Abstract
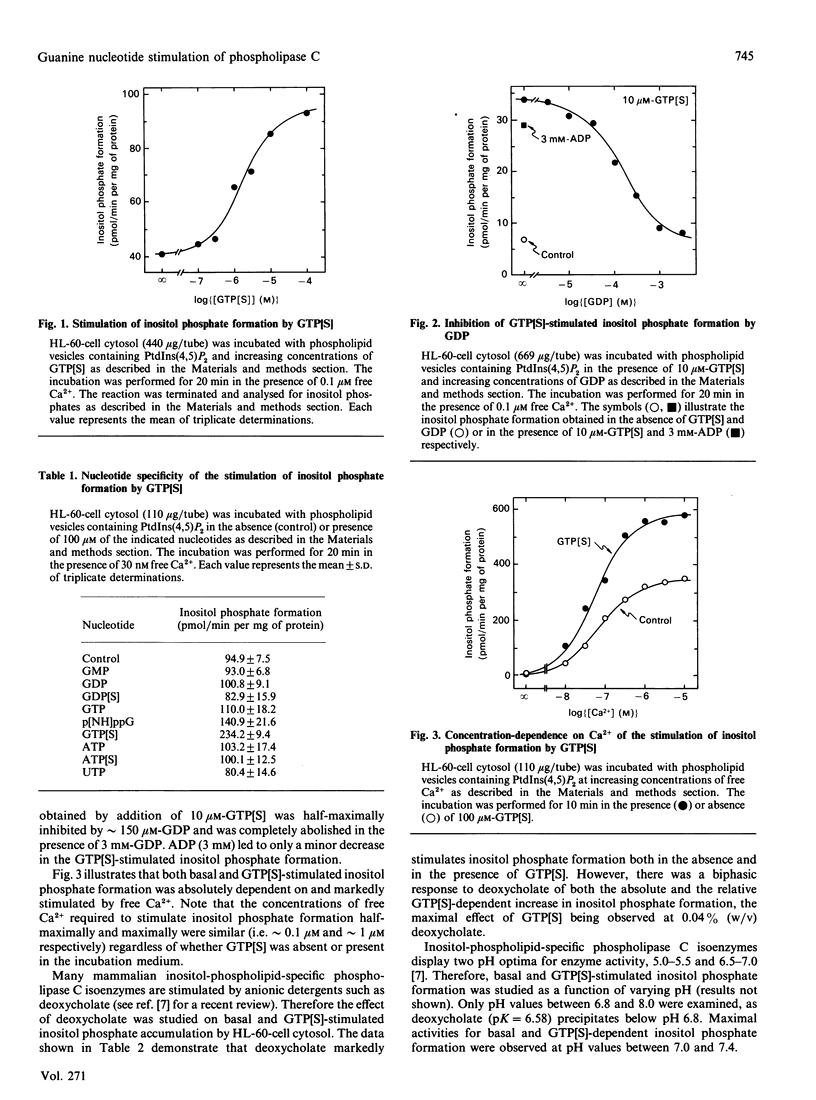
Myeloid differentiated human leukaemia (HL-60) cells contain a soluble phospholipase C that hydrolysed phosphatidylinositol 4.5-bisphosphate and was markedly stimulated by the metabolically stable GTP analogue guanosine 5'-[gamma-thio]triphosphate (GTP[S]). Half-maximal and maximal (up to 5-fold) stimulation of inositol phosphate formation by GTP[S] occurred at 1.5 microM and 30 microM respectively. Other nucleotides (GTP, GDP, GMP, guanosine 5'-[beta-thio]diphosphate. ATP, adenosine 5'-[gamma-thio]triphosphate, UTP) did not affect phospholipase C activity, GTP[S] stimulation of inositol phosphate accumulation was inhibited by excess GDP, but not by ADP. The effect of GTP[S] on inositol phosphate formation was absolutely dependent on and markedly stimulated by free Ca2+ (median effective concn. approximately 100 nM). Analysis of inositol phosphates by anion-exchange chromatography revealed InsP3 as the major product of GTP[S]-stimulated phospholipase C activity. In the absence of GTP[S], specific phospholipase C activity was markedly decreased when tested at high protein concentrations, whereas GTP[S] stimulation of the enzyme was markedly enhanced under these conditions. As both basal and GTP[S]-stimulated inositol phosphate formation were linear with time whether studied at low or high protein concentration, these results suggest that (a) phospholipase C is under an inhibitory constraint and (b) GTP[S] relieves this inhibition, most likely by activating a soluble GTP-binding protein.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anthes J. C., Eckel S., Siegel M. I., Egan R. W., Billah M. M. Phospholipase D in homogenates from HL-60 granulocytes: implications of calcium and G protein control. Biochem Biophys Res Commun. 1989 Aug 30;163(1):657–664. doi: 10.1016/0006-291x(89)92187-6. [DOI] [PubMed] [Google Scholar]
- Baldassare J. J., Fisher G. J. Regulation of membrane-associated and cytosolic phospholipase C activities in human platelets by guanosine triphosphate. J Biol Chem. 1986 Sep 15;261(26):11942–11944. [PubMed] [Google Scholar]
- Baldassare J. J., Knipp M. A., Henderson P. A., Fisher G. J. GTP gamma S-stimulated hydrolysis of phosphatidylinositol-4,5-bisphosphate soluble phospholipase C from human platelets requires soluble GTP-binding protein. Biochem Biophys Res Commun. 1988 Jul 15;154(1):351–357. doi: 10.1016/0006-291x(88)90692-4. [DOI] [PubMed] [Google Scholar]
- Balsinde J., Diez E., Mollinedo F. Phosphatidylinositol-specific phospholipase D: a pathway for generation of a second messenger. Biochem Biophys Res Commun. 1988 Jul 29;154(2):502–508. doi: 10.1016/0006-291x(88)90168-4. [DOI] [PubMed] [Google Scholar]
- Banno Y., Nakashima S., Tohmatsu T., Nozawa Y., Lapetina E. G. GTP and GDP will stimulate platelet cytosolic phospholipase C independently of Ca2+. Biochem Biophys Res Commun. 1986 Oct 30;140(2):728–734. doi: 10.1016/0006-291x(86)90792-8. [DOI] [PubMed] [Google Scholar]
- Bartfai T. Preparation of metal-chelate complexes and the design of steady-state kinetic experiments involving metal nucleotide complexes. Adv Cyclic Nucleotide Res. 1979;10:219–242. [PubMed] [Google Scholar]
- Berridge M. J., Dawson R. M., Downes C. P., Heslop J. P., Irvine R. F. Changes in the levels of inositol phosphates after agonist-dependent hydrolysis of membrane phosphoinositides. Biochem J. 1983 May 15;212(2):473–482. doi: 10.1042/bj2120473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol phosphates and cell signalling. Nature. 1989 Sep 21;341(6239):197–205. doi: 10.1038/341197a0. [DOI] [PubMed] [Google Scholar]
- Bojanic D., Wallace M. A., Wojcikiewicz R. J., Fain J. N. Guanine nucleotide and pyrophosphate activate exogenous phosphatidylinositol 4,5-bisphosphate hydrolysis in rat liver plasma membranes. Biochem Biophys Res Commun. 1987 Sep 30;147(3):1088–1094. doi: 10.1016/s0006-291x(87)80182-1. [DOI] [PubMed] [Google Scholar]
- Bone E. A., Fretten P., Palmer S., Kirk C. J., Michell R. H. Rapid accumulation of inositol phosphates in isolated rat superior cervical sympathetic ganglia exposed to V1-vasopressin and muscarinic cholinergic stimuli. Biochem J. 1984 Aug 1;221(3):803–811. doi: 10.1042/bj2210803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Bradford P. G., Rubin R. P. Guanine nucleotide regulation of phospholipase C activity in permeabilized rabbit neutrophils. Inhibition by pertussis toxin and sensitization to submicromolar calcium concentrations. Biochem J. 1986 Oct 1;239(1):97–102. doi: 10.1042/bj2390097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt S. J., Dougherty R. W., Lapetina E. G., Niedel J. E. Pertussis toxin inhibits chemotactic peptide-stimulated generation of inositol phosphates and lysosomal enzyme secretion in human leukemic (HL-60) cells. Proc Natl Acad Sci U S A. 1985 May;82(10):3277–3280. doi: 10.1073/pnas.82.10.3277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockcroft S., Stutchfield J. Effect of pertussis toxin and neomycin on G-protein-regulated polyphosphoinositide phosphodiesterase. A comparison between HL60 membranes and permeabilized HL60 cells. Biochem J. 1988 Dec 1;256(2):343–350. doi: 10.1042/bj2560343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockcroft S., Stutchfield J. G-proteins, the inositol lipid signalling pathway, and secretion. Philos Trans R Soc Lond B Biol Sci. 1988 Jul 26;320(1199):247–265. doi: 10.1098/rstb.1988.0075. [DOI] [PubMed] [Google Scholar]
- Crooke S. T., Bennett C. F. Mammalian phosphoinositide-specific phospholipase C isoenzymes. Cell Calcium. 1989 Jul;10(5):309–323. doi: 10.1016/0143-4160(89)90057-2. [DOI] [PubMed] [Google Scholar]
- Deckmyn H., Tu S. M., Majerus P. W. Guanine nucleotides stimulate soluble phosphoinositide-specific phospholipase C in the absence of membranes. J Biol Chem. 1986 Dec 15;261(35):16553–16558. [PubMed] [Google Scholar]
- Dillon S. B., Murray J. J., Uhing R. J., Snyderman R. Regulation of inositol phospholipid and inositol phosphate metabolism in chemoattractant-activated human polymorphonuclear leukocytes. J Cell Biochem. 1987 Dec;35(4):345–359. doi: 10.1002/jcb.240350409. [DOI] [PubMed] [Google Scholar]
- Downes C. P., Mussat M. C., Michell R. H. The inositol trisphosphate phosphomonoesterase of the human erythrocyte membrane. Biochem J. 1982 Apr 1;203(1):169–177. doi: 10.1042/bj2030169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Exton J. H. Mechanisms of action of calcium-mobilizing agonists: some variations on a young theme. FASEB J. 1988 Aug;2(11):2670–2676. doi: 10.1096/fasebj.2.11.2456243. [DOI] [PubMed] [Google Scholar]
- Fain J. N., Wallace M. A., Wojcikiewicz R. J. Evidence for involvement of guanine nucleotide-binding regulatory proteins in the activation of phospholipases by hormones. FASEB J. 1988 Jul;2(10):2569–2574. doi: 10.1096/fasebj.2.10.2838362. [DOI] [PubMed] [Google Scholar]
- Fisher G. J., Baldassare J. J., Voorhees J. J. GTP-dependent hydrolysis of phosphatidylinositol-4,5-bisphosphate by soluble phospholipase C from adult human epidermis. J Invest Dermatol. 1989 Jun;92(6):831–836. doi: 10.1111/1523-1747.ep12696846. [DOI] [PubMed] [Google Scholar]
- Gierschik P., Jakobs K. H. Receptor-mediated ADP-ribosylation of a phospholipase C-stimulating G protein. FEBS Lett. 1987 Nov 16;224(1):219–223. doi: 10.1016/0014-5793(87)80451-9. [DOI] [PubMed] [Google Scholar]
- Gierschik P., Sidiropoulos D., Jakobs K. H. Two distinct Gi-proteins mediate formyl peptide receptor signal transduction in human leukemia (HL-60) cells. J Biol Chem. 1989 Dec 25;264(36):21470–21473. [PubMed] [Google Scholar]
- Gierschik P., Sidiropoulos D., Spiegel A., Jakobs K. H. Purification and immunochemical characterization of the major pertussis-toxin-sensitive guanine-nucleotide-binding protein of bovine-neutrophil membranes. Eur J Biochem. 1987 May 15;165(1):185–194. doi: 10.1111/j.1432-1033.1987.tb11210.x. [DOI] [PubMed] [Google Scholar]
- Gierschik P., Steisslinger M., Sidiropoulos D., Herrmann E., Jakobs K. H. Dual Mg2+ control of formyl-peptide-receptor--G-protein interaction in HL 60 cells. Evidence that the low-agonist-affinity receptor interacts with and activates the G-protein. Eur J Biochem. 1989 Jul 15;183(1):97–105. doi: 10.1111/j.1432-1033.1989.tb14901.x. [DOI] [PubMed] [Google Scholar]
- Grzeskowiak M., Della Bianca V., Cassatella M. A., Rossi F. Complete dissociation between the activation of phosphoinositide turnover and of NADPH oxidase by formyl-methionyl-leucyl-phenylalanine in human neutrophils depleted of Ca2+ and primed by subthreshold doses of phorbol 12,myristate 13,acetate. Biochem Biophys Res Commun. 1986 Mar 28;135(3):785–794. doi: 10.1016/0006-291x(86)90997-6. [DOI] [PubMed] [Google Scholar]
- Jolles J., Zwiers H., Dekker A., Wirtz K. W., Gispen W. H. Corticotropin-(1--24)-tetracosapeptide affects protein phosphorylation and polyphosphoinositide metabolism in rat brain. Biochem J. 1981 Jan 15;194(1):283–291. doi: 10.1042/bj1940283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi A., Kozawa O., Kaibuchi K., Katada T., Ui M., Takai Y. Direct evidence for involvement of a guanine nucleotide-binding protein in chemotactic peptide-stimulated formation of inositol bisphosphate and trisphosphate in differentiated human leukemic (HL-60) cells. Reconstitution with Gi or Go of the plasma membranes ADP-ribosylated by pertussis toxin. J Biol Chem. 1986 Sep 5;261(25):11558–11562. [PubMed] [Google Scholar]
- Krause K. H., Schlegel W., Wollheim C. B., Andersson T., Waldvogel F. A., Lew P. D. Chemotactic peptide activation of human neutrophils and HL-60 cells. Pertussis toxin reveals correlation between inositol trisphosphate generation, calcium ion transients, and cellular activation. J Clin Invest. 1985 Oct;76(4):1348–1354. doi: 10.1172/JCI112109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lew P. D., Monod A., Krause K. H., Waldvogel F. A., Biden T. J., Schlegel W. The role of cytosolic free calcium in the generation of inositol 1,4,5-trisphosphate and inositol 1,3,4-trisphosphate in HL-60 cells. Differential effects of chemotactic peptide receptor stimulation at distinct Ca2+ levels. J Biol Chem. 1986 Oct 5;261(28):13121–13127. [PubMed] [Google Scholar]
- Lochrie M. A., Simon M. I. G protein multiplicity in eukaryotic signal transduction systems. Biochemistry. 1988 Jul 12;27(14):4957–4965. doi: 10.1021/bi00414a001. [DOI] [PubMed] [Google Scholar]
- Meisenhelder J., Suh P. G., Rhee S. G., Hunter T. Phospholipase C-gamma is a substrate for the PDGF and EGF receptor protein-tyrosine kinases in vivo and in vitro. Cell. 1989 Jun 30;57(7):1109–1122. doi: 10.1016/0092-8674(89)90048-2. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. The molecular heterogeneity of protein kinase C and its implications for cellular regulation. Nature. 1988 Aug 25;334(6184):661–665. doi: 10.1038/334661a0. [DOI] [PubMed] [Google Scholar]
- Omann G. M., Allen R. A., Bokoch G. M., Painter R. G., Traynor A. E., Sklar L. A. Signal transduction and cytoskeletal activation in the neutrophil. Physiol Rev. 1987 Jan;67(1):285–322. doi: 10.1152/physrev.1987.67.1.285. [DOI] [PubMed] [Google Scholar]
- Pike M. C., Bruck M. E., Arndt C., Lee C. S. Chemoattractants stimulate phosphatidylinositol-4-phosphate kinase in human polymorphonuclear leukocytes. J Biol Chem. 1990 Feb 5;265(4):1866–1873. [PubMed] [Google Scholar]
- Rhee S. G., Suh P. G., Ryu S. H., Lee S. Y. Studies of inositol phospholipid-specific phospholipase C. Science. 1989 May 5;244(4904):546–550. doi: 10.1126/science.2541501. [DOI] [PubMed] [Google Scholar]
- Rock C. O., Jackowski S. Thrombin- and nucleotide-activated phosphatidylinositol 4,5-bisphosphate phospholipase C in human platelet membranes. J Biol Chem. 1987 Apr 25;262(12):5492–5498. [PubMed] [Google Scholar]
- Ryu S. H., Cho K. S., Lee K. Y., Suh P. G., Rhee S. G. Purification and characterization of two immunologically distinct phosphoinositide-specific phospholipases C from bovine brain. J Biol Chem. 1987 Sep 15;262(26):12511–12518. [PubMed] [Google Scholar]
- Smith C. D., Chang K. J. Regulation of brain phosphatidylinositol-4-phosphate kinase by GTP analogues. A potential role for guanine nucleotide regulatory proteins. J Biol Chem. 1989 Feb 25;264(6):3206–3210. [PubMed] [Google Scholar]
- Stutchfield J., Cockcroft S. Guanine nucleotides stimulate polyphosphoinositide phosphodiesterase and exocytotic secretion from HL60 cells permeabilized with streptolysin O. Biochem J. 1988 Mar 1;250(2):375–382. doi: 10.1042/bj2500375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traynor-Kaplan A. E., Thompson B. L., Harris A. L., Taylor P., Omann G. M., Sklar L. A. Transient increase in phosphatidylinositol 3,4-bisphosphate and phosphatidylinositol trisphosphate during activation of human neutrophils. J Biol Chem. 1989 Sep 15;264(26):15668–15673. [PubMed] [Google Scholar]
- Urumow T., Wieland O. H. Stimulation of phosphatidylinositol 4-phosphate phosphorylation in human placenta membranes by GTP gamma S. FEBS Lett. 1986 Oct 27;207(2):253–257. doi: 10.1016/0014-5793(86)81499-5. [DOI] [PubMed] [Google Scholar]
- Wang P., Nishihata J., Takabori E., Yamamoto K., Toyoshima S., Osawa T. Purification and partial amino acid sequences of a phospholipase C-associated GTP-binding protein from calf thymocytes. J Biochem. 1989 Mar;105(3):461–466. doi: 10.1093/oxfordjournals.jbchem.a122687. [DOI] [PubMed] [Google Scholar]
- Wang P., Toyoshima S., Osawa T. Physical and functional association of cytosolic inositolphospholipid-specific phospholipase C of calf thymocytes with a GTP-binding protein. J Biochem. 1987 Nov;102(5):1275–1287. doi: 10.1093/oxfordjournals.jbchem.a122166. [DOI] [PubMed] [Google Scholar]
- Wang P., Toyoshima S., Osawa T. Properties of a novel GTP-binding protein which is associated with soluble phosphoinositides-specific phospholipase C. J Biochem. 1988 Jan;103(1):137–142. doi: 10.1093/oxfordjournals.jbchem.a122219. [DOI] [PubMed] [Google Scholar]


