Abstract
Metformin was developed from an offshoot of Guanidine. It is known to be the first-line medication for type 2 diabetes mellitus, polycystic ovarian syndrome, and weight reduction. Metformin has also been shown to have effectiveness in the management of non-alcoholic fatty liver disease (NAFLD), liver cirrhosis, and various carcinomas like hepatocellular, colorectal, prostate, breast, urinary bladder, blood, melanoma, bone, skin, lung and so on. This narrative review focuses on the effect of metformin on non-alcoholic fatty liver disease, liver cirrhosis, and hepatocellular carcinoma. The search platforms for the topic were PubMed, Scopus, and Google search engine. Critical words for searching included 'Metformin,' AND 'Indications of Metformin,' AND 'Non-Alcoholic Fatty Liver Disease,' AND 'Metformin mechanism of action,' AND 'NAFLD management,' AND 'NAFLD and inflammation,' AND 'Metformin and insulin,' AND 'Metformin and inflammation,' AND 'Liver cirrhosis,' AND 'Hepatocellular carcinoma.' Lifestyle modification and the use of hypoglycemic agents can help improve liver conditions. Metformin has several mechanisms that enhance liver health, including reducing reactive oxygen species, nuclear factor kappa beta (NF-κB), liver enzymes, improving insulin sensitivity, and improving hepatic cell lipophagy. Long-term use of metformin may cause some adverse effects like lactic acidosis and gastrointestinal disturbance. Metformin long-term overdose may lead to a rise in hydrogen sulfide in liver cells, which calls for pharmacovigilance. Drug regulating authorities should provide approval for further research, and national and international guidelines need to be developed for liver diseases, perhaps with the inclusion of metformin as part of the management regime.
Keywords: hepatic diseases and enzymes, inflammation and oxidative stress, insulin sensitivity, lifestyle, lipophagy, liver enzymes, metformin, nafld, novel treatment protective mechanism and healing, pharmacovigilance
Introduction and background
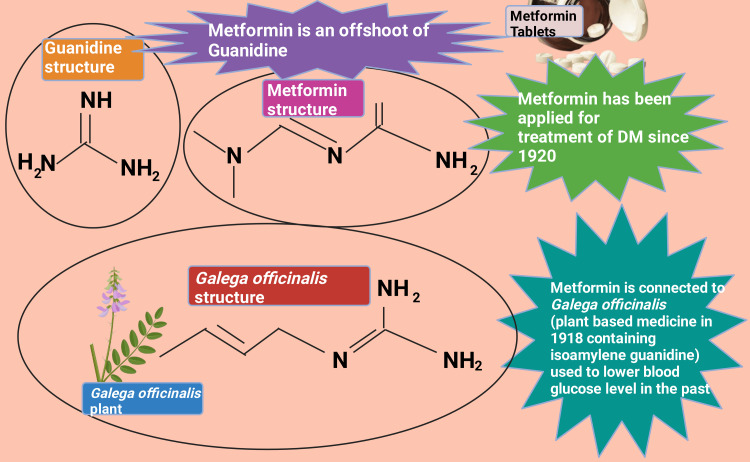
Metformin (dimethylbiguanide) was first introduced in France in 1957 by the French physician Jean Sterne (1909-1997) [1]. However, metformin was earliest portrayed in a scholarly peer-reviewed scientific journal by Emil Werner and James Bell in 1922 [2]. Metformin narration is connected to Galega officinalis (also known as Goat's rue, French lilac, Italian fitch, Spanish sainfoin, professor weed), a long-established plant-originated medicine in medieval Europe in 1918 [3-5]. Galega officinalis extract contained a considerable portion of isoamylene guanidine (galegine) and was demonstrated to lower blood glucose in 1918 [6-9]. Metformin, an offshoot of guanidine, was applied to treat type 2 diabetes mellitus (T2DM) from 1920 to 1930 (Figure 1) [1,5,6,10-12]. Nonetheless, Galega officinalis clinical utilization was ended due to adverse drug reaction (ADR) and the increased availability of insulin [1,13]. It was evidenced that metformin possesses antiviral potential [1,14-18]. This beneficial antiviral pharmacology was observed by scientists exploring anti-malarial medicine in the 1940s [1,19]. Nonetheless, metformin, from time to time, brings down blood glucose levels while treating influenza [1,14,20].
Figure 1. Metformin origin and history.
DM: diabetes mellitus.
This figure was drawn using the premium version of BioRender (https://biorender.com/), accessed on October 9, 2024, with the agreement license number PI27EIRU2R [10].
Image credit: Rahnuma Ahmad.
Diabetes mellitus (DM) is a persistent diverse metabolic disorder that has become a global epidemic and is principally caused by low synthesizing (availability) endogenous insulin from beta-cells of the pancreas and reduced sensitivity [21,22]. There are types of DM: type 1 (Insulin-dependent DM (IDDM)) and type 2 (non-insulin-dependent DM (NIDDM)) [23]. T2DM (NIDDM) is also acknowledged as adult-onset diabetes and comprises around 90-95% of all cases of DM [21,24,25]. T2DM is illustrated by two dominant insulin-associated incongruities: insulin resistance and β-cell dysfunction [26,27]. Globally, T2DM is considered the principal impelling force behind the death of 1.6 million individuals [28]. Largely, metformin is regarded as the first-line medication for T2DM [29]. Sharma et al. (2016) reported that 83.6% of British T2DM patients were prescribed metformin [30]. Pandya et al. (2023) reported that metformin occupies the bulk share (two-thirds) of the oral glucose-lowering medications in the USA. Additionally, metformin prescribed among T2DM cases receiving any rally consumed medication were 64%,66%,67%, 68%, and 68% in 2016, 2017, 2018, 2019, and 2020, respectively [31]. Overbeek et al. (2017) reported that metformin remains the most preferred blood glucose-lowering medication across all European countries, and utilization of this euglycemic agent has been observed to increase [32].
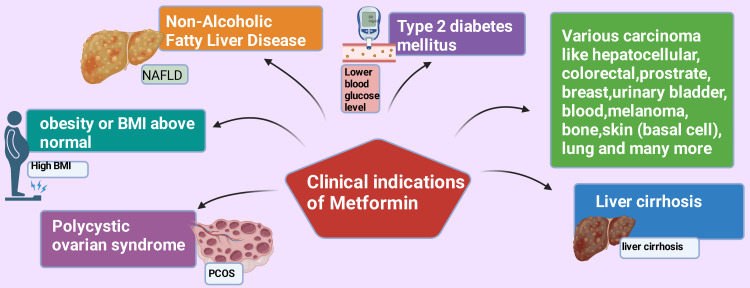
Naseri et al. (2022) reported that metformin is primarily prescribed for T2DM, PCOS, and weight reduction [33]. Various research studies are currently being conducted regarding metformin, and other reasonable clinical indications are transpiring that this medicine can be applied for purposes other than DM [34-38]. Those clinical indications include non-alcoholic fatty liver disease (NAFLD) [39], liver cirrhosis [40], various carcinoma, such as hepatocellular (HCC) [41,42], colorectal [43], prostate [44-46], breast [47,48], urinary bladder [49-51], blood [52,53], melanoma [54-56], bone [57-59], skin (basal cell) [60], lung [61,62], and many more (Figure 2). This narrative review paper will primarily concentrate on NAFLD, liver cirrhosis, and hepatocellular carcinoma.
Figure 2. Clinical indications of metformin.
BMI: body mass index.
This figure was drawn using the premium version of BioRender (https://biorender.com/), accessed on October 2, 2024, with license number AF27DL49UX [10].
Image credit: Rahnuma Ahmad.
Review
Materials and methods
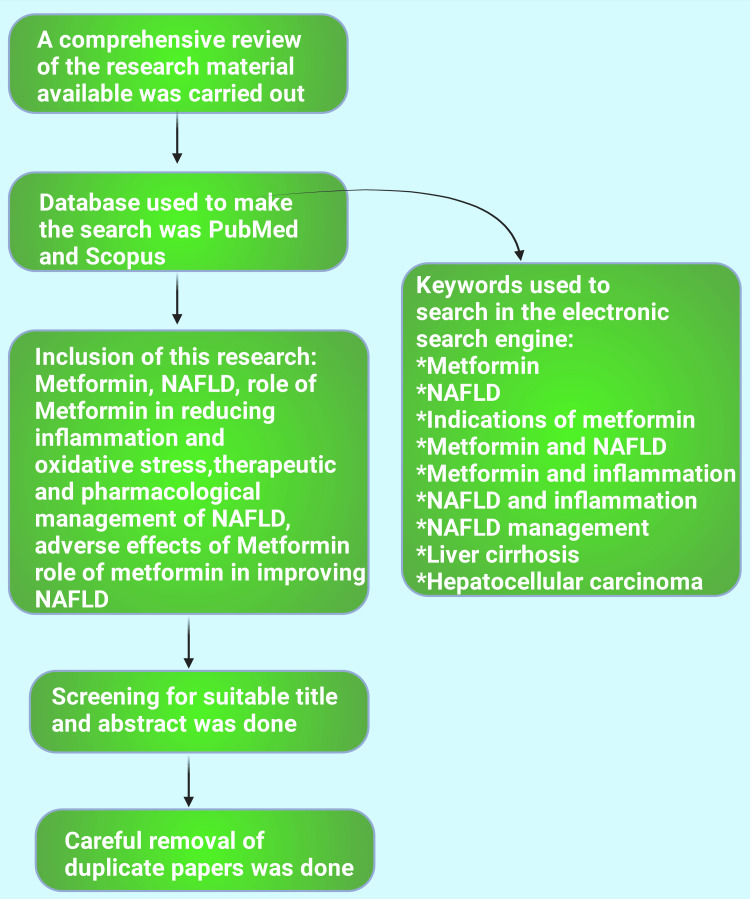
This narrative review delves into the role of metformin in managing conditions like non-alcoholic fatty liver disease. Research has also been carried out on the literature available regarding the current epidemiology of NAFLD and the possible therapeutic and pharmacological management of NAFLD. The role of metformin in reducing oxidative stress and inflammation and its effect on improving NAFLD have also been highlighted. The information needed for this research was gathered between July 2024 and September 2024, employing the data offered by Scopus, PubMed, and Google Scholar. Keywords for the search were 'Metformin,' AND 'Indications of Metformin,' AND 'Non-Alcoholic Fatty Liver Disease,' AND 'Metformin mechanism of action,' 'NAFLD management,' AND 'NAFLD and inflammation,' AND 'Metformin and insulin,' AND 'Metformin and inflammation,' AND 'Liver cirrhosis,' AND 'Hepatocellular carcinoma' (Figure 3).
Figure 3. Flowchart depicting the materials and method section of the current study.
NAFLD: Non-alcoholic fatty liver disease.
This figure was drawn using the premium version of BioRender (https://biorender.com/), accessed on September 20, 2024 with the agreement license number DV27CQSXP4 [10].
Image credit: Rahnuma Ahmad.
Review of the literature
Non-alcoholic Fatty Liver Disease
Global epidemiology of NAFLD: NAFLD is a comprehensive appellation for a range of disorders when fatty degeneration is detected through histopathological examination over 5% of hepatic cells and concurrently presence of metabolic syndrome precepting features (predominantly T2DM and obesity), disregard of excessive regular alcohol drinking or other long-lasting liver diseases [63-65]. Teng et al. (2023) reported that NAFLD is a prominent basis of hepatic disorders globally. It has been appraised that worldwide incidence among 1,000 populace 47 suffers from NAFLD and more seen among adults and males in comparison to pediatric community and females, respectively [66]. Riazi et al. recently published one systematic review and meta-analysis appraising that over 32% of adult people around the globe were stricken by NAFLD [67]. Another similar study by Younossi et al. 2023 revealed that over 30% of the population of our planet was suffering from NAFLD [68]. Multiple studies reported that in the past 30 years, the prevalence of NAFLD increased from 25 to 38% [68]. The maximum NAFLD frequency was in Latin America 44.37% (30.66%-59.00%), then the Middle East and North Africa (MENA) (36.53%, 28.63%-45.22%), South Asia (33.83%, 22.91%-46.79%), Southeast Asia (33.07%, 18.99%-51.03%), North America (31.20%, 25.86%-37.08%), East Asia (29.71%, 25.96%-33.76%), Asia Pacific 28.02% (24.69%-31.60%), and Western Europe 25.10% (20.55%-30.28%) [68]. Multiple studies reported that the worldwide occurrence of NAFLD was 38% [68-70]. It has been reported that the occurrence of NAFLD in the USA increased from 38 to 50% in the last three decennaries [70]. Ye et al. (2020) reported that in some nations, such as Malaysia and Pakistan, NAFLD was 25% or lower among non-obese subjects. Nonetheless, the prevalence was 50% or more in Mexico, Sweden, and Austria [71].
Asian epidemiology of non-alcoholic fatty liver disease: In Asia, for example, NAFLD-related health liability was detected in the uppermost (51.04%) and bottommost (22.28%) areas of Indonesia and Japan, respectively [72]. In India, the occurrence of NAFLD in both sexes was similar and generally pooled a commonness of 38.6% and 35.4% among adults and pediatric cases [73]. Various research groups reported that the Chinese mainland population had NAFLD ∼15% [74], 29.6% [75], 30% [76], 36.9% [77], and 44.39% [78]. However, another study conducted in Shanghai, China, reported that 5.07% of the pediatric population had fatty liver disease (FLD) [77]. The global prevalence of children and adolescents (below 18 years) NAFLD differs inter and intra-country. Among the pediatric obese population, it was 52.49% and 7.40% in non-obese pediatric cases. It has been estimated to reach up to 30.7% by 2040" [79]. The prevalence of primarily over ¼ of the Japanese population, men, was statistically (p<0.001) higher than women. It has been estimated that 39.3% and 44.8% of Japan's population will possibly be affected by NAFLD by 2030 and 2040, respectively [80]. A systematic review and meta-analysis were conducted among studies of the Kingdom of Saudi Arabia (KSA). Eight studies that included 4045 adult NAFLD cases were included. The pooled incidence of NAFLD among the study participants was 16.8% (11.1-22.5%). Additionally, 58% (45-70.9%) of these NAFLD were concurrently suffering from T2DM [81]. It has been estimated that NAFLD incidence in KSA will go beyond 30% by 2030 [82]. The prevalence rate of NAFLD in Indonesia is 51% [83]. In Malaysia, two studies published in 2013 and 2018 reported that NAFLD was 22.7% and 37.4%, respectively [84,85]. Proton-magnetic resonance spectroscopy and transient elastography are identified as exceedingly precise diagnostic devices to determine hepatic fatty degeneration in one most extensive population‐based analysis among the Asian population revealed that NAFLD is considerably increasing in this continent. In these studied populations, 80% and 5% had all five components without any features of metabolic syndrome (MetS) [86].
Therapeutic Intervention of NAFLD
Mayo Clinic of the United States of America recommended that medical intervention for NAFLD typically begins with reducing body weight. Consumption of a healthy nutritional diet, strictly avoiding energy-dense carbohydrate-containing food, and restraining amount of food and aerobic physical activity exercise. Reducing body weight and obesity often helps to minimize other potential health disorders that lead to NAFLD. Archetypally, it has been advised that lowering body weight by 10% or more has a beneficial impact on NAFLD [87]. It has been reported that more weight loss (10% or more) offers more benefits for NAFLD cases and possibly overthrows fatty liver hepatitis and even hepatic fibrosis [88]. Lifestyle intercessions constructed on modest to intense physical activity and a healthy eating plan and practice remain the principle of NAFLD non-pharmacological management [89-92]. It has advocated that "the Mediterranean diet is regarded as the diet of choice for the prevention/treatment of NAFLD and its complications, based on the available evidence" [93].
Dietary restriction and increased physical activity persist in the strategic remedial components to combat the worldwide health-related heavy impediment of hepatic fatty degeneration disorders [94-99]. Multiple studies reported that consuming low-energy-dense (strict avoidance carbohydrate) food, thereby limiting high-energy units, positively impacts MetS and minimizes the severity of NAFLD [100-103]. Various studies reported that sporadic energy-constraint food consumption (rigorous cutback of carbohydrate-rich foods) promotes ketogenesis and appears as the principal systematic feature of dietary interventions for managing NAFLD [104-110]. The ketogenic diet is an efficient intervention for the management of NAFLD. It is substantiated that liver "mitochondrial fluxes and redox state" are noticeably transformed throughout the ketogenic diet-persuaded improvement of NAFLD in humans [104].
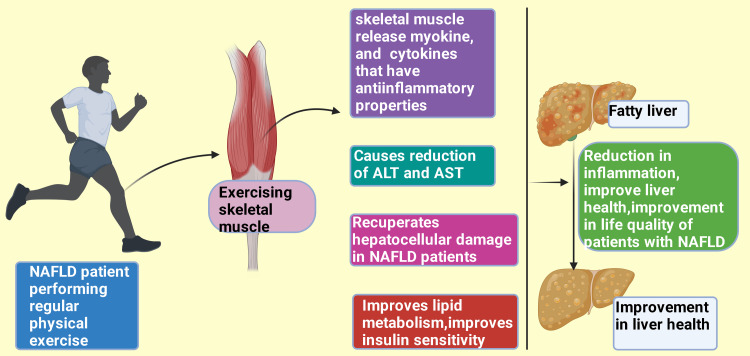
Adequate aerobic physical exercise of modest intensity (150-240 minutes per week) single-handedly salvages abnormal preservation of lipids (fat) in the liver and viscera, avert fibrosis and cirrhosis, and diminish fatal outcomes [111-113]. Health-enriching physical exercise drops the possibility of the development of equally obese NAFLD and non-obese NAFLD among Asians. In contrast, the likelihood of developing NAFLD among slim individuals was considerably minimal, even if they were nominally doing physical exercise compared to inactive lean cases [114,115]. Skeletal muscle often demarcated an endocrine organ [116,117] discharges cytokines and myokines while contracting or functioning. These cytokines and myokines possess anti-inflammatory properties, especially among hepatic and adipose tissue [118].
Furthermore, moderate to intense physical activity considerably reduces alanine aminotransferase (ALT) and aspartate aminotransferase (AST) and recuperates the hepatocellular damage among individuals with NAFLD [114,119]. High-level ALT and AST indicate hepatocellular injury (Figure 4) [120,121]. Xue et al. (2024) conducted one systematic review and meta-analysis and reported that regular physical activity reduces hepatic fat substances, fosters blood lipid metabolism, and improves the quality of life among patients suffering from NAFLD [122].
Figure 4. Physical exercise improves liver health in NAFLD patients.
NAFLD: Non-alcoholic fatty liver disease; ALT: Alanine aminotransferase; AST: Aspartate aminotransferase.
This figure was drawn using the premium version of BioRender (https://biorender.com/), accessed on September 21, 2024, with the agreement license number AB27C1IKPY [10].
Image credit: Rahnuma Ahmad
Pharmacological Intervention for the Management of NAFLD
To date, the United States Food and Drug Administration (USFDA) and European Medicines Agency (EMA) have not approved any medication for the management of NAFLD [123]. Consequently, any medication presently utilized for treating NAFLD must be considered "off-label use" [124,125]. Multiple systematic reviews and meta-analyses reported that medication for T2DM, hyperlipidemia, and other issues of MetS that USFDA and EMA approve for mentioned diseases is often used and improves NAFLD [126-131]. Insulin resistance (IR) is a foremost procedure in the evolution and progression of NAFLD [132-136]. Hence, medications possess potential pharmacodynamics to increase insulin sensitivity, congregating much attention for utilization for NAFLD or non-alcoholic steatohepatitis (NASH) (the most severe form of NAFLD) [137-141]. Kumar et al. reported that sustained high blood glucose levels promote diverse impediments comprising renal disorders, hepatic cirrhosis, and HCC [142]. Myriad aspects cause the development of liver-related disorders, including HCC involving IR and oxidative stress [143,144]. Oxidative stress remains a critical issue in the evolution of IR and DM, as well as many other impediments of DM, such as microvascular and cardiovascular issues [145]. Multiple studies reported that oxidative stress promotes the synthesis of insulin-degrading enzyme (IDE) and biliverdin reductase-A (BVR-A), thereby causing IRV [146,147].
Thiazolidinediones (TDZs), e.g., pioglitazone, rosiglitazone [148,149], glucagon-like peptide-1 (GLP-1) receptor agonists (GLP-1RAs), e.g., lixisenatide, liraglutide, dulaglutide, semaglutide [148,150,151] and sodium-glucose transport protein 2 (SGLT2) inhibitors, e.g., empagliflozin, dapagliflozin, canagliflozin, and ertugliflozin [152,153] are effective in controlling the blood glucose level, reducing risk of cardiovascular diseases, and giving the positive clinical outcome of diverse liver disorders including NAFLD [154-156]. Medications, TDZs [148], GLP-1RAs [150], and SGLT2 antagonists, control hyperglycemia, lower HbA1C [157], and improve T2DM and cardiovascular issues, favorable alteration of serum lipid profile, and additionally fatty degeneration of the liver. Multiple research projects revealed that diet control with a wholesome, balanced diet and increased physical activity remain predominant features for managing NAFLD [158-160].
Metformin and Non-alcoholic Fatty Liver Disease
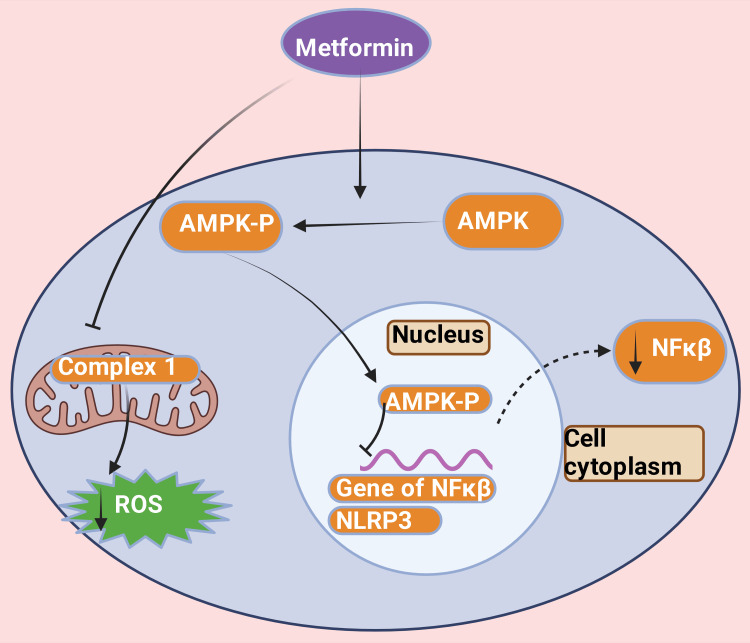
Metformin, a typical insulin sensitizer medication, has been extensively prescribed around the globe among T2DM cases [39,161,162]. By increasing insulin sensitivity, metformin reduces hyperglycemia IR and controls serum glucose level-induced T2DM [162,163]. Metformin impedes nuclear factor kappa B (NF-κB) signaling [164,165] and nucleotide-binding domain, leucine-rich-containing family, pyrin domain-containing-3 (NLRP3) inflammasome course and restricting reactive oxygen species (ROS) synthesis by macrophages through an AMP-activated protein kinase (AMPK) reliant or self-reliant, demeanor (Figure 5) [166-168]. Thereby, metformin deters the transformation of monocytes into macrophages [169,170]. This process escalates ATP cassette transporter type 1 (ABCA-1) endeavor [171], thereby fostering the dissemination of cholesterol from lipid-rich macrophages and enhancing high-density lipoprotein cholesterol (HDL-c) activity [172]. Therefore, it minimizes leukocyte-endothelium communication [173]. Hence, metformin decreases the inflammatory immune response and improves organ recovery induced by T2DM [174,175]. The long-standing second-rate inflammatory process plays a leading role in several non-communicable diseases because of unrelenting raised intensities of circulating pro-inflammatory cytokines throughout the lifetime [176-179]. These diseases included hepatic (severe form of NAFLD [39,180-182], NASH, fibrosis, cirrhosis, hepatocellular carcinoma) and extrahepatic (cardiovascular diseases, T2DM, renal disorders, etc.) [183-187].
Figure 5. Role of metformin in reducing the production of reactive oxygen species, nuclear factor kappa beta, and NLRP3.
Metformin phosphorylates AMPK, which enters the nucleus and impedes NF-κB signaling and nucleotide-binding domain, leucine-rich family, pyrin domain-containing-3 (NLRP3) inflammasome course and restricting reactive oxygen species (ROS) synthesis by inhibiting complex 1 in mitochondria.
NF-κB: nuclear factor kappa beta; NLRP3: nucleotide-binding domain, leucine-rich–containing family, pyrin domain–containing-3; ROS: reactive oxygen species; AMPK: AMP-activated protein kinase; AMPK-P: phosphorylated AMP-activated protein kinase.
This figure was drawn using the premium version of BioRender (https://biorender.com/), accessed on September 23, 2024, with the agreement license number OL27CBTPV1 [10].
Image credit: Rahnuma Ahmad
A Brief Portrayal of Metformin Action Regarding the Management of NAFLD
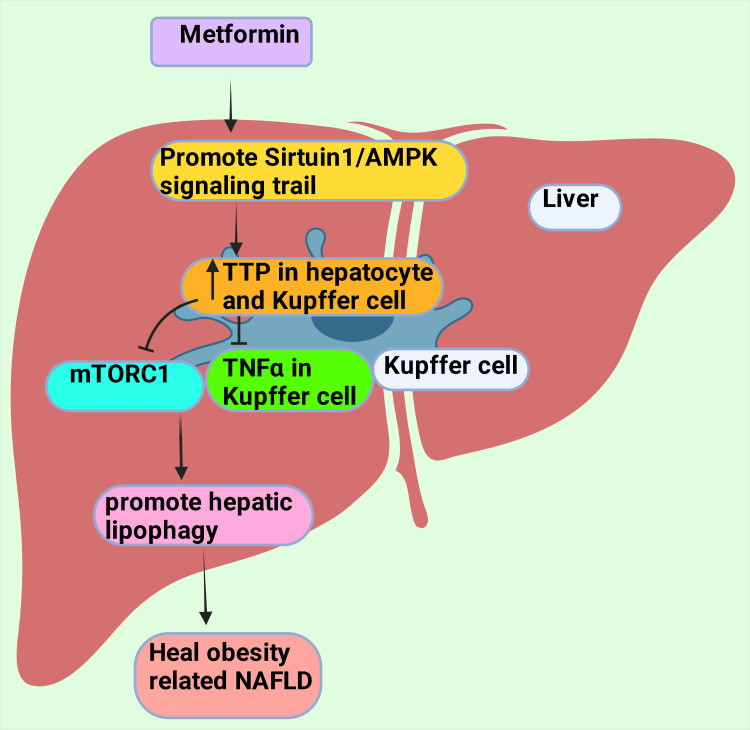
Metformin-provoked diminution of severity of NAFLD, possibly because of the amiable roles of Kupffer cells (KCs) and hepatocytes. This process is arbitrated by the existence of an mRNA-binding protein named tristetraprolin (TTP) [188,189]. Metformin actuates TTP in hepatocytes and KCs by the Sirtuin 1 (Sirt1)/AMPK signaling trail [190,191]. TTP inhibits the synthesis of tumor necrosis factor-alpha (TNF-α) in KCs, resulting in a drop in hepatocellular necroptosis [187]. Metformin stimulates TTP activation that deters the mammalian target of rapamycin complex-1 or mechanistic target of rapamycin complex 1 (mTORC1) through undermines Ras homolog enriched in the brain (RHEB) [192,193]. It ultimately upholds transcription factor EB (TFEB) and causes the nuclear transfer to foster hepatic cell lipophagy (a particular type of autophagy), healing obesity-related NAFLD (Figure 6) [188,190,194].
Figure 6. Metformin promotes lipophagy in hepatic cells by inhibiting TNFα and mTORC1 via actuation of TTP through the Sirtuin 1/AMPK signaling trail.
TNF α: Tumor necrosis factor-alpha; TTP: tristetraprolin; mTORC1: mammalian target of rapamycin complex 1; AMPK: AMP-activated protein kinase.
This figure was drawn using the premium version of BioRender (https://biorender.com/), accessed on September 25, 2024, with the agreement license number VX27CL769T [10].
Image credit: Rahnuma Ahmad
Multiple fatty hepatic genes patatin-like phospholipase domain-containing protein 3 (PNPLA3), transmembrane 6 superfamily member 2 (TM6SF2), hydroxysteroid 17-beta-dehydrogenase 13 (HSD17B13), and membrane-bound O-acyltransferase domain-containing protein 7 ((MBOAT7) also known as lysophospholipid acyltransferase 7) are responsible for the development NAFLD and NASH have been identified [195-197]. Multiple studies reported that the rs738409 variant of the PNPLA3 gene was responsible factor for progression of hepatic fibrosis, NAFLD/NASH, and higher risk of emerging HCC [198-200] among Japanese [201], Hmong population (currently Hmong people principally live in countries in Southeast Asia such as Myanmar, Thailand, Vietnam, Laos, and also in Southwest China) [202], Brazil (both among whites, blacks, and pardo) [203] multi-ethnic group of Malaysia (Malay, Chinese, and Indian) [204], Thailand [205], Guatemala [206], and many other countries. PNPLA3 polymorphism variant rs738409 plays a typical and mightiest gene in developing NAFLD [207,208]. Metformin modifies NAFLD in the development of gene expression [39,209]. These genes are responsible for inflammation in hepatic issues [208], thereby reducing hepatic fibrosis and stiffness and improving NAFLD [209]. It has been reported that metformin 500 mg 3 times daily for four months reduces hepatic transaminase (both ALT and AST) concentrations and improves hepatic insulin sensitivity [210]. Krakoff et al. (2010) reported that metformin steadily lowers serum ALT; nevertheless, body weight management and lifestyle alteration remain the principal priorities of the NAFLD therapeutic intervention strategy [211]. Multiple studies reported that metformin minimizes serum AST and ALT levels, improves hepatic physiology, vital body measurements, homeostatic model assessment for insulin resistance (HOMA-IR), body mass index (BMI), hepatic steatosis index (HSI), and metabolic variable among cases NAFLD with or without distinction DM [212-215]. However, Zhang et al., in their network meta-analysis, reported that metformin had a positive impact in minimizing ALT levels. Nevertheless, saroglitazar (a dual peroxisome proliferator-activated receptor (PPAR) α/γ agonist) efficacy was higher in comparison to metformin [216]. The Drug Controller General of India (DCGI) approved saroglitazar to treat NAFLD and NASH in March 2020, and earlier, it was permitted for diabetic dyslipidemia and hypertriglyceridemia [217-219]. Nevertheless, saroglitazar has not been allowed for NAFLD and NASH by drug regulatory authorities of several countries around the globe [220]. Saroglitazar, up to the present time, is in phase 2 trial in the USA and has yet to be approved by the FDA for NAFLD and NASH [221]. Huang et al. 2022 propose that metformin could be a repositioning medication for the treatment of NAFLD with high levels of ALT, AST, triglyceride (TG), total cholesterol (TC), and IR [182]. Another metanalysis revealed that TDZs, GLP-1RAs, and metformin (in particular, pioglitazone) were the most promising therapeutic appears for pharmacological therapeutic options for NAFLD management; nevertheless, considerable weight gain remains as ADRs [222]. Similarly, as Petrie (2024) reported in his review paper, metformin, regarding the management of metabolic dysfunction-associated steatotic liver disease ((MASLD) (previously designated as NAFLD), should be considered a repurposing medicine [223]. Gkiourtzis et al. (2023) in their meta-analysis utilized pediatric NAFLD patients, placebo, and metformin as control and experimental groups, respectively, revealed adequate safety issues or minimum ADRs. Metformin possesses pharmacodynamics in improving insulin and lipid-related parameters among pediatric obese NAFLD cases [224].
A Concise Depiction of Other Serum Glucose-Lowering Agents Except Insulin for the Management of Non-Alcoholic Fatty Liver Disease
It has been reported that worldwide, T2DM and NAFLD are increasingly living together as opposite sides of the same coin among several patients [225-227] because of the diverse bidirectional nexus [228] and drastically mortifies prognosis of these cases [225]. Dharmalingam et al. 2018 reported that among T2DM sufferers, 70% concurrently had NAFLD [229]. Scheen (2023) reported that medical doctors were disinclined to prescribe glucose-lowering agents other than insulin among patients with T2DM and fatty liver disease for many decades [230]. This study further reported that novel glucose-lowering medicines, such as GLP-1RAs and SGLT2 antagonists, lever up new horizon aspiration. These medications possess minimum (tolerable) ADRs and trigger weight loss, pleiotropic phenomenon (a distinct gene provides manifold phenotypic attributes), and safeguard cardiorenal physiology, as evidenced in efficacious therapeutic outcomes in managing MAFLD [230]. Jang et al. (2024) in their original research, reported that among multiple (TDZs, SGLT2 blockers, dipeptidyl peptidase 4 (DPP-4) antagonists, and sulfonylureas) orally prescribed antidiabetic medication, SGLT2 antagonists possibly would somewhat better choice among patients with NAFLD and T2DM. This study advocated more long-term research in this area to decide to shift in prescribing practices [231]. Park et al. (2023) reported that GLP-1RAs possess better pharmacodynamics in minimizing BMI, waist circumference, and hepatic fat portion among patients with NAFLD and NASH who are overweight or obese compared to TZDs [232].
Long-Term Adverse Drug Reactions of Metformin
Metformin is primarily considered a drug of choice and is heavily prescribed for T2DM [233,234]. Metformin is otherwise safe and well-tolerated medication if consumed for a prolonged period [235]. The most considerable ADRs of metformin allergies include lactic acidosis, vitamin B12 deficiency, metallic (altered) taste, and gastrointestinal disorders (nausea, vomiting, and diarrhea) [236]. Brand et al. (2022) reported that when prescribed among pregnant subjects, metformin singly or metformin + insulin does not produce additional ADRs or risk features compared to insulin [237]. Liu et al. (2024) revealed that prolonged consumption of metformin increased the possibility of ΔFosB degradation [238]. ΔFosB is a Fos close relative of transcription factor proteins [239,240]. Hence, degraded ΔFosB impairs the evolution of levodopa-induced dyskinesia (LID) synthesis by initiating the AMPK-facilitated autophagy route. This study furthermore provides evidence that the AMPK-persuaded autophagy passageway is a unique therapeutic goal for LID and signifies that conceivably repositing metformin is an advantageous therapeutic contestant for LID [238]. Long-term overdose of metformin could upregulate hydrogen sulfide (H2S) levels in the liver cells, causing hepatocellular damage in animal models [241,242].
Consequently, constant pharmacovigilance is an urgent necessity to monitor the H2S level in hepatic tissue; thereby, sharp diagnosis and pre can be executed [238]. Nevertheless, Conde et al. reported that metformin has novel protective mechanisms of metformin and indicated that repositioned metformin has the probability to be a novel treatment alternative for the management of oxidative stress-connected hepatic disorders [243]. Patients with renal and hepatic disorders develop lactic acidosis because of severe overdose of metformin and poor elimination [244-246]. These patients frequently and gradually develop symptoms like abdominal pain, nausea, hypotension, tachycardia, and tachypnea [244]. Furthermore, increased levels of lactic acid can lead to severe acidemia, tissue hypoperfusion, hypoxia, cardiopulmonary failure, acute renal damage, and hepatic dysfunction [244,247,248]. Metformin provokes vitamin B12 improper absorption from the gastrointestinal tract and raises the possibility of the risk of vitamin B12 scarcity among T2DM cases, especially after 12 to months of use [249-252]. Kim et al. (2019) reported taking metformin 1.5 gm daily or more principally related to vitamin B12 deficiency. It has been suggested that multivitamin supplementation frequently alleviates vitamin B12 insufficiency [253].
The Principal Findings of This Narrative Review
This narrative review highlights that metformin, which is typically used to control T2DM, polycystic ovarian syndrome (PCOS), and body weight, is now believed to be of use in improving several other conditions like NAFLD, liver cirrhosis, various carcinomas including liver carcinoma [33-62]. NAFLD is aggravated by inflammation, oxidative stress, and insulin resistance. NAFLD is now a global public health concern, with up to 32% of the worldwide adult population suffering from it [67]. Therapeutic management classically includes weight reduction through altering lifestyle, which includes regular physical exercise and adopting eating habits (nutritious food that excludes energy-dense, carbohydrate-rich) [87-93]. Research also suggests that hypoglycemic agents like Thiazolidinedione and sodium-glucose transporter protein inhibitors may improve liver health by lowering blood glucose levels [148,149]. Metformin shows several mechanisms by which it may promote liver health in conditions like NAFLD. It improves insulin sensitivity and inhibits nuclear factor kappa beta, NLRP3, and ROS. Conversion of monocyte to macrophage is deterred by metformin, and cholesterol is disseminated from lipid-rich macrophage with an increase in high-density lipoprotein (HDL). Metformin also promotes TTP that mTOR, TNFα and accelerates lipophagy, healing obesity-associated NAFLD [162-172, 188,190,194]. Metformin has been noted to suppress the expression of genes that aggravate fatty liver [39,209]. Pharmacovigilance is required while using metformin for a long time since there may be a formation of hydrogen sulfide, which may cause liver damage [238,241,242]. Other adverse reactions include gastrointestinal disorders, metallic taste in the mouth, lactic acidosis, and vitamin B12 deficiency [236].
Limitations of This Study
Narrative reviews have inbuilt constraints regarding neutrality, comprehensiveness of literature exploration, and clarification of results [254]. Nonetheless, Greenhalgh et al. (2018) reported that narrative reviews deliver clarification and appraisal, and strategic input snowballs conception and comprehension [255].
Future Research Perspectives
However, multiple studies have reported that metformin is a possible therapeutic contestant for the pharmacological intervention of NAFLD [39,182,224]. Nonetheless, these papers [39,182,224] recommend future research to get approval from several necessary drug regulatory authorities and national and international guidelines [88,126,256-258].
Conclusions
Although metformin is known for its role in managing T2DM, PCOS, and weight reduction, studies have emerged indicating its healing effect in cases of diseases like NAFLD, liver cirrhosis, and several carcinomas. NAFLD comprises a considerable portion of the world's adult population, and a much higher proportion exists among the pediatric obese population. The first steps in the management of NAFLD include adopting a healthy lifestyle like eating a nutritious diet, avoiding an energy-dense carbohydrate-rich diet, lowering food portions, and regular physical exercise to reduce weight and several hypoglycemic agents like thiazolidinedione and sodium-glucose cotransporter-2 blocker can be part of the management. Metformin plays several roles in improving liver health by inducing lipophagy and reducing oxidative stress and inflammation. It may even reduce gene expression that promotes fatty liver, hepatic fibrosis, and stiffness, like PNPLA3 polymorphism variant rs738409. Thus, metformin may reduce hepatic fibrosis and stiffness by reducing inflammation. There may be some complications due to long-term consumption of metformin that include lactic acidosis, vitamin B12 deficiency, metallic taste in the mouth, and gastrointestinal disorders like abdominal pain, nausea, and vomiting. Pharmacovigilance is required since a long-term overdose of metformin may raise hepatic cell hydrogen sulfide levels. Further research should be carried out to understand the protective mechanisms of metformin and the appropriate dosage for different liver diseases. It should be considered while forming national and international guidelines for managing NAFLD.
Disclosures
Conflicts of interest: In compliance with the ICMJE uniform disclosure form, all authors declare the following:
Payment/services info: All authors have declared that no financial support was received from any organization for the submitted work.
Financial relationships: All authors have declared that they have no financial relationships at present or within the previous three years with any organizations that might have an interest in the submitted work.
Other relationships: All authors have declared that there are no other relationships or activities that could appear to have influenced the submitted work.
Author Contributions
Concept and design: Mainul Haque, Rahnuma Ahmad
Acquisition, analysis, or interpretation of data: Mainul Haque, Rahnuma Ahmad
Drafting of the manuscript: Mainul Haque, Rahnuma Ahmad
Critical review of the manuscript for important intellectual content: Mainul Haque, Rahnuma Ahmad
Supervision: Mainul Haque, Rahnuma Ahmad
References
- 1.Metformin: historical overview. Bailey CJ. Diabetologia. 2017;60:1566–1576. doi: 10.1007/s00125-017-4318-z. [DOI] [PubMed] [Google Scholar]
- 2.CCXIV. — The preparation of methylguanidine, and of ββ-dimethylguanidine by the interaction of dicyanodiamide, and methylammonium and dimethylammonium chlorides, respectively. Werner EA, Bell J. J Chem Soc Trans. 1922;121:1790–1794. [Google Scholar]
- 3.Metformin: its botanical background. Bailey CF, Day C. https://onlinelibrary.wiley.com/doi/epdf/10.1002/pdi.606 Pract Diab Int. 2004;21:115–117. [Google Scholar]
- 4.Metformin: clinical use in type 2 diabetes. Sanchez-Rangel E, Inzucchi SE. Diabetologia. 2017;60:1586–1593. doi: 10.1007/s00125-017-4336-x. [DOI] [PubMed] [Google Scholar]
- 5.A bibliometrics analysis of metformin development from 1980 to 2019. Song Y, Ma P, Gao Y, Xiao P, Xu L, Liu H. Front Pharmacol. 2021;12:645810. doi: 10.3389/fphar.2021.645810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Metformin: therapeutic profile in the treatment of type 2 diabetes. Bailey CJ. Diabetes Obes Metab. 2024;26 Suppl 3:3–19. doi: 10.1111/dom.15663. [DOI] [PubMed] [Google Scholar]
- 7.The blooming of the French lilac. Witters LA. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC209536/pdf/JCI0114178.pdf. J Clin Invest. 2001;108:1105–1107. doi: 10.1172/JCI14178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Metformin: a review of its metabolic effects. Cusi K, Defronzo RA. https://scholars.uthscsa.edu/en/publications/metformin-a-review-of-its-metabolic-effects Diabetes Reviews. 1998;6:89–131. [Google Scholar]
- 9.Mechanisms underlying the metabolic actions of galegine that contribute to weight loss in mice. Mooney MH, Fogarty S, Stevenson C, et al. Br J Pharmacol. 2008;153:1669–1677. doi: 10.1038/bjp.2008.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.BioRender. [ September; 2024 ]. 2024. http://www.biorender.com. BioRender. [ Sep; 2024 ]. 2024. http://www.biorender.com http://www.biorender.com
- 11.Clinical pharmacokinetics of metformin. Graham GG, Punt J, Arora M, et al. Clin Pharmacokinet. 2011;50:81–98. doi: 10.2165/11534750-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 12.Metformin: current knowledge. Nasri H, Rafieian-Kopaei M. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4214027/pdf/JRMS-19-658.pdf. J Res Med Sci. 2014;19:658–664. [PMC free article] [PubMed] [Google Scholar]
- 13.A geroscience perspective on immune resilience and infectious diseases: a potential case for metformin. Justice JN, Gubbi S, Kulkarni AS, Bartley JM, Kuchel GA, Barzilai N. Geroscience. 2021;43:1093–1112. doi: 10.1007/s11357-020-00261-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Flumamine, a new synthetic analgesic and anti-flu drug. Garcia EY. https://pubmed.ncbi.nlm.nih.gov/14779282/ J Philipp Med Assoc. 1950;26:287–293. [PubMed] [Google Scholar]
- 15.Unveiling the potential pleiotropic effects of metformin in treating COVID-19: a comprehensive review. Petakh P, Kamyshna I, Kamyshnyi A. Front Mol Biosci. 2023;10:1260633. doi: 10.3389/fmolb.2023.1260633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Metformin reduces SARS-CoV-2 in a phase 3 randomized placebo controlled clinical trial (PREPRINT) Bramante CT, Beckman KB, Mehta T, et al. medRxiv. 2023 [Google Scholar]
- 17.A SARS-CoV-2 protein interaction map reveals targets for drug repurposing. Gordon DE, Jang GM, Bouhaddou M, et al. Nature. 2020;583:459–468. doi: 10.1038/s41586-020-2286-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.AMP-activated protein kinase restricts Zika virus replication in endothelial cells by potentiating innate antiviral responses and inhibiting glycolysis. Singh S, Singh PK, Suhail H, Arumugaswami V, Pellett PE, Giri S, Kumar A. J Immunol. 2020;204:1810–1824. doi: 10.4049/jimmunol.1901310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Metformin: is it a drug for all reasons and diseases? Triggle CR, Mohammed I, Bshesh K, et al. Metabolism. 2022;133:155223. doi: 10.1016/j.metabol.2022.155223. [DOI] [PubMed] [Google Scholar]
- 20.Blood sugar-lowering effect of 1,1-dimethylbiguanide (Article in French) Sterne J. https://pubmed.ncbi.nlm.nih.gov/13603402/ Therapie. 1958;13:650–659. [PubMed] [Google Scholar]
- 21.Pathophysiology of type 2 diabetes mellitus. Galicia-Garcia U, Benito-Vicente A, Jebari S, et al. Int J Mol Sci. 2020;21:6275. doi: 10.3390/ijms21176275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Diabetes mellitus: classification, mediators, and complications; a gate to identify potential targets for the development of new effective treatments. Antar SA, Ashour NA, Sharaky M, et al. Biomed Pharmacother. 2023;168:115734. doi: 10.1016/j.biopha.2023.115734. [DOI] [PubMed] [Google Scholar]
- 23.Pathophysiology of diabetes: an overview. Banday MZ, Sameer AS, Nissar S. Avicenna J Med. 2020;10:174–188. doi: 10.4103/ajm.ajm_53_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.2. Classification and diagnosis of diabetes: standards of care in diabetes-2023. ElSayed NA, Aleppo G, Aroda VR, et al. Diabetes Care. 2023;46:0–40. doi: 10.2337/dc23-S002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.The burden and risks of emerging complications of diabetes mellitus. Tomic D, Shaw JE, Magliano DJ. Nat Rev Endocrinol. 2022;18:525–539. doi: 10.1038/s41574-022-00690-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Insulin resistance the link between T2DM and CVD: basic mechanisms and clinical implications. Abdul-Ghani MA, Jayyousi A, DeFronzo RA, Asaad N, Al-Suwaidi J. Curr Vasc Pharmacol. 2019;17:153–163. doi: 10.2174/1570161115666171010115119. [DOI] [PubMed] [Google Scholar]
- 27.Pancreatic β-cell dysfunction in type 2 diabetes: implications of inflammation and oxidative stress. Dludla PV, Mabhida SE, Ziqubu K, et al. World J Diabetes. 2023;14:130–146. doi: 10.4239/wjd.v14.i3.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Type 2 diabetes mellitus, oxidative stress and inflammation: examining the links. Oguntibeju OO. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6628012/pdf/ijppp0011-0045.pdf. Int J Physiol Pathophysiol Pharmacol. 2019;11:45–63. [PMC free article] [PubMed] [Google Scholar]
- 29.Narrative review of data supporting alternate first-line therapies over metformin in type 2 diabetes. Andraos J, Smith SR, Tran A, Pham DQ. J Diabetes Metab Disord. 2024;23:385–394. doi: 10.1007/s40200-024-01406-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Trends in incidence, prevalence and prescribing in type 2 diabetes mellitus between 2000 and 2013 in primary care: a retrospective cohort study. Sharma M, Nazareth I, Petersen I. BMJ Open. 2016;6:0. doi: 10.1136/bmjopen-2015-010210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Medication prescribing for type 2 diabetes in the US long-term care setting: observational study. Pandya N, Jung M, Norfolk A, Goldblatt C, Trenery A, Sieradzan R. https://pubmed.ncbi.nlm.nih.gov/37094748/ J Am Med Dir Assoc. 2023;24:790–797. doi: 10.1016/j.jamda.2023.03.020. [DOI] [PubMed] [Google Scholar]
- 32.Type 2 diabetes mellitus treatment patterns across Europe: a population-based multi-database study. Overbeek JA, Heintjes EM, Prieto-Alhambra D, et al. Clin Ther. 2017;39:759–770. doi: 10.1016/j.clinthera.2017.02.008. [DOI] [PubMed] [Google Scholar]
- 33.Metformin: new applications for an old drug. Naseri A, Sanaie S, Hamzehzadeh S, et al. J Basic Clin Physiol Pharmacol. 2023;34:151–160. doi: 10.1515/jbcpp-2022-0252. [DOI] [PubMed] [Google Scholar]
- 34.The current and potential therapeutic use of metformin-the good old drug. Drzewoski J, Hanefeld M. Pharmaceuticals (Basel) 2021;14:122. doi: 10.3390/ph14020122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Metformin: a review of potential mechanism and therapeutic utility beyond diabetes. Dutta S, Shah RB, Singhal S, Dutta SB, Bansal S, Sinha S, Haque M. Drug Des Devel Ther. 2023;17:1907–1932. doi: 10.2147/DDDT.S409373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Metformin - a future therapy for neurodegenerative diseases: theme: drug discovery, development and delivery in Alzheimer's Disease Guest Editor: Davide Brambilla. Markowicz-Piasecka M, Sikora J, Szydłowska A, Skupień A, Mikiciuk-Olasik E, Huttunen KM. Pharm Res. 2017;34:2614–2627. doi: 10.1007/s11095-017-2199-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.The role of AMPK-dependent pathways in cellular and molecular mechanisms of metformin: a new perspective for treatment and prevention of diseases. Hasanvand A. Inflammopharmacology. 2022;30:775–788. doi: 10.1007/s10787-022-00980-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Metformin and breast cancer: molecular targets. Faria J, Negalha G, Azevedo A, Martel F. J Mammary Gland Biol Neoplasia. 2019;24:111–123. doi: 10.1007/s10911-019-09429-z. [DOI] [PubMed] [Google Scholar]
- 39.Effects of metformin on hepatic steatosis in adults with nonalcoholic fatty liver disease and diabetes: insights from the cellular to patient level. Pinyopornpanish K, Leerapun A, Pinyopornpanish K, Chattipakorn N. Gut Liver. 2021;15:827–840. doi: 10.5009/gnl20367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Management of type 2 diabetes in patients with compensated liver cirrhosis: short of evidence, plenty of potential. Arvanitakis K, Koufakis T, Kalopitas G, Papadakos SP, Kotsa K, Germanidis G. Diabetes Metab Syndr. 2024;18:102935. doi: 10.1016/j.dsx.2023.102935. [DOI] [PubMed] [Google Scholar]
- 41.Metformin in the prevention of hepatocellular carcinoma in diabetic patients: a systematic review. Cunha V, Cotrim HP, Rocha R, Carvalho K, Lins-Kusterer L. Ann Hepatol. 2020;19:232–237. doi: 10.1016/j.aohep.2019.10.005. [DOI] [PubMed] [Google Scholar]
- 42.The emerging role of metformin in the treatment of hepatocellular carcinoma: is there any value in repurposing metformin for HCC Immunotherapy? Papadakos SP, Ferraro D, Carbone G, et al. Cancers (Basel) 2023;15 doi: 10.3390/cancers15123161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.The effect of metformin on the survival of colorectal cancer patients with type 2 diabetes mellitus. Tarhini Z, Manceur K, Magne J, Mathonnet M, Jost J, Christou N. Sci Rep. 2022;12:12374. doi: 10.1038/s41598-022-16677-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Correlation between metformin intake and prostate cancer. Kim R, Song M, Shinn J, Kim HS. Cardiovasc Prev Pharmacother. 2023;5:91–97. [Google Scholar]
- 45.The relationship between metformin consumption and cancer risk: an updated umbrella review of systematic reviews and meta-analyses. Najafi F, Rajati F, Sarokhani D, Bavandpour M, Moradinazar M. Int J Prev Med. 2023;14:90. doi: 10.4103/ijpvm.ijpvm_62_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Risk analysis of metformin use in prostate cancer: a national population-based study. Jo JK, Song HK, Heo Y, Kim MJ, Kim YJ. Aging Male. 2023;26:2156497. doi: 10.1080/13685538.2022.2156497. [DOI] [PubMed] [Google Scholar]
- 47.Metformin and breast cancer: current findings and future perspectives from preclinical and clinical studies. Corleto KA, Strandmo JL, Giles ED. Pharmaceuticals (Basel) 2024;17:396. doi: 10.3390/ph17030396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Metformin and breast cancer: where are we now? Cejuela M, Martin-Castillo B, Menendez JA, Pernas S. Int J Mol Sci. 2022;23:2705. doi: 10.3390/ijms23052705. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 49.Association of metformin intake with bladder cancer risk and oncologic outcomes in type 2 diabetes mellitus patients: a systematic review and meta-analysis. Hu J, Chen JB, Cui Y, et al. Medicine (Baltimore) 2018;97:0. doi: 10.1097/MD.0000000000011596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Metformin use on incidence and oncologic outcomes of bladder cancer patients with T2DM: an updated meta-analysis. Liu CQ, Sun JX, Xu JZ, et al. Front Pharmacol. 2022;13:865988. doi: 10.3389/fphar.2022.865988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Metformin exerts an antitumor effect by inhibiting bladder cancer cell migration and growth, and promoting apoptosis through the PI3K/AKT/mTOR pathway. Shen Z, Xue D, Wang K, et al. BMC Urol. 2022;22:79. doi: 10.1186/s12894-022-01027-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Metformin and blood cancers. Cunha Júnior AD, Pericole FV, Carvalheira JB. Clinics (Sao Paulo) 2018;73:0. doi: 10.6061/clinics/2018/e412s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Metformin - its anti-cancer effects in hematologic malignancies. Podhorecka M. Oncol Rev. 2021;15:514. doi: 10.4081/oncol.2021.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Metformin inhibits melanoma cell metastasis by suppressing the miR-5100/SPINK5/STAT3 axis. Suwei D, Yanbin X, Jianqiang W, et al. Cell Mol Biol Lett. 2022;27:48. doi: 10.1186/s11658-022-00353-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Metformin: focus on melanoma. Jaune E, Rocchi S. Front Endocrinol (Lausanne) 2018;9:472. doi: 10.3389/fendo.2018.00472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Metformin is associated with improved clinical outcomes in patients with melanoma: a retrospective, multi-institutional study. Augustin RC, Huang Z, Ding F, et al. Front Oncol. 2023;13:1075823. doi: 10.3389/fonc.2023.1075823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Metformin and primary bone cancer risk in Taiwanese patients with type 2 diabetes mellitus. Tseng CH. Bone. 2021;151:116037. doi: 10.1016/j.bone.2021.116037. [DOI] [PubMed] [Google Scholar]
- 58.Effects of metformin on human bone-derived mesenchymal stromal cell-breast cancer cell line interactions. Teufelsbauer M, Lang C, Plangger A, et al. Med Oncol. 2022;39:54. doi: 10.1007/s12032-022-01655-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Metformin attenuates bone cancer pain by reducing TRPV1 and ASIC3 expression. Qian HY, Zhou F, Wu R, et al. Front Pharmacol. 2021;12:713944. doi: 10.3389/fphar.2021.713944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Metformin is associated with decreased risk of basal cell carcinoma: a whole-population case-control study from Iceland. Adalsteinsson JA, Muzumdar S, Waldman R, et al. J Am Acad Dermatol. 2021;85:56–61. doi: 10.1016/j.jaad.2021.02.042. [DOI] [PubMed] [Google Scholar]
- 61.Association of the metformin with the risk of lung cancer: a meta-analysis. Wang L, Song Y, Wu GN, Yuan DM. Transl Lung Cancer Res. 2013;2:259–263. doi: 10.3978/j.issn.2218-6751.2013.08.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Effects of metformin on survival outcomes of lung cancer patients with type 2 diabetes mellitus: a meta-analysis. Tian RH, Zhang YG, Wu Z, Liu X, Yang JW, Ji HL. Clin Transl Oncol. 2016;18:641–649. doi: 10.1007/s12094-015-1412-x. [DOI] [PubMed] [Google Scholar]
- 63.The prospect of non-alcoholic fatty liver disease in adult patients with metabolic syndrome: a systematic review. Zohara Z, Adelekun A, Seffah KD, et al. Cureus. 2023;15:0. doi: 10.7759/cureus.41959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Non-alcoholic fatty liver disease (NAFLD): pathogenesis and natural products for prevention and treatment. Guo X, Yin X, Liu Z, Wang J. Int J Mol Sci. 2022;23:15489. doi: 10.3390/ijms232415489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.NAFLD as a continuum: from obesity to metabolic syndrome and diabetes. Godoy-Matos AF, Silva Júnior WS, Valerio CM. Diabetol Metab Syndr. 2020;12:60. doi: 10.1186/s13098-020-00570-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Global incidence and prevalence of nonalcoholic fatty liver disease. Teng ML, Ng CH, Huang DQ, et al. Clin Mol Hepatol. 2023;29:0–42. doi: 10.3350/cmh.2022.0365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.The prevalence and incidence of NAFLD worldwide: a systematic review and meta-analysis. Riazi K, Azhari H, Charette JH, et al. Lancet Gastroenterol Hepatol. 2022;7:851–861. doi: 10.1016/S2468-1253(22)00165-0. [DOI] [PubMed] [Google Scholar]
- 68.The global epidemiology of nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH): a systematic review. Younossi ZM, Golabi P, Paik JM, Henry A, Van Dongen C, Henry L. Hepatology. 2023;77:1335–1347. doi: 10.1097/HEP.0000000000000004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Changing epidemiology, global trends and implications for outcomes of NAFLD. Wong VW, Ekstedt M, Wong GL, Hagström H. J Hepatol. 2023;79:842–852. doi: 10.1016/j.jhep.2023.04.036. [DOI] [PubMed] [Google Scholar]
- 70.Understanding the burden of nonalcoholic fatty liver disease: time for action. Younossi ZM, Henry L. Diabetes Spectr. 2024;37:9–19. doi: 10.2337/dsi23-0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Global prevalence, incidence, and outcomes of non-obese or lean non-alcoholic fatty liver disease: a systematic review and meta-analysis. Ye Q, Zou B, Yeo YH, et al. Lancet Gastroenterol Hepatol. 2020;5:739–752. doi: 10.1016/S2468-1253(20)30077-7. [DOI] [PubMed] [Google Scholar]
- 72.Prevalence, incidence, and outcome of non-alcoholic fatty liver disease in Asia, 1999-2019: a systematic review and meta-analysis. Li J, Zou B, Yeo YH, et al. Lancet Gastroenterol Hepatol. 2019;4:389–398. doi: 10.1016/S2468-1253(19)30039-1. [DOI] [PubMed] [Google Scholar]
- 73.Prevalence of non-alcoholic fatty liver disease in India: a systematic review and meta-analysis. Shalimar Shalimar, Elhence A, Bansal B, Gupta H, Anand A, Singh TP, Goel A. J Clin Exp Hepatol. 2022;12:818–829. doi: 10.1016/j.jceh.2021.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Epidemiology of non-alcoholic fatty liver disease in China. Fan JG, Farrell GC. J Hepatol. 2009;50:204–210. doi: 10.1016/j.jhep.2008.10.010. [DOI] [PubMed] [Google Scholar]
- 75.Epidemiological features of NAFLD From 1999 to 2018 in China. Zhou J, Zhou F, Wang W, et al. Hepatology. 2020;71:1851–1864. doi: 10.1002/hep.31150. [DOI] [PubMed] [Google Scholar]
- 76.The epidemiology of NAFLD in Mainland China with analysis by adjusted gross regional domestic product: a meta-analysis. Wu Y, Zheng Q, Zou B, et al. Hepatol Int. 2020;14:259–269. doi: 10.1007/s12072-020-10023-3. [DOI] [PubMed] [Google Scholar]
- 77.Prevalence and characteristics of MAFLD in Chinese adults aged 40 years or older: a community-based study. Zeng J, Qin L, Jin Q, et al. Hepatobiliary Pancreat Dis Int. 2022;21:154–161. doi: 10.1016/j.hbpd.2022.01.006. [DOI] [PubMed] [Google Scholar]
- 78.Prevalence of liver steatosis and fibrosis in the general population and various high-risk populations: a nationwide study with 5.7 million adults in China. Man S, Deng Y, Ma Y, et al. Gastroenterology. 2023;165:1025–1040. doi: 10.1053/j.gastro.2023.05.053. [DOI] [PubMed] [Google Scholar]
- 79.Meta-analysis: global prevalence, trend and forecasting of non-alcoholic fatty liver disease in children and adolescents, 2000-2021. Li J, Ha A, Rui F, et al. Aliment Pharmacol Ther. 2022;56:396–406. doi: 10.1111/apt.17096. [DOI] [PubMed] [Google Scholar]
- 80.The epidemiology of NAFLD and lean NAFLD in Japan: a meta-analysis with individual and forecasting analysis, 1995-2040. Ito T, Ishigami M, Zou B, et al. Hepatol Int. 2021;15:366–379. doi: 10.1007/s12072-021-10143-4. [DOI] [PubMed] [Google Scholar]
- 81.Prevalence of non-alcoholic fatty liver disease (NAFLD) in Saudi Arabia: systematic review and meta-analysis. Alenezi YM, Harris R, Morling J, Card T. Cureus. 2023;15:0. doi: 10.7759/cureus.40308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Prevalence and predictors of non-alcoholic fatty liver disease in tertiary care hospital of Taif, Saudi Arabia: a retrospective study. Alamri AS, Alhomrani M, Alsanie WF, et al. Saudi J Biol Sci. 2021;28:4921–4925. doi: 10.1016/j.sjbs.2021.05.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Development of non-alcoholic fatty liver disease scoring system among adult medical check-up patients: a large cross-sectional and prospective validation study. Lesmana CR, Pakasi LS, Inggriani S, Aidawati ML, Lesmana LA. Diabetes Metab Syndr Obes. 2015;8:213–218. doi: 10.2147/DMSO.S80364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Prevalence and risk factors of non-alcoholic fatty liver disease in a multiracial suburban Asian population in Malaysia. Goh SC, Ho EL, Goh KL. Hepatol Int. 2013;7:548–554. doi: 10.1007/s12072-012-9359-2. [DOI] [PubMed] [Google Scholar]
- 85.Prevalence and risk factors of sonographically detected non alcoholic fatty liver disease in a screening centre in Klang Valley, Malaysia: an observational cross-sectional study. Khammas AS, Hassan HA, Salih SQ, Kadir H, Ibrahim RM, Nasir NN, Mahmud R. Porto Biomed J. 2019;4:0. doi: 10.1016/j.pbj.0000000000000031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Prevalence of non-alcoholic fatty liver disease and advanced fibrosis in Hong Kong Chinese: a population study using proton-magnetic resonance spectroscopy and transient elastography. Wong VW, Chu WC, Wong GL, et al. Gut. 2012;61:409–415. doi: 10.1136/gutjnl-2011-300342. [DOI] [PubMed] [Google Scholar]
- 87.Mayo Clinic Staff. Mayo Clinic Staff. Nonalcoholic fatty liver disease. [ Aug; 2024 ]. 2024. https://www.mayoclinic.org/diseases-conditions/nonalcoholic-fatty-liver-disease/diagnosis-treatment/drc-20354573#:~:text=Treatment%20for%20NAFLD%20usually%20starts,weight%20or%20more%20is%20recommended https://www.mayoclinic.org/diseases-conditions/nonalcoholic-fatty-liver-disease/diagnosis-treatment/drc-20354573#:~:text=Treatment%20for%20NAFLD%20usually%20starts,weight%20or%20more%20is%20recommended
- 88.American Association of Clinical Endocrinology Clinical Practice Guideline for the Diagnosis and Management of Nonalcoholic Fatty Liver Disease in Primary Care and Endocrinology Clinical Settings: co-sponsored by the American Association for the Study of Liver Diseases (AASLD) Cusi K, Isaacs S, Barb D, et al. Endocr Pract. 2022;28:528–562. doi: 10.1016/j.eprac.2022.03.010. [DOI] [PubMed] [Google Scholar]
- 89.Mediterranean diet and nonalcoholic fatty liver disease. Anania C, Perla FM, Olivero F, Pacifico L, Chiesa C. World J Gastroenterol. 2018;24:2083–2094. doi: 10.3748/wjg.v24.i19.2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Non-alcoholic fatty liver disease and lifestyle modifications, focusing on physical activity. Kwak MS, Kim D. Korean J Intern Med. 2018;33:64–74. doi: 10.3904/kjim.2017.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lifestyle interventions for non-alcoholic fatty liver disease. Ahmed IA, Mikail MA, Mustafa MR, Ibrahim M, Othman R. Saudi J Biol Sci. 2019;26:1519–1524. doi: 10.1016/j.sjbs.2018.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lifestyle intervention in children with obesity and nonalcoholic fatty liver disease (NAFLD): study protocol for a randomized controlled trial in Ningbo city (the SCIENT study) Zhang PP, Wang YX, Shen FJ, et al. Trials. 2024;25:196. doi: 10.1186/s13063-024-08046-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Dietary patterns in non-alcoholic fatty liver disease (NAFLD): stay on the straight and narrow path! Katsiki N, Stoian AP, Rizzo M. Clin Investig Arterioscler. 2022;34 Suppl 1:0–31. doi: 10.1016/j.arteri.2021.10.001. [DOI] [PubMed] [Google Scholar]
- 94.Diet and exercise in NAFLD/NASH: beyond the obvious. Semmler G, Datz C, Reiberger T, Trauner M. Liver Int. 2021;41:2249–2268. doi: 10.1111/liv.15024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Eating, diet, and nutrition for the treatment of non-alcoholic fatty liver disease. Semmler G, Datz C, Trauner M. Clin Mol Hepatol. 2023;29:0–60. doi: 10.3350/cmh.2022.0364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Beyond the paradigm of weight loss in non-alcoholic fatty liver disease: from pathophysiology to novel dietary approaches. Armandi A, Schattenberg JM. Nutrients. 2021;13:1977. doi: 10.3390/nu13061977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Effects of dietary and lifestyle interventions on liver, clinical and metabolic parameters in children and adolescents with non-alcoholic fatty liver disease: a systematic review. Katsagoni CN, Papachristou E, Sidossis A, Sidossis L. Nutrients. 2020;12:2864. doi: 10.3390/nu12092864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Effect of aerobic exercise and diet on liver fat in pre-diabetic patients with non-alcoholic-fatty-liver-disease: a randomized controlled trial. Cheng S, Ge J, Zhao C, et al. Sci Rep. 2017;7:15952. doi: 10.1038/s41598-017-16159-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Responses of the serum lipid profile to exercise and diet interventions in nonalcoholic fatty liver disease. Qi Z, LE S, Cheng R, et al. Med Sci Sports Exerc. 2024;56:1036–1045. doi: 10.1249/MSS.0000000000003388. [DOI] [PubMed] [Google Scholar]
- 100.Impact of nutritional changes on nonalcoholic fatty liver disease. Perdomo CM, Frühbeck G, Escalada J. Nutrients. 2019;11:677. doi: 10.3390/nu11030677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Dietary patterns and components in nonalcoholic fatty liver disease (NAFLD): what key messages can health care providers offer? Riazi K, Raman M, Taylor L, Swain MG, Shaheen AA. Nutrients. 2019;11:2878. doi: 10.3390/nu11122878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Diet and non-alcoholic fatty liver disease, a short narrative review. Kwanten WJ. Acta Gastroenterol Belg. 2023;86:306–310. doi: 10.51821/86.2.11547. [DOI] [PubMed] [Google Scholar]
- 103.Different dietary approaches, non-alcoholic fatty liver disease, and cardiovascular disease: a literature review. Torres-Peña JD, Arenas-de Larriva AP, Alcala-Diaz JF, Lopez-Miranda J, Delgado-Lista J. Nutrients. 2023;15:1483. doi: 10.3390/nu15061483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Effect of a ketogenic diet on hepatic steatosis and hepatic mitochondrial metabolism in nonalcoholic fatty liver disease. Luukkonen PK, Dufour S, Lyu K, et al. Proc Natl Acad Sci U S A. 2020;117:7347–7354. doi: 10.1073/pnas.1922344117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Current evidence concerning effects of ketogenic diet and intermittent fasting in patients with nonalcoholic fatty liver. Sripongpun P, Churuangsuk C, Bunchorntavakul C. J Clin Transl Hepatol. 2022;10:730–739. doi: 10.14218/JCTH.2021.00494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Emerging role of hepatic ketogenesis in fatty liver disease. Mooli RG, Ramakrishnan SK. Front Physiol. 2022;13:946474. doi: 10.3389/fphys.2022.946474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Association between impaired ketogenesis and metabolic-associated fatty liver disease. Bae J, Lee BW. Biomolecules. 2023;13:1506. doi: 10.3390/biom13101506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Beneficial effects of the ketogenic diet on nonalcoholic fatty liver disease: a comprehensive review of the literature. Watanabe M, Tozzi R, Risi R, et al. Obes Rev. 2020;21:0. doi: 10.1111/obr.13024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Investigating the link between ketogenic diet, NAFLD, mitochondria, and oxidative stress: a narrative review. Paoli A, Cerullo G. Antioxidants (Basel) 2023;12:1065. doi: 10.3390/antiox12051065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ketogenic diet time-dependently prevents NAFLD through upregulating the expression of antioxidant protein metallothionein-2. You Y, Huang Y, Wang X, et al. Clin Nutr. 2024;43:1475–1487. doi: 10.1016/j.clnu.2024.04.029. [DOI] [PubMed] [Google Scholar]
- 111.Practical lifestyle management of nonalcoholic fatty liver disease for busy clinicians. Zelber-Sagi S, Moore JB. Diabetes Spectr. 2024;37:39–47. doi: 10.2337/dsi23-0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.The effects of physical exercise on fatty liver disease. van der Windt DJ, Sud V, Zhang H, Tsung A, Huang H. https://pubmed.ncbi.nlm.nih.gov/29212576/ Gene Expr. 2018;18:89–101. doi: 10.3727/105221617X15124844266408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Exercise in the management of metabolic-associated fatty liver disease (MAFLD) in adults: a position statement from exercise and sport science Australia. Keating SE, Sabag A, Hallsworth K, et al. Sports Med. 2023;53:2347–2371. doi: 10.1007/s40279-023-01918-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.NAFLD and physical exercise: ready, steady, go! Cigrovski Berkovic M, Bilic-Curcic I, Mrzljak A, Cigrovski V. Front Nutr. 2021;8:734859. doi: 10.3389/fnut.2021.734859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Independent association of physical activity with nonalcoholic fatty liver disease and alanine aminotransferase levels. Jang DK, Lee JS, Lee JK, Kim YH. J Clin Med. 2019;8:1013. doi: 10.3390/jcm8071013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Skeletal muscle as an endocrine organ: PGC-1α, myokines and exercise. Schnyder S, Handschin C. Bone. 2015;80:115–125. doi: 10.1016/j.bone.2015.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Muscle as an endocrine organ: focus on muscle-derived interleukin-6. Pedersen BK, Febbraio MA. Physiol Rev. 2008;88:1379–1406. doi: 10.1152/physrev.90100.2007. [DOI] [PubMed] [Google Scholar]
- 118.The search for exercise factors in humans. Catoire M, Kersten S. FASEB J. 2015;29:1615–1628. doi: 10.1096/fj.14-263699. [DOI] [PubMed] [Google Scholar]
- 119.Exercise-based interventions for nonalcoholic fatty liver disease: a meta-analysis and meta-regression. Orci LA, Gariani K, Oldani G, Delaune V, Morel P, Toso C. https://pdf.sciencedirectassets.com/273402/1-s2.0-S1542356516X00092/1-s2.0-S1542356516301495/main.pdf?X-Amz-Security-Token=IQoJb3JpZ2luX2VjED8aCXVzLWVhc3QtMSJHMEUCIQDd86UT5Suqnh%2FykF9woo02Xg55bibr2wyuNfo4VyapSQIgTeh99pd1UUrhnvWsGnuTK7LJiAlMGB63tPmCCKtkfnEquwUIh%2F%2F%2F%2F%2F%2F%2F%2F%2F%2F%2FARAFGgwwNTkwMDM1NDY4NjUiDEOh%2FaHOFgILmgelySqPBfpcBBSpcn1r6SkTZ2HYH0zx5NMBYY8pQOIMWYYiVedUdewcK86jb3HKPJx1k5JOsXY0m%2By%2FeCj%2BgN%2Byv%2F%2FXpGMNQ8kKVXTrD%2BYJN%2FM0d1xpdFOuZXKt5i4WJ1k%2FmI0BDYELtrNNWJs4xcuyDKC86GKPZmsDNpTPS1BimchkvzgkCRY05LBHsvBqsOfw8J4EYTGTqwWOCC%2FvNgH5Nx101X96cBxrfRlBq4fucNKbGHfPL%2FXZvqGvtW%2BxdCigZ2wv6a34H3y0xBMy1ENezdI5s8BkdG4cniV6yTBVtt1EJFX8MEj07JN%2FudSuasmKrxHHxtgkipu3M0NJRIIjGMMI80ZoFNcQtrTBxzcv1JPHF5YDFtO8lV83bVu%2FN9kviRsf2hjaIsUhLH89q25yfWK2ekkjb092f69psA8H5nDJWRFkbChuK%2BpMDqRXah%2FbyiHUi49V4eQVZnOrWNnOuIO96sV82wGz6%2FMk3Aae3DEZjzxSgrVv4cIgPiwf1SXfb7L5p2tOo8SIWzfcWuLoEJbUmn3IBJ3KtaIlO90dRM5Kw7JS8nfw0E2Ea1kxNVp%2F6LppBMGLD5%2Fp6NYtqQZAli4VM6GK8%2B68abo%2BaufysgLKw25%2BsQjEfkYNq2UCvLYNrGh%2Fb6F1JtCZGVHsrNn5oxRJL8FAYZ6OciSqVDm3aYi2kfU4nPCVGqhdK4gjCtAagUHvDWT1fxrZX9P%2Fc1sjKTJ4SgJkpz6lmlZNTxwrjKTK2jwQd5fCNsj2s%2B8IekSDFjKma%2Bp2OLH3ornW1XZOXVRzEux8mslmS6GBvpAM0lniHPiot0uRiOqK79%2F%2B1w5BO%2BXBy6%2BfWHKoFvZEl8SwrdYbmsef5sHCF59%2BEN7E5G7%2FF3ww6aTutwY6sQFDQpuj7QjazBY9XZvcxnKCvc03vbFOhN9kmPTiIOhHkLHzbCESFi%2FcQ5JxR1CMVZA42xlnqyXIWut16xmWNaHlBeb%2FdaYZWVqnNty3lptlByFU3Uix7KipdLORU426EQkuawnwv3Wf%2BqxbdLVhW3RdJbSl9YueLRB1gqki87kZE5Z3OrTfxPxmUcW1u%2FZ8fHOLutoXiUWlxuFoO%2B%2B90OoPPLjbYxqdSUyfNiXAl8zxVfo%3D&X-Amz-Algorithm=AWS4-HMAC-SHA256&X-Amz-Date=20241001T073822Z&X-Amz-SignedHeaders=host&X-Amz-Expires=300&X-Amz-Credential=ASIAQ3PHCVTYWJTYECUF%2F20241001%2Fus-east-1%2Fs3%2Faws4_request&X-Amz-Signature=c914ee7a10404339c2e3690e5ec32a775065263d72c4495c8eb06b8644685357&hash=72ff6fec73a5c155a93dffa32a3703acae3b905daca671d5fe296ea750afcaca&host=68042c943591013ac2b2430a89b270f6af2c76d8dfd086a07176afe7c76c2c61&pii=S1542356516301495&tid=spdf-16e8cb35-2029-4a8f-a1a6-2f37394a2b3f&sid=70fd8ddf692de741fd39e5555d308cf50067gxrqb&type=client&tsoh=d3d3LnNjaWVuY2VkaXJlY3QuY29t&ua=171f570105070d04095f00&rr=8cbacaf0bcedd436&cc=my. Clin Gastroenterol Hepatol. 2016;14:1398–1411. doi: 10.1016/j.cgh.2016.04.036. [DOI] [PubMed] [Google Scholar]
- 120.Serum alanine aminotransferase in skeletal muscle diseases. Nathwani RA, Pais S, Reynolds TB, Kaplowitz N. Hepatology. 2005;41:380–382. doi: 10.1002/hep.20548. [DOI] [PubMed] [Google Scholar]
- 121.Glucosinolate extract from radish (Raphanus sativus L.) seed attenuates high-fat diet-induced obesity: insights into gut microbiota and fecal metabolites. Zhu Q, Zhang P, Liu D, Tang L, Yu J, Zhang C, Jiang G. Front Nutr. 2024;11:1442535. doi: 10.3389/fnut.2024.1442535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Effect of different exercise modalities on nonalcoholic fatty liver disease: a systematic review and network meta-analysis. Xue Y, Peng Y, Zhang L, Ba Y, Jin G, Liu G. Sci Rep. 2024;14:6212. doi: 10.1038/s41598-024-51470-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.What are the current pharmacological therapies for nonalcoholic fatty liver disease? David D, Eapen CE. J Clin Exp Hepatol. 2021;11:232–238. doi: 10.1016/j.jceh.2020.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Efficacy of off-label therapy for non-alcoholic fatty liver disease in improving non-invasive and invasive biomarkers: a systematic review and network meta-analysis of randomized controlled trials. Luo Q, Wei R, Cai Y, Zhao Q, Liu Y, Liu WJ. Front Med (Lausanne) 2022;9:793203. doi: 10.3389/fmed.2022.793203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Non-alcoholic fatty liver disease, metabolic syndrome, and type 2 diabetes mellitus: where do we stand today? Mitrovic B, Gluvic ZM, Obradovic M, Radunovic M, Rizzo M, Banach M, Isenovic ER. Arch Med Sci. 2023;19:884–894. doi: 10.5114/aoms/150639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.New anti-diabetic agents for the treatment of non-alcoholic fatty liver disease: a systematic review and network meta-analysis of randomized controlled trials. Kongmalai T, Srinonprasert V, Anothaisintawee T, Kongmalai P, McKay G, Attia J, Thakkinstian A. Front Endocrinol (Lausanne) 2023;14:1182037. doi: 10.3389/fendo.2023.1182037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Effect of novel glucose lowering agents on non-alcoholic fatty liver disease: a systematic review and meta-analysis. Zafar Y, Rashid AM, Siddiqi AK, et al. Clin Res Hepatol Gastroenterol. 2022;46:101970. doi: 10.1016/j.clinre.2022.101970. [DOI] [PubMed] [Google Scholar]
- 128.Efficacy and safety of GLP-1 receptor agonists in patients with type 2 diabetes mellitus and non-alcoholic fatty liver disease: a systematic review and meta-analysis. Zhu Y, Xu J, Zhang D, et al. Front Endocrinol (Lausanne) 2021;12:769069. doi: 10.3389/fendo.2021.769069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Antidiabetic drugs and non-alcoholic fatty liver disease: a systematic review, meta-analysis and evidence map. Kumar J, Memon RS, Shahid I, et al. Dig Liver Dis. 2021;53:44–51. doi: 10.1016/j.dld.2020.08.021. [DOI] [PubMed] [Google Scholar]
- 130.Comparing the effectiveness of long-term use of daily and weekly glucagon-like peptide-1 receptor agonists treatments in patients with nonalcoholic fatty liver disease and type 2 diabetes mellitus: a network meta-analysis. Yuan X, Gao Z, Yang C, Duan K, Ren L, Song G. Front Endocrinol (Lausanne) 2023;14:1170881. doi: 10.3389/fendo.2023.1170881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Comparative efficacy of glucagon-like peptide 1 (GLP-1) receptor agonists, pioglitazone and vitamin E for liver histology among patients with nonalcoholic fatty liver disease: systematic review and pilot network meta-analysis of randomized controlled trials. Gu Y, Sun L, He Y, et al. Expert Rev Gastroenterol Hepatol. 2023;17:273–282. doi: 10.1080/17474124.2023.2172397. [DOI] [PubMed] [Google Scholar]
- 132.Current pharmacological therapies for nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. Takahashi Y, Sugimoto K, Inui H, Fukusato T. World J Gastroenterol. 2015;21:3777–3785. doi: 10.3748/wjg.v21.i13.3777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Insulin resistance in development and progression of nonalcoholic fatty liver disease. Alam S, Mustafa G, Alam M, Ahmad N. World J Gastrointest Pathophysiol. 2016;7:211–217. doi: 10.4291/wjgp.v7.i2.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.The intricate relationship between type 2 diabetes mellitus (T2DM), insulin resistance (IR), and nonalcoholic fatty liver disease (NAFLD) Tanase DM, Gosav EM, Costea CF, et al. J Diabetes Res. 2020;2020:3920196. doi: 10.1155/2020/3920196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.The role of insulin resistance and diabetes in nonalcoholic fatty liver disease. Fujii H, Kawada N, Japan Study Group Of Nafld Jsg-Nafld. Int J Mol Sci. 2020;21:3863. doi: 10.3390/ijms21113863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Molecular mechanisms of hepatic insulin resistance in nonalcoholic fatty liver disease and potential treatment strategies. Zhang CH, Zhou BG, Sheng JQ, Chen Y, Cao YQ, Chen C. Pharmacol Res. 2020;159:104984. doi: 10.1016/j.phrs.2020.104984. [DOI] [PubMed] [Google Scholar]
- 137.Liver-targeting drugs and their effect on blood glucose and hepatic lipids. Gastaldelli A, Stefan N, Häring HU. Diabetologia. 2021;64:1461–1479. doi: 10.1007/s00125-021-05442-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Glucagon-like peptide-1 receptor agonists for non-alcoholic fatty liver disease in type 2 diabetes: a meta-analysis. Wong C, Lee MH, Yaow CY, et al. Front Endocrinol (Lausanne) 2021;12:609110. doi: 10.3389/fendo.2021.609110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.PPAR-targeted therapies in the treatment of non-alcoholic fatty liver disease in diabetic patients. Lange NF, Graf V, Caussy C, Dufour JF. Int J Mol Sci. 2022;23 doi: 10.3390/ijms23084305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Pharmacological treatment for non-alcoholic fatty liver disease. Francque S, Vonghia L. Adv Ther. 2019;36:1052–1074. doi: 10.1007/s12325-019-00898-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Dietary and pharmacological treatment of nonalcoholic fatty liver disease. Jeznach-Steinhagen A, Ostrowska J, Czerwonogrodzka-Senczyna A, Boniecka I, Shahnazaryan U, Kuryłowicz A. Medicina (Kaunas) 2019;55 doi: 10.3390/medicina55050166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Therapeutic targets, novel drugs, and delivery systems for diabetes associated NAFLD and liver fibrosis. Kumar V, Xin X, Ma J, Tan C, Osna N, Mahato RI. Adv Drug Deliv Rev. 2021;176:113888. doi: 10.1016/j.addr.2021.113888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Oxidative stress management in chronic liver diseases and hepatocellular carcinoma. Uchida D, Takaki A, Oyama A, Adachi T, Wada N, Onishi H, Okada H. Nutrients. 2020;12:1576. doi: 10.3390/nu12061576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Role of oxidative stress in the pathogenesis of nonalcoholic fatty liver disease. Chen Z, Tian R, She Z, Cai J, Li H. Free Radic Biol Med. 2020;152:116–141. doi: 10.1016/j.freeradbiomed.2020.02.025. [DOI] [PubMed] [Google Scholar]
- 145.Oxidative stress in type 2 diabetes: impacts from pathogenesis to lifestyle modifications. Caturano A, D'Angelo M, Mormone A, et al. Curr Issues Mol Biol. 2023;45:6651–6666. doi: 10.3390/cimb45080420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.The interplay among oxidative stress, brain insulin resistance and AMPK dysfunction contribute to neurodegeneration in type 2 diabetes and Alzheimer disease. Barone E, Di Domenico F, Perluigi M, Butterfield DA. Free Radic Biol Med. 2021;176:16–33. doi: 10.1016/j.freeradbiomed.2021.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Structure, function, and regulation of insulin-degrading enzyme. Hulse RE, Ralat LA, Wei-Jen T. Vitam Horm. 2009;80:635–648. doi: 10.1016/S0083-6729(08)00622-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Thiazolidinediones for nonalcoholic steatohepatitis: a meta-analysis of randomized clinical trials. He L, Liu X, Wang L, Yang Z. Medicine (Baltimore) 2016;95:0. doi: 10.1097/MD.0000000000004947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.The role of thiazolidinediones in the amelioration of nonalcoholic fatty liver disease: a systematic review. Ndakotsu A, Vivekanandan G. Cureus. 2022;14:0. doi: 10.7759/cureus.25380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.GLP-1 receptor agonists in non-alcoholic fatty liver disease: current evidence and future perspectives. Nevola R, Epifani R, Imbriani S, et al. Int J Mol Sci. 2023;24:1703. doi: 10.3390/ijms24021703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Glucagon-like peptide-1 receptor agonists in patients with type 2 diabetes mellitus and nonalcoholic fatty liver disease-current background, hopes, and perspectives. Cazac GD, Lăcătușu CM, Ștefănescu G, Mihai C, Grigorescu ED, Onofriescu A, Mihai BM. Metabolites. 2023;13:581. doi: 10.3390/metabo13050581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Impact of sodium glucose cotransporter-2 inhibitors on liver steatosis/fibrosis/inflammation and redox balance in non-alcoholic fatty liver disease. Bellanti F, Lo Buglio A, Dobrakowski M, et al. World J Gastroenterol. 2022;28:3243–3257. doi: 10.3748/wjg.v28.i26.3243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.SGLT2 inhibitors: beyond glycemic control. Hasan I, Rashid T, Jaikaransingh V, Heilig C, Abdel-Rahman EM, Awad AS. J Clin Transl Endocrinol. 2024;35:100335. doi: 10.1016/j.jcte.2024.100335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Eggleton JS, Jialal I. StatPearls [Internet] Treasure Island (FL): StatPearls Publishing; 2024. Thiazolidinediones. [PubMed] [Google Scholar]
- 155.Response to pioglitazone in non-alcoholic fatty liver disease patients with vs. without type 2 diabetes: a meta-analysis of randomized controlled trials. Wang Z, Du H, Zhao Y, et al. Front Endocrinol (Lausanne) 2023;14:1111430. doi: 10.3389/fendo.2023.1111430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Pharmacological approaches to nonalcoholic fatty liver disease: current and future therapies. Genua I, Cusi K. Diabetes Spectr. 2024;37:48–58. doi: 10.2337/dsi23-0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Comparative effectiveness of GLP-1 receptor agonists on glycaemic control, body weight, and lipid profile for type 2 diabetes: systematic review and network meta-analysis. Yao H, Zhang A, Li D, Wu Y, Wang CZ, Wan JY, Yuan CS. BMJ. 2024;384:0. doi: 10.1136/bmj-2023-076410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Role of diet and lifestyle changes in nonalcoholic fatty liver disease. Nseir W, Hellou E, Assy N. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4110565/pdf/WJG-20-9338.pdf. World J Gastroenterol. 2014;20:9338–9344. doi: 10.3748/wjg.v20.i28.9338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Treatment of NAFLD with diet, physical activity and exercise. Romero-Gómez M, Zelber-Sagi S, Trenell M. J Hepatol. 2017;67:829–846. doi: 10.1016/j.jhep.2017.05.016. [DOI] [PubMed] [Google Scholar]
- 160.Lifestyle modifications in non-alcoholic fatty liver disease and non-alcoholic steatohepatitis. Stavropoulos K, Imprialos K, Pittaras A, Faselis C, Narayan P, Kokkinos P. Curr Vasc Pharmacol. 2018;16:239–245. doi: 10.2174/1570161115666170621080835. [DOI] [PubMed] [Google Scholar]
- 161.Metformin: an old drug for the treatment of diabetes but a new drug for the protection of the endothelium. Kinaan M, Ding H, Triggle CR. Med Princ Pract. 2015;24:401–415. doi: 10.1159/000381643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Mechanisms of action of metformin in type 2 diabetes: Effects on mitochondria and leukocyte-endothelium interactions. Apostolova N, Iannantuoni F, Gruevska A, Muntane J, Rocha M, Victor VM. Redox Biol. 2020;34:101517. doi: 10.1016/j.redox.2020.101517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Metformin acutely lowers blood glucose levels by inhibition of intestinal glucose transport. Horakova O, Kroupova P, Bardova K, Buresova J, Janovska P, Kopecky J, Rossmeisl M. Sci Rep. 2019;9:6156. doi: 10.1038/s41598-019-42531-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Metformin inhibits lithocholic acid-induced interleukin 8 upregulation in colorectal cancer cells by suppressing ROS production and NF-kB activity. Nguyen TT, Ung TT, Li S, Lian S, Xia Y, Park SY, Do Jung Y. Sci Rep. 2019;9:2003. doi: 10.1038/s41598-019-38778-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.NF-κB as the mediator of metformin's effect on ageing and ageing-related diseases. Kanigur Sultuybek G, Soydas T, Yenmis G. Clin Exp Pharmacol Physiol. 2019;46:413–422. doi: 10.1111/1440-1681.13073. [DOI] [PubMed] [Google Scholar]
- 166.Metformin Inhibits the NLRP3 inflammasome via AMPK/mTOR-dependent effects in diabetic cardiomyopathy. Yang F, Qin Y, Wang Y, et al. Int J Biol Sci. 2019;15:1010–1019. doi: 10.7150/ijbs.29680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Metformin inhibits NLR family pyrin domain containing 3 (NLRP)-relevant neuroinflammation via an adenosine-5'-monophosphate-activated protein kinase (AMPK)-dependent pathway to alleviate early brain injury after subarachnoid hemorrhage in mice. Jin L, Jin F, Guo S, et al. Front Pharmacol. 2022;13:796616. doi: 10.3389/fphar.2022.796616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Metformin inhibits the production of reactive oxygen species from NADH:ubiquinone oxidoreductase to limit induction of interleukin-1β (IL-1β) and boosts interleukin-10 (IL-10) in lipopolysaccharide (LPS)-activated macrophages. Kelly B, Tannahill GM, Murphy MP, O'Neill LA. J Biol Chem. 2015;290:20348–20359. doi: 10.1074/jbc.M115.662114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Metformin inhibits monocyte-to-macrophage differentiation via AMPK-mediated inhibition of STAT3 activation: potential role in atherosclerosis. Vasamsetti SB, Karnewar S, Kanugula AK, Thatipalli AR, Kumar JM, Kotamraju S. Diabetes. 2015;64:2028–2041. doi: 10.2337/db14-1225. [DOI] [PubMed] [Google Scholar]
- 170.Metformin inhibits ROS production by human M2 macrophages via the activation of AMPK. Nassif RM, Chalhoub E, Chedid P, et al. Biomedicines. 2022;10:319. doi: 10.3390/biomedicines10020319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Metformin promotes cholesterol efflux in macrophages by up-regulating FGF21 expression: a novel anti-atherosclerotic mechanism. Luo F, Guo Y, Ruan G, Li X. Lipids Health Dis. 2016;15:109. doi: 10.1186/s12944-016-0281-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Metformin in patients with and without diabetes: a paradigm shift in cardiovascular disease management. Luo F, Das A, Chen J, Wu P, Li X, Fang Z. Cardiovasc Diabetol. 2019;18:54. doi: 10.1186/s12933-019-0860-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Can metformin exert as an active drug on endothelial dysfunction in diabetic subjects? Salvatore T, Pafundi PC, Galiero R, et al. Biomedicines. 2020;9:3. doi: 10.3390/biomedicines9010003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Anti-inflammatory potential of the anti-diabetic drug metformin in the prevention of inflammatory complications and infectious diseases including COVID- 19: a narrative review. Plowman TJ, Christensen H, Aiges M, Fernandez E, Shah MH, Ramana KV. Int J Mol Sci. 2024;25:5190. doi: 10.3390/ijms25105190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Metformin: update on mechanisms of action and repurposing potential. Foretz M, Guigas B, Viollet B. Nat Rev Endocrinol. 2023;19:460–476. doi: 10.1038/s41574-023-00833-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Dietary inflammatory index and non-communicable disease risk: a narrative review. Phillips CM, Chen LW, Heude B, et al. Nutrients. 2019;11:1873. doi: 10.3390/nu11081873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Inflammation-nature's way to efficiently respond to all types of challenges: implications for understanding and managing "the epidemic" of chronic diseases. Bennett JM, Reeves G, Billman GE, Sturmberg JP. Front Med (Lausanne) 2018;5:316. doi: 10.3389/fmed.2018.00316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Health relevance of the modification of low grade inflammation in ageing (inflammageing) and the role of nutrition. Calder PC, Bosco N, Bourdet-Sicard R, et al. Ageing Res Rev. 2017;40:95–119. doi: 10.1016/j.arr.2017.09.001. [DOI] [PubMed] [Google Scholar]
- 179.Inflammation, metaflammation and immunometabolic disorders. Hotamisligil GS. Nature. 2017;542:177–185. doi: 10.1038/nature21363. [DOI] [PubMed] [Google Scholar]
- 180.Chronic inflammation-a link between nonalcoholic fatty liver disease (NAFLD) and dysfunctional adipose tissue. Petrescu M, Vlaicu SI, Ciumărnean L, et al. Medicina (Kaunas) 2022;58 doi: 10.3390/medicina58050641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Metformin and diammonium glycyrrhizinate enteric-coated capsule versus metformin alone versus diammonium glycyrrhizinate enteric-coated capsule alone in patients with nonalcoholic fatty liver disease and type 2 diabetes mellitus. Zhang R, Cheng K, Xu S, et al. Gastroenterol Res Pract. 2017;2017:8491742. doi: 10.1155/2017/8491742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Effect of metformin on nonalcoholic fatty liver based on meta-analysis and network pharmacology. Huang Y, Wang X, Yan C, et al. Medicine (Baltimore) 2022;101:0. doi: 10.1097/MD.0000000000031437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Metabolic inflammation - a role for hepatic inflammatory pathways as drivers of comorbidities in nonalcoholic fatty liver disease? Gehrke N, Schattenberg JM. Gastroenterology. 2020;158:1929–1947. doi: 10.1053/j.gastro.2020.02.020. [DOI] [PubMed] [Google Scholar]
- 184.MASLD: a systemic metabolic disorder with cardiovascular and malignant complications. Targher G, Byrne CD, Tilg H. Gut. 2024;73:691–702. doi: 10.1136/gutjnl-2023-330595. [DOI] [PubMed] [Google Scholar]
- 185.Pahwa R, Goyal A, Jialal I. StatPearls [Internet] Treasure Island (FL): StatPearls Publishing; 2024. Chronic inflammation. [PubMed] [Google Scholar]
- 186.Inflammation in obesity, diabetes, and related disorders. Rohm TV, Meier DT, Olefsky JM, Donath MY. Immunity. 2022;55:31–55. doi: 10.1016/j.immuni.2021.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Inflammatory mechanisms linking obesity and metabolic disease. Saltiel AR, Olefsky JM. J Clin Invest. 2017;127:1–4. doi: 10.1172/JCI92035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Metformin-induced TTP mediates communication between Kupffer cells and hepatocytes to alleviate hepatic steatosis by regulating lipophagy and necroptosis. Park J, Rah SY, An HS, et al. Metabolism. 2023;141:155516. doi: 10.1016/j.metabol.2023.155516. [DOI] [PubMed] [Google Scholar]
- 189.LncRNA AA465934 improves podocyte injury by promoting tristetraprolin-mediated HMGB1 downregulation in diabetic nephropathy. Yang N, Zhang Y, Ren P, Zhao L, Zheng D, Fu L, Jin J. Mol Cell Biol. 2024;44:87–102. doi: 10.1080/10985549.2024.2325527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Metformin: update on mechanisms of action on liver diseases. Ruan G, Wu F, Shi D, Sun H, Wang F, Xu C. Front Nutr. 2023;10:1327814. doi: 10.3389/fnut.2023.1327814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.The effects of metformin on autophagy. Lu G, Wu Z, Shang J, Xie Z, Chen C, Zhang C. Biomed Pharmacother. 2021;137:111286. doi: 10.1016/j.biopha.2021.111286. [DOI] [PubMed] [Google Scholar]
- 192.Metformin inhibits hepatic mTORC1 signaling via dose-dependent mechanisms involving AMPK and the TSC complex. Howell JJ, Hellberg K, Turner M, et al. Cell Metab. 2017;25:463–471. doi: 10.1016/j.cmet.2016.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Inhibition of mTORC1 through ATF4-induced REDD1 and Sestrin2 expression by metformin. Jang SK, Hong SE, Lee DH, et al. BMC Cancer. 2021;21:803. doi: 10.1186/s12885-021-08346-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.The regulation, function, and role of lipophagy, a form of selective autophagy, in metabolic disorders. Zhang S, Peng X, Yang S, et al. Cell Death Dis. 2022;13:132. doi: 10.1038/s41419-022-04593-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Genetic pathways in nonalcoholic fatty liver disease: insights from systems biology. Sookoian S, Pirola CJ, Valenti L, Davidson NO. Hepatology. 2020;72:330–346. doi: 10.1002/hep.31229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Review article: the emerging role of genetics in precision medicine for patients with non-alcoholic steatohepatitis. Carlsson B, Lindén D, Brolén G, Liljeblad M, Bjursell M, Romeo S, Loomba R. Aliment Pharmacol Ther. 2020;51:1305–1320. doi: 10.1111/apt.15738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Genetic and epigenetic factors determining NAFLD risk. Jonas W, Schürmann A. Mol Metab. 2021;50:101111. doi: 10.1016/j.molmet.2020.101111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Impact of PNPLA3 rs738409 polymorphism on the development of liver-related events in patients with nonalcoholic fatty liver disease. Rosso C, Caviglia GP, Birolo G, et al. Clin Gastroenterol Hepatol. 2023;21:3314–3321. doi: 10.1016/j.cgh.2023.04.024. [DOI] [PubMed] [Google Scholar]
- 199.Influence of the PNPLA3 rs738409 polymorphism on non-alcoholic fatty liver disease and renal function among normal weight subjects. Oniki K, Saruwatari J, Izuka T, et al. PLoS One. 2015;10:0. doi: 10.1371/journal.pone.0132640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Influence of type 2 diabetes in the association of PNPLA3 rs738409 and TM6SF2 rs58542926 polymorphisms in NASH advanced liver fibrosis. Gabriel-Medina P, Ferrer-Costa R, Rodriguez-Frias F, et al. Biomedicines. 2022;10 doi: 10.3390/biomedicines10051015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Differences in the genetic backgrounds of patients with alcoholic liver disease and non-alcoholic fatty liver disease. Yamamoto K, Kogiso T, Taniai M, Hashimoto E, Tokushige K. JGH Open. 2019;3:17–24. doi: 10.1002/jgh3.12097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.High frequency of the PNPLA3 rs738409 [G] single-nucleotide polymorphism in Hmong individuals as a potential basis for a predisposition to chronic liver disease. Tepper CG, Dang JH, Stewart SL, et al. Cancer. 2018;124 Suppl 7:1583–1589. doi: 10.1002/cncr.31122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.The rs738409 polymorphism of the PNPLA3 gene is associated with hepatic steatosis and fibrosis in Brazilian patients with chronic hepatitis C. Manchiero C, Nunes AK, Magri MC, Dantas BP, Mazza CC, Barone AA, Tengan FM. BMC Infect Dis. 2017;17:780. doi: 10.1186/s12879-017-2887-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.A multi-ethnic study of a PNPLA3 gene variant and its association with disease severity in non-alcoholic fatty liver disease. Zain SM, Mohamed R, Mahadeva S, Cheah PL, Rampal S, Basu RC, Mohamed Z. Hum Genet. 2012;131:1145–1152. doi: 10.1007/s00439-012-1141-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Associations of PNPLA3 and LEP genetic polymorphisms with metabolic-associated fatty liver disease in Thai people living with human immunodeficiency virus. Choochuay K, Kunhapan P, Puangpetch A, et al. World J Hepatol. 2024;16:366–378. doi: 10.4254/wjh.v16.i3.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Frequency of the PNPLA3 rs738409 polymorphism and other genetic loci for liver disease in a Guatemalan adult population. Lazo M, Xie J, Alvarez CS, et al. Liver Int. 2022;42:1470–1474. doi: 10.1111/liv.15268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.The PNPLA3 rs738409 C>G variant interacts with changes in body weight over time to aggravate liver steatosis, but reduces the risk of incident type 2 diabetes. Xia MF, Lin HD, Chen LY, et al. Diabetologia. 2019;62:644–654. doi: 10.1007/s00125-018-4805-x. [DOI] [PubMed] [Google Scholar]
- 208.Association between PNPLA3 rs738409 polymorphism and nonalcoholic fatty liver disease: a systematic review and meta-analysis. Salari N, Darvishi N, Mansouri K, Ghasemi H, Hosseinian-Far M, Darvishi F, Mohammadi M. BMC Endocr Disord. 2021;21:125. doi: 10.1186/s12902-021-00789-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Metformin treatment reverses high fat diet- induced non-alcoholic fatty liver diseases and dyslipidemia by stimulating multiple antioxidant and anti-inflammatory pathways. Yasmin T, Rahman MM, Khan F, et al. Biochem Biophys Rep. 2021;28:101168. doi: 10.1016/j.bbrep.2021.101168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Metformin in non-alcoholic steatohepatitis. Marchesini G, Brizi M, Bianchi G, Tomassetti S, Zoli M, Melchionda N. Lancet. 2001;358:893–894. doi: 10.1016/s0140-6736(01)06042-1. [DOI] [PubMed] [Google Scholar]
- 211.Effects of metformin and weight loss on serum alanine aminotransferase activity in the diabetes prevention program. Krakoff J, Clark JM, Crandall JP, et al. Obesity (Silver Spring) 2010;18:1762–1767. doi: 10.1038/oby.2010.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Metformin improves the hepatic steatosis index in non-obese patients with polycystic ovary syndrome. Riemann A, Blaschke M, Jauho-Ghadimi A, Siggelkow H, Gollisch KS. J Clin Med. 2022;11 doi: 10.3390/jcm11154294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.The effect of metformin on aminotransferase levels, metabolic parameters and body mass index in nonalcoholic fatty liver disease patients: a meta-analysis. Hu H, Wang J, Li X, Shen L, Shi D, Meng J. Curr Pharm Des. 2021;27:3235–3243. doi: 10.2174/1381612827666210315144821. [DOI] [PubMed] [Google Scholar]
- 214.The effects of metformin administration on liver enzymes and body composition in non-diabetic patients with non-alcoholic fatty liver disease and/or non-alcoholic steatohepatitis: an up-to-date systematic review and meta-analysis of randomized controlled trials. Jalali M, Rahimlou M, Mahmoodi M, et al. https://pdf.sciencedirectassets.com/272488/1-s2.0-S1043661820X00089/1-s2.0-S1043661820311075/main.pdf?X-Amz-Security-Token=IQoJb3JpZ2luX2VjEEAaCXVzLWVhc3QtMSJHMEUCIDJLKzdV%2B10n0P6Vl0CUdkjqap5DEtA14Bf6T2IrlqeIAiEAzFiquE2YHCbvoYFTkuMtgLjq2VcCB1VoAFU%2BprBmYzoquwUIiP%2F%2F%2F%2F%2F%2F%2F%2F%2F%2FARAFGgwwNTkwMDM1NDY4NjUiDEZlTPOWzFA4ZC5WkCqPBVsy0k8q7RAegu5Q8Yevoca2QFMqMbtFAGbwULwP%2FFIj2gl686PgcTKVEzhs%2FlP2haRd8LYikc3t3xbqynG0i%2Fs8fTeTzFZKN9WiiSbps%2FiBxtACzNypMDFgILKiO3p%2BnGU4QpJePEnLORIot07xN83cvEyzbgDmaAnpZVLnk4XfxqK9TGfVUBr4y9Pbtrn26jOIU%2FNkujdINjO5pFMHeEMwaENB5Ge79k1NCz%2BqJ7QMrmFAG2jZDb6%2BSDb8jM2t%2BQGX97akc%2BkFzyQqTjTjLv23kM%2BQRfUftWC1tNAHh9R7zOx2NSVF%2FnfOQqJA4tpXIlOTaxTR%2FGI1nwGqQWEfaBNkpRjODmOI481nt%2B4DxhJMLfdD2%2FlCB0rIRWr%2BhI5%2B%2B5rlaXGaAB%2BaYnbIrZHUJCubybmriB5EjGohL4pfKsaVJoBiBfmYY8wwQCKRzT7lWIjBbkQOjt2VCOBF1VN1qAsyexCKP8VKILzklM8Hj54Ds%2BlLjOVPs6Yw5%2Bd1QRX0GgC7rflv52rs0SoRdx2Ogv9PrDYBw7EGUoAiwKzp3lw92g4twThlyoCDinB34BTFqOybmK1EldcvZzBWiIEauqw%2BXwcEyZnQJzS6zqnRX17KRifXD6DJI3R%2BDKNDRawqCaboIFEqSlBfOMjOe4NJj2TWng7uk1mfDFeHDVFRn26FtdqPuDsu2itDWHEviOvKLLmqZybnJIy7rLe2wMvxSHBowCB0%2B%2FNLHNJ0IwgFELrFNSD6VTX9xRf1tuv51f%2FWSAU5ICaaaL9VMyVV80rIsVth%2FYmI3xcfhqG6L8kPmBfUz4Ru9w0yOmHFdm%2BDF1L2TUQ1Do%2B%2FcUmNLoHhebECEjwHOF1kQc%2FEGjgOAjgrfwkw1MLutwY6sQGpHKhT44EiTSSHQcUyjqxO5w4kUY2dqw71ky1g6ojwizurH8QvxH2RvARlMcL9oJ0m9F92fvTtHcOTxK9ZoSntYSICarWGrj5jufMbhUCaRwcCuxnELES0qr%2BrBN8GWK8yUud9TYv3%2FVN0HzkE1J7ZmCwEDVa0sVq7yGbqGnaIpWHKBJS7HC3jspn6xUe8jGYlDAVD5yINcY7URV1FxGipk4bugDpaEfUn0wve%2BiW%2FZ%2BQ%3D&X-Amz-Algorithm=AWS4-HMAC-SHA256&X-Amz-Date=20241001T080045Z&X-Amz-SignedHeaders=host&X-Amz-Expires=300&X-Amz-Credential=ASIAQ3PHCVTYU2Z4S4UN%2F20241001%2Fus-east-1%2Fs3%2Faws4_request&X-Amz-Signature=978f08cfbcaea7fd209b2cb55a269d3ffff3f5b23d3f467ec94ed38c575aac25&hash=fbac2dee2b9df689ca2a18032688d905c402fd7df525308986aab79bebb5e166&host=68042c943591013ac2b2430a89b270f6af2c76d8dfd086a07176afe7c76c2c61&pii=S1043661820311075&tid=spdf-2153af24-be42-4d3a-8b40-cb953ab1b9a0&sid=70fd8ddf692de741fd39e5555d308cf50067gxrqb&type=client&tsoh=d3d3LnNjaWVuY2VkaXJlY3QuY29t&ua=171f570105070b070d0d01&rr=8cbaebbbce0ed435&cc=my. Pharmacol Res. 2020;159:104799. doi: 10.1016/j.phrs.2020.104799. [DOI] [PubMed] [Google Scholar]
- 215.Metformin in non-alcoholic fatty liver disease: a systematic review and meta-analysis. Li Y, Liu L, Wang B, Wang J, Chen D. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3956897/pdf/br-01-01-0057.pdf. Biomed Rep. 2012;1:57–64. doi: 10.3892/br.2012.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.PPAR-alpha/gamma agonists, glucagon-like peptide-1 receptor agonists and metformin for non-alcoholic fatty liver disease: a network meta-analysis. Zhang ZY, Yan Q, Wu WH, Zhao Y, Zhang H, Li J. J Int Med Res. 2023;51 doi: 10.1177/03000605231177191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Saroglitazar for nonalcoholic fatty liver disease: a single centre experience in 91 patients. Padole P, Arora A, Sharma P, Chand P, Verma N, Kumar A. J Clin Exp Hepatol. 2022;12:435–439. doi: 10.1016/j.jceh.2021.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Saroglitazar in non-alcoholic fatty liver disease from bench to bedside: a comprehensive review and sub-group meta-analysis. Roy A, Tewari B, Giri S, Goenka M. Cureus. 2023;15:0. doi: 10.7759/cureus.47493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Effects of saroglitazar in the treatment of non-alcoholic fatty liver disease or non-alcoholic steatohepatitis: a systematic review and meta-analysis. Bandyopadhyay S, Samajdar SS, Das S. Clin Res Hepatol Gastroenterol. 2023;47:102174. doi: 10.1016/j.clinre.2023.102174. [DOI] [PubMed] [Google Scholar]
- 220.Current clinical trial status and future prospects of PPAR-targeted drugs for treating nonalcoholic fatty liver disease. Kamata S, Honda A, Ishii I. Biomolecules. 2023;13:1264. doi: 10.3390/biom13081264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.New advances in drug development for metabolic dysfunction-associated diseases and alcohol-associated liver disease. Zhang J, Li Y, Yang L, et al. Cell Biosci. 2024;14:90. doi: 10.1186/s13578-024-01267-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Efficacy of various hypoglycemic agents in the treatment of patients with nonalcoholic liver disease with or without diabetes: a network meta-analysis. Lian J, Fu J. Front Endocrinol (Lausanne) 2021;12:649018. doi: 10.3389/fendo.2021.649018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Metformin beyond type 2 diabetes: emerging and potential new indications. Petrie JR. Diabetes Obes Metab. 2024;26 Suppl 3:31–41. doi: 10.1111/dom.15756. [DOI] [PubMed] [Google Scholar]
- 224.The benefit of metformin in the treatment of pediatric non-alcoholic fatty liver disease: a systematic review and meta-analysis of randomized controlled trials. Gkiourtzis N, Michou P, Moutafi M, et al. Eur J Pediatr. 2023;182:4795–4806. doi: 10.1007/s00431-023-05169-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.The coexistence of nonalcoholic fatty liver disease and type 2 diabetes mellitus. Kosmalski M, Ziółkowska S, Czarny P, Szemraj J, Pietras T. J Clin Med. 2022;11:1375. doi: 10.3390/jcm11051375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Nonalcoholic fatty liver disease and its complex relation with type 2 diabetes mellitus-from prevalence to diagnostic approach and treatment strategies. Diaconu CT, Guja C. J Clin Med. 2022;11:5144. doi: 10.3390/jcm11175144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.NAFLD and diabetes: two sides of the same coin? Rationale for gene-based personalized NAFLD treatment . Xia MF, Bian H, Gao X. Front Pharmacol. 2019;10:877. doi: 10.3389/fphar.2019.00877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.NAFLD fibrosis progression and type 2 diabetes: the hepatic-metabolic interplay. Cernea S. Life (Basel) 2024;14 doi: 10.3390/life14020272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Nonalcoholic fatty liver disease and type 2 diabetes mellitus. Dharmalingam M, Yamasandhi PG. Indian J Endocrinol Metab. 2018;22:421–428. doi: 10.4103/ijem.IJEM_585_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Comparative effects between old and new antidiabetic agents on metabolic-associated fatty liver disease (MAFLD) Scheen AJ. Diabet Epidemiol Manag. 2023;11:100145. [Google Scholar]
- 231.Outcomes of various classes of oral antidiabetic drugs on nonalcoholic fatty liver disease. Jang H, Kim Y, Lee DH, et al. JAMA Intern Med. 2024;184:375–383. doi: 10.1001/jamainternmed.2023.8029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Comparison of glucagon-like peptide-1 receptor agonists and thiazolidinediones on treating nonalcoholic fatty liver disease: A network meta-analysis. Park MJ, Kim H, Kim MG, Kim K. Clin Mol Hepatol. 2023;29:693–704. doi: 10.3350/cmh.2022.0330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Drug treatment of type 2 diabetes mellitus in patients for whom metformin is contraindicated. Irons BK, Minze MG. Diabetes Metab Syndr Obes. 2014;7:15–24. doi: 10.2147/DMSO.S38753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Therapy of Type 2 diabetes: more gliflozines and less metformin? Verdecchia P, Murdolo G, Coiro S, Santucci A, Notaristefano F, Angeli F, Cavallini C. Eur Heart J Suppl. 2023;25:0–6. doi: 10.1093/eurheartjsupp/suad098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Corcoran C, Jacobs TF. StatPearls [Internet] Treasure Island (FL): StatPearls Publishing; 2024. Metformin. [PubMed] [Google Scholar]
- 236.Metformin-associated lactic acidosis: a case report and review. Ashraf S, Upreti P, Karki S, Khan M, Nasr R. Cureus. 2022;14:0. doi: 10.7759/cureus.24220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Metformin in pregnancy and risk of adverse long-term outcomes: a register-based cohort study. Brand KM, Saarelainen L, Sonajalg J, et al. BMJ Open Diabetes Res Care. 2022;10 doi: 10.1136/bmjdrc-2021-002363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.AMPK-mediated autophagy pathway activation promotes ΔFosB degradation to improve levodopa-induced dyskinesia. Liu K, Zhang Z, Xu Y, et al. Cell Signal. 2024;118:111125. doi: 10.1016/j.cellsig.2024.111125. [DOI] [PubMed] [Google Scholar]
- 239.∆FosB: a transcriptional regulator of stress and antidepressant responses. Nestler EJ. Eur J Pharmacol. 2015;753:66–72. doi: 10.1016/j.ejphar.2014.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Self-assembly of the bZIP transcription factor ΔFosB. Yin Z, Venkannagari H, Lynch H, et al. Curr Res Struct Biol. 2020;2:1–13. doi: 10.1016/j.crstbi.2019.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Ratiometric detection of H2S in liver injury by activated two-wavelength photoacoustic imaging. Wu R, Chen Z, Huo H, et al. Anal Chem. 2022;94:10797–10804. doi: 10.1021/acs.analchem.2c01571. [DOI] [PubMed] [Google Scholar]
- 242.Metformin raises hydrogen sulfide tissue concentrations in various mouse organs. Wiliński B, Wiliński J, Somogyi E, Piotrowska J, Opoka W. Pharmacol Rep. 2013;65:737–742. doi: 10.1016/s1734-1140(13)71053-3. [DOI] [PubMed] [Google Scholar]
- 243.Metformin protects primary rat hepatocytes against oxidative stress-induced apoptosis. Conde de la Rosa L, Vrenken TE, Buist-Homan M, Faber KN, Moshage H. Pharmacol Res Perspect. 2015;3:0. doi: 10.1002/prp2.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Metformin-induced lactic acidosis with emphasis on the anion gap. Blough B, Moreland A, Mora A Jr. Proc (Bayl Univ Med Cent) 2015;28:31–33. doi: 10.1080/08998280.2015.11929178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Treatment of metformin intoxication complicated by lactic acidosis and acute kidney injury: the role of prolonged intermittent hemodialysis. Regolisti G, Antoniotti R, Fani F, Greco P, Fiaccadori E. Am J Kidney Dis. 2017;70:290–296. doi: 10.1053/j.ajkd.2016.12.010. [DOI] [PubMed] [Google Scholar]
- 246.Dyatlova N, Tobarran NV, Kannan L, et al. StatPearls [Internet] Treasure Island (FL): StatPearls Publishing; 2024. Metformin-associated lactic acidosis (MALA) [PubMed] [Google Scholar]
- 247.Lactic acidosis related to pharmacotherapy and human diseases. Zanza C, Facelli V, Romenskaya T, et al. Pharmaceuticals (Basel) 2022;15:1496. doi: 10.3390/ph15121496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Metformin-associated lactic acidosis. Fadden EJ, Longley C, Mahambrey T. BMJ Case Rep. 2021;14 doi: 10.1136/bcr-2020-239154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Vitamin B12 deficiency in diabetic patients on metformin therapy: a cross-sectional study from Oman. Al-Hamdi A, Al-Gahhafi M, Al-Roshdi S, Jaju S, Al-Mamari A, Al Mahrezi AM. Sultan Qaboos Univ Med J. 2020;20:0–4. doi: 10.18295/squmj.2020.20.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Association of metformin use with vitamin B(12) deficiency in the institutionalized elderly. Wong CW, Leung CS, Leung CP, Cheng JN. Arch Gerontol Geriatr. 2018;79:57–62. doi: 10.1016/j.archger.2018.07.019. [DOI] [PubMed] [Google Scholar]
- 251.Vitamin B12 deficiency and clinical neuropathy with metformin use in type 2 diabetes. Farooq MD, Tak FA, Ara F, Rashid S, Mir IA. J Xenobiot. 2022;12:122–130. doi: 10.3390/jox12020011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Correlation between long-term use of metformin and incidence of NAFLD among patients with type 2 diabetes mellitus: a real-world cohort study. Huang KH, Lee CH, Cheng YD, Gau SY, Tsai TH, Chung NJ, Lee CY. Front Endocrinol (Lausanne) 2022;13:1027484. doi: 10.3389/fendo.2022.1027484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Association between metformin dose and vitamin B12 deficiency in patients with type 2 diabetes. Kim J, Ahn CW, Fang S, Lee HS, Park JS. Medicine (Baltimore) 2019;98:0. doi: 10.1097/MD.0000000000017918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Writing narrative literature reviews for peer-reviewed journals: secrets of the trade. Green BN, Johnson CD, Adams A. J Chiropr Med. 2006;5:101–117. doi: 10.1016/S0899-3467(07)60142-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Time to challenge the spurious hierarchy of systematic over narrative reviews? Greenhalgh T, Thorne S, Malterud K. Eur J Clin Invest. 2018;48:0. doi: 10.1111/eci.12931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Emerging therapeutic options for non-alcoholic fatty liver disease: a systematic review. Tidwell J, Balassiano N, Shaikh A, Nassar M. World J Hepatol. 2023;15:1001–1012. doi: 10.4254/wjh.v15.i8.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.The role of anti-diabetic drugs in NAFLD. Have we found the Holy Grail? A narrative review. Zachou M, Flevari P, Nasiri-Ansari N, Varytimiadis C, Kalaitzakis E, Kassi E, Androutsakos T. Eur J Clin Pharmacol. 2024;80:127–150. doi: 10.1007/s00228-023-03586-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. Diabetologia. 2016;59:1121–1140. doi: 10.1007/s00125-016-3902-y. [DOI] [PubMed] [Google Scholar]