. Hviid CVB, Knudsen CS, Parkner T. Reference interval and preanalytical properties of serum neurofilament light chain in Scandinavian adults. Scand J Clin Lab Invest. 2020;80:291–295. [DOI] [PubMed] [Google Scholar]
- 147. Yilmaz A, Blennow K, Hagberg L, et al. Neurofilament light chain protein as a marker of neuronal injury: Review of its use in HIV-1 infection and reference values for HIV-negative controls. Expert Rev Mol Diagn. 2017;17:761–770. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.