Abstract
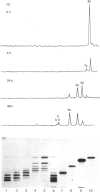
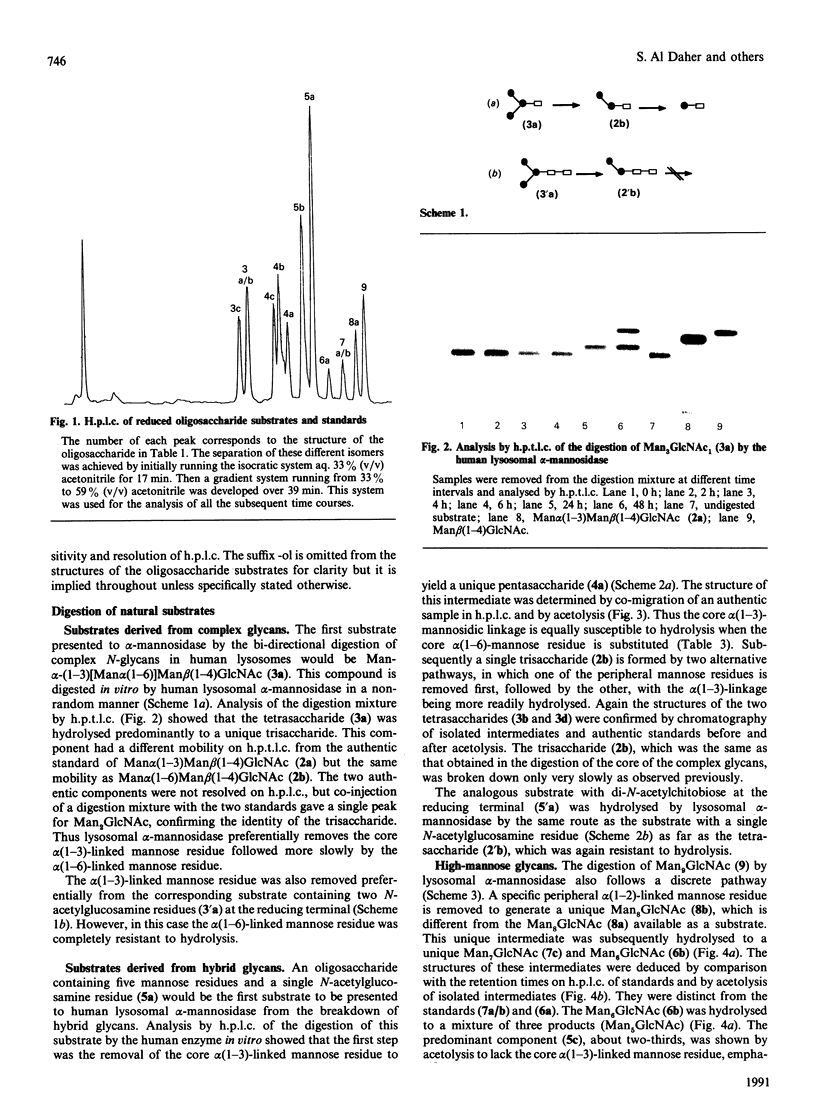
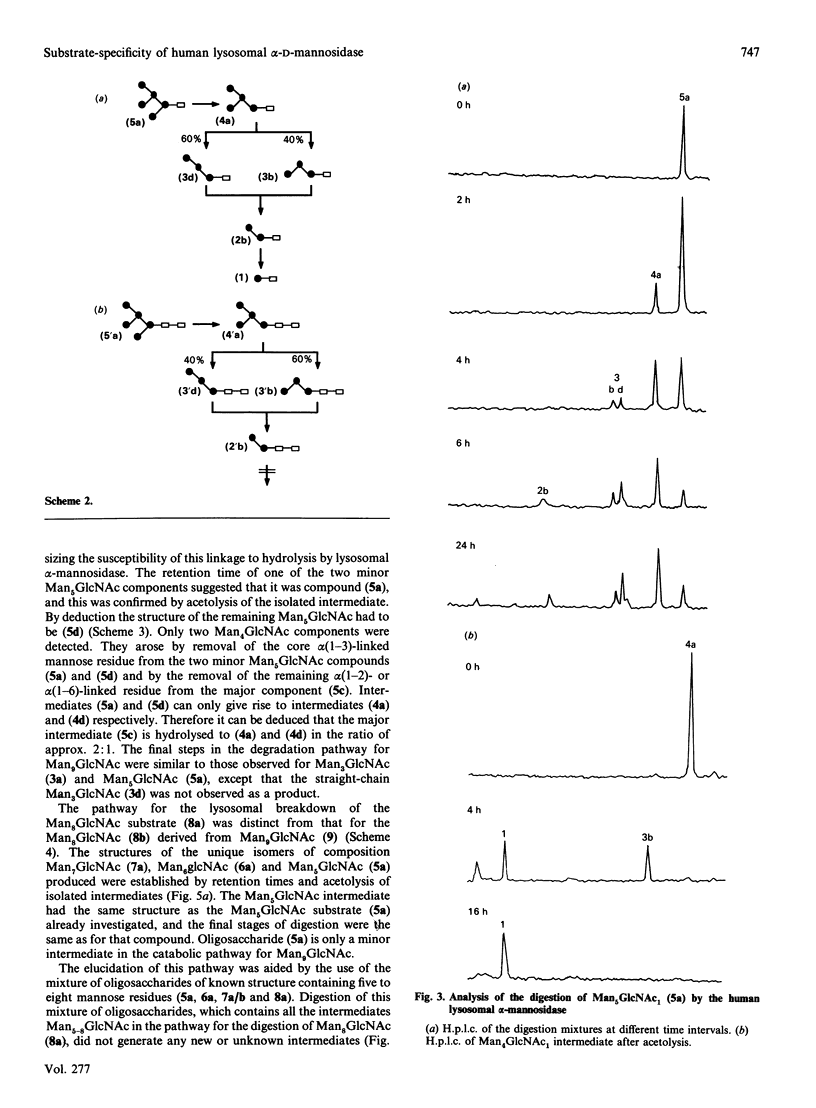
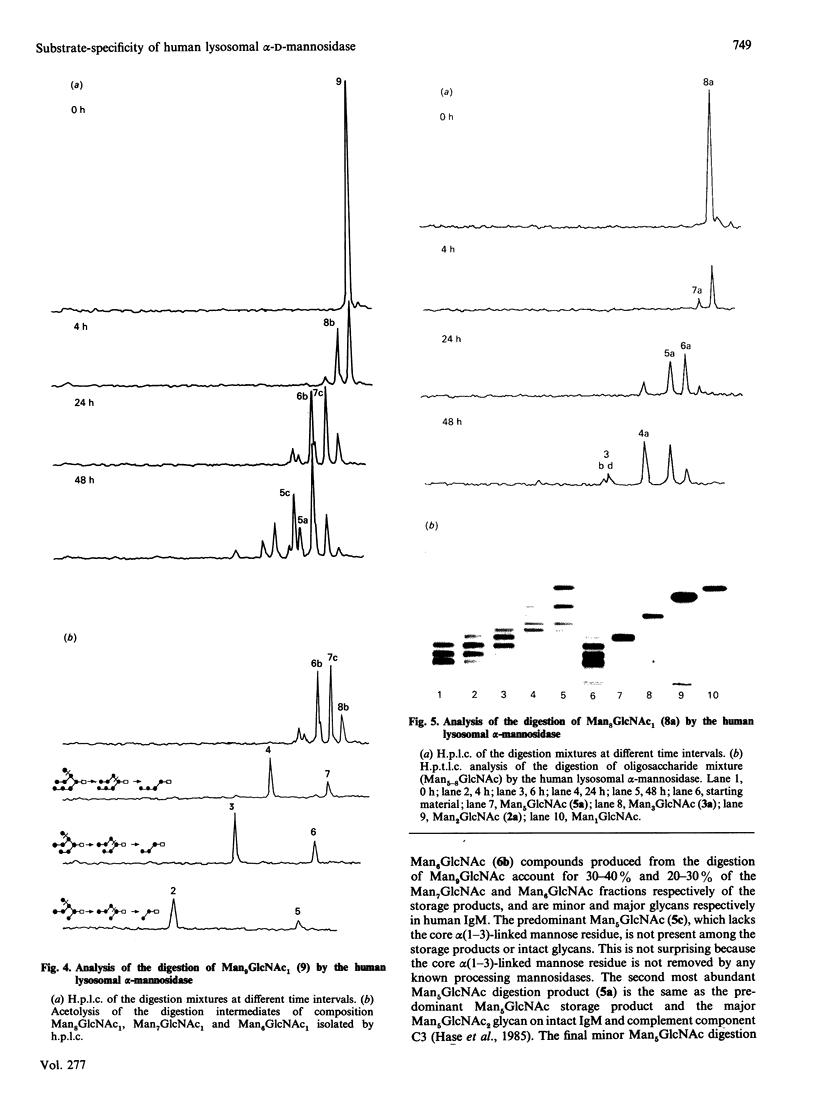
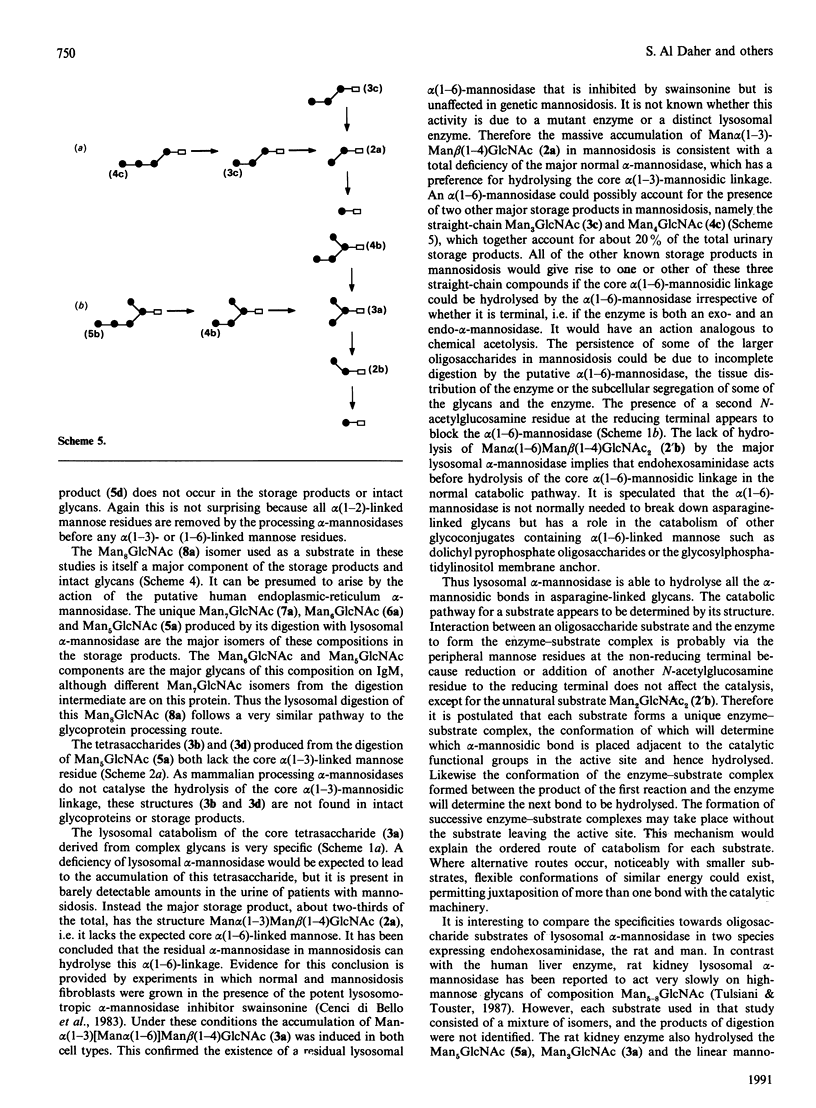
The specificity of human liver lysosomal alpha-mannosidase (EC 3.2.1.24) towards a series of oligosaccharide substrates derived from high-mannose, complex and hybrid asparagine-linked glycans and from the storage products in alpha-mannosidosis was investigated. The enzyme hydrolyses all alpha(1-2)-, alpha(1-3)- and alpha(1-6)-mannosidic linkages in these glycans without a requirement for added Zn2+, albeit at different rates. A major finding of this study is that all the substrates are hydrolysed by non-random pathways. These pathways were established by determining the structures of intermediates in the digestion mixtures by a combination of h.p.t.l.c. and h.p.l.c. before and after acetolysis. The catabolic pathway for a particular substrate appears to be determined by its structure, raising the possibility that degradation occurs by an uninterrupted sequence of steps within one active site. The structures of the digestion intermediates are compared with the published structures of the storage products in mannosidosis and of intact asparagine-linked glycans. Most but not all of the digestion intermediates derived from high-mannose glycans have structures found in intact asparagine-linked glycans of human glycoproteins or among the storage products in the urine of patients with mannosidosis. However, the relative abundances of these structures suggests that the catabolic pathway is not the same as the processing pathway. In contrast, the intermediates formed from the digestion of oligosaccharides derived from hybrid and complex N-glycans are completely different from any processing intermediates and also from the oligosaccharides of composition Man2-4GlcNAc that account for 80-90% of the storage products in alpha-mannosidosis. It is postulated that the structures of these major storage products arise from the action of an exo/endo-alpha(1-6)-mannosidase on the partially catabolized oligomannosides that accumulate in the absence of the main lysosomal alpha-mannosidase.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham D., Blakemore W. F., Jolly R. D., Sidebotham R., Winchester B. The catabolism of mammalian glycoproteins. Comparison of the storage products in bovine, feline and human mannosidosis. Biochem J. 1983 Dec 1;215(3):573–579. doi: 10.1042/bj2150573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronson N. N., Jr, Backes M., Kuranda M. J. Rat liver chitobiase: purification, properties, and role in the lysosomal degradation of Asn-linked glycoproteins. Arch Biochem Biophys. 1989 Aug 1;272(2):290–300. doi: 10.1016/0003-9861(89)90222-1. [DOI] [PubMed] [Google Scholar]
- Bischoff J., Liscum L., Kornfeld R. The use of 1-deoxymannojirimycin to evaluate the role of various alpha-mannosidases in oligosaccharide processing in intact cells. J Biol Chem. 1986 Apr 5;261(10):4766–4774. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Brassart D., Baussant T., Wieruszeski J. M., Strecker G., Montreuil J., Michalski J. C. Catabolism of N-glycosylprotein glycans: evidence for a degradation pathway of sialylglyco-asparagines resulting from the combined action of the lysosomal aspartylglucosaminidase and endo-N-acetyl-beta-D-glucosaminidase. A 400-MHz 1H-NMR study. Eur J Biochem. 1987 Nov 16;169(1):131–136. doi: 10.1111/j.1432-1033.1987.tb13589.x. [DOI] [PubMed] [Google Scholar]
- Burditt L. J., Chotai K., Hirani S., Nugent P. G., Winchester B. G., Blakemore W. F. Biochemical studies on a case of feline mannosidosis. Biochem J. 1980 Sep 1;189(3):467–473. doi: 10.1042/bj1890467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cenci di Bello I., Dorling P., Winchester B. The storage products in genetic and swainsonine-induced human mannosidosis. Biochem J. 1983 Dec 1;215(3):693–696. doi: 10.1042/bj2150693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman A., Kornfeld R. Structure of the high mannose oligosaccharides of a human IgM myeloma protein. I. The major oligosaccharides of the two high mannose glycopeptides. J Biol Chem. 1979 Feb 10;254(3):816–823. [PubMed] [Google Scholar]
- Chapman A., Kornfeld R. Structure of the high mannose oligosaccharides of a human IgM myeloma protein. II. The minor oligosaccharides of high mannose glycopeptide. J Biol Chem. 1979 Feb 10;254(3):824–828. [PubMed] [Google Scholar]
- DeGasperi R., Li Y. T., Li S. C. Presence of two endo-beta-N-acetylglucosaminidases in human kidney. J Biol Chem. 1989 Jun 5;264(16):9329–9334. [PubMed] [Google Scholar]
- Egge H., Michalski J. C., Strecker G. Heterogeneity of urinary oligosaccharides from mannosidosis: mass spectrometric analysis of permethylated Man9, Man8, and Man7 derivatives. Arch Biochem Biophys. 1982 Jan;213(1):318–326. doi: 10.1016/0003-9861(82)90468-4. [DOI] [PubMed] [Google Scholar]
- Hall N. A., Patrick A. D. A high-performance liquid chromatography method for the analysis of picomole amounts of oligosaccharides. Anal Biochem. 1989 May 1;178(2):378–384. doi: 10.1016/0003-2697(89)90656-8. [DOI] [PubMed] [Google Scholar]
- Hase S., Kikuchi N., Ikenaka T., Inoue K. Structures of sugar chains of the third component of human complement. J Biochem. 1985 Oct;98(4):863–874. doi: 10.1093/oxfordjournals.jbchem.a135366. [DOI] [PubMed] [Google Scholar]
- Hirani S., Winchester B. The multiple forms of alpha-D-mannosidase in human plasma. Biochem J. 1979 Jun 1;179(3):583–592. doi: 10.1042/bj1790583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hocking J. D., Jolly R. D., Batt R. D. Deficiency of alpha-mannosidase in Angus cattle. An inherited lysosomal storage disease. Biochem J. 1972 Jun;128(1):69–78. doi: 10.1042/bj1280069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuranda M. J., Aronson N. N., Jr A di-N-acetylchitobiase activity is involved in the lysosomal catabolism of asparagine-linked glycoproteins in rat liver. J Biol Chem. 1986 May 5;261(13):5803–5809. [PubMed] [Google Scholar]
- Matsuura F., Nunez H. A., Grabowski G. A., Sweeley C. C. Structural studies of urinary oligosaccharides from patients with mannosidosis. Arch Biochem Biophys. 1981 Apr 1;207(2):337–352. doi: 10.1016/0003-9861(81)90041-2. [DOI] [PubMed] [Google Scholar]
- Mellis S. J., Baenziger J. U. Separation of neutral oligosaccharides by high-performance liquid chromatography. Anal Biochem. 1981 Jul 1;114(2):276–280. doi: 10.1016/0003-2697(81)90480-2. [DOI] [PubMed] [Google Scholar]
- Michalski J. C., Haeuw J. F., Wieruszeski J. M., Montreuil J., Strecker G. In vitro hydrolysis of oligomannosyl oligosaccharides by the lysosomal alpha-D-mannosidases. Eur J Biochem. 1990 Apr 30;189(2):369–379. doi: 10.1111/j.1432-1033.1990.tb15498.x. [DOI] [PubMed] [Google Scholar]
- Nordén N. E., Lundblad A., Svensson S., Autio S. Characterization of two mannose-containing oligosaccharides isolated from the urine of patients with mannosidosis. Biochemistry. 1974 Feb 26;13(5):871–874. doi: 10.1021/bi00702a006. [DOI] [PubMed] [Google Scholar]
- Phillips N. C., Robinson D., Winchester B. G. Characterization of human liver alpha-D-mannosidase purified by affinity chromatography. Biochem J. 1976 Mar 1;153(3):579–587. doi: 10.1042/bj1530579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips N. C., Robinson D., Winchester B. G. Human liver alpha-D-mannosidase activity. Clin Chim Acta. 1974 Aug 30;55(1):11–19. doi: 10.1016/0009-8981(74)90328-3. [DOI] [PubMed] [Google Scholar]
- Song Z. W., Li S. C., Li Y. T. Absence of endo-beta-N-acetylglucosaminidase activity in the kidneys of sheep, cattle and pig. Biochem J. 1987 Nov 15;248(1):145–149. doi: 10.1042/bj2480145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabas I., Kornfeld S. Purification and characterization of a rat liver Golgi alpha-mannosidase capable of processing asparagine-linked oligosaccharides. J Biol Chem. 1979 Nov 25;254(22):11655–11663. [PubMed] [Google Scholar]
- Tulsiani D. R., Touster O. Substrate specificities of rat kidney lysosomal and cytosolic alpha-D-mannosidases and effects of swainsonine suggest a role of the cytosolic enzyme in glycoprotein catabolism. J Biol Chem. 1987 May 15;262(14):6506–6514. [PubMed] [Google Scholar]
- Warren C. D., Azaroff L. S., Bugge B., Jeanloz R. W., Daniel P. F., Alroy J. The accumulation of oligosaccharides in tissues and body fluids of cats with alpha-mannosidosis. Carbohydr Res. 1988 Sep 15;180(2):325–338. doi: 10.1016/0008-6215(88)80089-2. [DOI] [PubMed] [Google Scholar]
- Warren C. D., Daniel P. F., Bugge B., Evans J. E., James L. F., Jeanloz R. W. The structures of oligosaccharides excreted by sheep with swainsonine toxicosis. J Biol Chem. 1988 Oct 15;263(29):15041–15049. [PubMed] [Google Scholar]
- Warren C. D., Schmit A. S., Jeanloz R. W. Chromatographic separation of oligosaccharides from mannosidosis urine. Carbohydr Res. 1983 Jun 1;116(2):171–182. doi: 10.1016/0008-6215(83)88107-5. [DOI] [PubMed] [Google Scholar]
- Yamashita K., Tachibana Y., Mihara K., Okada S., Yabuuchi H., Kobata A. Urinary oligosaccharides of mannosidosis. J Biol Chem. 1980 Jun 10;255(11):5126–5133. [PubMed] [Google Scholar]