Abstract
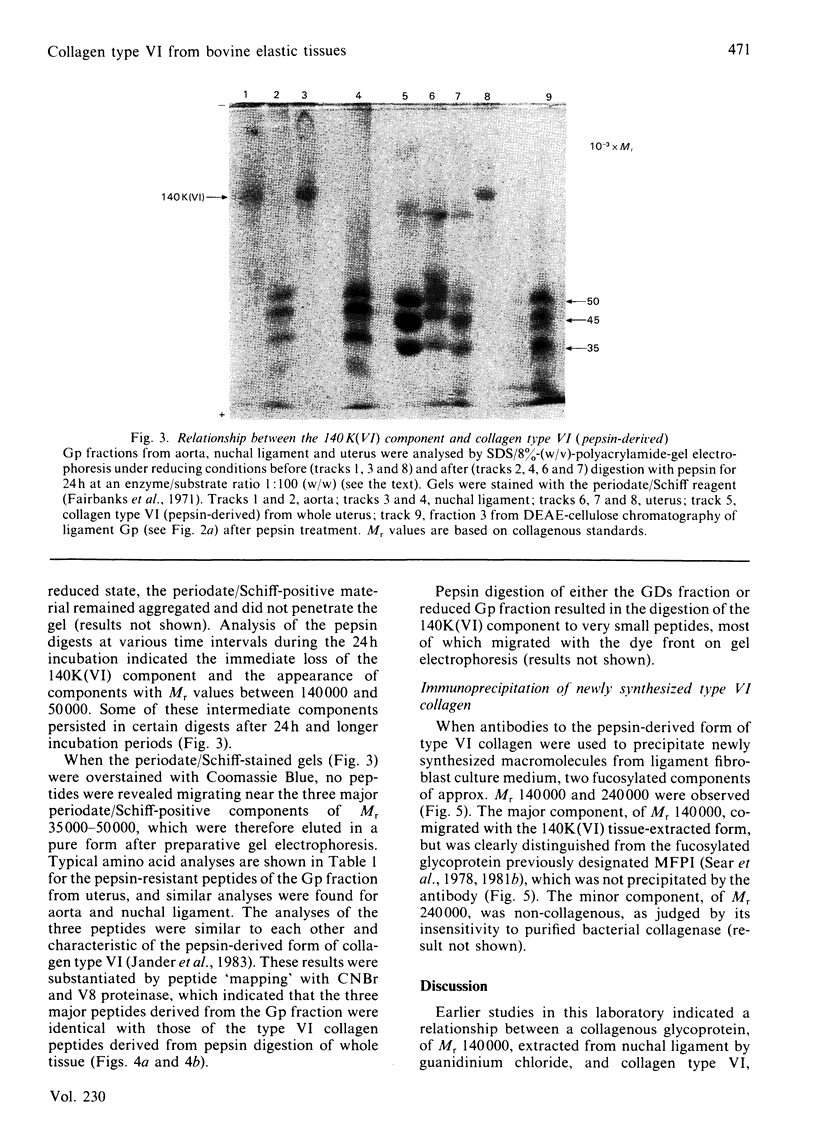
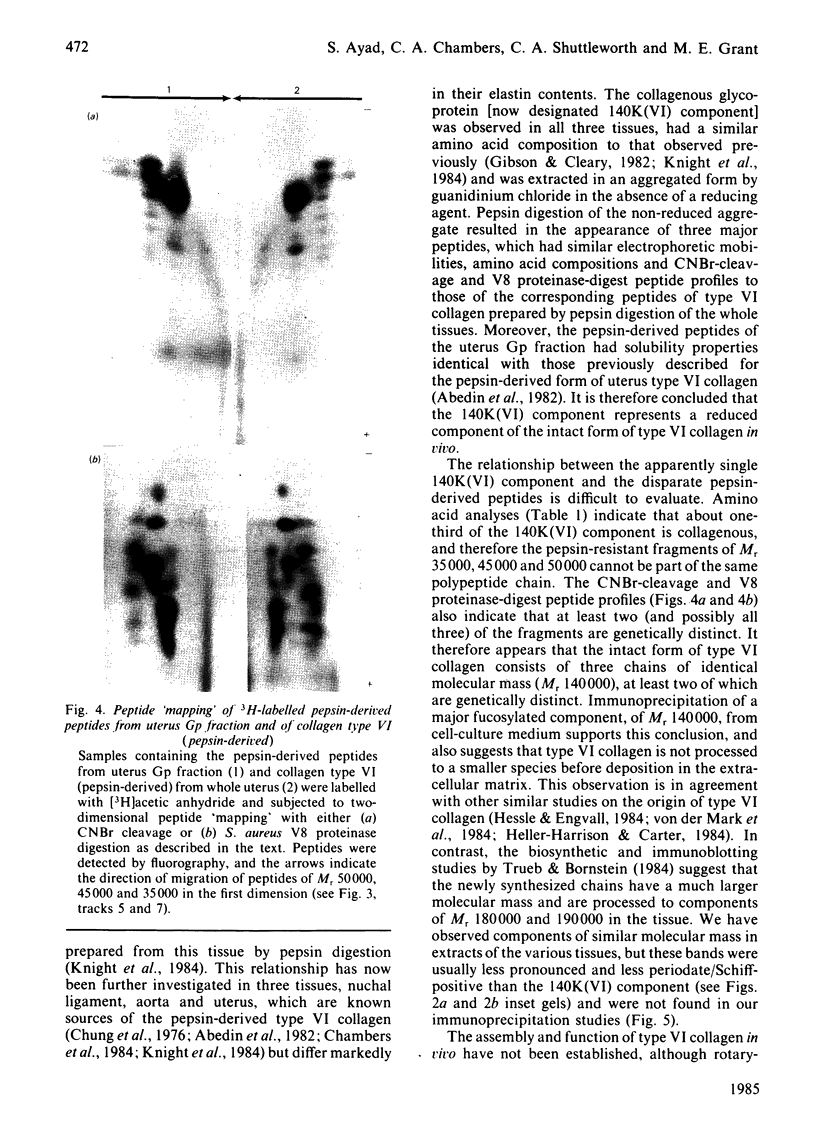
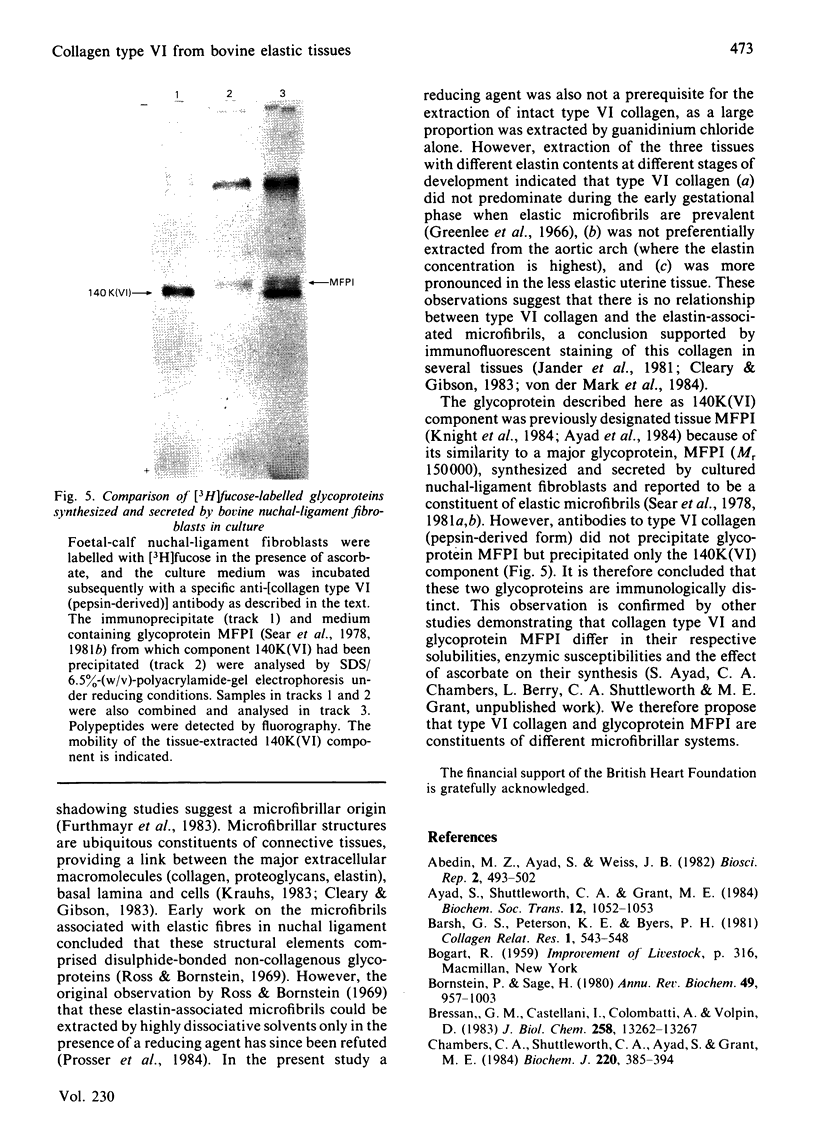
Foetal-bovine nuchal ligament and aorta, together with adult-bovine aorta and pregnant uterus, were extracted under dissociative conditions in the absence and in the presence of a reducing agent. A collagenous glycoprotein of Mr 140000 [designated component 140K(VI)], identified in these extracts as the major periodate/Schiff-positive component, was shown to be related to collagen type VI. Digestion of non-reduced extracts with pepsin yielded periodate/Schiff-positive peptides that, on the basis of their electrophoretic mobilities, amino acid analyses and peptide 'maps', were identical with type VI collagen fragments prepared by standard procedures. It is concluded that collagen type VI occurs in vivo as molecule comprising three chains of Mr 140000 in which the helical domains account for about one-third of each polypeptide. Biosynthetic experiments with nuchal-ligament fibroblasts in culture demonstrated that a bacterial-collagenase-sensitive [3H]fucose-labelled glycoprotein, Mr 140000, was immunoprecipitated from culture medium by a specific antibody to the pepsin-derived form of collagen type VI. This result suggests that the collagenous polypeptides [140K(VI) components] represent the biosynthetic precursors of type VI collagen that do not undergo processing to smaller species before deposition in the extracellular matrix. Analyses of 5M-guanidinium chloride extracts of tissues with markedly different elastin contents and at different stages of development suggested that there was no relationship between collagen type VI and elastic-fibre microfibrils, a conclusion supported by the observation that the immunoprecipitated glycoprotein, Mr 140000, was distinct from the glycoprotein MFPI, Mr 150000, believed to be a constituent of these microfibrils [Sear, Grant & Jackson (1981) Biochem. J. 194, 587-598].
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abedin M. Z., Ayad S., Weiss J. B. Isolation and native characterization of cysteine-rich collagens from bovine placental tissues and uterus and their relationship to types IV and V collagens. Biosci Rep. 1982 Jul;2(7):493–502. doi: 10.1007/BF01115247. [DOI] [PubMed] [Google Scholar]
- Barsh G. S., Peterson K. E., Byers P. H. Peptide mapping of collagen chains using CNBr cleavage of proteins within polyacrylamide gels. Coll Relat Res. 1981 Nov;1(6):543–548. doi: 10.1016/s0174-173x(81)80035-0. [DOI] [PubMed] [Google Scholar]
- Bornstein P., Sage H. Structurally distinct collagen types. Annu Rev Biochem. 1980;49:957–1003. doi: 10.1146/annurev.bi.49.070180.004521. [DOI] [PubMed] [Google Scholar]
- Bressan G. M., Castellani I., Colombatti A., Volpin D. Isolation and characterization of a 115,000-dalton matrix-associated glycoprotein from chick aorta. J Biol Chem. 1983 Nov 10;258(21):13262–13267. [PubMed] [Google Scholar]
- Chambers C. A., Shuttleworth C. A., Ayad S., Grant M. E. Collagen heterogeneity and quantification in developing bovine nuchal ligament. Biochem J. 1984 Jun 1;220(2):385–394. doi: 10.1042/bj2200385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung E., Rhodes K., Miller E. J. Isolation of three collagenous components of probable basement membrane origin from several tissues. Biochem Biophys Res Commun. 1976 Aug 23;71(4):1167–1174. doi: 10.1016/0006-291x(76)90776-2. [DOI] [PubMed] [Google Scholar]
- Cleary E. G., Gibson M. A. Elastin-associated microfibrils and microfibrillar proteins. Int Rev Connect Tissue Res. 1983;10:97–209. doi: 10.1016/b978-0-12-363710-9.50009-5. [DOI] [PubMed] [Google Scholar]
- Evans H. B., Ayad S., Abedin M. Z., Hopkins S., Morgan K., Walton K. W., Weiss J. B., Holt P. J. Localisation of collagen types and fibronectin in cartilage by immunofluorescence. Ann Rheum Dis. 1983 Oct;42(5):575–581. doi: 10.1136/ard.42.5.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Furthmayr H., Wiedemann H., Timpl R., Odermatt E., Engel J. Electron-microscopical approach to a structural model of intima collagen. Biochem J. 1983 May 1;211(2):303–311. doi: 10.1042/bj2110303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuto D. K., Miller E. J. Characterization of a unique collagenous fraction from limited pepsin digests of human placental tissue: molecular organization of the native aggregate. Biochemistry. 1981 Mar 17;20(6):1635–1640. doi: 10.1021/bi00509a035. [DOI] [PubMed] [Google Scholar]
- Furuto D. K., Miller E. J. Isolation of a unique collagenous fraction from limited pepsin digests of human placental tissue. Characterization of one of the constituent polypeptide chains. J Biol Chem. 1980 Jan 10;255(1):290–295. [PubMed] [Google Scholar]
- Gibson G. J., Kielty C. M., Garner C., Schor S. L., Grant M. E. Identification and partial characterization of three low-molecular-weight collagenous polypeptides synthesized by chondrocytes cultured within collagen gels in the absence and in the presence of fibronectin. Biochem J. 1983 May 1;211(2):417–426. doi: 10.1042/bj2110417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson M. A., Cleary E. G. A collagen-like glycoprotein from elastin-rich tissues. Biochem Biophys Res Commun. 1982 Apr 29;105(4):1288–1295. doi: 10.1016/0006-291x(82)90926-3. [DOI] [PubMed] [Google Scholar]
- Gisslow M. T., McBride B. C. A rapid sensitive collagenase assay. Anal Biochem. 1975 Sep;68(1):70–78. doi: 10.1016/0003-2697(75)90680-6. [DOI] [PubMed] [Google Scholar]
- Greenlee T. K., Jr, Ross R., Hartman J. L. The fine structure of elastic fibers. J Cell Biol. 1966 Jul;30(1):59–71. doi: 10.1083/jcb.30.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller-Harrison R. A., Carter W. G. Pepsin-generated type VI collagen is a degradation product of GP140. J Biol Chem. 1984 Jun 10;259(11):6858–6864. [PubMed] [Google Scholar]
- Hessle H., Engvall E. Type VI collagen. Studies on its localization, structure, and biosynthetic form with monoclonal antibodies. J Biol Chem. 1984 Mar 25;259(6):3955–3961. [PubMed] [Google Scholar]
- Higgins R. C., Dahmus M. E. Rapid visualization of protein bands in preparative SDS-polyacrylamide gels. Anal Biochem. 1979 Mar;93(2):257–260. doi: 10.1016/s0003-2697(79)80148-7. [DOI] [PubMed] [Google Scholar]
- Jander R., Rauterberg J., Glanville R. W. Further characterization of the three polypeptide chains of bovine and human short-chain collagen (intima collagen). Eur J Biochem. 1983 Jun 1;133(1):39–46. doi: 10.1111/j.1432-1033.1983.tb07427.x. [DOI] [PubMed] [Google Scholar]
- Jander R., Rauterberg J., Voss B., von Bassewitz D. B. A cysteine-rich collagenous protein from bovine placenta. Isolation of its constituent polypeptide chains and some properties of the non-denatured protein. Eur J Biochem. 1981;114(1):17–25. [PubMed] [Google Scholar]
- Knight K. R., Ayad S., Shuttleworth C. A., Grant M. E. A collagenous glycoprotein found in dissociative extracts of foetal bovine nuchal ligament. Evidence for a relationship with type VI collagen. Biochem J. 1984 Jun 1;220(2):395–403. doi: 10.1042/bj2200395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krauhs J. M. Microfibrils in the aorta. Connect Tissue Res. 1983;11(2-3):153–167. doi: 10.3109/03008208309004851. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Laurain G., Delvincourt T., Szymanowicz A. G. Isolation of a macromolecular collagenous fraction and AB2 collagen from calf skin. FEBS Lett. 1980 Oct 20;120(1):44–48. doi: 10.1016/0014-5793(80)81042-8. [DOI] [PubMed] [Google Scholar]
- Miller E. J. Biochemical characteristics and biological significance of the genetically-distinct collagens. Mol Cell Biochem. 1976 Dec 10;13(3):165–192. doi: 10.1007/BF01731779. [DOI] [PubMed] [Google Scholar]
- Miller E. J., Gay S. Collagen: an overview. Methods Enzymol. 1982;82(Pt A):3–32. doi: 10.1016/0076-6879(82)82058-2. [DOI] [PubMed] [Google Scholar]
- Ninomiya Y., Olsen B. R. Synthesis and characterization of cDNA encoding a cartilage-specific short collagen. Proc Natl Acad Sci U S A. 1984 May;81(10):3014–3018. doi: 10.1073/pnas.81.10.3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odermatt E., Risteli J., van Delden V., Timpl R. Structural diversity and domain composition of a unique collagenous fragment (intima collagen) obtained from human placenta. Biochem J. 1983 May 1;211(2):295–302. doi: 10.1042/bj2110295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prosser I. W., Gibson M. A., Cleary E. G. Microfibrillar protein from elastic tissue: a critical evaluation. Aust J Exp Biol Med Sci. 1984 Aug;62(Pt 4):485–505. doi: 10.1038/icb.1984.46. [DOI] [PubMed] [Google Scholar]
- Risteli J., Bächinger H. P., Engel J., Furthmayr H., Timpl R. 7-S collagen: characterization of an unusual basement membrane structure. Eur J Biochem. 1980;108(1):239–250. doi: 10.1111/j.1432-1033.1980.tb04717.x. [DOI] [PubMed] [Google Scholar]
- Rojkind M., Giambrone M. A., Biempica L. Collagen types in normal and cirrhotic liver. Gastroenterology. 1979 Apr;76(4):710–719. [PubMed] [Google Scholar]
- Ross R., Bornstein P. The elastic fiber. I. The separation and partial characterization of its macromolecular components. J Cell Biol. 1969 Feb;40(2):366–381. doi: 10.1083/jcb.40.2.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanes J. R. Laminin, fibronectin, and collagen in synaptic and extrasynaptic portions of muscle fiber basement membrane. J Cell Biol. 1982 May;93(2):442–451. doi: 10.1083/jcb.93.2.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuppan D., Timpl R., Glanville R. W. Discontinuities in the triple helical sequence Gly-X-Y of basement membrane (type IV) collagen. FEBS Lett. 1980 Jun 30;115(2):297–300. doi: 10.1016/0014-5793(80)81191-4. [DOI] [PubMed] [Google Scholar]
- Sear C. H., Grant M. E., Jackson D. S. The nature of the microfibrillar glycoproteins of elastic fibres. A biosynthetic study. Biochem J. 1981 Feb 15;194(2):587–598. doi: 10.1042/bj1940587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sear C. H., Kewley M. A., Jones C. J., Grant M. E., Jackson D. S. The identification of glycoproteins associated with elastic-tissue microfibrils. Biochem J. 1978 Mar 15;170(3):715–718. doi: 10.1042/bj1700715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trüeb B., Bornstein P. Characterization of the precursor form of type VI collagen. J Biol Chem. 1984 Jul 10;259(13):8597–8604. [PubMed] [Google Scholar]
- van der Rest M., Mayne R., Ninomiya Y., Seidah N. G., Chretien M., Olsen B. R. The structure of type IX collagen. J Biol Chem. 1985 Jan 10;260(1):220–225. [PubMed] [Google Scholar]
- von der Mark H., Aumailley M., Wick G., Fleischmajer R., Timpl R. Immunochemistry, genuine size and tissue localization of collagen VI. Eur J Biochem. 1984 Aug 1;142(3):493–502. doi: 10.1111/j.1432-1033.1984.tb08313.x. [DOI] [PubMed] [Google Scholar]







