Abstract
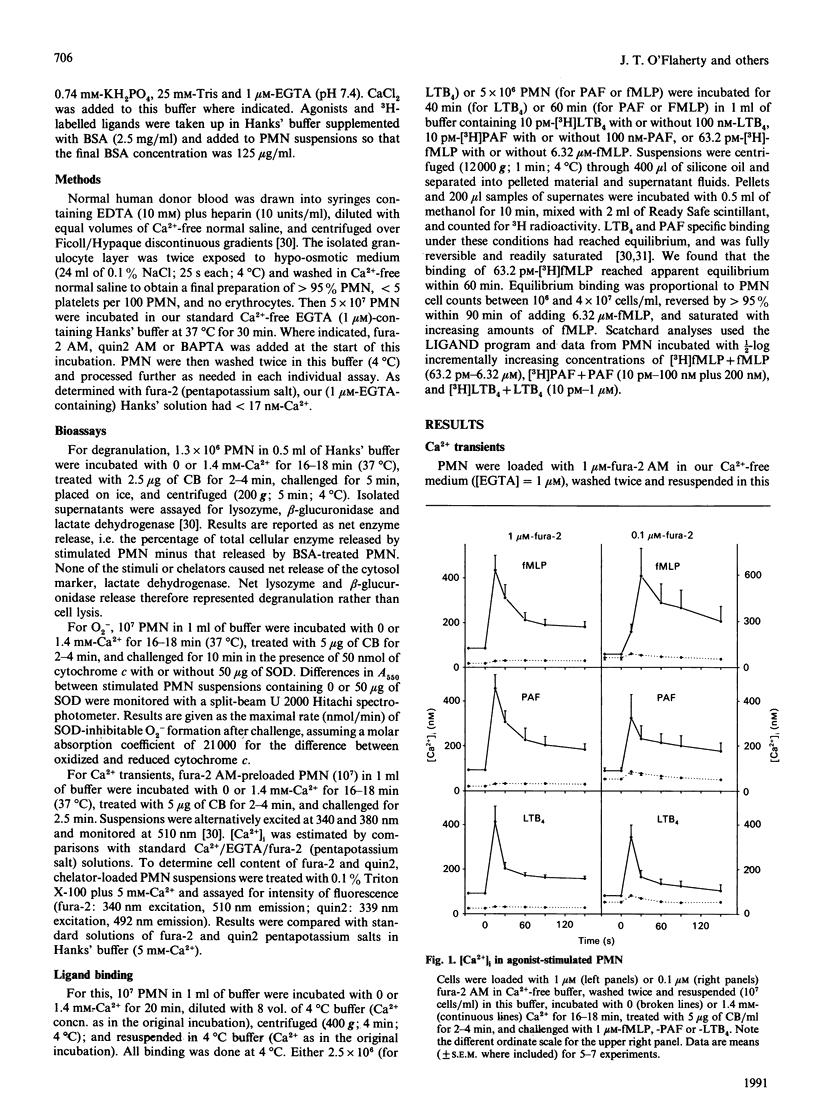
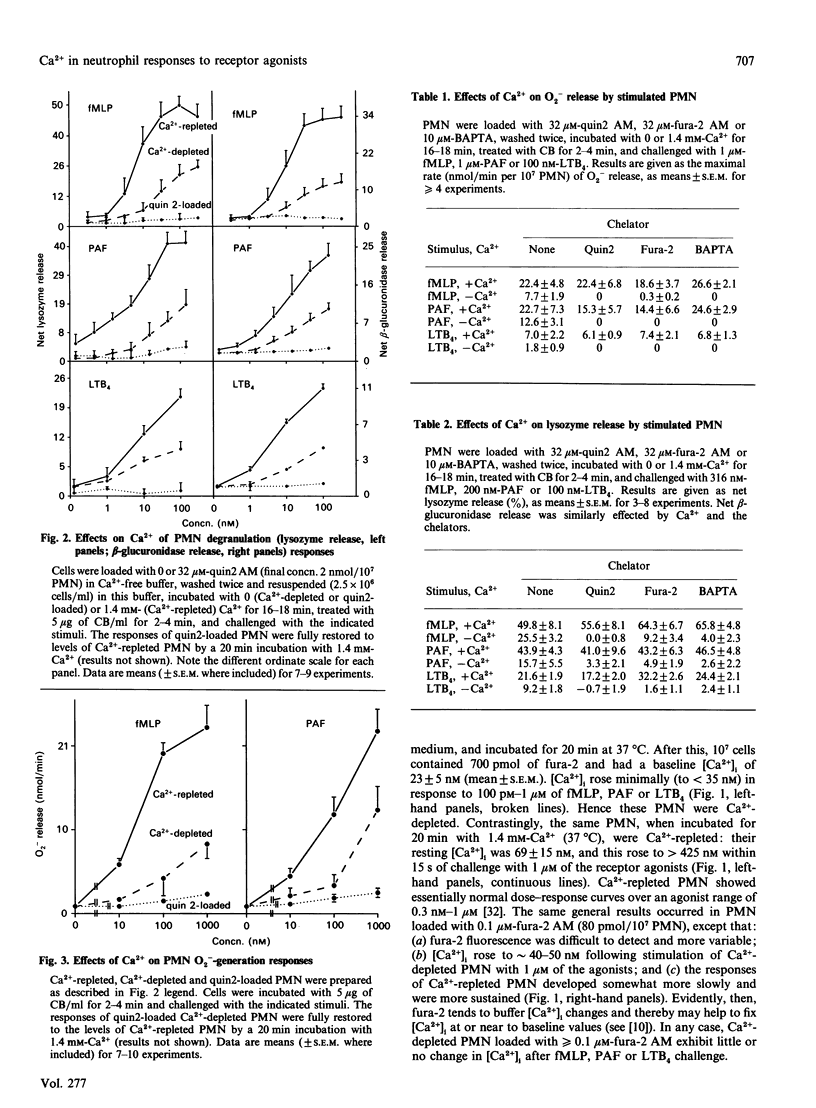
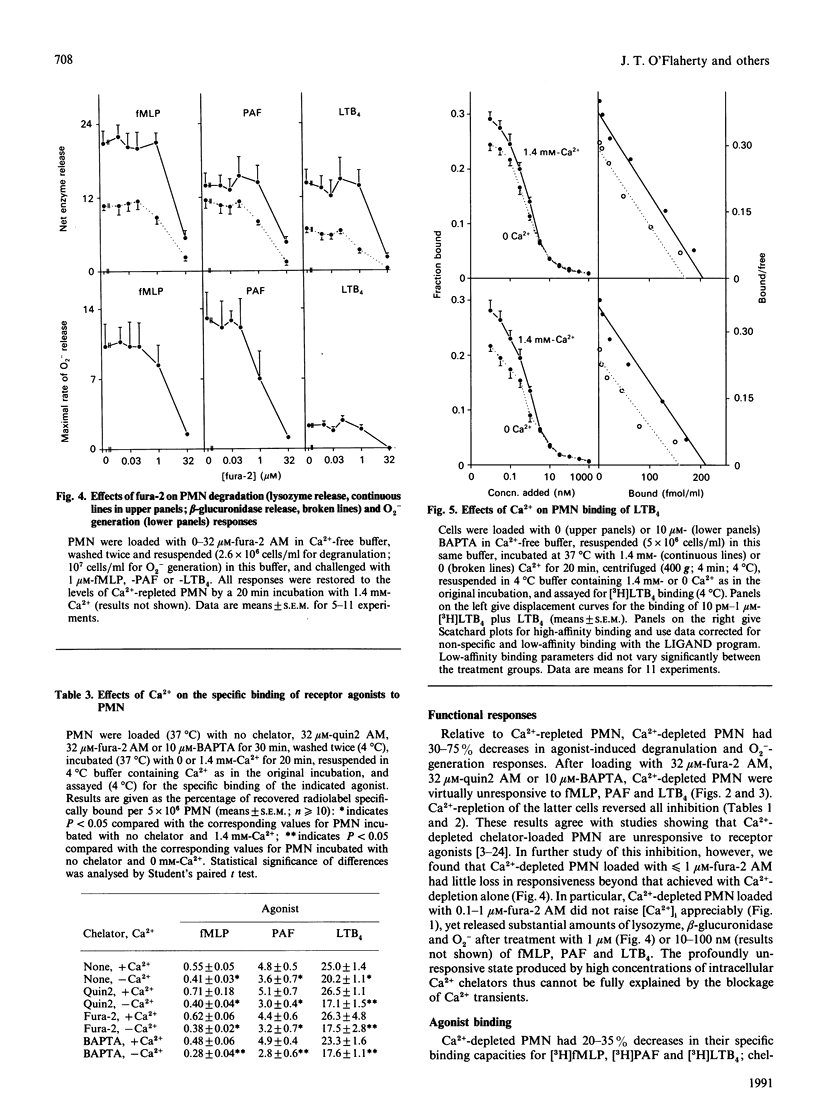
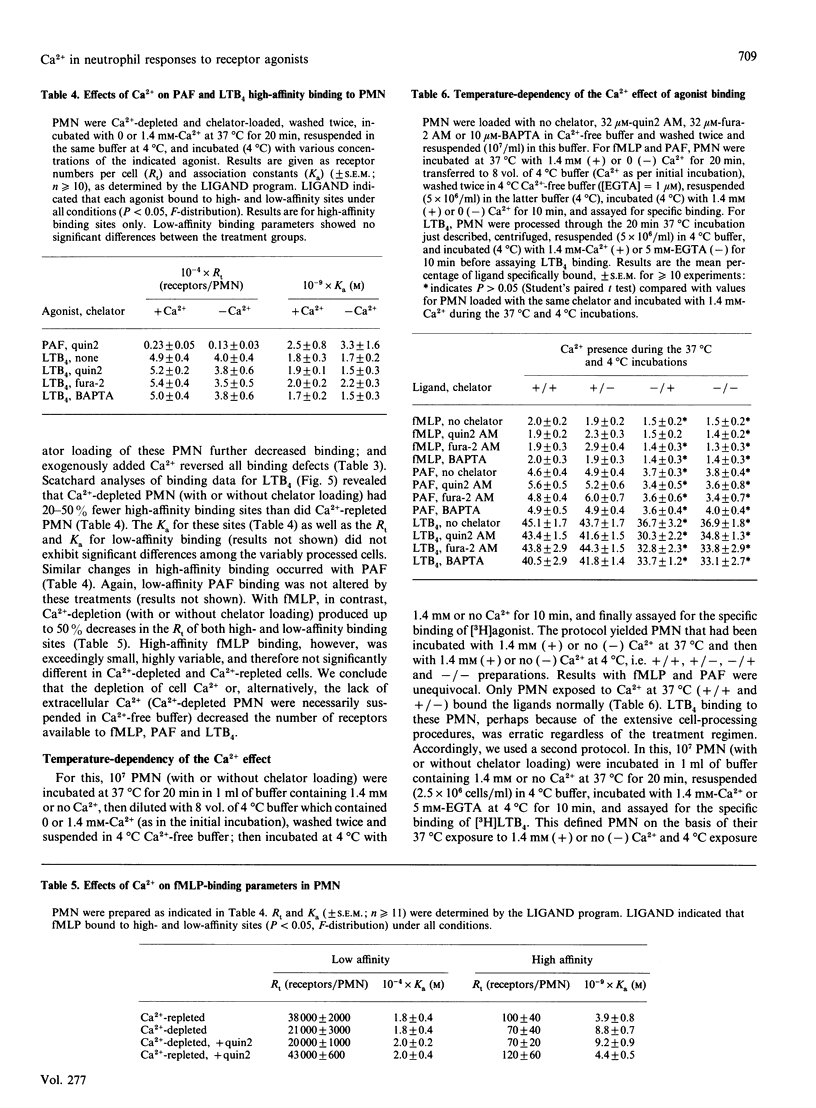
Previous studies have concluded that cytosolic Ca2+ [( Ca2+]i) transients are essential for neutrophils (PMN) to degranulate and make superoxide anion when challenged with the receptor agonists N-formyl-methionyl-leucyl-phenylalanine, platelet-activating factor and leukotriene B4. This view is based on the profound unresponsiveness of PMN that have their [Ca2+]i fixed at resting levels by removing storage Ca2+ and loading the cells with greater than or equal to 20 microM of a Ca2+ chelator, quin2 AM. We too observed this unresponsive state in PMN loaded with 10-32 microM-quin2 AM, fura-2 AM or 1,2-bis-(2-aminophenoxy) ethane-NNN'N'-tetra-acetic acid (BAPTA). When loaded with less than or equal to 1 microM fura-2 AM, however, Ca(2+)-depleted PMN failed to alter [Ca2+]i appreciably, yet still had substantial degranulation and superoxide-anion-generating responses to the receptor agonists. Function thus did not require [Ca2+]i transients. Moreover, Ca(2+)-depleted PMN had 20-35% decreases in receptor numbers for each of the three agonists, and chelator loading of these cells decreased receptor availability by 30-50%. All receptor losses were reversed by incubating PMN with Ca2+ at 37 degrees C, but not at 4 degrees C, and agonist binding at 4 degrees C was not influenced by the presence or absence of extracellular Ca2+. Ca2+ thus caused PMN to up-regulate their agonist receptors at 37 degrees C, and the effect persisted at 4 degrees C regardless of ambient Ca2+. We conclude that Ca2+ acts in at least three ways to regulate responses to receptor agonists. First, some pool of (probably cellular) Ca2+ maintains receptor expression. Second, [Ca2+]i transients potentiate, but are not required for, function. The [Ca2+]i pool may or may not be the same as that influencing receptors. Finally, another pool(s) of Ca2+ signals or permits responses. This last pool, rather than [Ca2+]i transients, appears essential for the bioactions of standard Ca(2+)-mobilizing stimuli.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abramson S. N., Molinoff P. B. In vitro interactions of agonists and antagonists with beta-adrenergic receptors. Biochem Pharmacol. 1984 Mar 15;33(6):869–875. doi: 10.1016/0006-2952(84)90440-4. [DOI] [PubMed] [Google Scholar]
- Andersson T., Dahlgren C., Lew P. D., Stendahl O. Cell surface expression of fMet-Leu-Phe receptors on human neutrophils. Correlation to changes in the cytosolic free Ca2+ level and action of phorbol myristate acetate. J Clin Invest. 1987 Apr;79(4):1226–1233. doi: 10.1172/JCI112941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apfeldorf W. J., Melnick D. A., Meshulam T., Rasmussen H., Malech H. L. A transient rise in intracellular free calcium is not a sufficient stimulus for respiratory burst activation in human polymorphonuclear leukocytes. Biochem Biophys Res Commun. 1985 Oct 30;132(2):674–680. doi: 10.1016/0006-291x(85)91185-4. [DOI] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol phosphates and cell signalling. Nature. 1989 Sep 21;341(6239):197–205. doi: 10.1038/341197a0. [DOI] [PubMed] [Google Scholar]
- Campbell A. K., Hallett M. B. Measurement of intracellular calcium ions and oxygen radicals in polymorphonuclear leucocyte-erythrocyte 'ghost' hybrids. J Physiol. 1983 May;338:537–550. doi: 10.1113/jphysiol.1983.sp014688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewald B., Thelen M., Baggiolini M. Two transduction sequences are necessary for neutrophil activation by receptor agonists. J Biol Chem. 1988 Nov 5;263(31):16179–16184. [PubMed] [Google Scholar]
- Di Virgilio F., Lew D. P., Pozzan T. Protein kinase C activation of physiological processes in human neutrophils at vanishingly small cytosolic Ca2+ levels. Nature. 1984 Aug 23;310(5979):691–693. doi: 10.1038/310691a0. [DOI] [PubMed] [Google Scholar]
- Doyle D., Baumann H. Turnover of the plasma membrane of mammalian cells. Life Sci. 1979 Mar 12;24(11):951–966. doi: 10.1016/0024-3205(79)90313-8. [DOI] [PubMed] [Google Scholar]
- Exton J. H. Role of calcium and phosphoinositides in the actions of certain hormones and neurotransmitters. J Clin Invest. 1985 Jun;75(6):1753–1757. doi: 10.1172/JCI111886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher M. P., Gallin J. I. Degranulating stimuli increase the availability of receptors on human neutrophils for the chemoattractant f-met-leu-phe. J Immunol. 1980 Apr;124(4):1585–1588. [PubMed] [Google Scholar]
- Fletcher M. P., Gallin J. I. Human neutrophils contain an intracellular pool of putative receptors for the chemoattractant N-formyl-methionyl-leucyl-phenylalanine. Blood. 1983 Oct;62(4):792–799. [PubMed] [Google Scholar]
- Fällman M., Lew D. P., Stendahl O., Andersson T. Receptor-mediated phagocytosis in human neutrophils is associated with increased formation of inositol phosphates and diacylglycerol. Elevation in cytosolic free calcium and formation of inositol phosphates can be dissociated from accumulation of diacylglycerol. J Clin Invest. 1989 Sep;84(3):886–891. doi: 10.1172/JCI114249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gennaro R., Pozzan T., Romeo D. Monitoring of cytosolic free Ca2+ in C5a-stimulated neutrophils: loss of receptor-modulated Ca2+ stores and Ca2+ uptake in granule-free cytoplasts. Proc Natl Acad Sci U S A. 1984 Mar;81(5):1416–1420. doi: 10.1073/pnas.81.5.1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinstein S., Furuya W. Receptor-mediated activation of electropermeabilized neutrophils. Evidence for a Ca2+- and protein kinase C-independent signaling pathway. J Biol Chem. 1988 Feb 5;263(4):1779–1783. [PubMed] [Google Scholar]
- Grzeskowiak M., Della Bianca V., Cassatella M. A., Rossi F. Complete dissociation between the activation of phosphoinositide turnover and of NADPH oxidase by formyl-methionyl-leucyl-phenylalanine in human neutrophils depleted of Ca2+ and primed by subthreshold doses of phorbol 12,myristate 13,acetate. Biochem Biophys Res Commun. 1986 Mar 28;135(3):785–794. doi: 10.1016/0006-291x(86)90997-6. [DOI] [PubMed] [Google Scholar]
- Heidenreich K. A., Weiland G. A., Molinoff P. B. Effects of magnesium and N-ethylmaleimide on the binding of 3H-hydroxybenzylisoproterenol to beta-adrenergic receptors. J Biol Chem. 1982 Jan 25;257(2):804–810. [PubMed] [Google Scholar]
- Hwang S. B. Identification of a second putative receptor of platelet-activating factor from human polymorphonuclear leukocytes. J Biol Chem. 1988 Mar 5;263(7):3225–3233. [PubMed] [Google Scholar]
- Hwang S. B. Specific receptor sites for platelet activating factor on rat liver plasma membranes. Arch Biochem Biophys. 1987 Sep;257(2):339–344. doi: 10.1016/0003-9861(87)90574-1. [DOI] [PubMed] [Google Scholar]
- Jaconi M. E., Lew D. P., Carpentier J. L., Magnusson K. E., Sjögren M., Stendahl O. Cytosolic free calcium elevation mediates the phagosome-lysosome fusion during phagocytosis in human neutrophils. J Cell Biol. 1990 May;110(5):1555–1564. doi: 10.1083/jcb.110.5.1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo C., Lefkowitz R. J., Snyderman R. Guanine nucleotides modulate the binding affinity of the oligopeptide chemoattractant receptor on human polymorphonuclear leukocytes. J Clin Invest. 1983 Sep;72(3):748–753. doi: 10.1172/JCI111045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korchak H. M., Vienne K., Rutherford L. E., Wilkenfeld C., Finkelstein M. C., Weissmann G. Stimulus response coupling in the human neutrophil. II. Temporal analysis of changes in cytosolic calcium and calcium efflux. J Biol Chem. 1984 Apr 10;259(7):4076–4082. [PubMed] [Google Scholar]
- Korchak H. M., Vosshall L. B., Haines K. A., Wilkenfeld C., Lundquist K. F., Weissmann G. Activation of the human neutrophil by calcium-mobilizing ligands. II. Correlation of calcium, diacyl glycerol, and phosphatidic acid generation with superoxide anion generation. J Biol Chem. 1988 Aug 15;263(23):11098–11105. [PubMed] [Google Scholar]
- Lagast H., Lew P. D., Waldvogel F. A. Adenosine triphosphate-dependent calcium pump in the plasma membrane of guinea pig and human neutrophils. J Clin Invest. 1984 Jan;73(1):107–115. doi: 10.1172/JCI111180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lew D. P., Andersson T., Hed J., Di Virgilio F., Pozzan T., Stendahl O. Ca2+-dependent and Ca2+-independent phagocytosis in human neutrophils. Nature. 1985 Jun 6;315(6019):509–511. doi: 10.1038/315509a0. [DOI] [PubMed] [Google Scholar]
- Lew P. D., Monod A., Waldvogel F. A., Dewald B., Baggiolini M., Pozzan T. Quantitative analysis of the cytosolic free calcium dependency of exocytosis from three subcellular compartments in intact human neutrophils. J Cell Biol. 1986 Jun;102(6):2197–2204. doi: 10.1083/jcb.102.6.2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lew P. D., Monod A., Waldvogel F. A., Pozzan T. Role of cytosolic free calcium and phospholipase C in leukotriene-B4-stimulated secretion in human neutrophils. Comparison with the chemotactic peptide formyl-methionyl-leucyl-phenylalanine. Eur J Biochem. 1987 Jan 2;162(1):161–168. doi: 10.1111/j.1432-1033.1987.tb10556.x. [DOI] [PubMed] [Google Scholar]
- Lew P. D., Wollheim C. B., Waldvogel F. A., Pozzan T. Modulation of cytosolic-free calcium transients by changes in intracellular calcium-buffering capacity: correlation with exocytosis and O2-production in human neutrophils. J Cell Biol. 1984 Oct;99(4 Pt 1):1212–1220. doi: 10.1083/jcb.99.4.1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miki I., Watanabe T., Nakamura M., Seyama Y., Ui M., Sato F., Shimizu T. Solubilization and characterization of leukotriene B4 receptor-GTP binding protein complex from porcine spleen. Biochem Biophys Res Commun. 1990 Jan 15;166(1):342–348. doi: 10.1016/0006-291x(90)91951-n. [DOI] [PubMed] [Google Scholar]
- Naccache P. H., Molski T. F., Borgeat P., Sha'afi R. I. Intracellular calcium redistribution and its relationship to fMet-Leu-Phe, leukotriene B4, and phorbol ester induced rabbit neutrophil degranulation. J Cell Physiol. 1985 Feb;122(2):273–280. doi: 10.1002/jcp.1041220217. [DOI] [PubMed] [Google Scholar]
- Ng D. S., Wong K. GTP regulation of platelet-activating factor binding to human neutrophil membranes. Biochem Biophys Res Commun. 1986 Nov 26;141(1):353–359. doi: 10.1016/s0006-291x(86)80376-x. [DOI] [PubMed] [Google Scholar]
- Niedel J., Wilkinson S., Cuatrecasas P. Receptor-mediated uptake and degradation of 125I-chemotactic peptide by human neutrophils. J Biol Chem. 1979 Nov 10;254(21):10700–10706. [PubMed] [Google Scholar]
- O'Flaherty J. T., Chabot M. C., Redman J., Jr, Jacobson D., Wykle R. L. Receptor-independent metabolism of platelet-activating factor by myelogenous cells. FEBS Lett. 1989 Jul 3;250(2):341–344. doi: 10.1016/0014-5793(89)80751-3. [DOI] [PubMed] [Google Scholar]
- O'Flaherty J. T., Jacobson D. Protein kinase C blockers and neutrophil receptors for leukotriene B4. Biochem Biophys Res Commun. 1989 Sep 29;163(3):1456–1460. doi: 10.1016/0006-291x(89)91142-x. [DOI] [PubMed] [Google Scholar]
- O'Flaherty J. T., Jacobson D., Redman J. Mechanism involved in the mobilization of neutrophil calcium by 5-hydroxyeicosatetraenoate. J Immunol. 1988 Jun 15;140(12):4323–4328. [PubMed] [Google Scholar]
- O'Flaherty J. T., Redman J. F., Jacobson D. P. Cyclical binding, processing, and functional interactions of neutrophils with leukotriene B4. J Cell Physiol. 1990 Feb;142(2):299–308. doi: 10.1002/jcp.1041420212. [DOI] [PubMed] [Google Scholar]
- O'Flaherty J. T., Redman J. F., Jacobson D. P., Rossi A. G. Stimulation and priming of protein kinase C translocation by a Ca2+ transient-independent mechanism. Studies in human neutrophils challenged with platelet-activating factor and other receptor agonists. J Biol Chem. 1990 Dec 15;265(35):21619–21623. [PubMed] [Google Scholar]
- O'Flaherty J. T., Surles J. R., Redman J., Jacobson D., Piantadosi C., Wykle R. L. Binding and metabolism of platelet-activating factor by human neutrophils. J Clin Invest. 1986 Aug;78(2):381–388. doi: 10.1172/JCI112588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Flaherty J., Kosfeld S., Nishihira J. Binding and metabolism of leukotriene B4 by neutrophils and their subcellular organelles. J Cell Physiol. 1986 Mar;126(3):359–370. doi: 10.1002/jcp.1041260306. [DOI] [PubMed] [Google Scholar]
- Pozzan T., Lew D. P., Wollheim C. B., Tsien R. Y. Is cytosolic ionized calcium regulating neutrophil activation? Science. 1983 Sep 30;221(4618):1413–1415. doi: 10.1126/science.6310757. [DOI] [PubMed] [Google Scholar]
- Richter J., Andersson T., Olsson I. Effect of tumor necrosis factor and granulocyte/macrophage colony-stimulating factor on neutrophil degranulation. J Immunol. 1989 May 1;142(9):3199–3205. [PubMed] [Google Scholar]
- Rossi A. G., McMillan R. M., MacIntyre D. E. Agonist-induced calcium flux, phosphoinositide metabolism, aggregation and enzyme secretion in human neutrophils. Agents Actions. 1988 Jul;24(3-4):272–282. doi: 10.1007/BF02028283. [DOI] [PubMed] [Google Scholar]
- Rossi F., Grzeskowiak M., Della Bianca V. Double stimulation with FMLP and Con A restores the activation of the respiratory burst but not of the phosphoinositide turnover in Ca2+-depleted human neutrophils. A further example of dissociation between stimulation of the NADPH oxidase and phosphoinositide turnover. Biochem Biophys Res Commun. 1986 Oct 15;140(1):1–11. doi: 10.1016/0006-291x(86)91050-8. [DOI] [PubMed] [Google Scholar]
- Sherman J. W., Goetzl E. J., Koo C. H. Selective modulation by guanine nucleotides of the high affinity subset of plasma membrane receptors for leukotriene B4 on human polymorphonuclear leukocytes. J Immunol. 1988 Jun 1;140(11):3900–3904. [PubMed] [Google Scholar]
- Sklar L. A., Mueller H., Omann G., Oades Z. Three states for the formyl peptide receptor on intact cells. J Biol Chem. 1989 May 25;264(15):8483–8486. [PubMed] [Google Scholar]
- Sklar L. A., Oades Z. G. Signal transduction and ligand-receptor dynamics in the neutrophil. Ca2+ modulation and restoration. J Biol Chem. 1985 Sep 25;260(21):11468–11475. [PubMed] [Google Scholar]
- Smolen J. E., Stoehr S. J., Boxer L. A. Human neutrophils permeabilized with digitonin respond with lysosomal enzyme release when exposed to micromolar levels of free calcium. Biochim Biophys Acta. 1986 Apr 8;886(1):1–17. doi: 10.1016/0167-4889(86)90205-3. [DOI] [PubMed] [Google Scholar]
- Smolen J. E., Stoehr S. J. Micromolar concentrations of free calcium provoke secretion of lysozyme from human neutrophils permeabilized with saponin. J Immunol. 1985 Mar;134(3):1859–1865. [PubMed] [Google Scholar]
- Smolen J. E., Stoehr S. J., Traynor A. E., Sklar L. A. The kinetics of secretion from permeabilized human neutrophils: release of elastase and correlations with other granule constituents and right angle light scatter. J Leukoc Biol. 1987 Jan;41(1):8–13. doi: 10.1002/jlb.41.1.8. [DOI] [PubMed] [Google Scholar]
- Truett A. P., 3rd, Verghese M. W., Dillon S. B., Snyderman R. Calcium influx stimulates a second pathway for sustained diacylglycerol production in leukocytes activated by chemoattractants. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1549–1553. doi: 10.1073/pnas.85.5.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valone F. H., Ruis N. M. Platelet-activating factor binding to human platelet membranes. Biotechnol Appl Biochem. 1986 Oct;8(5):465–470. [PubMed] [Google Scholar]
- Ward P. A., Cunningham T. W., McCulloch K. K., Phan S. H., Powell J., Johnson K. J. Platelet enhancement of O2-. responses in stimulated human neutrophils. Identification of platelet factor as adenine nucleotide. Lab Invest. 1988 Jan;58(1):37–47. [PubMed] [Google Scholar]
- von Tscharner V., Deranleau D. A., Baggiolini M. Calcium fluxes and calcium buffering in human neutrophils. J Biol Chem. 1986 Aug 5;261(22):10163–10168. [PubMed] [Google Scholar]


