Abstract
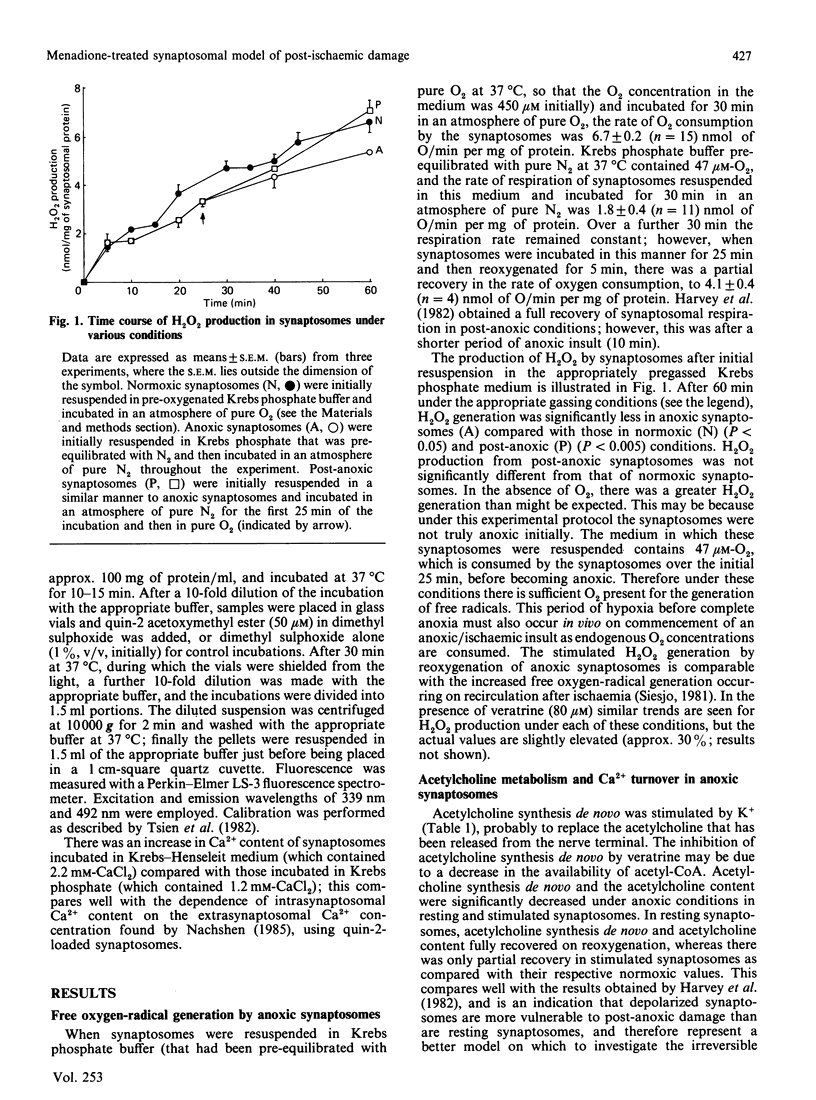
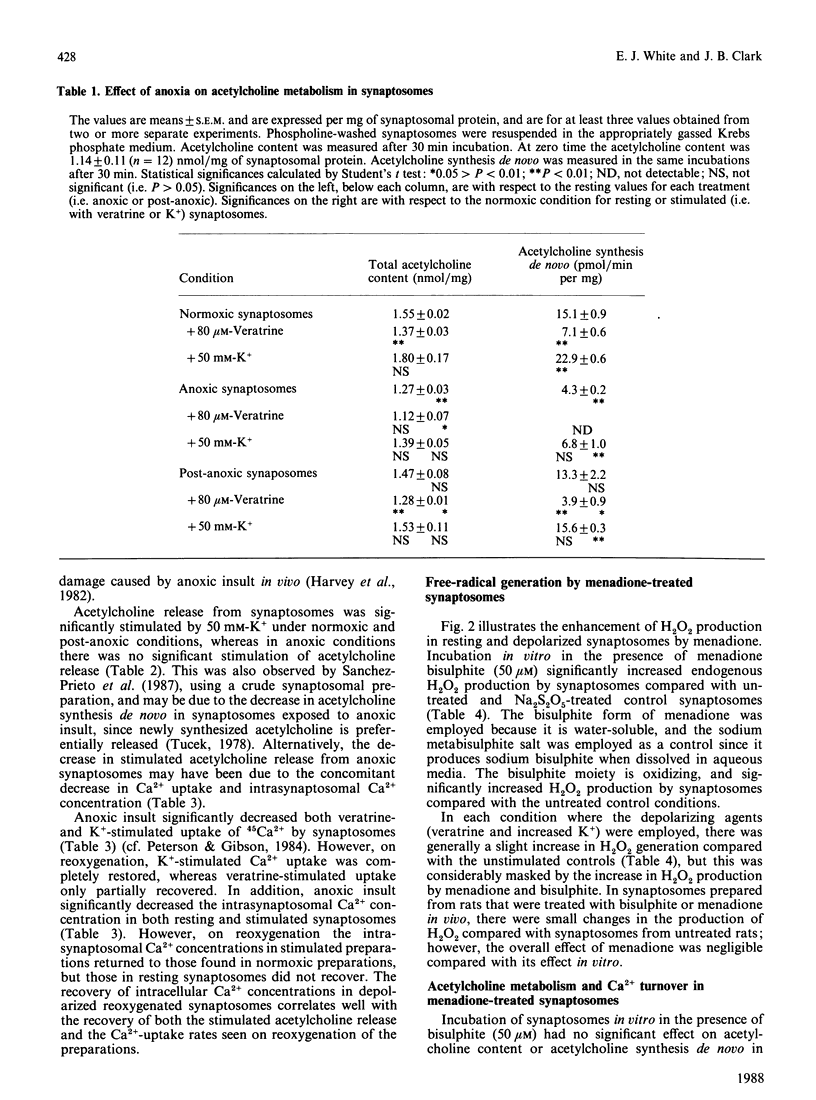
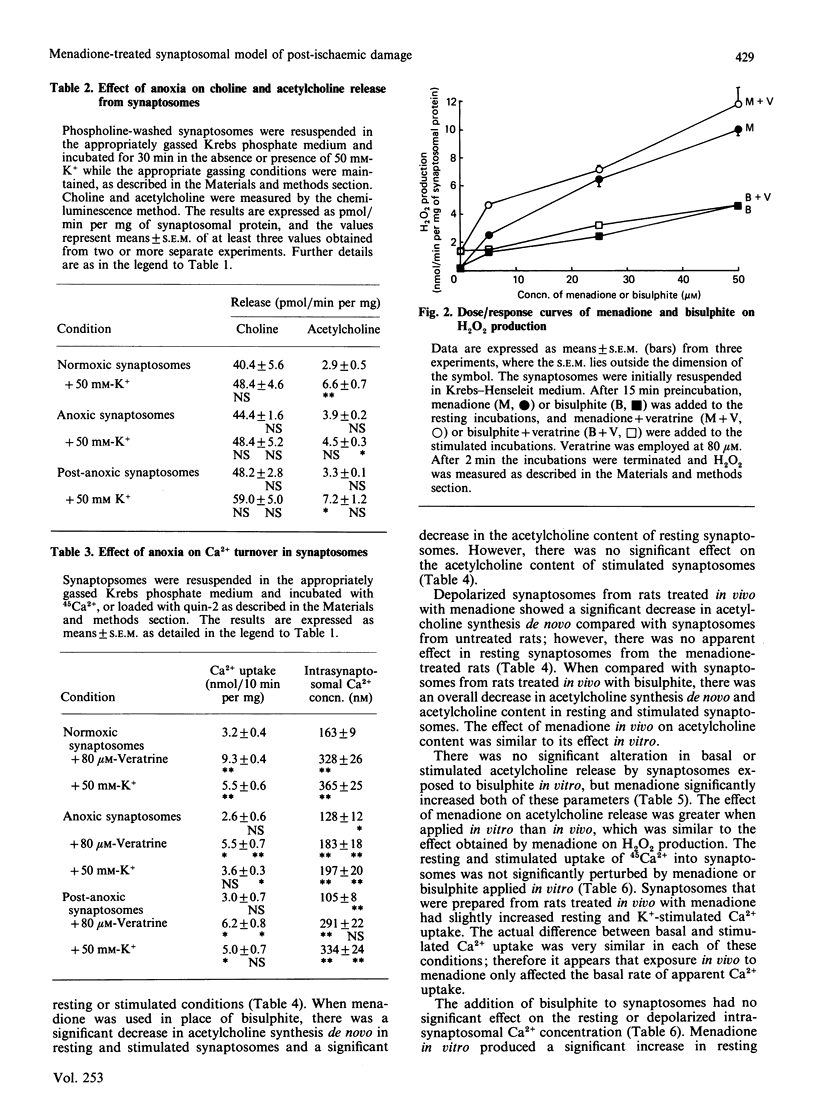
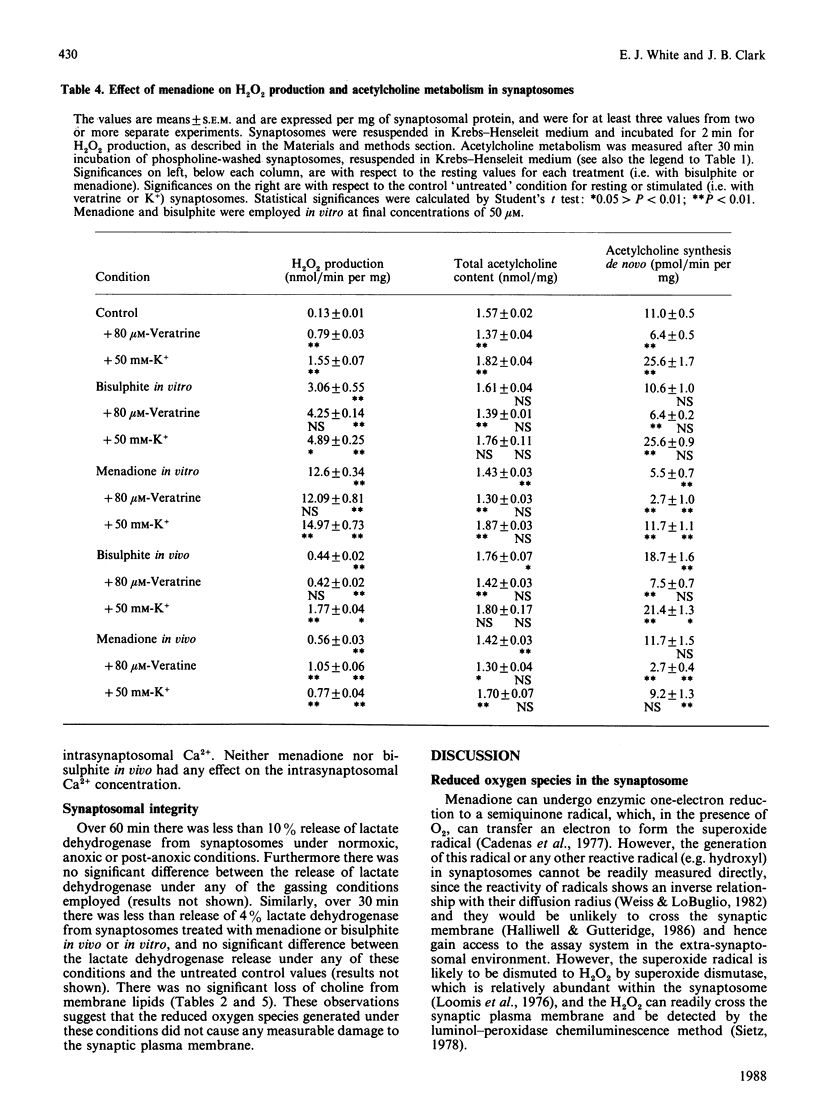
Menadione bisulphite increased endogenous oxygen-radical production by rat brain synaptosomes, as indicated by H2O2 generation. Increased oxygen-radical production was also demonstrated in synaptosomes prepared from menadione-treated rats and synaptosomes reoxygenated after an anoxic insult. Acetylcholine synthesis de novo was inhibited in synaptosomes incubated with menadione in vitro, in synaptosomes prepared from menadione-treated animals in vivo, and in depolarized post-anoxic synaptosomes. Intrasynaptosomal free Ca2+ was increased by menadione in vitro (50 microM), but this increase was not due to stimulation of Ca2+ entry into the nerve terminals. Acetylcholine release was stimulated by menadione in vitro, possibly as a consequence of the elevated intrasynaptosomal Ca2+ content. The Ca2+ contents of synaptosomes prepared from menadione (10 mg/kg)-treated animals in vivo and synaptosomes reoxygenated after anoxia were unchanged. In synaptosomes prepared from menadione-treated animals, acetylcholine release was no longer significantly stimulated by K+, whereas it was unchanged from control (normoxic) values in synaptosomes reoxygenated after anoxia. None of these treatments caused any measurable damage to the synaptic plasma membrane (as judged by the release of lactate dehydrogenase), or to synaptosomal phospholipases (as judged by choline release from membrane phospholipids). Synaptosomes prepared from menadione-treated rats were found to be a good model for the study of post-anoxic damage to nerve-terminal function.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashley R. H., Brammer M. J., Marchbanks R. Measurement of intrasynaptosomal free calcium by using the fluorescent indicator quin-2. Biochem J. 1984 Apr 1;219(1):149–158. doi: 10.1042/bj2190149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth R. F., Clark J. B. A rapid method for the preparation of relatively pure metabolically competent synaptosomes from rat brain. Biochem J. 1978 Nov 15;176(2):365–370. doi: 10.1042/bj1760365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth R. F., Harvey S. A., Clark J. B. Effects of in vivo hypoxia on acetylcholine synthesis by rat brain synaptosomes. J Neurochem. 1983 Jan;40(1):106–110. doi: 10.1111/j.1471-4159.1983.tb12659.x. [DOI] [PubMed] [Google Scholar]
- Braughler J. M., Duncan L. A., Goodman T. Calcium enhances in vitro free radical-induced damage to brain synaptosomes, mitochondria, and cultured spinal cord neurons. J Neurochem. 1985 Oct;45(4):1288–1293. doi: 10.1111/j.1471-4159.1985.tb05555.x. [DOI] [PubMed] [Google Scholar]
- Cadenas E., Boveris A., Ragan C. I., Stoppani A. O. Production of superoxide radicals and hydrogen peroxide by NADH-ubiquinone reductase and ubiquinol-cytochrome c reductase from beef-heart mitochondria. Arch Biochem Biophys. 1977 Apr 30;180(2):248–257. doi: 10.1016/0003-9861(77)90035-2. [DOI] [PubMed] [Google Scholar]
- Di Monte D., Bellomo G., Thor H., Nicotera P., Orrenius S. Menadione-induced cytotoxicity is associated with protein thiol oxidation and alteration in intracellular Ca2+ homeostasis. Arch Biochem Biophys. 1984 Dec;235(2):343–350. doi: 10.1016/0003-9861(84)90207-8. [DOI] [PubMed] [Google Scholar]
- Halliwell B., Gutteridge J. M. Oxygen free radicals and iron in relation to biology and medicine: some problems and concepts. Arch Biochem Biophys. 1986 May 1;246(2):501–514. doi: 10.1016/0003-9861(86)90305-x. [DOI] [PubMed] [Google Scholar]
- Harvey S. A., Booth R. F., Clark J. B. The effects in vitro of hypoglycaemia and recovery from anoxia on synaptosomal metabolism. Biochem J. 1982 Sep 15;206(3):433–439. doi: 10.1042/bj2060433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillered L., Ernster L. Respiratory activity of isolated rat brain mitochondria following in vitro exposure to oxygen radicals. J Cereb Blood Flow Metab. 1983 Jun;3(2):207–214. doi: 10.1038/jcbfm.1983.28. [DOI] [PubMed] [Google Scholar]
- Israël M., Lesbats B. Application to mammalian tissues of the chemiluminescent method for detecting acetylcholine. J Neurochem. 1982 Jul;39(1):248–250. doi: 10.1111/j.1471-4159.1982.tb04727.x. [DOI] [PubMed] [Google Scholar]
- Ksiezak H. J., Gibson G. E. Oxygen dependence of glucose and acetylcholine metabolism in slices and synaptosomes from rat brain. J Neurochem. 1981 Aug;37(2):305–314. doi: 10.1111/j.1471-4159.1981.tb00456.x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Loomis T. C., Yee G., Stahl W. L. Regional and subcellular distribution of superoxide dismutase in brain. Experientia. 1976 Nov 15;32(11):1374–1376. doi: 10.1007/BF01937382. [DOI] [PubMed] [Google Scholar]
- Meldrum B., Evans M., Griffiths T., Simon R. Ischaemic brain damage: the role of excitatory activity and of calcium entry. Br J Anaesth. 1985 Jan;57(1):44–46. doi: 10.1093/bja/57.1.44. [DOI] [PubMed] [Google Scholar]
- Mizuno Y., Ohta K. Regional distributions of thiobarbituric acid-reactive products, activities of enzymes regulating the metabolism of oxygen free radicals, and some of the related enzymes in adult and aged rat brains. J Neurochem. 1986 May;46(5):1344–1352. doi: 10.1111/j.1471-4159.1986.tb01745.x. [DOI] [PubMed] [Google Scholar]
- Nachshen D. A. Regulation of cytosolic calcium concentration in presynaptic nerve endings isolated from rat brain. J Physiol. 1985 Jun;363:87–101. doi: 10.1113/jphysiol.1985.sp015697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patole M. S., Swaroop A., Ramasarma T. Generation of H2O2 in brain mitochondria. J Neurochem. 1986 Jul;47(1):1–8. doi: 10.1111/j.1471-4159.1986.tb02823.x. [DOI] [PubMed] [Google Scholar]
- Peterson C., Gibson G. E. Synaptosomal calcium metabolism during hypoxia and 3,4-diaminopyridine treatment. J Neurochem. 1984 Jan;42(1):248–253. doi: 10.1111/j.1471-4159.1984.tb09725.x. [DOI] [PubMed] [Google Scholar]
- Rehncrona S., Smith D. S., Akesson B., Westerberg E., Siesjö B. K. Peroxidative changes in brain cortical fatty acids and phospholipids, as characterized during Fe2+- and ascorbic acid-stimulated lipid peroxidation in vitro. J Neurochem. 1980 Jun;34(6):1630–1638. doi: 10.1111/j.1471-4159.1980.tb11254.x. [DOI] [PubMed] [Google Scholar]
- Sanchez-Prieto J., Harvey S. A., Clark J. B. Effects of in vitro anoxia and low pH on acetylcholine release by rat brain synaptosomes. J Neurochem. 1987 Apr;48(4):1278–1284. doi: 10.1111/j.1471-4159.1987.tb05658.x. [DOI] [PubMed] [Google Scholar]
- Siesjö B. K. Cell damage in the brain: a speculative synthesis. J Cereb Blood Flow Metab. 1981;1(2):155–185. doi: 10.1038/jcbfm.1981.18. [DOI] [PubMed] [Google Scholar]
- Thor H., Smith M. T., Hartzell P., Bellomo G., Jewell S. A., Orrenius S. The metabolism of menadione (2-methyl-1,4-naphthoquinone) by isolated hepatocytes. A study of the implications of oxidative stress in intact cells. J Biol Chem. 1982 Oct 25;257(20):12419–12425. [PubMed] [Google Scholar]
- Tsien R. Y., Pozzan T., Rink T. J. Calcium homeostasis in intact lymphocytes: cytoplasmic free calcium monitored with a new, intracellularly trapped fluorescent indicator. J Cell Biol. 1982 Aug;94(2):325–334. doi: 10.1083/jcb.94.2.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss S. J., LoBuglio A. F. Phagocyte-generated oxygen metabolites and cellular injury. Lab Invest. 1982 Jul;47(1):5–18. [PubMed] [Google Scholar]
- Willoughby J., Harvey S. A., Clark J. B. Compartmentation and regulation of acetylcholine synthesis at the synapse. Biochem J. 1986 Apr 1;235(1):215–223. doi: 10.1042/bj2350215. [DOI] [PMC free article] [PubMed] [Google Scholar]


