Abstract
The addition to intact cells of an inositol phospho-oligosaccharide (POS), which is the polar head-group of an insulin-sensitive glycosylphosphatidylinositol, mimics and may mediate some of the biological effects of this hormone. Here we report the existence of a POS transport system in hepatocytes. This POS transport system is specific and time- and dose-dependent. Insulin-resistance caused by dexamethasone administration to rats was accompanied by a decrease in the hepatocyte POS transport system. In contrast, bilateral adrenalectomy provoked a significant increase in the transport of POS. Both the temporal uptake of POS and the regulation of this process by conditions known to modify the sensitivity to insulin suggest that this novel transport system might be involved in the insulin signalling mechanism.
Full text
PDF
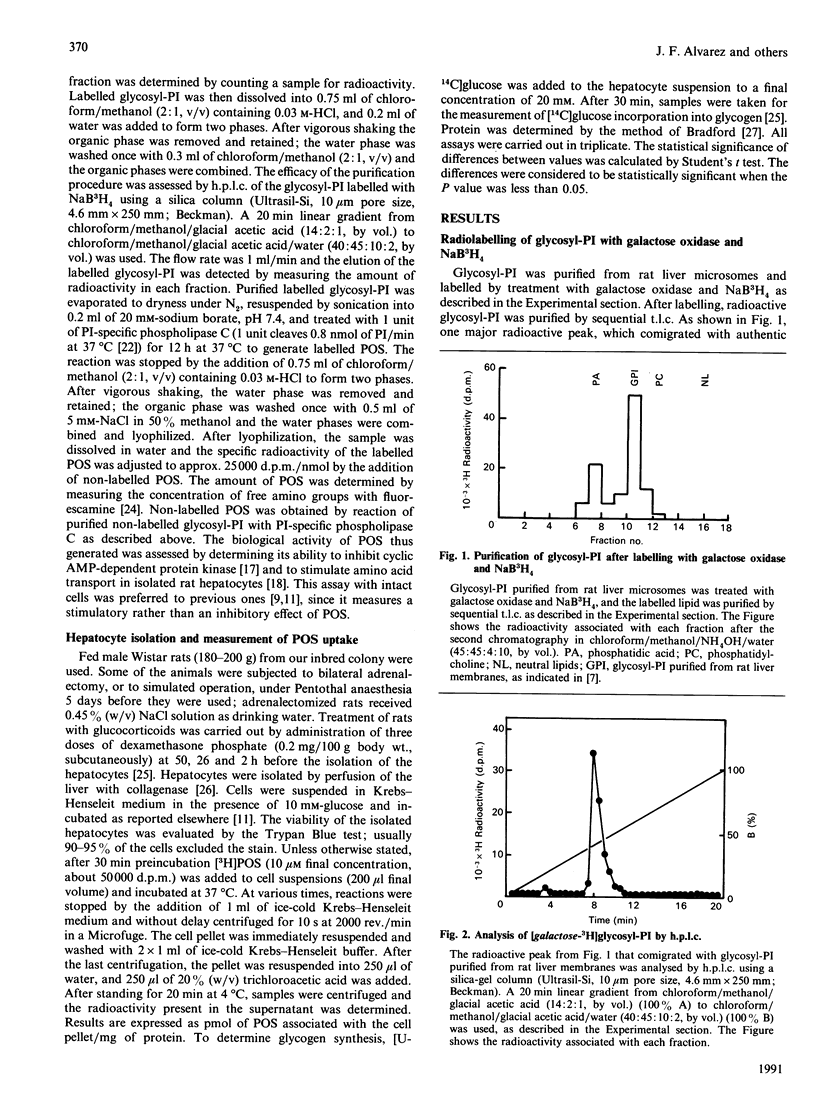
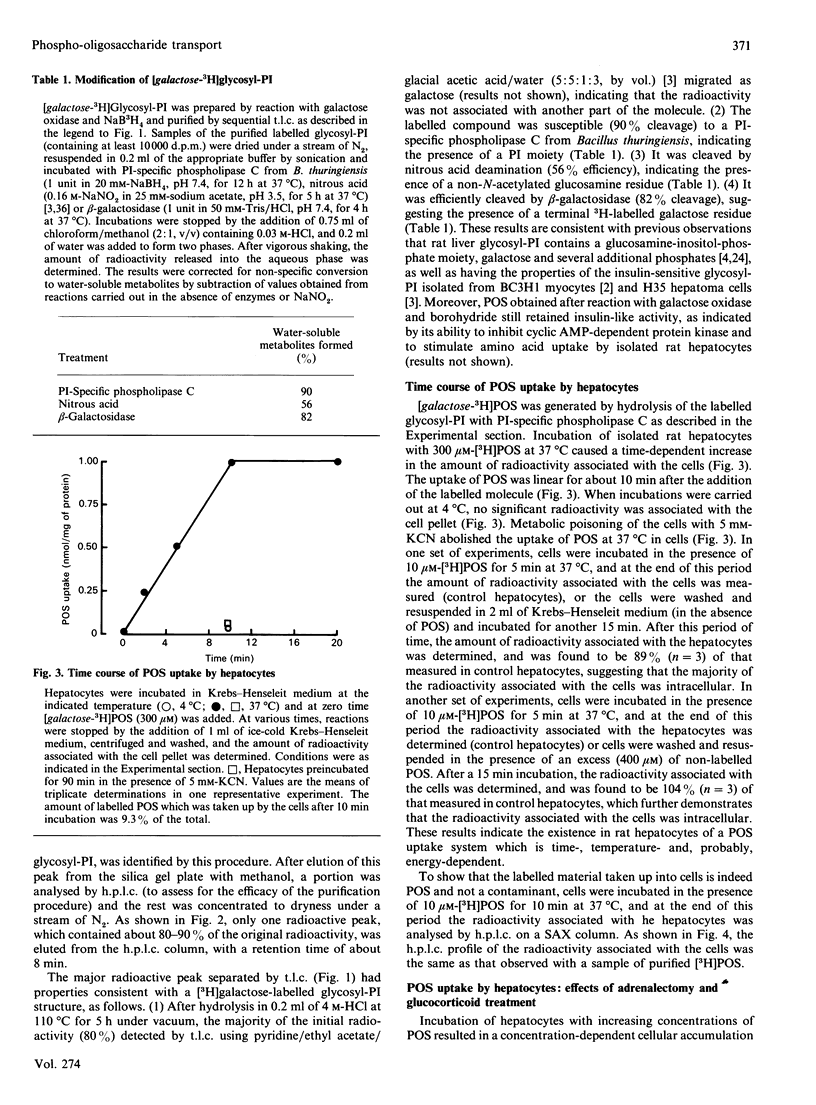
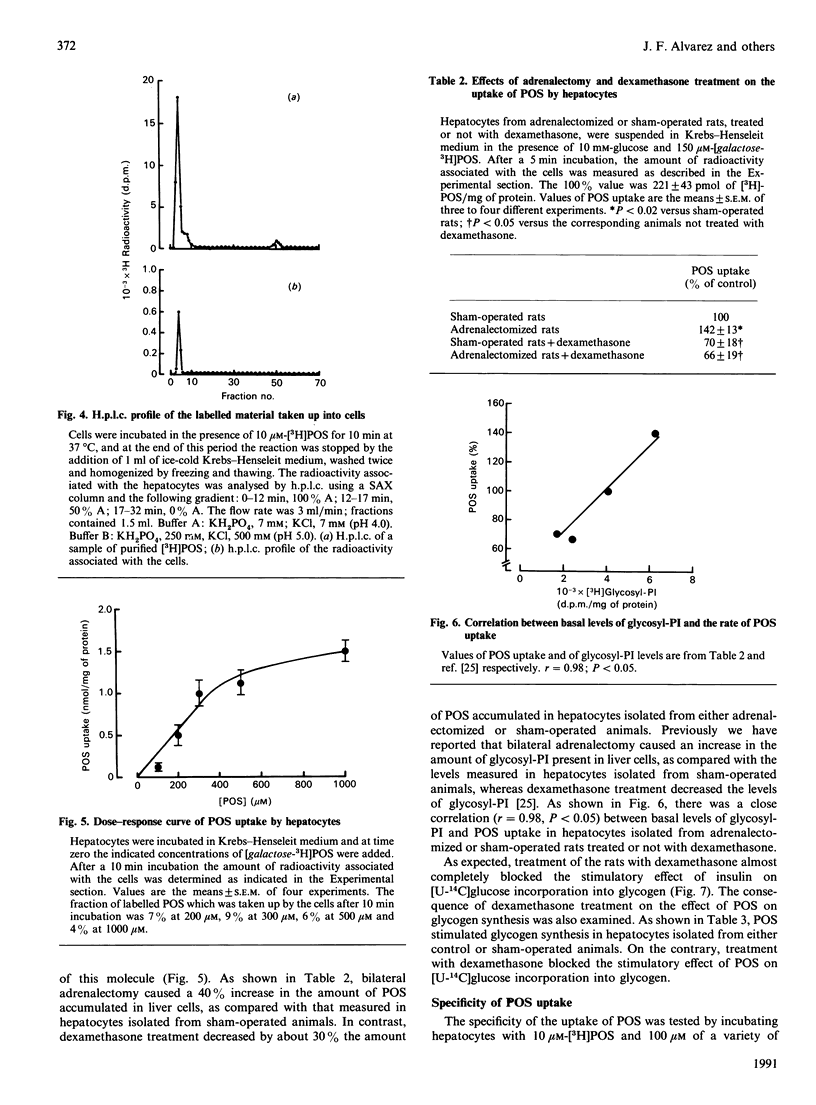
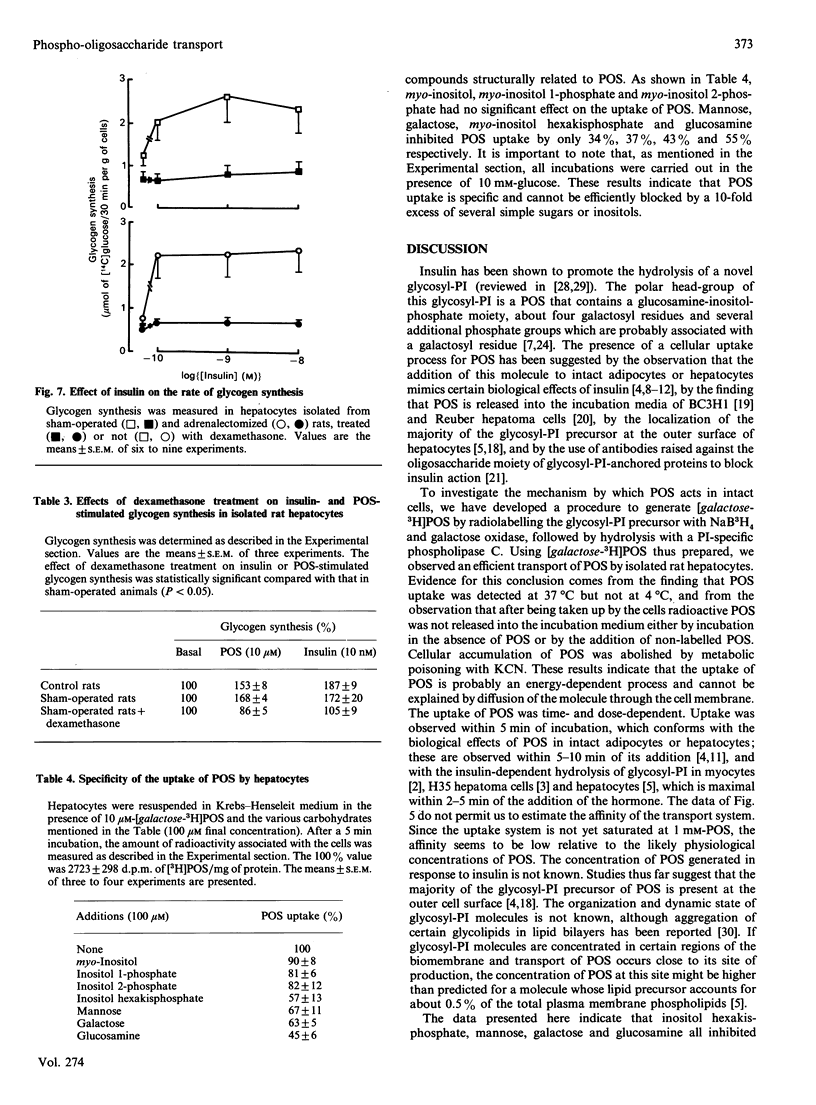

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alemany S., Mato J. M., Strålfors P. Phospho-dephospho-control by insulin is mimicked by a phospho-oligosaccharide in adipocytes. Nature. 1987 Nov 5;330(6143):77–79. doi: 10.1038/330077a0. [DOI] [PubMed] [Google Scholar]
- Alvarez J. F., Cabello M. A., Felíu J. E., Mato J. M. A phospho-oligosaccharide mimics insulin action on glycogen phosphorylase and pyruvate kinase activities in isolated rat hepatocytes. Biochem Biophys Res Commun. 1987 Sep 15;147(2):765–771. doi: 10.1016/0006-291x(87)90996-x. [DOI] [PubMed] [Google Scholar]
- Alvarez J. F., Varela I., Ruiz-Albusac J. M., Mato J. M. Localisation of the insulin-sensitive phosphatidylinositol glycan at the outer surface of the cell membrane. Biochem Biophys Res Commun. 1988 May 16;152(3):1455–1462. doi: 10.1016/s0006-291x(88)80449-2. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Felíu J. E., Mojena M., Silvestre R. A., Monge L., Marco J. Stimulatory effect of vasoactive intestinal peptide on glycogenolysis and gluconeogenesis in isolated rat hepatocytes: antagonism by insulin. Endocrinology. 1983 Jun;112(6):2120–2127. doi: 10.1210/endo-112-6-2120. [DOI] [PubMed] [Google Scholar]
- Ferguson M. A., Haldar K., Cross G. A. Trypanosoma brucei variant surface glycoprotein has a sn-1,2-dimyristyl glycerol membrane anchor at its COOH terminus. J Biol Chem. 1985 Apr 25;260(8):4963–4968. [PubMed] [Google Scholar]
- Gaulton G. N., Kelly K. L., Pawlowski J., Mato J. M., Jarett L. Regulation and function of an insulin-sensitive glycosyl-phosphatidylinositol during T lymphocyte activation. Cell. 1988 Jun 17;53(6):963–970. doi: 10.1016/s0092-8674(88)90509-0. [DOI] [PubMed] [Google Scholar]
- Gottschalk W. K., Jarett L. The insulinomimetic effects of the polar head group of an insulin-sensitive glycophospholipid on pyruvate dehydrogenase in both subcellular and whole cell assays. Arch Biochem Biophys. 1988 Feb 15;261(1):175–185. doi: 10.1016/0003-9861(88)90116-6. [DOI] [PubMed] [Google Scholar]
- Hakomori S. Glycosphingolipids in cellular interaction, differentiation, and oncogenesis. Annu Rev Biochem. 1981;50:733–764. doi: 10.1146/annurev.bi.50.070181.003505. [DOI] [PubMed] [Google Scholar]
- Howard A. D., Berger J., Gerber L., Familletti P., Udenfriend S. Characterization of the phosphatidylinositol-glycan membrane anchor of human placental alkaline phosphatase. Proc Natl Acad Sci U S A. 1987 Sep;84(17):6055–6059. doi: 10.1073/pnas.84.17.6055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly K. L., Mato J. M., Jarett L. The polar head group of a novel insulin-sensitive glycophospholipid mimics insulin action on phospholipid methyltransferase. FEBS Lett. 1986 Dec 15;209(2):238–242. doi: 10.1016/0014-5793(86)81119-x. [DOI] [PubMed] [Google Scholar]
- Kelly K. L., Mato J. M., Merida I., Jarett L. Glucose transport and antilipolysis are differentially regulated by the polar head group of an insulin-sensitive glycophospholipid. Proc Natl Acad Sci U S A. 1987 Sep;84(18):6404–6407. doi: 10.1073/pnas.84.18.6404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly K. L., Merida I., Wong E. H., DiCenzo D., Mato J. M. A phospho-oligosaccharide mimics the effect of insulin to inhibit isoproterenol-dependent phosphorylation of phospholipid methyltransferase in isolated adipocytes. J Biol Chem. 1987 Nov 5;262(31):15285–15290. [PubMed] [Google Scholar]
- Larner J., Huang L. C., Schwartz C. F., Oswald A. S., Shen T. Y., Kinter M., Tang G. Z., Zeller K. Rat liver insulin mediator which stimulates pyruvate dehydrogenase phosphate contains galactosamine and D-chiroinositol. Biochem Biophys Res Commun. 1988 Mar 30;151(3):1416–1426. doi: 10.1016/s0006-291x(88)80520-5. [DOI] [PubMed] [Google Scholar]
- Low M. G., Saltiel A. R. Structural and functional roles of glycosyl-phosphatidylinositol in membranes. Science. 1988 Jan 15;239(4837):268–275. doi: 10.1126/science.3276003. [DOI] [PubMed] [Google Scholar]
- Machicao F., Mushack J., Seffer E., Ermel B., Häring H. U. Mannose, glucosamine and inositol monophosphate inhibit the effects of insulin on lipogenesis. Further evidence for a role for inositol phosphate-oligosaccharides in insulin action. Biochem J. 1990 Mar 15;266(3):909–916. [PMC free article] [PubMed] [Google Scholar]
- Mato J. M. Insulin mediators revisited. Cell Signal. 1989;1(2):143–146. doi: 10.1016/0898-6568(89)90003-x. [DOI] [PubMed] [Google Scholar]
- Mato J. M., Kelly K. L., Abler A., Jarett L., Corkey B. E., Cashel J. A., Zopf D. Partial structure of an insulin-sensitive glycophospholipid. Biochem Biophys Res Commun. 1987 Jul 31;146(2):764–770. doi: 10.1016/0006-291x(87)90595-x. [DOI] [PubMed] [Google Scholar]
- Mato J. M., Kelly K. L., Abler A., Jarett L. Identification of a novel insulin-sensitive glycophospholipid from H35 hepatoma cells. J Biol Chem. 1987 Feb 15;262(5):2131–2137. [PubMed] [Google Scholar]
- McMahon M., Gerich J., Rizza R. Effects of glucocorticoids on carbohydrate metabolism. Diabetes Metab Rev. 1988 Feb;4(1):17–30. doi: 10.1002/dmr.5610040105. [DOI] [PubMed] [Google Scholar]
- Merida I., Corrales F. J., Clemente R., Ruiz-Albusac J. M., Villalba M., Mato J. M. Different phosphorylated forms of an insulin-sensitive glycosylphosphatidylinositol from rat hepatocytes. FEBS Lett. 1988 Aug 15;236(1):251–255. doi: 10.1016/0014-5793(88)80325-9. [DOI] [PubMed] [Google Scholar]
- Rizza R. A., Mandarino L. J., Gerich J. E. Cortisol-induced insulin resistance in man: impaired suppression of glucose production and stimulation of glucose utilization due to a postreceptor detect of insulin action. J Clin Endocrinol Metab. 1982 Jan;54(1):131–138. doi: 10.1210/jcem-54-1-131. [DOI] [PubMed] [Google Scholar]
- Romero G., Gámez G., Huang L. C., Lilley K., Luttrell L. Anti-inositolglycan antibodies selectively block some of the actions of insulin in intact BC3H1 cells. Proc Natl Acad Sci U S A. 1990 Feb;87(4):1476–1480. doi: 10.1073/pnas.87.4.1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero G., Luttrell L., Rogol A., Zeller K., Hewlett E., Larner J. Phosphatidylinositol-glycan anchors of membrane proteins: potential precursors of insulin mediators. Science. 1988 Apr 22;240(4851):509–511. doi: 10.1126/science.3282305. [DOI] [PubMed] [Google Scholar]
- Saltiel A. R., Cuatrecasas P. Insulin stimulates the generation from hepatic plasma membranes of modulators derived from an inositol glycolipid. Proc Natl Acad Sci U S A. 1986 Aug;83(16):5793–5797. doi: 10.1073/pnas.83.16.5793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saltiel A. R., Fox J. A., Sherline P., Cuatrecasas P. Insulin-stimulated hydrolysis of a novel glycolipid generates modulators of cAMP phosphodiesterase. Science. 1986 Aug 29;233(4767):967–972. doi: 10.1126/science.3016898. [DOI] [PubMed] [Google Scholar]
- Saltiel A. R. Insulin generates an enzyme modulator from hepatic plasma membranes: regulation of adenosine 3',5'-monophosphate phosphodiesterase, pyruvate dehydrogenase, and adenylate cyclase. Endocrinology. 1987 Mar;120(3):967–972. doi: 10.1210/endo-120-3-967. [DOI] [PubMed] [Google Scholar]
- Saltiel A. R., Sorbara-Cazan L. R. Inositol glycan mimics the action of insulin on glucose utilization in rat adipocytes. Biochem Biophys Res Commun. 1987 Dec 31;149(3):1084–1092. doi: 10.1016/0006-291x(87)90519-5. [DOI] [PubMed] [Google Scholar]
- Steck T. L., Dawson G. Topographical distribution of complex carbohydrates in the erythrocyte membrane. J Biol Chem. 1974 Apr 10;249(7):2135–2142. [PubMed] [Google Scholar]
- Strålfors P., Alemany S. Phosphorylation control by insulin in adipocytes is interfered with at a post-receptor step by phosphoinositol and glucosamine. FEBS Lett. 1990 Jul 30;268(1):169–172. doi: 10.1016/0014-5793(90)81000-e. [DOI] [PubMed] [Google Scholar]
- Varela I., Alvarez J. F., Clemente R., Ruiz-Albusac J. M., Mato J. M. Asymmetric distribution of the phosphatidylinositol-linked phospho-oligosaccharide that mimics insulin action in the plasma membrane. Eur J Biochem. 1990 Mar 10;188(2):213–218. doi: 10.1111/j.1432-1033.1990.tb15392.x. [DOI] [PubMed] [Google Scholar]
- Varela I., Avila M., Mato J. M., Hue L. Insulin-induced phospho-oligosaccharide stimulates amino acid transport in isolated rat hepatocytes. Biochem J. 1990 Apr 15;267(2):541–544. doi: 10.1042/bj2670541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villalba M., Kelly K. L., Mato J. M. Inhibition of cyclic AMP-dependent protein kinase by the polar head group of an insulin-sensitive glycophospholipid. Biochim Biophys Acta. 1988 Jan 18;968(1):69–76. doi: 10.1016/0167-4889(88)90045-6. [DOI] [PubMed] [Google Scholar]
- Witters L. A., Watts T. D. An autocrine factor from Reuber hepatoma cells that stimulates DNA synthesis and acetyl-CoA carboxylase. Characterization of biologic activity and evidence for a glycan structure. J Biol Chem. 1988 Jun 15;263(17):8027–8036. [PubMed] [Google Scholar]


