Abstract
Objective
The superficial zone cells in articular cartilage (SFZCs) have been identified as stem/progenitor chondrocytes and promoted cell self-renewal in the osteoarthritis (OA). Several studies emphasized the involvement of senescence and autophagy in OA. Interleukin-1β (IL-1β) is one of the main inflammatory mediators of OA, and whether it induces senescence and autophagy in SFZCs remains unclear. The present study aimed to investigate autophagy flux, mitochondrial function, and intracellular reactive oxygen species (ROS) that resulted in senescence in SFZCs induced by IL-1β.
Methods
Using western blotting, reverse transcription-quantitative PCR, immunofluorescence, intracellular ROS detection, mitochondrial staining, and determination of mitochondrial membrane potential, we tested senescence and autophagy markers in SFZCs induced by IL-1β in vitro. The consequences of mitochondrial function and ROS were also studied with IL-1β-induced senescence.
Results
IL-1β treatment decreased SFZC proliferation, induced SFZC senescence, and reduced SFZCs’ chondrogenic differentiation capacity. Moreover, IL-1β impaired autophagy flux, and the autophagy activator, rapamycin, attenuated the senescence of SFZCs. IL-1β-induced autophagy defect resulted in mitochondrial dysfunction and overproduction of ROS, and autophagy activation notably protected against mitochondrial dysfunction and reduced the levels of ROS. Moreover, antioxidant N-acetylcysteine reversed the senescence of IL-1β in SFZCs.
Conclusion
IL-1β promotes autophagy impairment and subsequently results in dysfunctional mitochondria and overproduction of ROS, which finally causes SFZC senescence.
Keywords: IL-1β, articular cartilage superficial zone cells, autophagy, senescence, reactive oxygen species
Introduction
Articular cartilage includes the superficial, middle, deep, and calcified cartilage zones, characterized by different structures, compositions, and biomechanical features.1,2 The superficial zone (SFZ) is the first structure to be destroyed in the progression of osteoarthritis (OA). 2 The previous studies demonstrated that SFZ cells (SFZCs) are self-renewal cartilage stem/progenitor cells, which play a key role in the formation of adult cartilage and cartilage homeostasis.2 -4 However, the specific mechanisms underlying the phenotypes of SFZCs remain to be fully elucidated in the OA environment.
Cellular senescence is a stable terminal state in which cells irreversibly enter growth arrest while remaining metabolically active. 5 In recent years, numerous studies have demonstrated that cellular senescence plays a significant role in the occurrence of OA.6 -8 Senescent cells (SnCs) accumulated in articular cartilage, subchondral bone, synovial membrane, and sub-patellar fat pads with age, thus facilitating the development of OA. 8 SnCs secrete senescence-associated secretory phenotype (SASP) factors, including a variety of inflammatory factors (e.g., IL-1, IL-6, IL-8) and matrix-degrading enzymes (e.g., metallopeptidase [MMP]-1, MMP-3, MMP-13), and cause imbalances in the anabolic and catabolic activities of the extracellular matrix, resulting in cartilage degradation and destruction. 8
Autophagy is a cytoprotective process in which damaged intracellular components, such as long-lived proteins and dysfunctional mitochondria, are captured by autophagosomes and transported to lysosomes to be scavenged to maintain intracellular homeostasis. 9 Autophagy dysfunction is associated with aging and numerous types of degenerative diseases, including OA. The results of previous studies have demonstrated that autophagy dysfunction leads to the occurrence of OA, and activation of autophagy alleviates the degeneration of articular cartilage.10 -12
The association between autophagy and senescence has been widely studied, and the results of numerous previous studies suggested that autophagy is positively associated with senescence.13 -16 Previous studies have demonstrated that basal autophagy is essential for sustaining the stem cell capacity. Garcia-Prat et al. 14 reported that autophagy dysfunction induced senescence in young satellite cells by accelerated mitochondrial dysfunction and reactive oxygen species (ROS) overproduction, resulting in a decline in the function and number of satellite cells. Ma et al. 13 demonstrated that autophagy controlled mesenchymal stem cell function and senescence during bone aging, and activation of autophagy may alleviate mesenchymal stem cell senescence and partially reverse bone loss in elderly mice.
Inflammatory cytokine interleukin-1β (IL-1β) plays crucial roles in cartilage catabolism and OA progression. 17 IL-1β participates in numerous cellular activities that induce chondrocyte oxidative stress and senescence.18,19 However, the effects of IL-1β on SFZCs and the specific associated mechanisms remain to be fully clarified. We hypothesized that IL-1β may lead to autophagy impairment, thus resulting in the excessive accumulation of dysfunctional mitochondria and the overproduction of ROS, causing senescence of SFZCs.
Materials and Methods
Isolation and Culture of SFZCs and Chondrocyte
SFZCs were isolated as previously described. 20 Briefly, the knee joints were dissected from a neonatal mouse, and soft tissue surrounding the articular cartilage was removed under a stereomicroscope. Articular cartilage was digested using 0.25% trypsin (Gibco; Thermo Fisher Scientific, Inc., Shanghai, China) for 1 hour and subsequently incubated using 173-U/ml type Ι collagenase (Gibco; Thermo Fisher Scientific, Inc) for 1.5 hours. A total of 5 × 105 isolated cells were plated on 10-cm2 culture dishes, which were pre-coated using 0.1% fibronectin (Sigma-Aldrich, Shanghai, China) for 2 hours, and subsequently blocked using 3% bovine serum albumin for 30 minutes. Unattached cells were removed twice following washing with DMEM/high glucose for 20 minutes after seeding, and the attached cells were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM)/high glucose containing 10% fetal bovine serum (Gibco; Thermo Fisher Scientific, Inc). SFZCs were subcultured into uncoated culture dishes when sub-confluent at a 1:10 ratio. Chondrocytes were isolated by additional overnight collagenase II digestion of residual cartilage tissue as previously described. 21 In short, after further incubation with 0.1% collagenase II (Gibco; Thermo Fisher Scientific, Inc) overnight at 37 °C, isolated chondrocytes were plated into 12-well plates in DMEM/F12 containing 10% fetal bovine serum. All studies were carried out in accordance with the policies and approval to the Institutional Animal Care and Use Committee of the General Hospital of Western Theater Command (no. 2021EC1-14). The studies of this article were performed and reported in accordance with the ARRIVE (Animal Research: Reporting of In Vivo Experiments) guidelines.
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazoliumbromide Assay
SFZCs were cultured in 96-well plates at a density of 5 × 103 cells per well. Following incubation for 12 hours, cells were treated with rapamycin (100 nM; Sigma-Aldrich) or IL-1β (20 ng/ml; Peprotech; New Jersey, USA) for 5 days. Subsequently, a total of 20 μl of 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazoliumbromide solution (5 mg/ml; Sigma-Aldrich) was added to each well, and the cultures were incubated at 37 °C for 4 hours. The culture medium was discarded, and 150 μl of DMSO (Dimethyl sulfoxide) was added to each well for the complete solubilization of the purple formazan crystals. The optical density of the samples was measured using a microplate reader at 490 nm (Thermo Fisher Scientific, Inc.).
Senescence-Associated β-Galactosidase Staining
SFZCs were cultured in 12-well plates at a density of 1 × 105 cells per well. At 50%-60% fusion, cells were treated with IL-1β (20 ng/ml; Peprotech), rapamycin (100 nM; Sigma-Aldrich), antioxidant N-acetylcysteine (NAC, 2 μM; Beyotime Institute of Biotechnology; Shanghai, China), or hydroxychloroquine (HCQ, 10 μM; Sigma-Aldrich) for the indicated times. Senescence-associated β-galactosidase (SA-β-gal) activity was assessed using an SA-β-gal staining kit (cat. no. 9860S; Cell Signaling Technology; Boston, USA). The number of SnCs was calculated using ImageJ software (National Institutes of Health; version 1.47).
Western Blotting
SFZCs were lysed using (Radio Immunoprecipitation Assay Lysis buffer) lysis buffer with protease inhibitors (Roche Diagnostics GmbH; Basel, Switzerland), and total protein concentration was assessed using a BCA protein assay reagent (Beyotime Institute of Biotechnology). The primary antibodies were as follows: p53 (cat. no. 2524S; dilution 1:1000; Cell Signaling Technology), p62 (cat. no. 23214S; dilution 1:1000; Cell Signaling Technology), microtubule-associated light-chain 1 protein 3 (LC3; cat. no. L7543; dilution 1:1000; Sigma-Aldrich), and GAPDH (glyceraldehyde-3-phosphate dehydrogenase) (cat. no. 5174S; dilution 1:2000; Cell Signaling Technology). Following primary incubation, membranes were incubated with horseradish peroxidase-labeled secondary antibodies (cat. no. ZB-2301; dilution 1:5000; Zhongshan Jinqiao Biotechnology; Beijing, China) at 37 °C for 30 minutes. Signals were detected using ECL (enhanced chemiluminescence) reagent (Thermo Fisher Scientific, Inc.).
Reverse Transcription-Quantitative PCR
Total RNA was extracted from SFZCs and chondrocytes using the TRIzol reagent (Invitrogen, Thermo Fisher Scientific, Inc.). Subsequently, single-stranded complementary DNA was synthesized from total RNA using the PrimeScript RT Reagent Kit containing gDNA Eraser (Takara Bio, Inc. Kyoto, Japan). qPCR Was carried out using a Max3000 PCR machine (Agilent Technologies, Inc. California, USA) and SYBR Premix Ex Taq Kit (Takara Bio, Inc.). Thermocycling conditions were used as follows: initial denaturation for 30 seconds at 95 °C, followed by 40 cycles at 95 °C for 30 seconds, 60 °C for 30 seconds, and 72 °C for 30 seconds, followed by the final extension at 95 °C for 30 seconds, 60 °C for 30 seconds, and 95 °C for 30 seconds. Sequences for the primers used were as follows: Tenascin C forward 5’-TTTGCCCTCACTCCCGAAG-3’ and reverse 5’-AGGGTCATGTTTAGCCCACTC-3’; Ets-related gene (Erg) forward 5’-AGCGTCCTCAGTTAGATCCTTAC-3’ and reverse 5’-TCATGTTGGGCTTGCTCTTCC-3’; Matrix metallopeptidase-3 (Mmp-3) forward 5’-TTAAAGACAGGCACTTTTGGCG-3’ and reverse 5’-CCCTCGTATAGCCCAGAACT-3’; Mmp-13 forward 5’-CTTCTTCTTGTTGAGCTGGACTC-3’ and reverse 5’-CTGTGGAGGTCACTGTAGACT-3’; Sox9 forward 5’-CACAAGAAAGACCACCCCGA-3’ and reverse 5’-ATGTGAGTCTGTTCCGTGGC-3’; Aggrecan forward 5’-CCTGCTACTTCATCGACCCC-3’ and reverse 5’-AGATGC TGTTGACTCGAACCT-3’; type II collagen (Col II) forward 5’-CTGGTGGAGCAGCAAGAGCAA-3’ and reverse 5’-CAGTGGACAGTAGACGGAGGAAAG-3’; proteoglycan 4 (Prg4) forward 5’-GAA AATACTTCCCGTCTGCTTGT-3’ and reverse 5’-ACTCCATGTAGTGCTGACAG TTA-3’; autophagy-related gene 7 (Atg7) forward 5’-ACCTCGCTGGGACTTGTGC-3’ and reverse 5’-GGTGAATCCTTCTCGCTCGT-3’; Beclin1 forward 5’-CAGTACCAGCGGGAGTATAGTGA-3’ and reverse 5’-TGTGGAAGGTGGCATTGAAGA-3’; serine/threonine protein-kinase Ulk1 (Ulk1) forward 5’-AAGTTCGAGTTCTCTCGCAAG-3’ and reverse 5’-ACCTCCAGGTCGTGCTTCT-3’; and Cyclophili A forward 5’-CGAGCTCTGAGCACTGGAGA-3’ and reverse 5’-TGGCGTGTA AAGTCACCACC-3’. Cyclophili A was used as an internal control as previously described.1,21 The experiment was independently repeated three times for all samples.
Immunofluorescence
SFZCs were cultured on 12-well slides and treated with IL-1β (20 ng/ml; Peprotech) or rapamycin (100 nM; Sigma-Aldrich) for 5 days and in the absence or presence of bafilomycin (10 nM; Sigma-Aldrich). Cells were blocked in 0.8% BSA (Bovine Serum Albumin) in PBS (Phosphate buffered saline) for 30 minutes and incubated overnight at 4 °C with LC3 antibody (cat. no. L7543; dilution 1:200; Sigma-Aldrich) or IgG controls. A fluorescent dye-conjugated secondary antibody (Thermo Fisher Scientific, Inc.) was incubated. The slides were mounted using ProLong Gold Antifade Reagent (Thermo Fisher Scientific, Inc.) and observed using fluorescence microscopy (Olympus Corporation, Tokyo, Japan). The number of LC3 puncta was quantified using the Image software (National Institutes of Health; version 1.47).
Intracellular ROS Detection
ROS was assessed using an ROS Assay Kit (cat. no. S0033S; Beyotime Institute of Biotechnology). Briefly, 2’-7’dichlorofluorescin diacetate (DCFH-DA; final concentration 10 μM) was added, and the cells were incubated for 20 minutes at 37 °C. Subsequently, DCFH-DA fluorescence was detected using fluorescence microscopy. Alternatively, the fluorescence intensity was measured by flow cytometry at an excitation wavelength of 488 nm and an emission wavelength of 525 nm.
Mitochondrial Staining
Mitochondrial staining was performed following cell incubation at 37 °C with 100-nM MitoTracker Red CMXRos (cat. no. M7512; Invitrogen, Thermo Fisher Scientific, Inc.). Samples were directly analyzed using confocal microscopy without fixing.
Determination of Mitochondrial Membrane Potential
Mitochondrial membrane potential was monitored using a Mitochondrial Membrane Potential Assay Kit with JC-1 (cat. no. C2006; Beyotime Institute of Biotechnology). Mitochondrial membrane potential was expressed as a ratio of red to green fluorescence.
Statistical Analysis
Numerical data are presented as mean ± standard deviation. Differences between two groups were evaluated using unpaired Student t test. One-way analysis of variance was used for comparisons between three or more groups, followed by a Tukey’s post hoc test. Statistical analysis was carried out using SPSS (version, 18.0; IBM Corp., Armonk, NY). P < 0.05 was considered to indicate a statistically significant difference.
Result
IL-1β Induces Senescence of SFZCs with Decreased Proliferation and Chondrogenic Differentiation
First, in order to verify the phenotypic characterization of SFZCs in this study, total RNAs extracted from SFZCs and chondrocyte cultures were processed for reverse transcription-quantitative PCR analysis. Prg4, Erg, and Tenascin C are abundantly expressed in SFZCs.20,22,23 The gene expression levels of Prg4, Erg, and Tenascin C were significantly higher in SFZC cultures than in chondrocyte cultures ( Suppl. Fig. S1 ). These findings indicated that the isolated SFZC phenotype is conserved in this study.
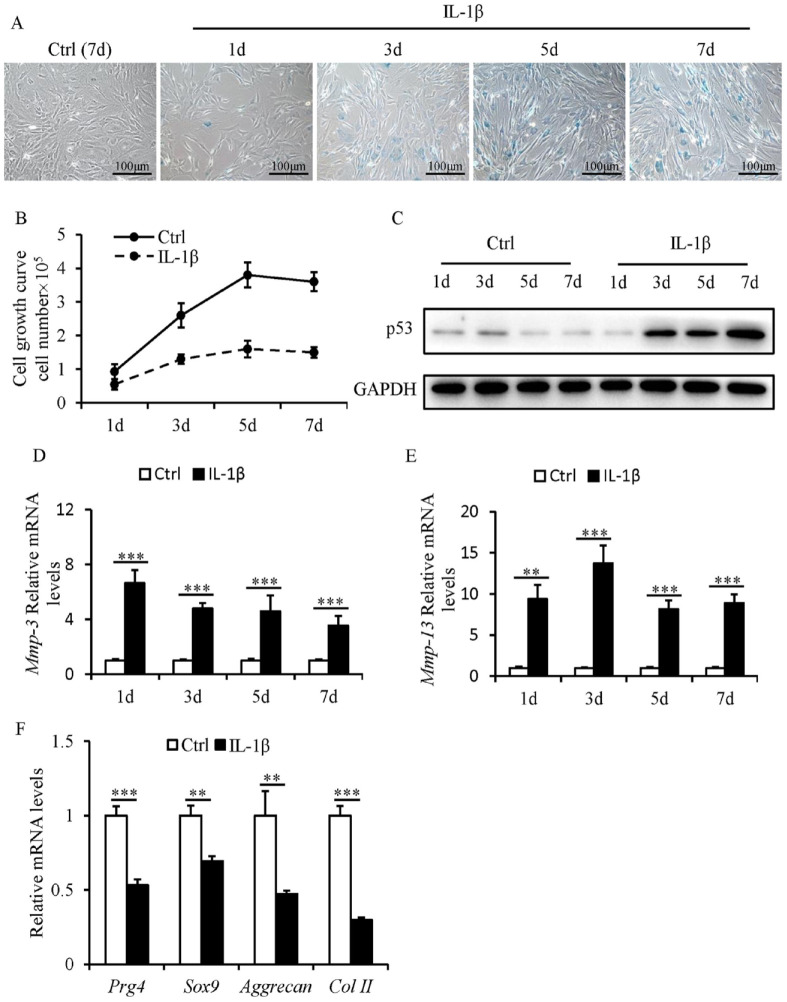
SnCs accumulate in the SFZ and contribute to OA-associated pathologies. 24 To investigate the role of IL-1β in the induction of senescence in SFZCs, SA-β-gal staining analysis was carried out. Most IL-1β-treated SFZCs were positive for SA-β-gal staining from day 3 ( Fig. 1A ). IL-1β led to a significant reduction in the cell proliferation of SFZCs ( Fig. 1B ). P53 protein expression levels were increased in IL-1β-treated SFZCs from day 3 ( Fig. 1C ). The mRNA expression levels of Mmp-3 and Mmp-13 were markedly increased in IL-1β-treated SFZCs (Fig. 1D and E). The gene expression levels of four chondrogenic markers (Sox9, Prg4, Col II, and Aggrecan) were quantified to investigate the chondrogenic differentiation capacity of SFZCs. The results demonstrated reduced mRNA expression levels of chondrogenic marker genes in IL-1β-treated SFZCs ( Fig. 1F ). These findings indicated that IL-1β induces senescence of SFZCs with decreased proliferation and chondrogenic differentiation.
Figure 1.
Effects of IL-1β on the proliferation, chondrogenic differentiation, and senescence of SFZCs. SFZCs were treated with IL-1β (20 ng/ml) for the indicated number of days. (A) Cellular morphology and SA-β-gal staining. (B) Cell proliferation capacity was evaluated using cell counting and cell growth curves. (C) Protein levels of p53 were determined using western blot analysis. (D and E) mRNA Levels of SASP factors Mmp-3 and Mmp-13 were determined using RT-qPCR. (F) RT-qPCR analysis of four chondrogenic differentiation markers Sox9, Prg4, Aggrecan, and Col II at day 5. IL-1β = interleukin-1β; SFZCs = superficial zone cells in articular cartilage; SA-β-gal = senescence-associated β-galactosidase; mRNA = messenger RNA; SASP = senescence-associated secretory phenotype; RT-qPCR = reverse transcription-quantitative PCR. **P < 0.01 and ***P < 0.001.
Autophagy Levels Decline in SFZCs Following IL-1β Treatment
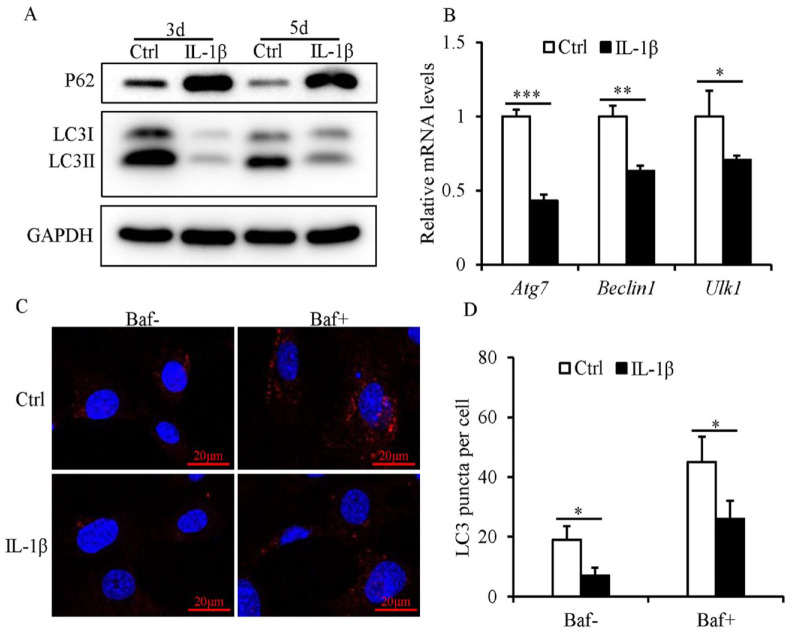
A reduction in the protein expression levels of LC3II and an increase in the protein expression levels of p62 were detected in IL-1β-treated SFZCs, compared with those of the control ( Fig. 2A ). Moreover, the mRNA expression levels of key autophagy genes Atg7, Beclin1, and Ulk1 were reduced in IL-1β-treated SFZCs, compared with the control ( Fig. 2B ). The number of LC3 puncta was significantly reduced in IL-1β-treated SFZCs with or without bafilomycin (Fig. 2C and D).
Figure 2.
Defective autophagy in SFZCs following IL-1β. (A and B) SFZCs were treated with IL-1β (20 ng/ml) for 3 or 5 days. (A) Protein expression levels of autophagy markers, p62 and LC3, were determined using western blotting. (B) RT-qPCR analysis of autophagy genes Atg7, Beclin1, and Ulk1 at day 5. (C and D) SFZCs were treated with IL-1β (20 ng/ml) for 5 days and in the absence or presence of bafilomycin for 4 hours. Immunofluorescent staining of LC3 and quantitative analysis of LC3 puncta. SFZCs = superficial zone cells in articular cartilage; IL-1β = interleukin-1β; LC3 = light-chain 1 protein 3; RT-qPCR = reverse transcription-quantitative PCR; Baf = bafilomycin; GAPDH = glyceraldehyde-3-phosphate dehydrogenase. *P < 0.05, **P < 0.01, and ***P < 0.001.
Inhibition of Autophagy Induces Senescence in SFZCs
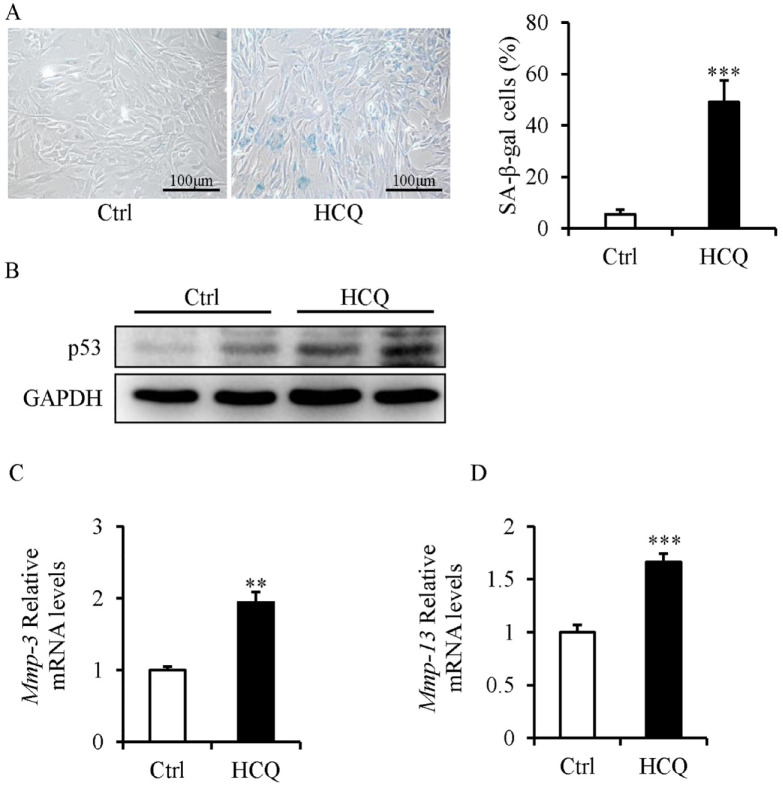
SFZCs were treated with HCQ, a lysosome inhibitor that inhibits autophagy, to examine its effects on the senescence of SFZCs. HCQ markedly increased the percentage of SA-β-gal-positive cells ( Fig. 3A ), increased the protein expression levels of p53 ( Fig. 3B ), and increased the mRNA expression levels of Mmp-3 and Mmp-13 ( Fig. 3C and D ).
Figure 3.
Inhibition of autophagy induces senescence in SFZCs. SFZCs were treated with HCQ (10 μM) for 5 days. (A) SA-β-gal staining and quantitative analysis of SA-β-gal positive cells. (B) Western blot analysis of senescence marker p53. (C and D) mRNA Expression levels of SASP factor Mmp-3 and Mmp-13 were determined using RT-qPCR. SFZCs = superficial zone cells in articular cartilage; HCQ = hydroxychloroquine; SA-β-gal = senescence-associated β-galactosidase; mRNA = messenger RNA; SASP = senescence-associated secretory phenotype; RT-qPCR = reverse transcription-quantitative PCR; GAPDH = glyceraldehyde-3-phosphate dehydrogenase. **P < 0.01 and ***P < 0.001.
Activation of Autophagy Using Rapamycin Attenuates Senescence and Restores Proliferation and Chondrogenic Differentiation of IL-1β-Treated SFZCs
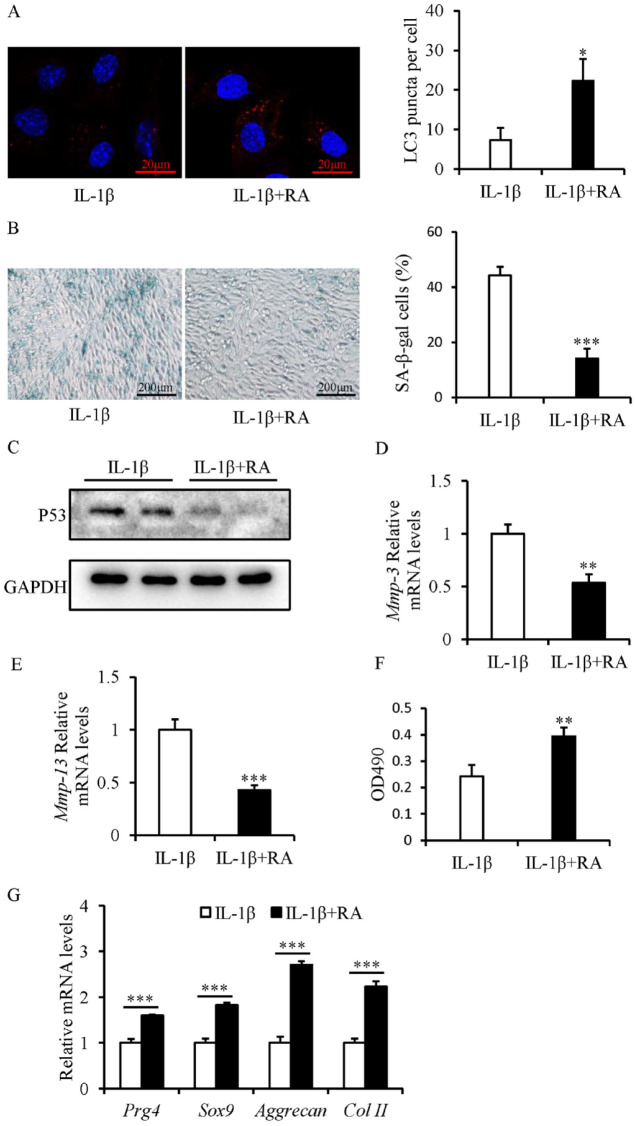
The LC3 immunofluorescent staining suggested that rapamycin treatment resulted in restoration of autophagy flux in IL-1β-treated SFZCs, as treatment with rapamycin markedly increased the LC3 puncta number ( Fig. 4A ).
Figure 4.
Rapamycin restores the proliferation and chondrogenic differentiation capacity and attenuates senescence of IL-1β-treated SFZCs. SFZCs were treated with IL-1β (20 ng/ml) and (or) RA (100 nM) for 5 days. (A) Immunofluorescent staining of LC3 and quantitative analysis of LC3 puncta. (B) SA-β-gal staining and quantitative analysis of SA-β-gal-positive cells. (C) Western blot analysis of senescence marker p53. (D and E) mRNA Expression levels of SASP factors Mmp-3 and Mmp-13 were determined using RT-qPCR. (F) Cell proliferation capacity was evaluated using a 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazoliumbromide assay. (G) RT-qPCR analysis of four chondrogenic differentiation markers: Sox9, Prg4, Aggrecan, and Col II. IL-1β = interleukin-1β; SFZCs = superficial zone cells in articular cartilage; LC3 = light-chain 1 protein 3; SA-β-gal = senescence-associated β-galactosidase; mRNA = messenger RNA; SASP = senescence-associated secretory phenotype; RT-qPCR = reverse transcription-quantitative PCR; RA = rapamycin. *P < 0.05, **P < 0.01, and ***P < 0.001.
Rapamycin attenuated the senescence of IL-1β-treated SFZCs, and rapamycin treatment markedly reduced the percentage of SA-β-gal-positive cells ( Fig. 4B ), decreased the protein expression levels of p53 ( Fig. 4C ), and decreased the mRNA expression levels of Mmp-3 and Mmp-13 ( Fig. 4D and E ). Moreover, the rapamycin promoted the proliferation of IL-1β-treated SFZCs ( Fig. 4F ). Furthermore, treatment with rapamycin significantly restored the chondrogenic differentiation capacity of IL-1β-treated SFZCs, demonstrated using RT-qPCR detection of chondrogenic markers, such as Sox9, Prg4, Col II, and Aggrecan ( Fig. 4G ).
Autophagy Activation Attenuates Mitochondrial Dysfunction and Decreases ROS Production in IL-1β-Treated Senescent SFZCs
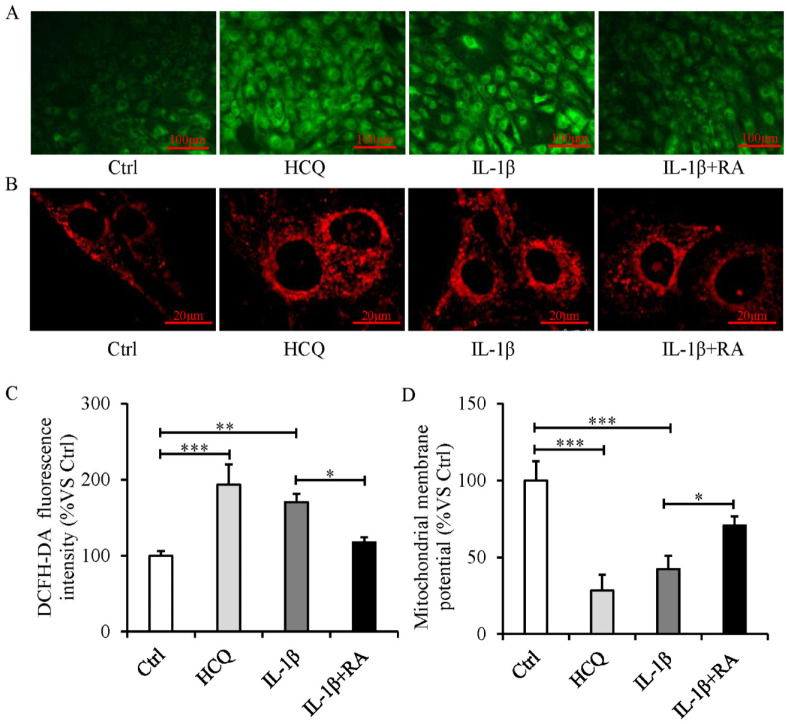
The excessive production of ROS ( Fig. 5A and C ), accumulation of mitochondria ( Fig. 5B ), and decreased mitochondrial membrane potential ( Fig. 5D ) occurred in IL-1β-treated SFZCs. In addition, pharmacological disruption of autophagy by HCQ in SFZCs resulted in a phenotype similar to that observed in IL-1β-treated SFZCs with mitochondrial dysfunction and ROS overproduction ( Fig. 5A-D ). Furthermore, autophagy activation following treatment with rapamycin resulted in a significantly decreased production of ROS, a decreased accumulation of mitochondria, and an increased mitochondrial membrane potential ( Fig. 5A-D ).
Figure 5.
Mitochondrial dysfunction and intracellular ROS elevation occur during autophagy defect, and autophagy activation attenuates mitochondrial dysfunction and decreases ROS production in IL-1β-treated SFZCs. SFZCs were treated with HCQ (10 μM), RA (100 nM), or IL-1β (20 ng/ml) for 5 days. (A) ROS assessment using DCFH-DA fluorescence. (B) Mitochondrial staining using MitoTracker. (C) DCFH-DA fluorescence quantified using flow cytometry. (D) Mitochondrial membrane potentials were measured using JC-1 staining, expressed as the ratio of red to green fluorescence. ROS = reactive oxygen species; IL-1β = interleukin-1β; SFZCs = superficial zone cells in articular cartilage; HCQ = hydroxychloroquine; RA = rapamycin; DCFH-DA = 2’-7’dichlorofluorescin diacetate. *P < 0.05, **P < 0.01, and ***P < 0.001.
ROS Inhibition Alleviates Senescence in IL-1β-Treated SFZC
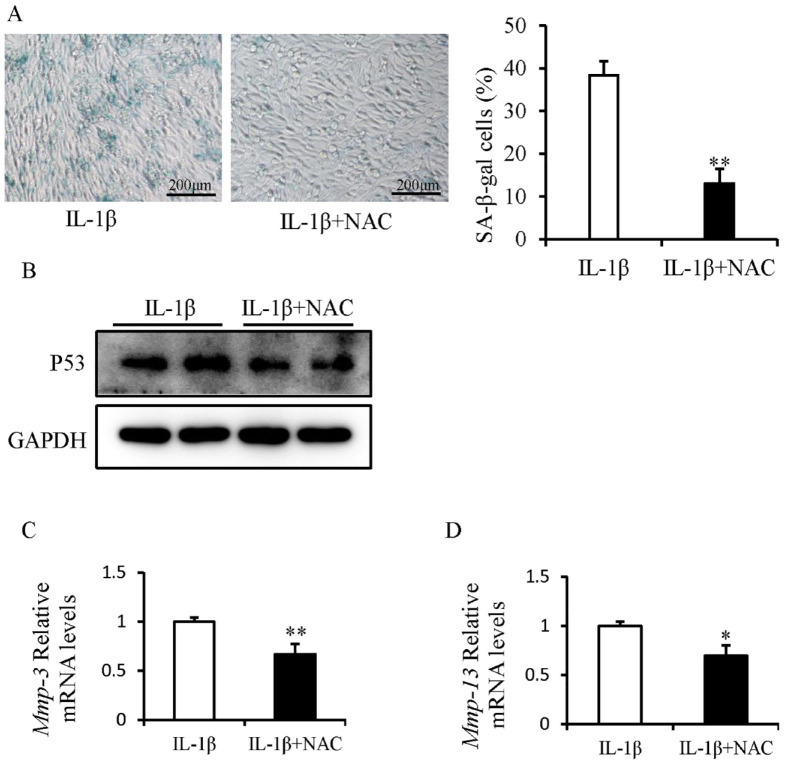
The effects of the antioxidant NAC on the development of IL-1β-induced senescence in SFZCs were investigated. IL-1β-induced SFZC senescence was partially attenuated following NAC treatment ( Fig. 6A ). NAC treatment reduced the protein expression levels of p53 ( Fig. 6B ). NAC treatment decreased the mRNA expression levels of Mmp-3 and Mmp-13 ( Fig. 6C and D ).
Figure 6.
ROS inhibition alleviates senescence in IL-1β-treated SFZCs. SFZCs were treated with IL-1β (20 ng/ml) and (or) NAC (2 μM) for 5 days. (A) SA-β-gal staining and quantitative analysis of SA-β-gal positive cells. (B) Western blot analysis of senescence marker p53. (C and D) mRNA Expression levels of SASP factors Mmp-3 and Mmp-13 were determined using RT-qPCR. ROS = reactive oxygen species; IL-1β = interleukin-1β; SFZCs = superficial zone cells in articular cartilage; NAC = N-acetylcysteine; SA-β-gal = senescence-associated β-galactosidase; mRNA = messenger RNA; SASP = senescence-associated secretory phenotype; RT-qPCR = reverse transcription-quantitative PCR; GAPDH = glyceraldehyde-3-phosphate dehydrogenase. *P < 0.05 and **P < 0.01.
Discussion
OA is a degenerative disease that causes irreversible destruction of articular cartilage, and at present, there is no effective treatment method. Although articular cartilage exhibits a poor ability to self-repair following injuries, numerous previous studies have verified the presence of chondrogenic stem cells in the SFZ of articular cartilage. Kozhemyakina et al. 4 reported that Prg4-positive cells in the SFZ of articular cartilage served as progenitor cells for all deeper layers of the mature chondrocytes. Li et al. 2 further demonstrated that SFZCs were self-renewal progenitor cells which were able to sustain their own population, thus fulfilling the criteria of unipotent adult stem cells. SFZCs play critical roles in cartilage homeostasis and OA progression. In SFZ-specific β-catenin-knockout mice, OA progression was markedly accelerated, and this was accompanied by reduced levels of Prg4 expression and SFZ degradation. 13 Moreover, Tan et al. 1 demonstrated that deficiency of activin receptor-like kinase 5 (ALK5) in SFZCs promoted the development of OA. Inflammatory cytokine IL-1β exerts a profound impact on cartilage catabolism and OA progression. 17 Previous studies revealed that human SFZCs were more susceptible to IL-1-induced damage than the chondrocytes from deep cartilage, 25 and in situ hybridization of cartilage organ cultures revealed that IL-1 stimulated human SFZC transcribed monocyte chemoattractant protein-1 mRNA. 26 However, the underlying mechanism of IL-1β in regulating the biological functions of SFZCs is poorly understood.
First, the results demonstrated that IL-1β induced senescence in SFZCs with decreased proliferation and chondrogenic differentiation. Moreover, autophagic flux was impaired in IL-1β-induced senescent SFZCs, and upregulation of autophagy using rapamycin attenuated senescence and restored the chondrogenic differentiation of IL-1β-treated SFZCs. In addition, mitochondrial dysfunction and ROS overproduction occurred during senescence of IL-1β-induced SFZCs. These findings suggested that IL-1β leads to autophagy impairment, resulting in excessive accumulation of dysfunctional mitochondria and ROS. In turn, this causes senescence of SFZCs. Thus, autophagy activation and antioxidants may be potential strategies to counteract cellular senescence and OA.
Second, we found that IL-1β induced senescence in SFZCs with decreased proliferation and chondrogenic differentiation. SnCs exist in most tissues of the knee joint, and these SnCs exhibit common senescence-related characteristics, such as telomere shortening, upregulated cyclin-dependent kinase inhibitors (p21, p16, and p53), mitochondrial dysfunction, and increased production of ROS and SASP.8,27,28 The results of previous studies demonstrated that cellular senescence is responsible for OA development, and selectively eliminating SnCs reduces cartilage degradation. Xu et al. 29 reported that intra-articular injection of SnCs induced an OA-like condition in mice. Carbone and Rodeo 30 revealed that SnCs accumulated in the articular cartilage and synovium following anterior cruciate ligament transection, and selectively eliminating these cells attenuated cartilage degradation of post-traumatic OA, reduced pain, and promoted cartilage development. Tan et al. 1 demonstrated that ALK5 deficiency in SFZCs induced senescence of SFZCs, which was characterized by a decreased proliferation and differentiation, an increase in SA-β-gal-positive cells, and ROS overproduction. The results of the present study indicated that senescence of SFZCs under an inflammatory environment may play a role in OA development; however, further investigations are required.
In addition, these findings demonstrate that autophagy exerts a protective effect against senescence of SFZCs under an inflammatory environment. Autophagy is a highly conserved catabolic process used to sustain cellular homeostasis and promote cellular survival and function. Autophagy is crucial for maintaining the homeostasis and function of chondrocytes. In the early stages of OA, autophagy flux is enhanced in OA chondrocytes as an adaptive reaction to cellular stress; however, in the later stages of OA, autophagy flux is decreased. 10 The results of previous studies demonstrated that rapamycin activated autophagy, decreasing the catabolic events of human chondrocytes and alleviating articular cartilage destruction in post-traumatic OA models.31,32 The results of the present study demonstrated that treatment with IL-1β blocked the autophagic flux in SFZCs, and upregulation of autophagy using rapamycin restored the IL-1β-induced disruption of autophagic flux and attenuated senescence in SFZCs.
Notably, the results of the present study indicated that ROS inhibition using antioxidant NAC alleviated senescence in IL-1β-treated SFZCs. Autophagy dysfunction is inadequate in eliminating damaged and dysfunctional organelles and proteins, leading to cellular senescence. However, the underlying mechanisms by which autophagy dysfunction contributes to cellular senescence under inflammatory environments and its implications into human diseases remain to be fully clarified.33,34 ROS is a vital marker and regulator of cellular senescence. 15 The mitochondrion is the main origin of ROS, and mitochondrial dysfunction contributes to excessive production of ROS. This gives rise to further mitochondrial dysfunction and subsequent cellular senescence. 15 Autophagy impairment in chondrocytes resulted in ROS overproduction and increased oxidative stress. 35 Guillen et al. 19 reported that autophagy defects induced the senescence of young satellite cells using mitochondrial dysfunction and ROS overproduction. The present study aimed to explore the effects of mitochondrial function and ROS production in the autophagy process during senescence of IL-1β-induced SFZCs. The results of the present study demonstrated that mitochondrial dysfunction and excessive production of ROS occurred in IL-1β-treated SFZCs, and autophagy activation removed dysfunctional mitochondria and decreased the production of ROS.
SFZCs are perfect cell source for progenitor cell-based cartilage regeneration and OA therapy, as they have mighty chondrogenic capacity, extensive proliferative potential, and apparent resistance to hypertrophy.36,37 Several studies have applied SFZCs for in vivo cartilage regeneration.36,37 However, our results showed that SFZCs exhibit senescence phenotype with decreased proliferation and chondrogenic capacity under an inflammatory environment. This drawback could prevent further application of SFZCs for cell-based cartilage regeneration and OA therapy. Finding methods to prevent SFZC senescence under an inflammatory environment could be of considerable value for the application of SFZCs in cartilage regeneration and OA therapy. Our results indicated that autophagy activation and ROS inhibition alleviated senescence in IL-1β-treated SFZCs, which provides an experimental basis for the prevention of SFZC senescence from the perspective of autophagy activation and ROS inhibition.
Conclusion
In summary, autophagy impairment and ROS overproduction are crucial for IL-1β-induced senescence of SFZCs. IL-1β promotes autophagy impairment and subsequently results in dysfunctional mitochondria and overproduction of ROS, which finally causes senescence in SFZCs. The present study furthers the current understanding of the association among autophagy, oxidative stress, and senescence in SFZCs under an inflammatory environment.
Supplemental Material
Supplemental material, sj-docx-1-car-10.1177_19476035231194771 for Il-1β Promotes Superficial Zone Cells Senescence in Articular Cartilage by Inhibiting Autophagy by Wei Xu, Juan Wang, Lin Cui, Chen Huang, Ning Xia, Meiming Xie, Da Liu and Dongfa Liao in CARTILAGE
Footnotes
Author Contributions: W.X., M.X., and D.L. contributed to the design of the study. W.X., J.W., L.C., N.X., and C.H. contributed to the acquisition, collection, and assembly of data. W.X., M.X., D.L., and Df.L. contributed to reagents/materials/analysis tools. W.X., M.X., and D.L. wrote the main manuscript text. All authors contributed in revising the manuscript and approved the final version to be submitted.
Acknowledgments and Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was supported by the Science Foundation of General Hospital of Western Theater Command (No. 2021-XZYG-C11), Key research and development Plan of Sichuan Province (No. 23ZDYF0941), and Southwest Jiaotong University Medical Engineering combination project (No. 2682022TPY031).
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical Approval: All studies were carried out in accordance with the policies and approval to the Institutional Animal Care and Use Committee of the General Hospital of Western Theater Command.
ORCID iD: Meiming Xie  https://orcid.org/0000-0002-9786-5486
https://orcid.org/0000-0002-9786-5486
Availability of Data and Materials: The data sets analyzed during the current study are available from the corresponding author on reasonable request.
Supplementary material for this article is available on the Cartilage website at http://cart.sagepub.com/supplemental.
References
- 1. Tan Q, Wang Q, Kuang L, Zhang J, Peng X, Liang S, et al. TGF-beta/Alk5 signaling prevents osteoarthritis initiation via regulating the senescence of articular cartilage stem cells. J Cell Physiol. 2021;236(7):5278-92. doi: 10.1002/jcp.30231. [DOI] [PubMed] [Google Scholar]
- 2. Li L, Newton PT, Bouderlique T, Sejnohova M, Zikmund T, Kozhemyakina E, et al. Superficial cells are self-renewing chondrocyte progenitors, which form the articular cartilage in juvenile mice. FASEB J. 2017;31(3):1067-84. doi: 10.1096/fj.201600918R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Decker RS, Um HB, Dyment NA, Cottingham N, Usami Y, Enomoto-Iwamoto M, et al. Cell origin, volume and arrangement are drivers of articular cartilage formation, morphogenesis and response to injury in mouse limbs. Dev Biol. 2017;426(1):56-68. doi: 10.1016/j.ydbio.2017.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kozhemyakina E, Zhang M, Ionescu A, Ayturk UM, Ono N, Kobayashi A, et al. Identification of a Prg4-expressing articular cartilage progenitor cell population in mice. Arthritis Rheumatol. 2015;67(5):1261-73. doi: 10.1002/art.39030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Di Micco R, Krizhanovsky V, Baker D, d’Adda di Fagagna F. Cellular senescence in ageing: from mechanisms to therapeutic opportunities. Nat Rev Mol Cell Biol. 2021;22(2):75-95. doi: 10.1038/s41580-020-00314-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Coryell PR, Diekman BO, Loeser RF. Mechanisms and therapeutic implications of cellular senescence in osteoarthritis. Nat Rev Rheumatol. 2021;17(1):47-57. doi: 10.1038/s41584-020-00533-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mobasheri A. The future of osteoarthritis therapeutics: targeted pharmacological therapy. Curr Rheumatol Rep. 2013;15(10):364. doi: 10.1007/s11926-013-0364-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jeon OH, David N, Campisi J, Elisseeff JH. Senescent cells and osteoarthritis: a painful connection. J Clin Invest. 2018;128(4):1229-37. doi: 10.1172/JCI95147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kitada M, Koya D. Autophagy in metabolic disease and ageing. Nat Rev Endocrinol. 2021;17(11):647-61. doi: 10.1038/s41574-021-00551-9. [DOI] [PubMed] [Google Scholar]
- 10. Li YS, Zhang FJ, Zeng C, Luo W, Xiao WF, Gao SG, et al. Autophagy in osteoarthritis. Joint Bone Spine. 2016;83(2):143-8. doi: 10.1016/j.jbspin.2015.06.009. [DOI] [PubMed] [Google Scholar]
- 11. Duan R, Xie H, Liu ZZ. The role of autophagy in osteoarthritis. Front Cell Dev Biol. 2020;8:608388. doi: 10.3389/fcell.2020.608388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Valenti MT, Dalle Carbonare L, Zipeto D, Mottes M. Control of the autophagy pathway in osteoarthritis: key regulators, therapeutic targets and therapeutic strategies. Int J Mol Sci. 2021;22(5). doi: 10.3390/ijms22052700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ma Y, Qi M, An Y, Zhang L, Yang R, Doro DH, et al. Autophagy controls mesenchymal stem cell properties and senescence during bone aging. Aging Cell. 2018;17(1). doi: 10.1111/acel.12709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Garcia-Prat L, Martinez-Vicente M, Perdiguero E, Ortet L, Rodriguez-Ubreva J, Rebollo E, et al. Autophagy maintains stemness by preventing senescence. Nature. 2016;529(7584):37-42. doi: 10.1038/nature16187. [DOI] [PubMed] [Google Scholar]
- 15. Tai H, Wang Z, Gong H, Han X, Zhou J, Wang X, et al. Autophagy impairment with lysosomal and mitochondrial dysfunction is an important characteristic of oxidative stress-induced senescence. Autophagy. 2017;13(1):99-113. doi: 10.1080/15548627.2016.1247143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kang HT, Lee KB, Kim SY, Choi HR, Park SC. Autophagy impairment induces premature senescence in primary human fibroblasts. PLoS ONE. 2011;6(8):e23367. doi: 10.1371/journal.pone.0023367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Vincent TL. IL-1 in osteoarthritis: time for a critical review of the literature. F1000Res. 2019;8:F1000 Faculty Rev-934. doi: 10.12688/f1000research.18831.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Huang Z, Lan J, Gao X. Feprazone mitigates IL-1beta-induced cellular senescence in chondrocytes. ACS Omega. 2021;6(14):9442-8. doi: 10.1021/acsomega.0c06066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Guillén MI, Megías J, Clérigues V, Gomar F, Alcaraz MJ. The CO-releasing molecule CORM-2 is a novel regulator of the inflammatory process in osteoarthritic chondrocytes. Rheumatology. 2008;47(9):1323-8. doi: 10.1093/rheumatology/ken264. [DOI] [PubMed] [Google Scholar]
- 20. Yasuhara R, Ohta Y, Yuasa T, Kondo N, Hoang T, Addya S, et al. Roles of beta-catenin signaling in phenotypic expression and proliferation of articular cartilage superficial zone cells. Lab Invest. 2011;91(12):1739-52. doi: 10.1038/labinvest.2011.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wang X, Qi H, Wang Q, Zhu Y, Wang X, Jin M, et al. FGFR3/fibroblast growth factor receptor 3 inhibits autophagy through decreasing the ATG12-ATG5 conjugate, leading to the delay of cartilage development in achondroplasia. Autophagy. 2015;11(11):1998-2013. doi: 10.1080/15548627.2015.1091551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Iwamoto M, Tamamura Y, Koyama E, Komori T, Takeshita N, Williams JA, et al. Transcription factor ERG and joint and articular cartilage formation during mouse limb and spine skeletogenesis. Dev Biol. 2007;305(1):40-51. doi: 10.1016/j.ydbio.2007.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Koyama E, Shibukawa Y, Nagayama M, Sugito H, Young B, Yuasa T, et al. A distinct cohort of progenitor cells participates in synovial joint and articular cartilage formation during mouse limb skeletogenesis. Dev Biol. 2008;316(1):62-73. doi: 10.1016/j.ydbio.2008.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jeon OH, Kim C, Laberge RM, Demaria M, Rathod S, Vasserot AP, et al. Local clearance of senescent cells attenuates the development of post-traumatic osteoarthritis and creates a pro-regenerative environment. Nat Med. 2017;23(6):775-81. doi: 10.1038/nm.4324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hauselmann HJ, Flechtenmacher J, Michal L, Thonar EJ, Shinmei M, Kuettner KE, et al. The superficial layer of human articular cartilage is more susceptible to interleukin-1-induced damage than the deeper layers. Arthritis Rheum. 1996;39(3):478-88. doi: 10.1002/art.1780390316. [DOI] [PubMed] [Google Scholar]
- 26. Villiger PM, Terkeltaub R, Lotz M. Monocyte chemoattractant protein-1 (MCP-1) expression in human articular cartilage. Induction by peptide regulatory factors and differential effects of dexamethasone and retinoic acid. J Clin Invest. 1992;90(2):488-96. doi: 10.1172/JCI115885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Del Rey MJ, Valín Á, Usategui A, Ergueta S, Martín E, Municio C, et al. Senescent synovial fibroblasts accumulate prematurely in rheumatoid arthritis tissues and display an enhanced inflammatory phenotype. Immun Ageing. 2019;16:29. doi: 10.1186/s12979-019-0169-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. McCulloch K, Litherland GJ, Rai TS. Cellular senescence in osteoarthritis pathology. Aging Cell. 2017;16(2):210-8. doi: 10.1111/acel.12562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Xu M, Bradley EW, Weivoda MM, Hwang SM, Pirtskhalava T, Decklever T, et al. Transplanted senescent cells induce an osteoarthritis-like condition in mice. J Gerontol A Biol Sci Med Sci. 2017;72(6):780-5. doi: 10.1093/gerona/glw154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Carbone A, Rodeo S. Review of current understanding of post-traumatic osteoarthritis resulting from sports injuries. J Orthop Res. 2017;35(3):397-405. [DOI] [PubMed] [Google Scholar]
- 31. Sasaki H, Takayama K, Matsushita T, Ishida K, Kubo S, Matsumoto T, et al. Autophagy modulates osteoarthritis-related gene expression in human chondrocytes. Arthritis Rheum. 2012;64(6):1920-8. doi: 10.1002/art.34323. [DOI] [PubMed] [Google Scholar]
- 32. Caramés B, Hasegawa A, Taniguchi N, Miyaki S, Blanco FJ, Lotz M. Autophagy activation by rapamycin reduces severity of experimental osteoarthritis. Ann Rheum Dis. 2012;71(4):575-81. doi: 10.1136/annrheumdis-2011-200557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rajendran P, Alzahrani AM, Hanieh HN, Kumar SA, Ben Ammar R, Rengarajan T, et al. Autophagy and senescence: a new insight in selected human diseases. J Cell Physiol. 2019;234(12):21485-92. doi: 10.1002/jcp.28895. [DOI] [PubMed] [Google Scholar]
- 34. Tabibzadeh S. Role of autophagy in aging: the good, the bad, and the ugly. Aging Cell. 2023;22(1):e13753. doi: 10.1111/acel.13753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. López de Figueroa P, Lotz MK, Blanco FJ, Caramés B. Autophagy activation and protection from mitochondrial dysfunction in human chondrocytes. Arthritis Rheumatol. 2015;67(4):966-76. doi: 10.1002/art.39025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rikkers M, Korpershoek JV, Levato R, Malda J, Vonk LA. The clinical potential of articular cartilage-derived progenitor cells: a systematic review. npj Regen Med. 2022;7(1):2. doi: 10.1038/s41536-021-00203-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Xu W, Wang W, Liu D, Liao D. Roles of cartilage-resident stem/progenitor cells in cartilage physiology, development, repair and osteoarthritis. Cells. 2022;11(15):2305. doi: 10.3390/cells11152305. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-car-10.1177_19476035231194771 for Il-1β Promotes Superficial Zone Cells Senescence in Articular Cartilage by Inhibiting Autophagy by Wei Xu, Juan Wang, Lin Cui, Chen Huang, Ning Xia, Meiming Xie, Da Liu and Dongfa Liao in CARTILAGE