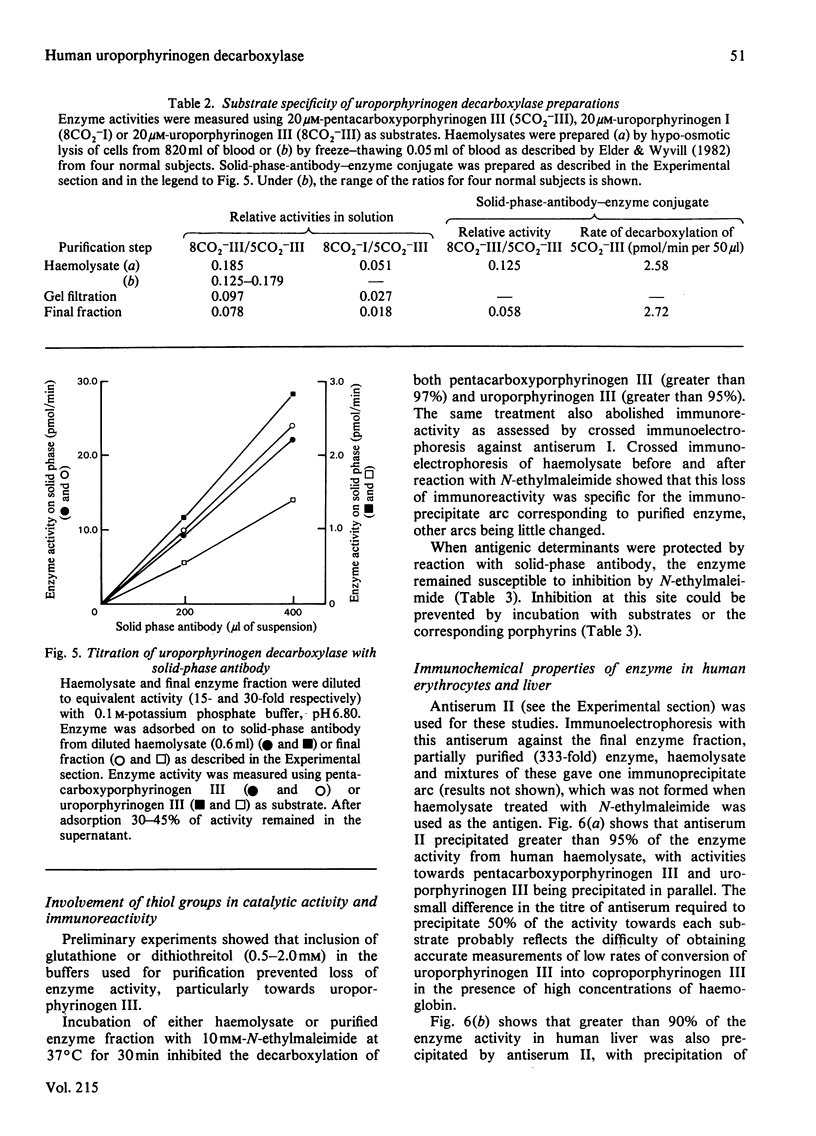
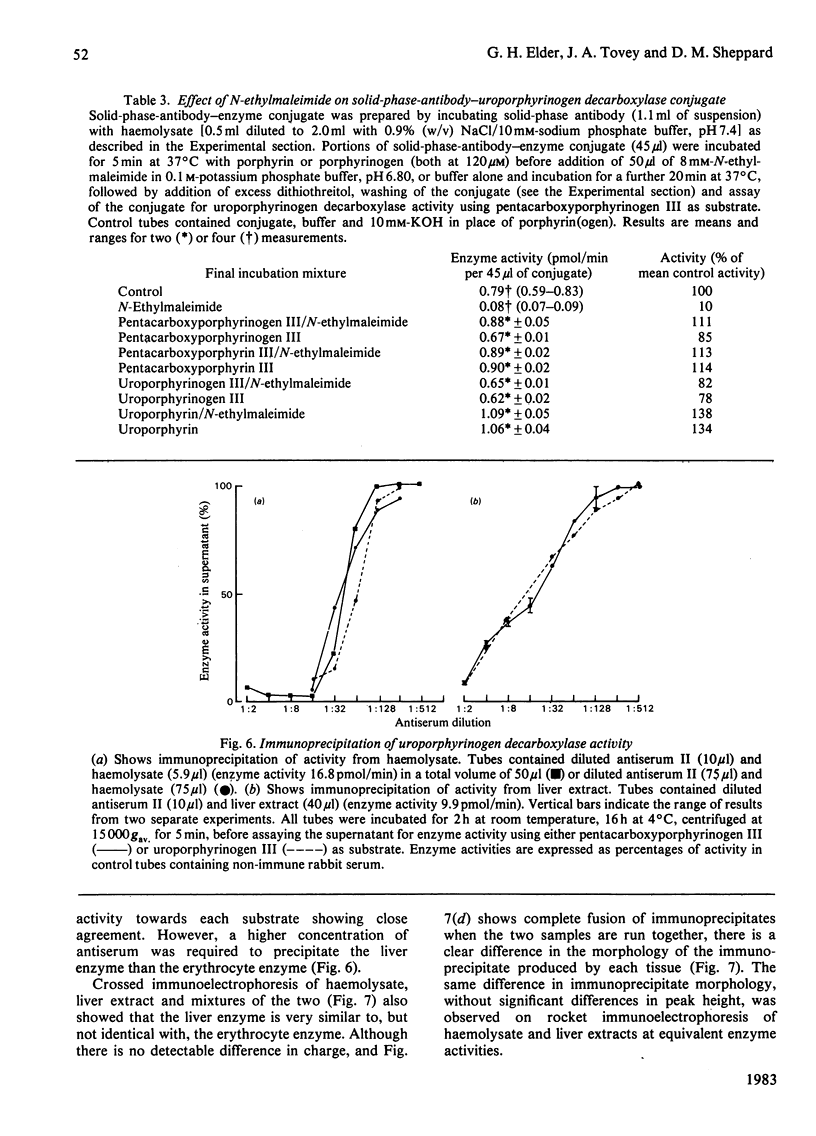
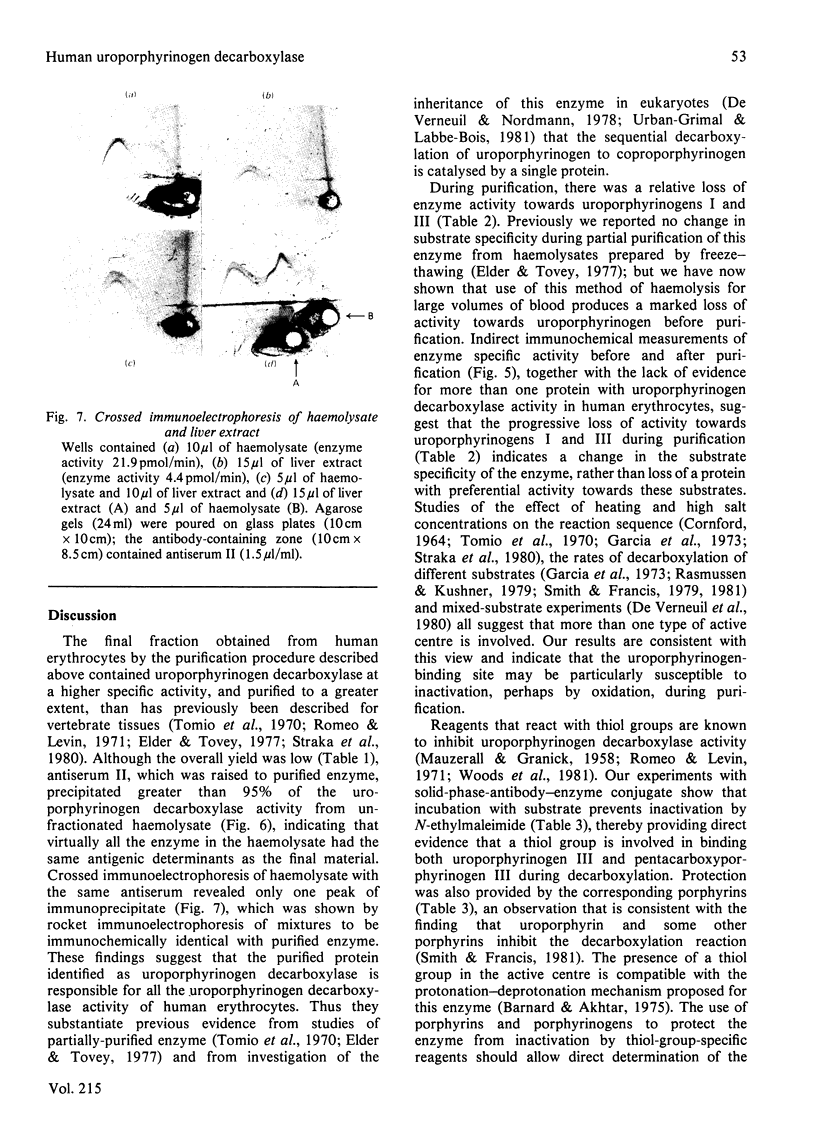
Abstract
Uroporphyrinogen decarboxylase (EC 4.1.1.37) has been purified 4419-fold to a specific activity of 58.3 nmol of coproporphyrinogen III formed/min per mg of protein (with pentacarboxyporphyrinogen III as substrate) from human erythrocytes by adsorption to DEAE-cellulose, (NH4)2SO4 fractionation, gel filtration, phenyl-Sepharose chromatography and polyacrylamide-gel electrophoresis. Progressive loss of activity towards uroporphyrinogens I and III occurred during purification. Experiments employing immunoprecipitation, immunoelectrophoresis and titration with solid-phase antibody indicated that all the uroporphyrinogen decarboxylase activity of human erythrocytes resides in one protein, and that the substrate specificity of this protein had changed during purification. The purified enzyme had a minimum mol.wt. of 39 500 on sodium dodecyl sulphate/polyacrylamide-gel electrophoresis. Gel filtration gave a mol.wt. of 58 000 for the native enzyme. Isoelectric focusing showed a single band with a pI of 4.60. Reaction with N-ethylmaleimide abolished both catalytic activity and immunoreactivity. Incubation with substrates or porphyrins prevented inactivation by N-ethylmaleimide. An antiserum raised against purified erythrocyte enzyme precipitated more than 90% of the uroporphyrinogen decarboxylase activity from human liver. Quantitative immunoprecipitation and crossed immunoelectrophoresis showed that the erythrocyte and liver enzymes are very similar but not identical. The differences observed may reflect secondary modification of enzyme structure by proteolysis or oxidation of thiol groups, rather than a difference in primary structure.
Full text
PDF



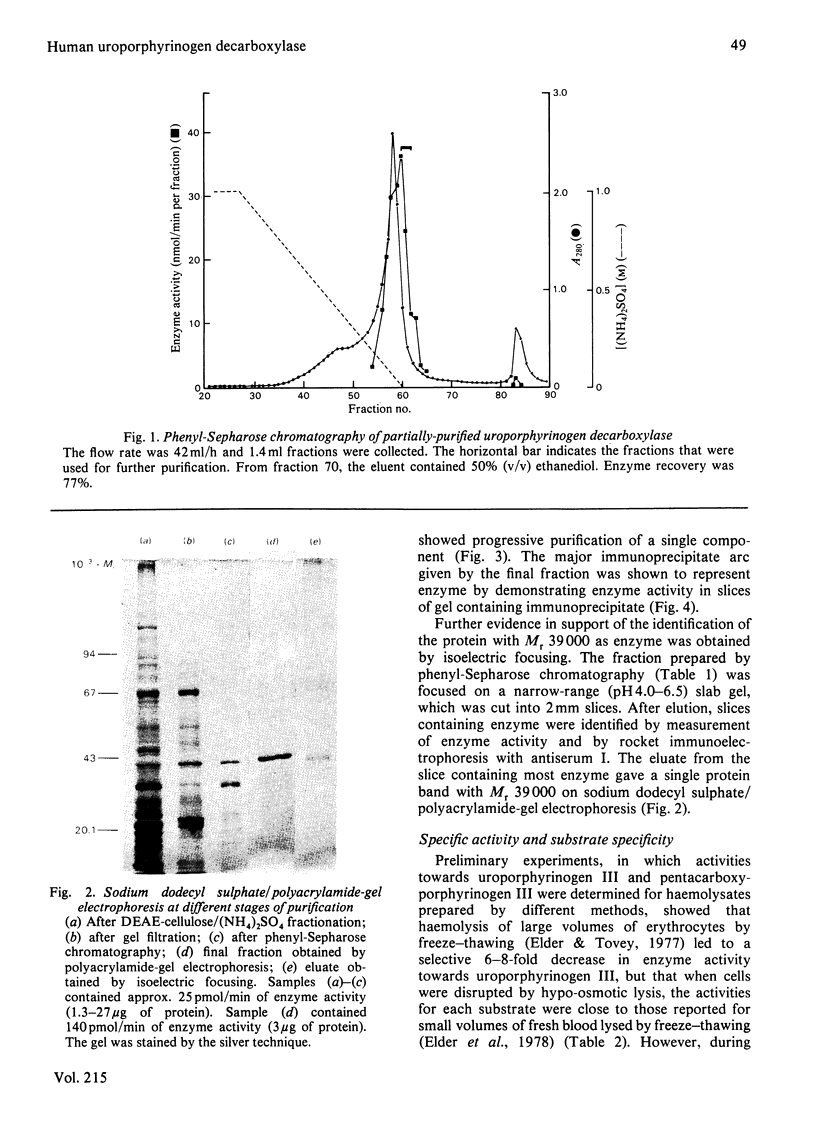
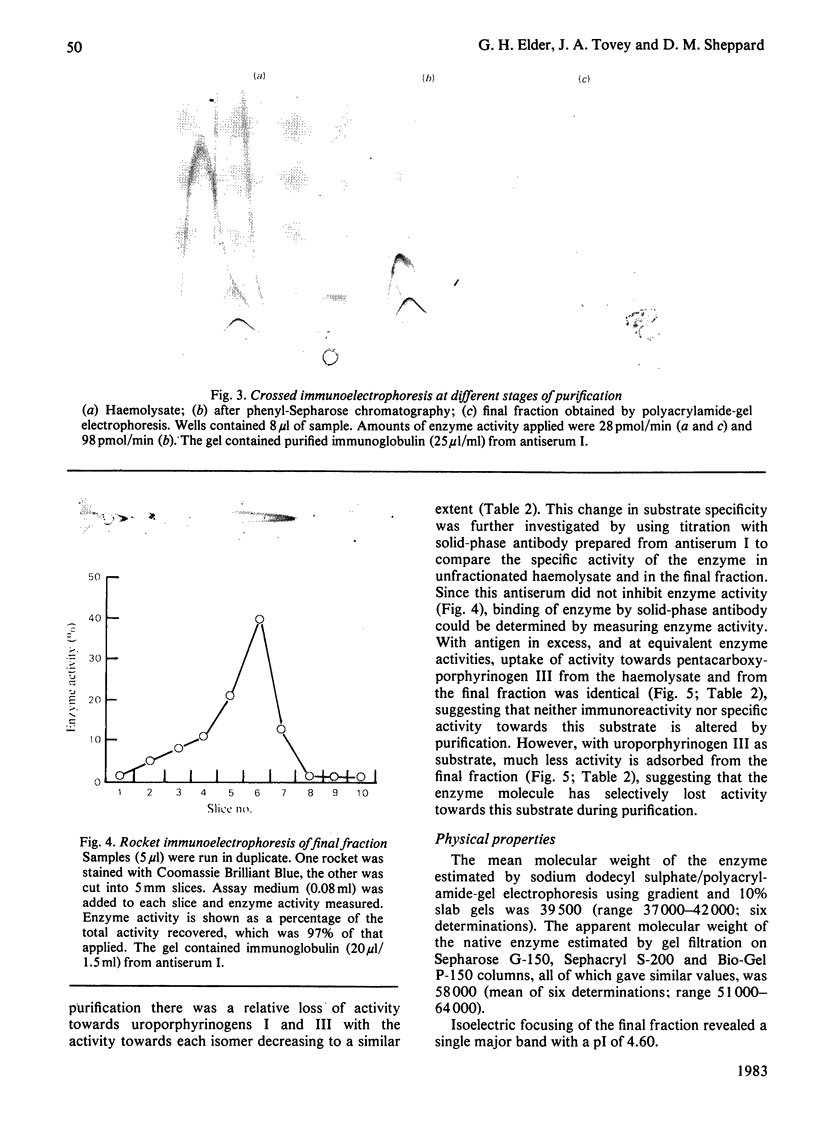


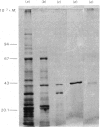
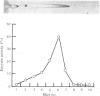
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames G. F. Resolution of bacterial proteins by polyacrylamide gel electrophoresis on slabs. Membrane, soluble, and periplasmic fractions. J Biol Chem. 1974 Jan 25;249(2):634–644. [PubMed] [Google Scholar]
- Arosio P., Yokota M., Drysdale J. W. Characterization of serum ferritin in iron overload: possible identity to natural apoferritin. Br J Haematol. 1977 Jun;36(2):199–207. doi: 10.1111/j.1365-2141.1977.tb00640.x. [DOI] [PubMed] [Google Scholar]
- Bensadoun A., Weinstein D. Assay of proteins in the presence of interfering materials. Anal Biochem. 1976 Jan;70(1):241–250. doi: 10.1016/s0003-2697(76)80064-4. [DOI] [PubMed] [Google Scholar]
- Cornford P. Transformation of porphobilinogen into porphyrins by preparations from human erythrocytes. Biochem J. 1964 Apr;91(1):64–73. doi: 10.1042/bj0910064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elder G. H., Lee G. B., Tovey J. A. Decreased activity of hepatic uroporphyrinogen decarboxylase in sporadic porphyria cutanea tarda. N Engl J Med. 1978 Aug 10;299(6):274–278. doi: 10.1056/NEJM197808102990603. [DOI] [PubMed] [Google Scholar]
- Elder G. H., Sheppard D. M. Immunoreactive uroporphyrinogen decarboxylase is unchanged in porphyria caused by TCDD and hexachlorobenzene. Biochem Biophys Res Commun. 1982 Nov 16;109(1):113–120. doi: 10.1016/0006-291x(82)91573-x. [DOI] [PubMed] [Google Scholar]
- Elder G. H., Smith S. G., Herrero C., Lecha M., Mascaro J. M., Muniesa A. M., Czarnecki D. B., Brenan J., Poulos V., DE Salamanca R. E. Hepatoerythropoietic porphyria: a new uroporphyrinogen decarboxylase defect or homozygous porphyria cutanea tarda? Lancet. 1981 Apr 25;1(8226):916–919. doi: 10.1016/s0140-6736(81)91615-9. [DOI] [PubMed] [Google Scholar]
- Elder G. H., Tovey J. A. Uroporphyrinogen decarboxylase activity of human tissues [proceedings]. Biochem Soc Trans. 1977;5(5):1470–1472. doi: 10.1042/bst0051470. [DOI] [PubMed] [Google Scholar]
- Elder G. H., Wyvill P. C. Measurement of uroporphyrinogen decarboxylase using porphyrinogens prepared by chemical reduction. Enzyme. 1982;28(2-3):186–195. doi: 10.1159/000459101. [DOI] [PubMed] [Google Scholar]
- Garcia R. C., San Martin de Viale L. C., Tomio J. M., Grinstein M. Porphyrin biosynthesis. X. Porphyrinogen carboxy-lyase from avian erythrocytes: further properties. Biochim Biophys Acta. 1973 May 5;309(1):203–210. doi: 10.1016/0005-2744(73)90332-x. [DOI] [PubMed] [Google Scholar]
- Goldstein J. A., Linko P., Bergman H. Induction of porphyria in the rat by chronic versus acute exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin. Biochem Pharmacol. 1982 Apr 15;31(8):1607–1613. doi: 10.1016/0006-2952(82)90388-4. [DOI] [PubMed] [Google Scholar]
- Hales C. N., Woodhead J. S. Labeled antibodies and their use in the immunoradiometric assay. Methods Enzymol. 1980;70(A):334–355. doi: 10.1016/s0076-6879(80)70063-0. [DOI] [PubMed] [Google Scholar]
- Jackson A. H., Sancovich H. A., Ferramola A. M., Evans N., Games D. E., Matlin S. A., Elder G. H., Smith S. G. Macrocyclic intermediates in the biosynthesis of porphyrins. Philos Trans R Soc Lond B Biol Sci. 1976 Feb 5;273(924):191–206. doi: 10.1098/rstb.1976.0009. [DOI] [PubMed] [Google Scholar]
- Kushner J. P., Barbuto A. J., Lee G. R. An inherited enzymatic defect in porphyria cutanea tarda: decreased uroporphyrinogen decarboxylase activity. J Clin Invest. 1976 Nov;58(5):1089–1097. doi: 10.1172/JCI108560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAUZERALL D., GRANICK S. Porphyrin biosynthesis in erythrocytes. III. Uroporphyrinogen and its decarboxylase. J Biol Chem. 1958 Jun;232(2):1141–1162. [PubMed] [Google Scholar]
- McColl K. E., Thompson G. G., Moore M. R., Goldberg A. Acute ethanol ingestion and haem biosynthesis in healthy subjects. Eur J Clin Invest. 1980 Apr;10(2 Pt 1):107–112. doi: 10.1111/j.1365-2362.1980.tb02068.x. [DOI] [PubMed] [Google Scholar]
- Merril C. R., Goldman D. Quantitative two-dimensional protein electrophoresis for studies of inborn errors of metabolism. Clin Chem. 1982 Apr;28(4 Pt 2):1015–1020. [PubMed] [Google Scholar]
- Rasmussen G. L., Kushner J. P. The enzymatic decarboxylation of the naturally occurring isomers of uroporphyrinogen by human erythrocytes. J Lab Clin Med. 1979 Jan;93(1):54–59. [PubMed] [Google Scholar]
- Romeo G., Levin E. Y. Uroporphyrinogen decarboxylase from mouse spleen. Biochim Biophys Acta. 1971 Feb 23;230(2):330–341. doi: 10.1016/0304-4165(71)90220-0. [DOI] [PubMed] [Google Scholar]
- Smith A. G., Francis J. E. Decarboxylation of porphyrinogens by rat liver uroporphyrinogen decarboxylase. Biochem J. 1979 Nov 1;183(2):455–458. doi: 10.1042/bj1830455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A. G., Francis J. E. Investigations of rat liver uroporphyrinogen decarboxylase. Comparisons of porphyrinogens I and III as substrates and the inhibition by porphyrins. Biochem J. 1981 Apr 1;195(1):241–250. doi: 10.1042/bj1950241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Săsărman A., Chartrand P., Lavoie M., Tardif D., Proschek R., Lapointe C. Mapping of a new hem gene in Escherichia coli K12. J Gen Microbiol. 1979 Aug;113(2):297–303. doi: 10.1099/00221287-113-2-297. [DOI] [PubMed] [Google Scholar]
- Tomio J. M., García R. C., San Martín de Viale L. C., Grinstein M. Porphyrin biosynthesis. VII. Porphyrinogen carboxy-lyase from avian erythrocytes. Purification and properties. Biochim Biophys Acta. 1970 Feb 11;198(2):353–363. doi: 10.1016/0005-2744(70)90068-9. [DOI] [PubMed] [Google Scholar]
- Urban-Grimal D., Labbe-Bois R. Genetic and biochemical characterization of mutants of Saccharomyces cerevisiae blocked in six different steps of heme biosynthesis. Mol Gen Genet. 1981;183(1):85–92. doi: 10.1007/BF00270144. [DOI] [PubMed] [Google Scholar]
- Woods J. S., Kardish R., Fowler B. A. Studies on the action of porphyrinogenic trace metals on the activity of hepatic uroporphyrinogen decarboxylase. Biochem Biophys Res Commun. 1981 Nov 16;103(1):264–271. doi: 10.1016/0006-291x(81)91688-0. [DOI] [PubMed] [Google Scholar]
- de Verneuil H., Aitken G., Nordmann Y. Familial and sporadic porphyria cutanea: two different diseases. Hum Genet. 1978 Oct 31;44(2):145–151. doi: 10.1007/BF00295407. [DOI] [PubMed] [Google Scholar]
- de Verneuil H., Grandchamp B., Nordmann Y. Some kinetic properties of human red cell uroporphyrinogen decarboxylase. Biochim Biophys Acta. 1980 Jan 11;611(1):174–186. doi: 10.1016/0005-2744(80)90053-4. [DOI] [PubMed] [Google Scholar]