Abstract
Protein hydrolysates from the goat placenta provide multiple benefits, such as immune system enhancement, antioxidant activities, and reductions in uric acid levels. Despite these benefits, their industrial applications have been underexplored. This study aimed to prepare extract protein hydrolysates (GPERPs) from residual goat placenta extract (GPER) and assess their functional properties, focusing on how different drying methods influence these properties. The essential amino acid contents were 30.94% for the GPER and 34.11% for the GPERPs. Moreover, all the essential amino acids were present, and the amino acid score (AAS) for each exceeded 1.0 in the GPERPs. The foaming properties of the spray-dried GPERPs (95.56 ± 5.89%) were significantly greater than those of the freeze-dried GPERPs (49.13 ± 4.17%) at pH values of 4.0~10.0. The emulsion stability (ES) of the spray-dried GPERPs (453.44 ± 8.13 min) was notably greater than that of the freeze-dried GPERPs (245.58 ± 7.12 min). Furthermore, the water retention capacity (WRC) of the freeze-dried GPERPs (201.49 ± 6.12%) was significantly greater than that of the spray-dried GPERPs (103.35 ± 7.13%), except at pH 10.0 (101.44 ± 8.13%). Similarly, at pH values of 6.0, 8.0, and 10.0, the oil retention capacity (ORC) of the freeze-dried GPERPs (715.58 ± 12.15%) was significantly greater than that of the spray-dried GPERPs (560.56 ± 11.15%), although the opposite trend was noted under acidic conditions. In terms of the antioxidant activity, the ability of the goat placenta extract residual protein hydrolysates (GPERPs) to scavenge DPPH radicals and superoxide anion radicals increased with the increasing peptide powder concentration, and the maximum scavenging rates of the DPPH radicals (39.5 ± 0.56%) and superoxide anions (81.2 ± 0.54%) in the freeze-dried peptide powder were greater than those in the spray-dried peptide powder. These findings contribute to the understanding of the physicochemical and antioxidant properties of GPERPs under various drying methods and provide fundamental data for the development of functional foods based on GPERPs.
Keywords: goat placenta, protein hydrolysates, drying process, physicochemical properties, antioxidant properties
1. Introduction
The goat placenta is known to contain a variety of bioactive compounds, including growth factors, proteins, peptides, and vitamins, and it facilitates material exchange between the ewe and the fetus during pregnancy. It is particularly abundant in protein (more than 80% of its dry weight), 17 amino acids, 14 trace elements, and other components [1]. In China, the goat placenta is a potent component of Chinese medicine that is widely used as a traditional alternative therapy. The goat placenta is documented in the ancient Chinese medical book Compendium of Materia Medica as a traditional tonic [2,3]. Chou et al. [4] found that sheep placenta extract could significantly reduce the aging index, attenuate oxidative stress damage, enhance the antioxidant capacity, and effectively delay aging in mice experiments. Hou et al. [5] conducted an in vitro immunoreactivity assay using goat placental proteins and examined the changes in the immunoreactivity at different temperatures and pH values; the proteins retained their maximal immunoreactivity at 30 °C and demonstrated strong pH adaptability.
Protein hydrolysates, defined as sources of releasable mixtures of bioactive peptides [6], are complex mixtures of oligopeptides, peptides, and free amino acids generated by the enzymatic, chemical, or microbial hydrolysis of whole proteins [7,8]. Active peptides offer significant health benefits, including immune enhancement, cell regeneration, improved skin elasticity, antioxidant effects, and age-delay properties [9,10,11]. Simultaneously, peptides can be used as additives to improve food physicochemical properties, such as the solubility, emulsifying capabilities, thickening abilities, water-holding capacity, and oil-binding abilities, which are crucial for their application in the food, pharmaceutical, and cosmetic industries [12,13,14,15].
The drying methods employed during the production of proteins or protein hydrolysates can significantly impact their functional properties and stability [16,17,18]. Dong et al. [19] found that the drying method significantly influences the physicochemical properties of fish skin protein hydrolysate (SPH), with spray-dried SPH (SPH-SD) exhibiting a higher antioxidant activity and unique structural characteristics compared with freeze-dried SPH (SPH-FD). Soraiyay et al. [20] investigated the effects of spray drying (SD at 180 °C), freeze drying (FD at −35 °C), and foam-mat electrohydrodynamic drying (EHD) on egg white; while the gel hardness and water-holding capacity showed no significant differences, foam-mat EHD produced powders with the highest protein content of 66.1% and a foaming capacity of 725%, closely resembling FD powders in their microstructure and properties. Freeze drying, which is conducted at a low temperature of −30 °C, is widely acclaimed for producing high-quality, high-value products [21]. Zeng et al. [22] employed freeze-drying, spray-drying, and hot-air-drying processes to produce collagen peptide powder from chicken skin, and the freeze-dried collagen peptide powder was better than the hot-air-dried and spray-dried peptide powders in terms of the solubility, emulsion stability, water retention, oil absorption, and water absorption. In a study on the biochemical and emulsification properties of two gluten hydrolysates prepared with the same protease to the same degree of hydrolysis (1.4%) but subjected to different drying processes, E. Linarès et al. [23] reported that while the drying process did not significantly affect the molecular size distribution, hydrophobicity, or solubility of the gluten hydrolysates, the freeze-dried dispersions showed superior emulsifying properties compared with the spray-dried ones, suggesting that the insoluble fraction behavior during drying influences the emulsification performance. Kleekayai et al. [24] compared spray-dried (SD) and freeze-dried (FD) whey protein hydrolysates (WPHs) made with Alcalase® and Prolyve® (Sigma-Aldrich, Dublin, Ireland), finding that the SD-WPHs had higher antioxidative properties due to a greater proportion of peptides (<1 kDa), with the most potent WPH showing oxygen radical absorbance capacity and Trolox equivalent values of 1132 and 686 µmol TE/g, respectively. The application of heat can alter the functional properties of proteins by inducing denaturation and aggregation, which can be mediated by hydrophobic and sulfhydryl–disulfide bond exchange reactions [25]. Protein hydrolysates (peptides, oligopeptides, and amino acids) serve as essential components of food systems, fulfilling both structural and functional roles. They act as structural building blocks, regulating gel properties and enhancing biocompatibility, which are of particular importance for their application in food systems [26]. Consequently, the drying method employed for protein hydrolysates is a critical consideration during the construction of food systems.
Currently, most studies on the goat placenta focus on the preparation and efficacy of peptides and do not investigate the effects of the drying processes on the properties of goat placenta protein hydrolysates [27]. Concurrently, the expansion of the dairy goat industry and the concomitant increase in the goat population have significantly elevated the demand for efficacious goat placenta processing technology [28]. The aim of this study was to investigate the effects of drying methods (freeze drying and spray drying) on the functional properties of GPERPs. Evaluating the effects of different drying methods on the functional properties and stability of protein hydrolysates is essential for optimizing their industrial application. In this study, alterations in the amino acid composition and the distribution of essential amino acids during the reaction process were analyzed. Furthermore, to investigate the potential application of GPERPs in food systems, the foaming performance, emulsification capacity, oil retention capacity, and antioxidative properties of GPERPs under different drying methods were comparatively analyzed. This study aimed to provide a preliminary analysis of how different drying processes influence peptide preparation and their application in food systems.
2. Materials and Methods
2.1. Materials and Chemicals
Goat placenta was obtained from ewes at parturition and preserved by freezing at −45 °C. The neutral protease (3.97 × 103 U/g) and flavored protease (4.69 × 103 U/L) were purchased from Novozymes (China) Biotechnology Co., Ltd (Tianjin, China). 1,1-Biphenyl-2-pierylhydrazyl (DPPH), Pyrogallol, and 1,10-Phenanthroline were purchased from Beijing Solarbio Science & Technology Co., Ltd. (Beijing, China). All other reagents used in this study were of analytical grade.
2.2. Preparation of Protein Hydrolysates from Goat Placenta Extraction Residue
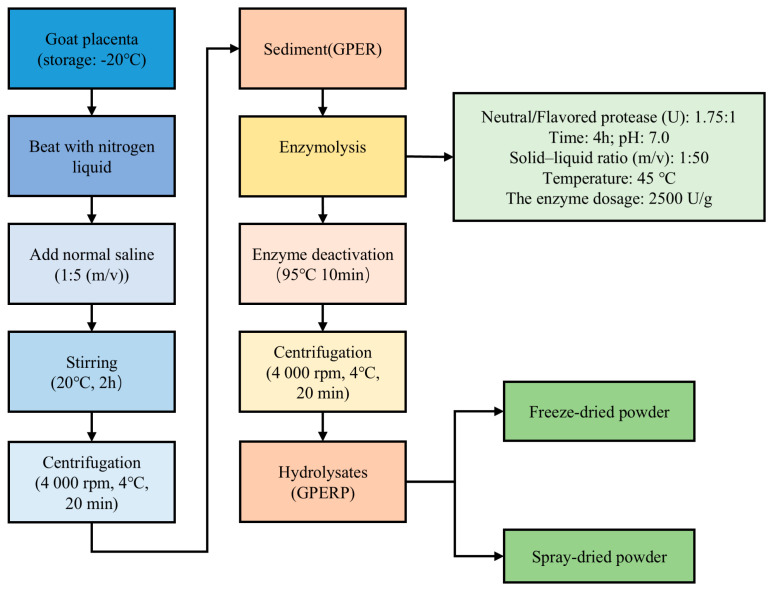
The goat placenta was collected immediately after delivery, frozen at −45 °C with immersion freezing for 12 min, and preserved at −20 °C [29]. The process of producing GPERPs from GPER is illustrated in Figure 1. Two drying methods were employed: freeze drying and spray drying. Both are the most commonly used drying methods for bioactive ingredients in industry [30]. The hydrolysis conditions of the GPERPs were as follows: the enzyme dosage was 2500 U/g; the neutrase and flavorzyme complex ratio was 1.75:1; the hydrolysis time was 4 h; the hydrolysis pH was 7.0; the temperature was 45 °C; and the solid–liquid ratio was 1:50 [31]. The spray-drying process parameters for the inlet air temperature were as follows: 165 ± 1 °C; exhaust air temperature: 98 ± 2 °C; pump speed: 8.0 mL/min; exhaust air pressure: −100~150 Pa; and atomization frequency: 25 Hz. The freeze-drying process parameters for the drying bin temperature were as follows: 30 °C; drying bin pressure: 20 Pa; cold trap temperature: maintained at −50 °C. Under these conditions, the degree of hydrolysis was 39.60%, and the peptide yield was 67.85%.
Figure 1.
Process for producing protein hydrolysates from goat placenta extraction residue (GPER).
2.3. Analysis of Amino Acid Profiles and Free Amino Acids
An amino acid composition analysis of the GPERPs and GPER was performed using an amino acid analyzer (S433D, Sykam, Germany) [32]. The protein quality was characterized by the amino acid score (AAS). The AAS determines the efficiency with which absorbed dietary nitrogen can meet essential amino acid requirements at safe levels of protein intake. It is calculated as described in FAO/WHO/UNU (2007) [33].
2.4. Functional Properties
Among the characteristics of technological ingredients, the foaming properties, emulsifying capacity, and water and oil retention capacities are of the greatest importance in food formulation [34]. The functional properties under different drying methods, including the foaming capacity (FC), foaming stability (FS), water retention capacity (WRC), and oil retention capacity (ORC), were measured.
2.4.1. Foaming Properties
The FC and FS of the hydrolysates were determined as described previously with slight modifications [35]. Aliquots (30 mL) of sample solution (1%, m/v) at various pH values (2.0~10.0) were blended at high speed (10,000 rpm) in a homogenizer (Y50, Shanghai Yuldor Machinery Equipment Co., Ltd., Shanghai, China) for 1 min. The resulting suspensions were rapidly transferred into calibrated tubes, and the total volume of the resultant mixtures was determined after 0.5 and 20 min. The FC and FS (%) were estimated as follows:
| (1) |
| (2) |
2.4.2. Emulsifying Properties
The emulsion properties were determined according to the method described by Wang [36]. To prepare the emulsion, 10 mL of soybean oil and 30 mL of sample solution (0.2%, w/v) at different pH values were shaken together and homogenized at 12,000× g and 20 °C for 1 min. A 50 μL sample of the emulsion was taken from the bottom of the container at different times and diluted with 5 mL of a 0.1% (w/v) sodium dodecyl sulfate (SDS) solution. The absorbance of the diluted emulsion was determined at 500 nm. The emulsifying activity was determined from the absorbance measured immediately after emulsion formation. The emulsifying activity index (EAI) and emulsion stability (ES) were calculated as follows:
| (3) |
| (4) |
where A denotes the absorbance of the emulsion; n denotes the dilution ratio; ρ denotes the mass concentration (0.002 g/mL); φ denotes the ratio of the oil phase in the emulsion (0.25); A0 denotes the initial absorbance of the emulsion; and A10min is the absorbance of the emulsion after 10 min.
2.4.3. Water Retention Capacity (WRC)
The WRC was determined according to the procedure described by Zhang [35]. Briefly, the dried sample (1.0 g) was first mixed with distilled water (20 mL) for 24 h. The mixed sample was subsequently centrifuged (6000 rpm for 15 min) to collect the residue, which was subsequently weighed. The RWC was calculated via Equation (5):
| (5) |
where W2 is the weight of the aqueous residue (g) and W1 is the weight of the dry sample (g).
2.4.4. Oil Retention Capacity (ORC)
The ORC analysis was performed as previously described [37]. The sample (1.0 g) was mixed with soybean oil in a centrifuge tube and allowed to stand at room temperature (RT) (25 °C) for 1 h. The mixture was then centrifuged at 1500× g for 10 min, the supernatant was poured off, and the solid particles were recovered by filtration. The ORC was calculated via Equation (6):
| (6) |
where W2 is the pellet weight (g) and W1 is the dry weight (g).
2.5. Antioxidant Properties
2.5.1. Measurement of Superoxide Radical-Scavenging Capacity
The superoxide radical-scavenging ability of GPERPs treated with different drying methods was tested via the o-triol oxidation method [38], and the Tris-HCl buffer solution and sample solution to be tested were added to different tubes according to the requirements in Table 1. The reaction was terminated by adding 1 mL of 10 mol/L HCl each after 10 min of reaction at room temperature for A0, A1, A2, and A. The absorbance values were measured at 320 nm, and the scavenging rate was determined via Equation (7):
| (7) |
Table 1.
The amount of reagent added was used to determine the superoxide radical-scavenging capacity.
| Tris-HCl (50 mM) |
Sample (50 g/L) | HCl (10 M) |
Catechoroglucinol (3 mM) |
|
|---|---|---|---|---|
| A0 | 5 mL | 1 mL | 1 mL | 1 mL |
| A | 5 mL | 1 mL | - | 1 mL |
| A1 | 5 mL | - | - | 1 mL |
| A2 | 5 mL | - | 1 mL | 1 mL |
2.5.2. Determination of DPPH Free Radical-Scavenging Capacity
Two milliliters of the sample was mixed with 2 mL of 0.1 mmol/L DPPH–ethanol solution in a test tube and, after standing for 30 min, the absorbance value (A1) was determined using anhydrous ethanol as a reference; in the control solution, 2 mL of anhydrous ethanol was used instead of the hydrolyzed solution and, after standing for 30 min, the absorbance value (A0) was determined using anhydrous ethanol as a reference; 2 mL of the same concentration of the hydrolyzed solution was mixed with 2 mL of anhydrous ethanol, and the background absorbance value (A2) was measured with anhydrous ethanol as a reference [39]. The absorbance values were determined at 517 nm, and the scavenging rate was determined via Equation (8):
| (8) |
2.6. Statistical Analysis
The results are expressed as the mean ± standard deviation of information obtained via triplicate calculations. Analysis of variance (ANOVA) was performed at a p value < 0.05. Multiple comparisons were compared via Duncan’s test.
3. Results and Discussion
3.1. Preparation of GPER and GPERPs
3.1.1. The Basic Components of the GPER
The basic chemical compositions of the GPER are shown in Table 2. The GPER exhibited a high moisture content (93.12%). On a dry basis, the GPER contained the highest amount of protein in the solid phase at 97.73%. A previous study has shown that freeze-dried and supercritical CO2-defatted goat placenta powder contained 91.2% protein [40]. GPER, characterized by its high protein content and low fat content, is well suited for the preparation of bioactive peptides.
Table 2.
Proximate composition of the extracted goat placenta residue.
| Composition | Content (%) |
|---|---|
| Moisture | 93.121 ± 0.761 |
| Protein | 6.723 ± 0.213 |
| Fat | 0.062 ± 0.005 |
| Total Carbohydrates | 0.017 ± 0.002 |
| Ash | 0.023 ± 0.009 |
3.1.2. Composition and Analysis of Amino Acids
The amino acid composition affects the structure and hydrophobicity of proteins, thereby affecting their biological activity and physicochemical properties [41,42]. The amino acid compositions and amino acid scores (AASs) of the GPER and GPERPs are shown in Table 3 and Table 4. In both the GPER and GPERPs, glutamic acid (Glu), aspartic acid (Asp), and glycine (Gly) were the most abundant amino acids. Ren et al. [43] reported similar results in Tibetan goat placenta peptides, with Glu, Gly, and Asp being the most abundant amino acids. In the GPERPs, the contents of essential amino acids (including threonine (Thr), valine (Val), isoleucine (Ile), phenylalanine (Phe), lysine (Lys), and tryptophan (Trp) were greater than those in the GPER. The process of protein breakdown leads to the formation of amino acids and small peptide chains, thereby increasing the levels of amino acids in the resulting substance. There was minimal difference between the GPER and GPERPs regarding the content of hydrophobic amino acids.
Table 3.
Amino acid composition of GPER and GPERPs.
| Amino Acid | GPER/% | GPERPs/% |
|---|---|---|
| Asp | 8.59 | 7.88 |
| *Thr | 4.18 | 4.61 |
| Ser | 4.41 | 3.94 |
| Glu | 13.41 | 11.64 |
| Ala | 6.83 | 6.97 |
| Cys | 1.85 | 1.79 |
| *Val | 4.62 | 5.31 |
| Met | 2.14 | 2.10 |
| *Ile | 3.69 | 4.29 |
| *Leu | 7.15 | 6.97 |
| Tyr | 3.08 | 4.34 |
| *Phe | 3.86 | 4.26 |
| *Lys | 6.30 | 6.92 |
| His | 2.10 | 2.70 |
| Arg | 7.40 | 7.66 |
| Pro | 7.09 | 6.42 |
| Gly | 12.46 | 11.65 |
| *Trp | 1.14 | 1.75 |
| Essential amino acids | 30.94 | 34.11 |
| Hydrophobic amino acids | 45.7 | 45.87 |
Note: * represents essential amino acids.
Table 4.
Essential amino acid compositions (AASs) of GPER and GPERPs.
| Amino Acid | FAO/WHO | GPER | GPERPs | ||
|---|---|---|---|---|---|
| Content | AAS | Content | AAS | ||
| Ile | 40 | 36.9 | 0.92 | 42.9 | 1.07 |
| Leu | 70 | 71.5 | 1.02 | 69.7 | 1.00 |
| Lys | 55 | 63.0 | 1.15 | 69.2 | 1.26 |
| Met + Cys | 35 | 39.9 | 1.14 | 38.9 | 1.11 |
| Phe + Tyr | 60 | 69.4 | 1.16 | 86.0 | 1.43 |
| Thr | 40 | 41.8 | 1.05 | 46.1 | 1.15 |
| Trp | 10 | 11.4 | 1.14 | 17.5 | 1.75 |
| Val | 50 | 46.2 | 0.92 | 53.1 | 1.06 |
| Value | 360 | 380.1 | 423.4 | ||
Note: content represents the amount (mg) of essential amino acids per gram of protein.
The percentages of essential amino acids in the GPER (30.94%) and GPERPs (34.11%) were compared with the FAO/WHO recommendations. Ren et al. [44] prepared Tibetan goat placenta peptides using a complex enzyme method with papain and neutral protease, with essential amino acids comprising 34.54% of the total amino acids. According to Table 3, all essential amino acids were present, with most having an amino acid score (AAS) equal to or exceeding 1.0. Notably, all the essential amino acid AAS values for the GPERPs were greater than 1, indicating superior nutritional quality compared with the GPER. An AAS greater than 1 indicates that the dietary protein provides the amino acid at levels surpassing the body’s basic requirements, which serves as a positive nutritional indicator, suggesting that this protein offers an advantage in providing essential amino acids [45]. These findings suggest that GPERPs may serve as a valuable nutrient in functional foods, with greater potential for application.
3.2. Physicochemical Properties of GPERPs
3.2.1. Foaming Capacity (FC) and Foaming Stability (FS)
The FC and FS represent the increase in the volume of a foam after mixing and the volume remaining over time, respectively. A higher FC requires proteins or peptides to disperse easily in water, migrate quickly to the water–air boundary, and expand to form a protective layer around air bubbles [46]. Numerous factors influence the foaming capacity of proteins or their hydrolysates, including their concentration, molecular weight, ratio of hydrophobic amino acids, and capacity to reduce surface tension [47,48,49]. Proteins with flexible molecules and loose structures generally exhibit better foaming abilities and stabilities than those with rigid structures. The foaming stability is typically highest near the isoelectric point, provided that the solubility remains relatively constant [50].
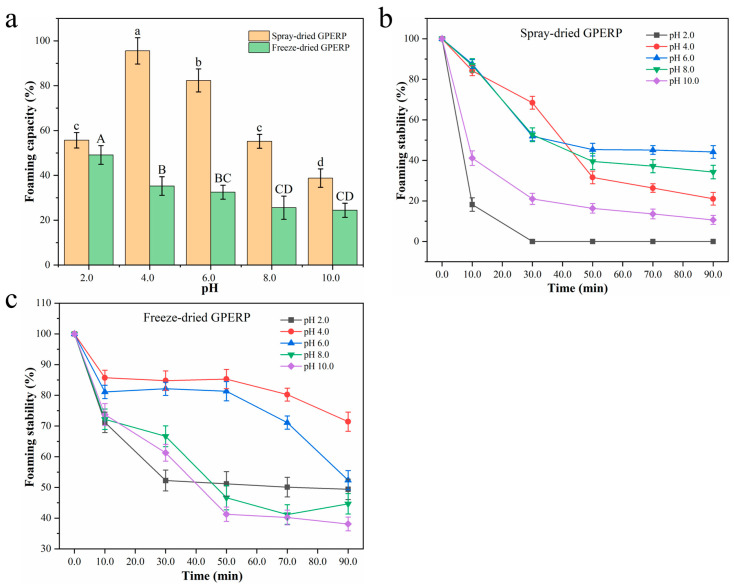
As shown in Figure 2a, the foaming capacity (FC) values of the spray-dried GPERPs were significantly greater than those of the freeze-dried GPERPs across various pH values (p < 0.05), except at pH 2.0. At pH 2.0, there was no significant difference in the foaming abilities between the spray-dried and freeze-dried GPERP samples. The inferior foam formation in the freeze-dried GPERPs compared with the spray-dried GPERPs may be attributed to the spherical nature of protein hydrolysates, which hinders their ability to form surface membranes around air bubbles. The foaming properties of GPERPs are more favorable under neutral conditions than under highly acidic or alkaline conditions [51]. Both drying methods yield GPERPs with foaming properties, likely due to the large number of peptides produced during enzymatic hydrolysis. This process reduces the molecular weight and allows more air to enter the molecular interior, increasing the surface activity [52]. Kanwate et al. [53] reported similar results for gelatin extracted from the swim bladder of Labeo rohita. As shown in Figure 2b, the FS of the spray-dried GPERPs decreased significantly over time. At both pH 2.0 and pH 10.0, the stability of the spray-dried GPERPs was significantly compromised, as evidenced by the complete absence of foam at pH 2.0 after 30 min and a marked reduction in the foam stability at pH 10, indicating that the FS is markedly influenced by highly acidic or alkaline conditions. The low FS of spray-dried GPERPs may result from conformational changes in the peptide chain, leading to a brittle liquid film that cannot effectively encase air bubbles [54]. As depicted in Figure 2c, the freeze-dried GPERPs exhibited greater stability for the first 50 min within the pH range of 4–6, which may be attributed to the isoelectric point of the peptide. For the foaming properties of goat placenta residue hydrolysates, spray drying clearly offers more advantages.
Figure 2.
Effects of different drying processes on the foaming characteristics of GPERPs: foaming capacity (a), foaming stability of spray-dried GPERPs (b), and foaming stability of freeze-dried GPERPs (c). Lowercase letters denote comparisons between the foaming capacity of GPERPs under different pH conditions in the spray-dried group, and uppercase letters denote comparisons between the foaming capacity of GPERPs under different pH conditions in the freeze-dried group. Different letters indicate significant differences (p < 0.05).
3.2.2. Emulsifying Activity Index (EAI) and Emulsion Stability (ES)
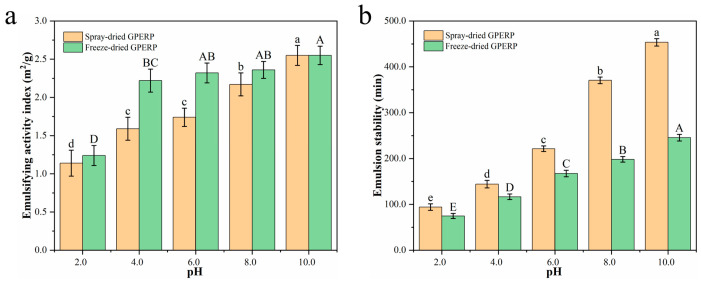
Peptides present in self-assembled gel systems, and Pickering emulsions can modulate the microstructures and overall properties of both emulsions and gels because of their distinct structural characteristics and hydrophilic/hydrophobic properties. Moreover, peptides exhibit remarkable surface activity and interfacial stability within these systems. The emulsification activity indexes (EAIs) and emulsion stabilities (ESs) of the spray-dried and freeze-dried GPERPs are shown in Figure 3a,b. The EAIs and ESs of the goat placenta protein hydrolysates from the two drying methods showed different trends. Among them, the EAI of the freeze-dried samples was significantly greater than that of the spray-dried samples, whereas the emulsification stability exhibited the opposite trend. The EAI of the GPERPs increased with the increasing pH (p < 0.05); at pH values of 4.0 and 6.0, the EAI of the freeze-dried GPERPs was significantly greater than that of the spray-dried GPERPs, whereas at pH values of 8.0 and 10.0, there was no significant difference in the EAI. At lower pH values, the hydrolysate has a positive charge, causing electrostatic repulsion between molecules and hindering the formation of an emulsification system. Under alkaline conditions, the hydrolysate promotes oil and water interface diffusion, resulting in effective emulsification [28]. The impact of the pH on the ES of the GPERPs is depicted in Figure 3b. Both the spray-dried and freeze-dried GPERPs exhibited increased ESs with the increasing pH (p < 0.05). The ES for the spray-dried GPERPs was 94.13 min at pH 2.0, which significantly increased to 453.44 min at pH 10. Similarly, the freeze-dried GPERPs showed a significant increase in their ES (p < 0.05), from 74.67 min at pH 2.0 to 245.58 min at pH 10.0.
Figure 3.
Effect of spray and freeze drying on the emulsifying properties of GPERPs: emulsifying activity index (a) and emulsifying stability (b). Lowercase letters denote comparisons between the emulsifying activity index and emulsion stability of GPERPs under different pH conditions in the spray-dried group, and uppercase letters denote comparisons between the emulsifying activity index and emulsion stability of GPERPs under different pH conditions in the freeze-dried group. Different letters indicate significant differences (p < 0.05).
The presence of more hydrophobic amino acids may be linked to the observed effects. Du et al. [55] performed a functional characterization of freeze-dried, vacuum-dried, and spray-dried egg white peptide powders and reported that the emulsifications and emulsion stabilities of the vacuum-dried and spray-dried samples were significantly lower than those of the freeze-dried samples. During spray drying and heating, protein molecules re-aggregate through hydrogen and disulfide bonds, reducing their flexibility and significantly impacting their emulsification and emulsification stability. Proteins rich in hydrophobic amino acids within the emulsion–gel matrix can enhance both the stability and emulsification characteristics [56,57].
3.2.3. Water Retention Capacity (WRC) and Oil Retention Capacity (ORC)
The water retention capacity refers to the ability of proteins or their hydrolysates to absorb water at room temperature, which is affected by the pH, temperature, surface charge, ionic strength, protein structure, and amino acid composition [58]. For proteins to exhibit a good water retention capacity, three conditions must be met: protein or hydrolysate particles fully swell after rehydration but do not dissolve; protein or hydrolysate particles have good viscosity after rehydration; and proteins or hydrolysates form gel network structures [59].
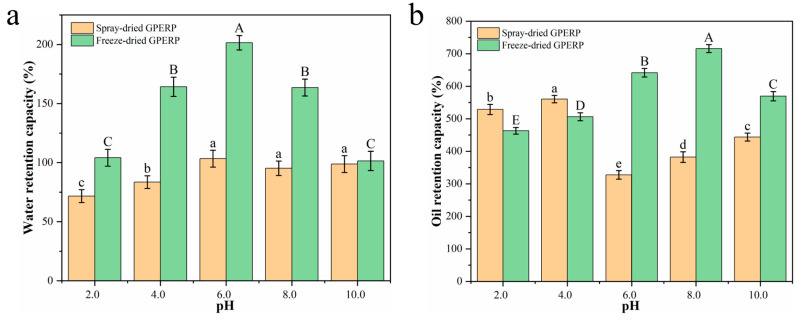
The effects of different pH values on the WRC of the GPERPs are shown in Figure 4a. The WRC of the freeze-dried GPERPs was significantly greater than that of the spray-dried GPERPs (p < 0.05), except at pH 10.0. The WRC of the freeze-dried GPERPs reached its maximum value of 201.49% when the pH was 6. At pH values between 6.0 and 8.0, the WRCs of the spray-dried GPERP samples were not significantly different but were significantly greater than those at pH 2.0 and 4.0 (p < 0.05). Overall, the WRC of the freeze-dried GPERPs was greater than that of the spray-dried GPERPs, possibly because the spray-dried WGPERP particles were fine, dissolved quickly in water, and had difficulty swelling, whereas the freeze-dried powder particles were coarse, had poor solubility, and swelled easily, so the WRC was greater than that of the spray-dried WGPERPs.
Figure 4.
Effects of spray and freeze drying on the water and oil retention capacities of GPERPs: water retention capacity (a) and oil retention capacity (b). Lowercase letters denote comparisons between the water and oil retention capacities of GPERPs under different pH conditions in the spray-dried group, and uppercase letters denote comparisons between the water and oil retention capacities of GPERPs under different pH conditions in the freeze-dried group. Different letters indicate significant differences (p < 0.05).
The oil retention capacity (ORC) is another crucial functional property of proteins or hydrolysates in food systems. The lipophilic properties of proteins are related to the nature of the lipophilic groups on the surfaces of their molecules [60]. The nonpolar amino acid side chains of proteins can form hydrophobic interactions with the hydrocarbon chains of lipids, which influence their oil-binding capacity [61]. The effect of the pH on the ORC of the GPERPs is shown in Figure 4b. The figure indicates that the ORC of the spray-dried GPERPs under acidic conditions exceeds that under alkaline conditions, with a maximum ORC of 560% at pH 4.0. Compared with that of the spray-dried GPERPs, the ORC of the freeze-dried GPERPs exhibited the opposite trend. Under alkaline conditions (pH 8.0), the ORC of the freeze-dried GPERPs was 715%, which was significantly greater than that of the spray-dried GPERPs (382%) (p < 0.05).
3.3. Antioxidant Properties of GPERPs
Antioxidant peptides can prevent oxidation by reacting with free radicals, transforming them into more stable products, and aborting the chain reaction of free radicals. O2− is the primary reactive oxygen radical in living organisms; although it is not highly active, it can produce H2O2 and hydroxyl radicals through disproportionation reactions and other pathways. Many studies have shown that peptides effectively scavenge free radicals and that the scavenging effect of peptides on free radicals is closely related to the amino acids they contain [62,63].
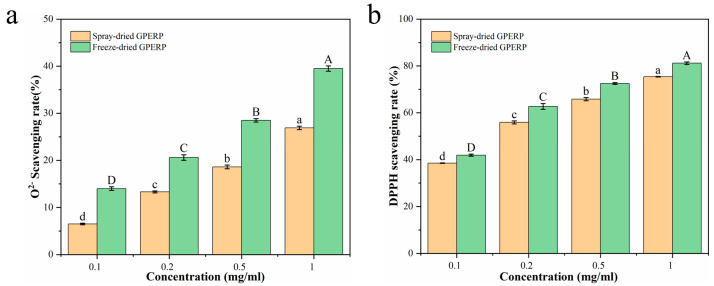
The O2−-scavenging effects of the different concentrations of the goat placenta peptide powders obtained via the two drying methods are shown in Figure 5a. The O2−-scavenging ability of the peptide powder is related to its solution concentration; as the concentration increases, its scavenging ability improves for peptide powders produced via different processes. At the same concentration, the magnitude of the O2−-scavenging rate was as follows: that of the freeze-dried peptide powder was greater than that of the spray-dried peptide powder. Certain factors inherent to the spray-drying process—including the shear force of the nozzle, the thermal stress generated during droplet drying, and the adsorption of proteins or peptides at the air–liquid interface—can potentially damage these biomolecules [64]. The DPPH-scavenging activities of the placental peptides obtained via different drying methods are shown in Figure 5b. The DPPH-scavenging activity of the peptides obtained via freeze drying at the same concentration was slightly greater than that of the peptides obtained via spray drying. DPPH, a stable free radical with a single electron, participates in oxidation reactions. After the addition of free radical scavengers, the DPPH lone pair of electrons is paired, resulting in a decrease in the absorbance value of its ethanol solution at a wavelength of 517 nm, and the degree of discoloration is quantitatively related to the number of electrons it accepts. Wang et al. demonstrated that the spray-drying parameters also influence the antioxidant capacity of peptide powder. These findings indicate that with an increasing inlet temperature, the antioxidant capacity decreases. However, up to a certain threshold, an increased feed rate can positively affect the antioxidant capacity, possibly by safeguarding heat-sensitive components [65,66]. Guo et al. [67] reported that the presence of amino acids such as Try, Phe, and Lys at the C or N terminus of a peptide exhibits strong antioxidant activity. However, it is not only the presence of some favorable amino acids in the peptide sequence that is crucial for the activity of the peptide but also their correct position in the peptide sequence. However, we do not know the exact position of these amino acids in goat placenta peptides. Therefore, determining the sequence of the antioxidant peptides is the direction of future research, which can be facilitated by further purification of the peptides by ultrafiltration to facilitate the discovery.
Figure 5.
Effects of spray and freeze drying on the antioxidant properties of GPERPs: O2− free radicals (a) and DPPH free radicals (b). Lowercase letters denote comparisons between the O2− or DPPH free radicals of GPERPs at different concentrations in the spray-dried group, and uppercase letters denote comparisons between the O2− or DPPH free radicals of GPERPs at different concentrations in the freeze-dried group. Different letters indicate significant differences (p < 0.05).
4. Conclusions
Studying the changes in the physicochemical properties of GPERPs under different drying conditions is essential for understanding them and applying them in food systems. From the investigations of the present work, it can be concluded that the different drying methods (freeze drying and spray drying) of the GPERPs had an impact on the physicochemical and antioxidant properties. Moreover, GPERPs contain more essential amino acids than GPER, and the AAS values of all the essential amino acids of GPERPs are greater than 1, indicating that its nutritional quality is superior to that of GPER. The functional property results revealed that the spray-dried GPERPs have superior foaming properties and emulsifying activity, whereas freeze-dried GPERPs have a better water retention capacity, and oil retention capacity at most pH values. GPERPs have good antioxidant properties, and freeze-dried powder generally outperforms spray-dried peptide powder. Overall, freeze drying or spray drying could be appropriate drying methods for the preparation of hydrolysates from residual goat placenta extract with better functionalities. The findings of this study will enhance our understanding of the functional properties of GPERPs, guiding their application in self-assembly, Pickering colloids, and other food ingredients, thereby expanding the use of goat placenta residues and increasing the economic value of this byproduct.
Author Contributions
Y.H. and X.C.: methodology and writing—original draft preparation. Q.S., M.Z. and S.Y.: methodology, conceptualization, investigation. L.P.: data curation, software. Q.L. and Y.F.: data curation, software, resources. R.Q.: writing—review and editing. A.L.: writing—review and editing, supervision, project administration. All authors have read and agreed to the published version of the manuscript.
Data Availability Statement
The datasets generated for this study are available upon request to the first author.
Conflicts of Interest
The authors declare that they have no competing interests.
Funding Statement
The authors would like to thank the following for funding this research: the Henan Provincial Key Science and Technology Special Project: 232102111060, 232102111072; the Key Research and Development Project of Henan Province: 231111310700; and the Key Research and Development Project of Henan Province: 241111310500.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Wang L.H., Rudolph A.M., Benet L.Z. Pharmacokinetic studies of the disposition of acetaminophen in the sheep maternal-placental-fetal unit. J. Pharmacol. Exp. Ther. 1986;238:198–205. [PubMed] [Google Scholar]
- 2.Compaoré A., Dierickx S., Jaiteh F., Nahum A., Bohissou T.F.E., Tinto H., Scott S., D’Alessandro U., Schallig H., Grietens K.P. Fear and rumours regarding placental biopsies in a malaria-in-pregnancy trial in Benin. Malar. J. 2018;17:425. doi: 10.1186/s12936-018-2578-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Deng Z., Wang M., Fan Y., Liu M. Salviae miltiorrhizae and ligustrazine hydrochloride injection combined with mecobalamin for treating diabetic peripheral neuropathy: A protocol for systematic review and meta-analysis. Medicine. 2021;100:e24103. doi: 10.1097/MD.0000000000024103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chou M.-Y., Yang C.-P.O., Li W.-C., Yang Y.-M., Huang Y.-J., Wang M.-F., Lin W.-T. Evaluation of Antiaging Effect of Sheep Placenta Extract Using SAMP8 Mice. Processes. 2022;10:2242. doi: 10.3390/pr10112242. [DOI] [Google Scholar]
- 5.Hou Y.N., Yang S.R., Huang J.H., Xu Q.Y., Liao A.M., Zhong Q.F., Li M.X. Nutritional profile and in vitro immunomodulatory activity of protein extract from goat placenta and fermented extraction residual. J. Food Process Eng. 2021;44:e13576. doi: 10.1111/jfpe.13576. [DOI] [Google Scholar]
- 6.Abeer M.M., Trajkovic S., Brayden D.J. Measuring the oral bioavailability of protein hydrolysates derived from food sources: A critical review of current bioassays. Biomed. Pharmacother. 2021;144:112275. doi: 10.1016/j.biopha.2021.112275. [DOI] [PubMed] [Google Scholar]
- 7.Li W., Xi Y., Wang J., Zhang Y., Li H., Liu X. Food-derived protein hydrolysates and peptides: Anxiolytic and antidepressant activities, characteristics, and mechanisms. Food Sci. Hum. Wellness. 2024;13:1168–1185. doi: 10.26599/FSHW.2022.9250097. [DOI] [Google Scholar]
- 8.Nakayama K., Sanbongi C., Ikegami S. Effects of Whey Protein Hydrolysate Ingestion on Postprandial Aminoacidemia Compared with a Free Amino Acid Mixture in Young Men. Nutrients. 2018;10:507. doi: 10.3390/nu10040507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jeong S., Yoon S., Kim S., Jung J., Kor M., Shin K., Lim C., Han H.S., Lee H., Park K.Y., et al. Anti-Wrinkle Benefits of Peptides Complex Stimulating Skin Basement Membrane Proteins Expression. Int. J. Mol. Sci. 2019;21:73. doi: 10.3390/ijms21010073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Muluye R.A., Bian Y., Wang L., Alemu P.N., Cui H., Peng X., Li S. Placenta Peptide Can Protect Mitochondrial Dysfunction through Inhibiting ROS and TNF-α Generation, by Maintaining Mitochondrial Dynamic Network and by Increasing IL-6 Level during Chronic Fatigue. Front. Pharmacol. 2016;7:328. doi: 10.3389/fphar.2016.00328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Choi H.Y., Kim S.W., Kim B., Lee H.N., Kim S.J., Song M., Kim S., Kim J., Kim Y.B., Kim J.H., et al. Alpha-fetoprotein, identified as a novel marker for the antioxidant effect of placental extract, exhibits synergistic antioxidant activity in the presence of estradiol. PLoS ONE. 2014;9:e99421. doi: 10.1371/journal.pone.0099421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Teng D., Fang Y., Song X., Gao Y. Optimization of enzymatic hydrolysis parameters for antioxidant capacity of peptide from goat placenta. Food Bioprod. Process. 2011;89:202–208. doi: 10.1016/j.fbp.2010.05.001. [DOI] [Google Scholar]
- 13.Karami Z., Akbari-adergani B. Bioactive food derived peptides: A review on correlation between structure of bioactive peptides and their functional properties. J. Food Sci. Technol.-Mysore. 2019;56:535–547. doi: 10.1007/s13197-018-3549-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang Y., Li Z.Q., Li H., Selomulya C. Effect of hydrolysis on the emulsification and antioxidant properties of plant-sourced proteins. Curr. Opin. Food Sci. 2022;48:100949. doi: 10.1016/j.cofs.2022.100949. [DOI] [Google Scholar]
- 15.Kristinsson H.G., Rasco B.A. Fish protein hydrolysates: Production, biochemical, and functional properties. Crit. Rev. Food Sci. Nutr. 2000;40:43–81. doi: 10.1080/10408690091189266. [DOI] [PubMed] [Google Scholar]
- 16.Hernández-García S., Salazar-Montoya J.A., Totosaus A. Emulsifying Properties of Food Proteins Conjugated with Glucose or Lactose by Two Methods (Spray-Drying Or Freeze-Drying) Int. J. Food Prop. 2016;19:526–536. doi: 10.1080/10942912.2015.1033551. [DOI] [Google Scholar]
- 17.Shen Y.T., Tang X., Li Y.H. Drying methods affect physicochemical and functional properties of quinoa protein isolate. Food Chem. 2021;339:127823. doi: 10.1016/j.foodchem.2020.127823. [DOI] [PubMed] [Google Scholar]
- 18.Lin N., Liu B., Liu Z., Qi T. Effects of different drying methods on the structures and functional properties of phosphorylated Antarctic krill protein. J. Food Sci. 2020;85:3690–3699. doi: 10.1111/1750-3841.15503. [DOI] [PubMed] [Google Scholar]
- 19.Dong Y., Yan W., Zhang Y.Q. Effects of Spray Drying and Freeze Drying on Physicochemical Properties, Antioxidant and ACE Inhibitory Activities of Bighead Carp (Aristichthys nobilis) Skin Hydrolysates. Foods. 2022;11:2083. doi: 10.3390/foods11142083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Soraiyay Zafar H., Asefi N., Siahpoush V., Roufegarinejad L., Alizadeh A. Preparation of egg white powder using electrohydrodynamic drying method and its effect on quality characteristics and functional properties. Food Chem. 2023;426:136567. doi: 10.1016/j.foodchem.2023.136567. [DOI] [PubMed] [Google Scholar]
- 21.Gong K.J., Shi A.M., Liu H.Z., Liu L., Hu H., Adhikari B., Wang Q. Emulsifying properties and structure changes of spray and freeze-dried peanut protein isolate. J. Food Eng. 2016;170:33–40. doi: 10.1016/j.jfoodeng.2015.09.011. [DOI] [Google Scholar]
- 22.Zeng Q.R., Zhang M., Adhikari B.P., Mujumdar A.S. Effect of Drying Processes on the Functional Properties of Collagen Peptides Produced from Chicken Skin. Dry. Technol. 2013;31:1653–1660. doi: 10.1080/07373937.2013.790826. [DOI] [Google Scholar]
- 23.Linarès E., Larré C., Popineau Y. Freeze- or spray-dried gluten hydrolysates.: 1.: Biochemical and emulsifying properties as a function of drying process. J. Food Eng. 2001;48:127–135. doi: 10.1016/S0260-8774(00)00148-5. [DOI] [Google Scholar]
- 24.Kleekayai T., O’Neill A., Clarke S., Holmes N., O’Sullivan B., FitzGerald R.J. Contribution of Hydrolysis and Drying Conditions to Whey Protein Hydrolysate Characteristics and In Vitro Antioxidative Properties. Antioxidants. 2022;11:399. doi: 10.3390/antiox11020399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Katekhong W., Charoenrein S. Color and gelling properties of dried egg white: Effect of drying methods and storage conditions. Int. J. Food Prop. 2017;20:2157–2168. doi: 10.1080/10942912.2016.1233429. [DOI] [Google Scholar]
- 26.Du Y.N., Xue S., Yan J.N., Jiang X.Y., Wu H.T. Gelation and microstructural properties of ternary composite gel of scallop (Patinopecten yessoensis) protein hydrolysates/κ-carrageenan/xanthan gum. J. Food Sci. 2022;87:302–311. doi: 10.1111/1750-3841.15987. [DOI] [PubMed] [Google Scholar]
- 27.Wang L., Song X.-B., Cui H.-T., Man S.-S., Li W., Muluye R.A., Bian Y.-H., Chu X.-Q., Yan D.-D., Cai Y.-Z. Antifatigue effects of peptide isolated from sheep placenta. Chin. Herb. Med. 2018;10:279–284. doi: 10.1016/j.chmed.2018.06.005. [DOI] [Google Scholar]
- 28.Hymes-Fecht U.C., Casper D.P. Adaptation and withdrawal of feeding dried Aspergillus oryzae fermentation product to dairy cattle and goats on in vitro NDF digestibility of selected forage sources. Transl. Anim. Sci. 2021;5:txab051. doi: 10.1093/tas/txab051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liang D., Lin F., Yang G., Yue X., Zhang Q., Zhang Z., Chen H. Advantages of immersion freezing for quality preservation of litchi fruit during frozen storage. LWT-Food Sci. Technol. 2015;60:948–956. doi: 10.1016/j.lwt.2014.10.034. [DOI] [Google Scholar]
- 30.Li M., Zhang F., Liu Z., Guo X., Wu Q., Qiao L. Controlled release system by active gelatin film incorporated with β-cyclodextrin-thymol inclusion complexes. Food Bioprocess Technol. 2018;11:1695–1702. doi: 10.1007/s11947-018-2134-1. [DOI] [Google Scholar]
- 31.Zhou J.J., Hou Y.C., Liu W.W., Li G.Y., Yang G.M. Optimization of immune peptides production from goat placenta. Food Ferment. Ind. 2015;41:129–134. [Google Scholar]
- 32.Liu K.-L., Zheng J.-B., Chen F.-S. Relationships between degree of milling and loss of Vitamin B, minerals, and change in amino acid composition of brown rice. LWT-Food Sci. Technol. 2017;82:429–436. doi: 10.1016/j.lwt.2017.04.067. [DOI] [Google Scholar]
- 33.World Health Organization. Food and Agriculture Organization. United Nations University . Protein and Amino Acid Requirements in Human Nutrition Report of a Joint WHO/FAO/UNU Expert Consultation. WHO; Geneva, Switzerland: 2007. (WHO Technical Report Series No. 935). [Google Scholar]
- 34.de Queiroz A.L.M., Bezerra T.K.A., de Freitas Pereira S., da Silva M.E.C., de Almeida Gadelha C.A., Gadelha T.S., Pacheco M.T.B., Madruga M.S. Functional protein hydrolysate from goat by-products: Optimization and characterization studies. Food Biosci. 2017;20:17–27. doi: 10.1016/j.fbio.2017.07.009. [DOI] [Google Scholar]
- 35.Zhang M.-Y., Liao A.-M., Thakur K., Huang J.-H., Zhang J.-G., Wei Z.-J. Modification of wheat bran insoluble dietary fiber with carboxymethylation, complex enzymatic hydrolysis and ultrafine comminution. Food Chem. 2019;297:124983. doi: 10.1016/j.foodchem.2019.124983. [DOI] [PubMed] [Google Scholar]
- 36.Wang J., Zhao M., Yang X., Jiang Y. Improvement on functional properties of wheat gluten by enzymatic hydrolysis and ultrafiltration. J. Cereal Sci. 2006;44:93–100. doi: 10.1016/j.jcs.2006.04.002. [DOI] [Google Scholar]
- 37.Zheng Y., Li Y. Physicochemical and functional properties of coconut (Cocos nucifera L) cake dietary fibres: Effects of cellulase hydrolysis, acid treatment and particle size distribution. Food Chem. 2018;257:135–142. doi: 10.1016/j.foodchem.2018.03.012. [DOI] [PubMed] [Google Scholar]
- 38.Zhang Q.A., Wang X., Song Y., Fan X.H., García-Martín J.F. Optimization of Pyrogallol Autoxidation Conditions and Its Application in Evaluation of Superoxide Anion Radical Scavenging Capacity for Four Antioxidants. J. AOAC Int. 2016;99:504–511. doi: 10.5740/jaoacint.15-0223. [DOI] [PubMed] [Google Scholar]
- 39.Kaur G., Jabbar Z., Athar M., Alam M. Punica granatum (pomegranate) flower extract possesses potent antioxidant activity and abrogates Fe-NTA induced hepatotoxicity in mice. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2006;44:984–993. doi: 10.1016/j.fct.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 40.Xie H.-Y., Ji Y.-L., Gao W.-D., Xie X.-D., He X. Determination of Antioxidant Activity of Enzymatic Hydrolysate of Yak Casein by Phenanthroline-Fe2+ Method. Food Ferment. Ind. 2012;38:175–178. [Google Scholar]
- 41.Adewumi O.O., Felix-Minnaar J.V., Jideani V.A. Functional Properties and Amino Acid Profile of Bambara Groundnut and Moringa oleifera Leaf Protein Complex. Processes. 2022;10:205. doi: 10.3390/pr10020205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lu Z.X., He J.F., Zhang Y.C., Bing D.J. Composition, physicochemical properties of pea protein and its application in functional foods. Crit. Rev. Food Sci. Nutr. 2020;60:2593–2605. doi: 10.1080/10408398.2019.1651248. [DOI] [PubMed] [Google Scholar]
- 43.Ren H.W., Shi J.F., Cai Y.L., Fan W.G., Jiang Q.X., Li Z.Z., Pei J.W., Wang Y.R. Optimization of ultrasound-assisted enzymatic preparation of Tibetan goat placenta peptides by response surface methodology and analysis of antioxidant capacity. Food Sci. 2019;40:265–273. [Google Scholar]
- 44.Ren H.W., Shi J.F., Wang M.Q., Fan W.G., Li Z.Z. Antioxidant capacity and structural characterization of goat placenta peptides from Tibetan sheep. Food Mach. 2020;36:162–169. [Google Scholar]
- 45.Yang J., Huang J., Zhu Z., Huang M. Investigation of optimal conditions for production of antioxidant peptides from duck blood plasma: Response surface methodology. Poult. Sci. 2020;99:7159–7168. doi: 10.1016/j.psj.2020.08.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wouters A., Schaefer S., Joye I.J., Delcour J.A. Relating the structural, air-water interfacial and foaming properties of wheat (Triticum aestivum L.) gliadin and maize (Zea mays L.) zein based nanoparticle suspensions. Colloids Surf. A Physicochem. Eng. Asp. 2019;567:249–259. doi: 10.1016/j.colsurfa.2019.01.071. [DOI] [Google Scholar]
- 47.Ivanova P., Kalaydzhiev H., Dessev T.T., Silva C.L.M., Rustad T., Chalova V.I. Foaming properties of acid-soluble protein-rich ingredient obtained from industrial rapeseed meal. J. Food Sci. Technol. 2018;55:3792–3798. doi: 10.1007/s13197-018-3311-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shimoyama A., Kido S., Kinekawa Y., Doi Y. Guar foaming albumin: A low molecular mass protein with high foaming activity and foam stability isolated from guar meal. J. Agric. Food Chem. 2008;56:9200–9205. doi: 10.1021/jf8010323. [DOI] [PubMed] [Google Scholar]
- 49.Bhinder S., Kaur A., Singh B., Yadav M.P., Singh N. Proximate composition, amino acid profile, pasting and process characteristics of flour from different Tartary buckwheat varieties. Food Res. Int. 2020;130:108946. doi: 10.1016/j.foodres.2019.108946. [DOI] [PubMed] [Google Scholar]
- 50.Jia F., Zhang C., Wang Q., Li J., Wang J. Physicochemical and structural characteristics of the Venn components of wheat gliadin. Grain Oil Sci. Technol. 2020;3:18–24. doi: 10.1016/j.gaost.2020.01.003. [DOI] [Google Scholar]
- 51.Man D. Enzymic hydrolysis of food proteins. J. Food Eng. 1989;9:165–166. doi: 10.1016/0260-8774(89)90015-0. [DOI] [Google Scholar]
- 52.Kuehler C.A., Stine C.M. Effect of enzymatic hydrolysis on some functional properties of whey protein. J. Food Sci. 2010;39:379–382. doi: 10.1111/j.1365-2621.1974.tb02899.x. [DOI] [Google Scholar]
- 53.Kanwate B.W., Ballari R.V., Kudre T.G. Influence of spray-drying, freeze-drying and vacuum-drying on physicochemical and functional properties of gelatin from Labeo rohita swim bladder. Int. J. Biol. Macromol. 2019;121:135–141. doi: 10.1016/j.ijbiomac.2018.10.015. [DOI] [PubMed] [Google Scholar]
- 54.Ye J., Deng L., Wang Y., McClements D.J., Luo S., Liu C. Impact of rutin on the foaming properties of soybean protein: Formation and characterization of flavonoid-protein complexes. Food Chem. 2021;362:130238. doi: 10.1016/j.foodchem.2021.130238. [DOI] [PubMed] [Google Scholar]
- 55.Du T.Y., Xu J.C., Zhu S.N., Yao X.J., Guo J., Lv W.Q. Effects of spray drying, freeze drying, and vacuum drying on physicochemical and nutritional properties of protein peptide powder from salted duck egg white. Front. Nutr. 2022;9:1026903. doi: 10.3389/fnut.2022.1026903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li J., Geng S., Liu B., Wang H., Liang G. Self-assembled mechanism of hydrophobic amino acids and β-cyclodextrin based on experimental and computational methods. Food Res. Int. 2018;112:136–142. doi: 10.1016/j.foodres.2018.06.017. [DOI] [PubMed] [Google Scholar]
- 57.Wan C., Yu S., Dang P., Gao L., Ge J., Li Y., Yang H., Yang P., Feng B., Gao J. Nitrogen regulates the synthesis of hydrophobic amino acids to improve protein structural and gel properties in common buckwheat. Int. J. Biol. Macromol. 2023;253:126871. doi: 10.1016/j.ijbiomac.2023.126871. [DOI] [PubMed] [Google Scholar]
- 58.Rajpurohit B., Li Y. Overview on pulse proteins for future foods: Ingredient development and novel applications. J. Future Foods. 2023;3:340–356. doi: 10.1016/j.jfutfo.2023.03.005. [DOI] [Google Scholar]
- 59.Darewicz M., Dziuba J., Dziuba M. Functional properties and biological activities of bovine casein proteins and peptides. Pol. J. Food Nutr. Sci. 2006;15:79–86. [Google Scholar]
- 60.Sefa-Dedeh S., Stanley D. Cowpea proteins. 1. Use of response surface methodology in predicting cowpea (Vigna unguiculata) protein extractability. J. Agric. Food Chem. 1979;27:1238–1243. doi: 10.1021/jf60226a063. [DOI] [PubMed] [Google Scholar]
- 61.Wu G., Hui X., Brennan M.A., Zeng X.A., Guo X., Brennan C.S. Combination of rehydrated sodium caseinate aqueous solution with blackcurrant concentrate and the formation of encapsulates via spray drying and freeze drying: Alterations to the functional properties of protein. J. Food Process. Preserv. 2021;45:e15406. doi: 10.1111/jfpp.15406. [DOI] [PubMed] [Google Scholar]
- 62.Kalita C., Mehta U., Aayush K., Sawant P., Chavan P., Rasane P., Sharma S., Singh G.P., Nawghare G.K., Dhruv, et al. Recent trends in antioxidative peptides derived from soybean and other soy-based products: A comprehensive review. Process Biochem. 2024;136:311–323. doi: 10.1016/j.procbio.2023.11.027. [DOI] [Google Scholar]
- 63.Rebollo-Hernanz M., Kusumah J., Bringe N.A., Shen Y., Gonzalez de Mejia E. Peptide release, radical scavenging capacity, and antioxidant responses in intestinal cells are determined by soybean variety and gastrointestinal digestion under simulated conditions. Food Chem. 2023;405:134929. doi: 10.1016/j.foodchem.2022.134929. [DOI] [Google Scholar]
- 64.Sarabandi K., Jafari S.M. Improving the antioxidant stability of flaxseed peptide fractions during spray drying encapsulation by surfactants: Physicochemical and morphological features. J. Food Eng. 2020;286:110131. doi: 10.1016/j.jfoodeng.2020.110131. [DOI] [Google Scholar]
- 65.Anderson R.F., Amarasinghe C., Fisher L.J., Mak W.B., Packer J.E. Reduction in free-radical-induced DNA strand breaks and base damage through fast chemical repair by flavonoids. Free Radic. Res. 2000;33:91–103. doi: 10.1080/10715760000300651. [DOI] [PubMed] [Google Scholar]
- 66.Ramalingam M., Yong-Ki P. Free radical scavenging activities of Cnidium officinale Makino and Ligusticum chuanxiong Hort. methanolic extracts. Pharmacogn. Mag. 2010;6:323–330. doi: 10.4103/0973-1296.71794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Guo H., Kouzuma Y., Yonekura M. Structures and Properties of Antioxidative Peptides Derived from Royal Jelly Protein. Food Chem. 2009;113:238–245. doi: 10.1016/j.foodchem.2008.06.081. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated for this study are available upon request to the first author.