Abstract
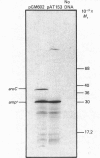
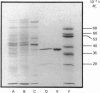
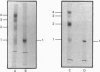
The enzyme chorismate synthase was purified in milligram quantities from an overproducing strain of Escherichia coli. The amino acid sequence was deduced from the nucleotide sequence of the aroC gene and confirmed by determining the N-terminal amino acid sequence of the purified enzyme. The complete polypeptide chain consists of 357 amino acid residues and has a calculated subunit Mr of 38,183. Cross-linking and gel-filtration experiments show that the enzyme is tetrameric. An improved purification of chorismate synthase from Neurospora crassa is also described. Cross-linking and gel-filtration experiments on the N. crassa enzyme show that it is also tetrameric with a subunit Mr of 50,000. It is proposed that the subunits of the N. crassa enzyme are larger because they contain a diaphorase domain that is absent from the E. coli enzyme.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anton I. A., Coggins J. R. Sequencing and overexpression of the Escherichia coli aroE gene encoding shikimate dehydrogenase. Biochem J. 1988 Jan 15;249(2):319–326. doi: 10.1042/bj2490319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann B. J. Linkage map of Escherichia coli K-12, edition 7. Microbiol Rev. 1983 Jun;47(2):180–230. doi: 10.1128/mr.47.2.180-230.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernofsky C., Mills R. C. Diaphorases from Aerobacter aerogenes. J Bacteriol. 1966 Nov;92(5):1404–1414. doi: 10.1128/jb.92.5.1404-1414.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biggin M. D., Gibson T. J., Hong G. F. Buffer gradient gels and 35S label as an aid to rapid DNA sequence determination. Proc Natl Acad Sci U S A. 1983 Jul;80(13):3963–3965. doi: 10.1073/pnas.80.13.3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Carter P. E., Dunbar B., Fothergill J. E. The serine proteinase chain of human complement component C1s. Cyanogen bromide cleavage and N-terminal sequences of the fragments. Biochem J. 1983 Dec 1;215(3):565–571. doi: 10.1042/bj2150565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke L., Carbon J. A colony bank containing synthetic Col El hybrid plasmids representative of the entire E. coli genome. Cell. 1976 Sep;9(1):91–99. doi: 10.1016/0092-8674(76)90055-6. [DOI] [PubMed] [Google Scholar]
- Coggins J. R., Boocock M. R., Chaudhuri S., Lambert J. M., Lumsden J., Nimmo G. A., Smith D. D. The arom multifunctional enzyme from Neurospora crassa. Methods Enzymol. 1987;142:325–341. doi: 10.1016/s0076-6879(87)42044-2. [DOI] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Davies G. E., Stark G. R. Use of dimethyl suberimidate, a cross-linking reagent, in studying the subunit structure of oligomeric proteins. Proc Natl Acad Sci U S A. 1970 Jul;66(3):651–656. doi: 10.1073/pnas.66.3.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan K., Chaudhuri S., Campbell M. S., Coggins J. R. The overexpression and complete amino acid sequence of Escherichia coli 3-dehydroquinase. Biochem J. 1986 Sep 1;238(2):475–483. doi: 10.1042/bj2380475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan K., Lewendon A., Coggins J. R. The purification of 5-enolpyruvylshikimate 3-phosphate synthase from an overproducing strain of Escherichia coli. FEBS Lett. 1984 Jan 2;165(1):121–127. doi: 10.1016/0014-5793(84)80027-7. [DOI] [PubMed] [Google Scholar]
- Floss H. G., Onderka D. K., Carroll M. Stereochemistry of the 3-deoxy-D-arabino-heptulosonate 7-phosphate synthetase reaction and the chorismate synthetase reaction. J Biol Chem. 1972 Feb 10;247(3):736–744. [PubMed] [Google Scholar]
- Gaertner F. H., Cole K. W. The protease problem in Neurospora. Structural modification of the arom multienzyme system during its extraction and isolation. Arch Biochem Biophys. 1976 Dec;177(2):566–573. doi: 10.1016/0003-9861(76)90468-9. [DOI] [PubMed] [Google Scholar]
- Grosjean H., Fiers W. Preferential codon usage in prokaryotic genes: the optimal codon-anticodon interaction energy and the selective codon usage in efficiently expressed genes. Gene. 1982 Jun;18(3):199–209. doi: 10.1016/0378-1119(82)90157-3. [DOI] [PubMed] [Google Scholar]
- Hagervall T. G., Björk G. R. Genetic mapping and cloning of the gene (trmC) responsible for the synthesis of tRNA (mnm5s2U)methyltransferase in Escherichia coli K12. Mol Gen Genet. 1984;196(2):201–207. doi: 10.1007/BF00328051. [DOI] [PubMed] [Google Scholar]
- Hasan N., Nester E. W. Dehydroquinate synthase in Bacillus subtilis. An enzyme associated with chorismate synthase and flavin reductase. J Biol Chem. 1978 Jul 25;253(14):4999–5004. [PubMed] [Google Scholar]
- Hawley D. K., McClure W. R. Compilation and analysis of Escherichia coli promoter DNA sequences. Nucleic Acids Res. 1983 Apr 25;11(8):2237–2255. doi: 10.1093/nar/11.8.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lampkin S. L., 4th, Cole K. W., Vitto A., Gaertner F. H. The protease problem in Neurospora. Variable stability of enzymes in aromatic amino acid metabolism. Arch Biochem Biophys. 1976 Dec;177(2):561–565. doi: 10.1016/0003-9861(76)90467-7. [DOI] [PubMed] [Google Scholar]
- Lumsden J., Coggins J. R. The subunit structure of the arom multienzyme complex of Neurospora crassa. A possible pentafunctional polypeptide chain. Biochem J. 1977 Mar 1;161(3):599–607. doi: 10.1042/bj1610599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumsden J., Coggins J. R. The subunit structure of the arom multienzyme complex of Neurospora crassa. Evidence from peptide 'maps' for the identity of the subunits. Biochem J. 1978 Feb 1;169(2):441–444. doi: 10.1042/bj1690441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing J., Vieira J. A new pair of M13 vectors for selecting either DNA strand of double-digest restriction fragments. Gene. 1982 Oct;19(3):269–276. doi: 10.1016/0378-1119(82)90016-6. [DOI] [PubMed] [Google Scholar]
- Millar G., Coggins J. R. The complete amino acid sequence of 3-dehydroquinate synthase of Escherichia coli K12. FEBS Lett. 1986 May 5;200(1):11–17. doi: 10.1016/0014-5793(86)80501-4. [DOI] [PubMed] [Google Scholar]
- Millar G., Lewendon A., Hunter M. G., Coggins J. R. The cloning and expression of the aroL gene from Escherichia coli K12. Purification and complete amino acid sequence of shikimate kinase II, the aroL-gene product. Biochem J. 1986 Jul 15;237(2):427–437. doi: 10.1042/bj2370427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morell H., Clark M. J., Knowles P. F., Sprinson D. B. The enzymic synthesis of chorismic and prephenic acids from 3-enolpyruvylshikimic acid 5-phosphate. J Biol Chem. 1967 Jan 10;242(1):82–90. [PubMed] [Google Scholar]
- Mulligan M. E., Brosius J., McClure W. R. Characterization in vitro of the effect of spacer length on the activity of Escherichia coli RNA polymerase at the TAC promoter. J Biol Chem. 1985 Mar 25;260(6):3529–3538. [PubMed] [Google Scholar]
- Pittard J., Wallace B. J. Distribution and function of genes concerned with aromatic biosynthesis in Escherichia coli. J Bacteriol. 1966 Apr;91(4):1494–1508. doi: 10.1128/jb.91.4.1494-1508.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell G. A., Dunbar B., Fothergill-Gilmore L. A. The complete amino acid sequence of chicken skeletal-muscle enolase. Biochem J. 1986 May 15;236(1):115–126. doi: 10.1042/bj2360115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shine J., Dalgarno L. Determinant of cistron specificity in bacterial ribosomes. Nature. 1975 Mar 6;254(5495):34–38. doi: 10.1038/254034a0. [DOI] [PubMed] [Google Scholar]
- Smith M. A., Gerrie L. M., Dunbar B., Fothergill J. E. Primary structure of bovine complement activation fragment C4a, the third anaphylatoxin. Purification and complete amino acid sequence. Biochem J. 1982 Nov 1;207(2):253–260. doi: 10.1042/bj2070253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staden R. A new computer method for the storage and manipulation of DNA gel reading data. Nucleic Acids Res. 1980 Aug 25;8(16):3673–3694. doi: 10.1093/nar/8.16.3673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinoco I., Jr, Borer P. N., Dengler B., Levin M. D., Uhlenbeck O. C., Crothers D. M., Bralla J. Improved estimation of secondary structure in ribonucleic acids. Nat New Biol. 1973 Nov 14;246(150):40–41. doi: 10.1038/newbio246040a0. [DOI] [PubMed] [Google Scholar]
- Tribe D. E., Camakaris H., Pittard J. Constitutive and repressivle enzymes of the common pathway of aromatic biosynthesis in Escherichia coli K-12: regulation of enzyme synthesis at different growth rates. J Bacteriol. 1976 Sep;127(3):1085–1097. doi: 10.1128/jb.127.3.1085-1097.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twigg A. J., Sherratt D. Trans-complementable copy-number mutants of plasmid ColE1. Nature. 1980 Jan 10;283(5743):216–218. doi: 10.1038/283216a0. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Welch G. R., Cole K. W., Gaertner F. H. Chorismate synthase of Neurospora crassa: a flavoprotein. Arch Biochem Biophys. 1974 Dec;165(2):505–518. doi: 10.1016/0003-9861(74)90276-8. [DOI] [PubMed] [Google Scholar]