Abstract
Tin-based perovskite solar cells have garnered attention for their biocompatibility, narrow bandgap, and long thermal carrier lifetime. However, nip-type tin-based perovskite solar cells have underperformed largely due to the indiscriminate use of metal oxide electron transport layers originally designed for nip-type lead-based perovskite solar cells. Here, we reveal that this underperformance is caused by oxygen vacancies and deeper energy levels in metal oxide. To address these issues, we propose a metal chalcogenide electron transport layer, specifically Sn(S0.92Se0.08)2, which circumvents the oxygen molecules desorption and impedes the Sn2+ oxidation. As a result, tin-based perovskite solar cells with Sn(S0.92Se0.08)2 demonstrate a VOC increase from 0.48 – 0.73 V and a power conversion efficiency boost from 6.98 – 11.78%. Additionally, these cells exhibit improved stability, retaining over 95% of their initial efficiency after 1632 h. Our findings showcase metal chalcogenides as promising candidates for future nip-type tin-based perovskite solar cell applications.
Subject terms: Solar cells, Solar cells
nip-Type tin-based perovskite solar cells have underperformed largely due to the metal oxide electron transport layers originally designed for lead-based devices. Here, authors employ metal chalcogenide as the electron transport layer, achieving enhanced efficiency up to 11.78% for stable devices.
Introduction
Organic-inorganic metal halide perovskite solar cells (PSCs) have attracted attention as a result of the meteoric rise in their power conversion efficiencies (PCEs) over the past several years1–7. In particular, lead (Pb)-based perovskites, including methylammonium lead iodide (CH3NH3PbI3, MAPbI3), formamidinium lead iodide (CH(NH2)2PbI3, FAPbI3), and their mixtures, were considered as highly attractive solar light-harvesting materials because of their high absorption coefficient, adjustable optical bandgap, high charge carrier mobility, and simple solution processing8–17. However, the toxicity of Pb to human beings and the environment cannot be ignored, which significantly hinders the future commercialization of Pb-based PSCs18–22. Therefore, considerable efforts have been made to explore environmentally friendly less-toxic or nontoxic perovskite materials to replace Pb, such as tin (Sn)23–25, germanium (Ge)26,27, bismuth (Bi)28,29, and antimony (Sb)30,31. Among these candidates, tin-based PSCs (TPSCs) are recognized as the promising Pb alternative for several reasons. First, TPSCs possess a higher theoretical PCE than LPSCs according to the Shockley-Queisser (S-Q) limit32–39. Second, the most promising path toward the commercialization of PSCs involves developing all-perovskite tandem solar cells, which require narrow bandgap perovskites40–48. Tin-based and tin-lead-mixed perovskites are promising candidates for these narrow-bandgap perovskite materials. Finally, to date, TPSCs have achieved the highest PCE, exceeding 15%, among all Pb-free PSCs32,36,49–58.
Similar to the lead-based PSCs (LPSCs), TPSCs can also be classified into two architecture types, nip and pin, where the difference is defined by the order of deposition of the electron and hole transport layers relative to the transparent conductive oxide (TCO) substrate32–38,40. The highest performing TPSCs are currently achieved in the pin architecture32,36. However, nip-type TPSCs are also of significant interest due to their low-cost electron transport materials and high thermal operational stability37–58. Moreover, it is well known that nearly all record PCEs that LPSCs were certified by the National Renewable Energy Laboratory (NREL) were achieved in the nip architectures59. As such, nip architecture should be the preferred platform for high-performing TPSCs as well although their PCEs are not satisfied so far37–58. A key contributing factor for the unsatisfied performance of nip-type TPSCs is the indiscriminate adoption of functional materials from nip-type LPSCs, lacking tailored design for the former. For instance, nip-type TPSCs to date all use metal oxides (TiO2 and SnO2) as electron transport layers (ETL), all of which were originally used in LPSCs37–58. However, they are no longer the optimal choice for TPSCs as the corresponding nip-type TPSCs show much lower performance than pin-type LPSCs and even most open-circuit voltages (VOCs) of reported nip-type TPSCs were <0.5 V, as shown in Table S1. Theoretically, such low VOCs can be attributed to the mismatched band structure between the metal oxide ETLs and Sn-based perovskite layers25. Moreover, the presence of oxygen vacancies in metal oxides is another possible reason for the poor performance60–63. Therefore, it is crucial to identify the reasons of poor performance caused by metal oxides and to explore novel ETLs to enable efficient, reliable, and scalable TPSCs.
In this work, we elucidate the origin of poor photovoltaic performance in nip-type TPSCs and the underlying mechanism by which metal oxide ETLs influence this performance. Specifically, we demonstrate that the desorption of oxygen molecules from oxygen vacancies in TiO2 triggers the oxidation process from Sn2+ to Sn4+ in Sn-based perovskites. Additionally, the deeper energy levels of TiO2 ETLs result in a reduction in VOC. To address these challenges, we introduce an ETL, a metal mixed-chalcogenide of Sn(S0.92Se0.08)2, into the nip-type TPSC. Unlike conventional metal oxide ETLs, the Sn(S0.92Se0.08)2 ETL not only circumvents the desorption of O2 molecules but also hinders the reaction between Sn2+ ions within Sn-based perovskites and O2 molecules present in air. Moreover, the Sn(S0.92Se0.08)2 ETL exhibits a shallower CBM position compared to metal oxide counterparts. Additionally, it boasts several other advantageous properties, including improved morphology, heightened conductivity, and increased electron mobility. These attributes confer upon the nip-type TPSC with the Sn(S0.92Se0.08)2 ETL a significantly elevated VOC, increasing from 0.48 to 0.73 V, and enhancing the PCE from 6.98 to 11.78%, representing a >65% improvement. Furthermore, our ETL substantially enhances the operational stability of the nip-type TPSC dramatically.
Results
To explore the impact of metal oxide ETLs on nip-type TPSCs, our study focused on the widely used FTO/TiO2/Sn-based perovskite/PTAA/Ag configuration. The TiO2 ETL was deposited onto the FTO substrate using a titanium butoxide solution and subsequently annealed at 450 °C. The Sn-based perovskite PEA0.15FA0.85SnI2.85Br0.15 was then deposited on the TiO2 ETL utilizing a one-step deposition approach. Supplementary Table 1 provides a summary of representative photovoltaic performance for this specific structure37–58. Remarkably, Supplementary Fig. 1 highlights a significant disparity between the experimental and theoretical values of the PCE and VOC in nip-type TPSCs. We speculate that the primary reasons to such significant discrepancy can be ascribed to the presence of oxygen vacancies and deeper band positions within the TiO2 ETLs in nip-type TPSCs.
Oxygen vacancy
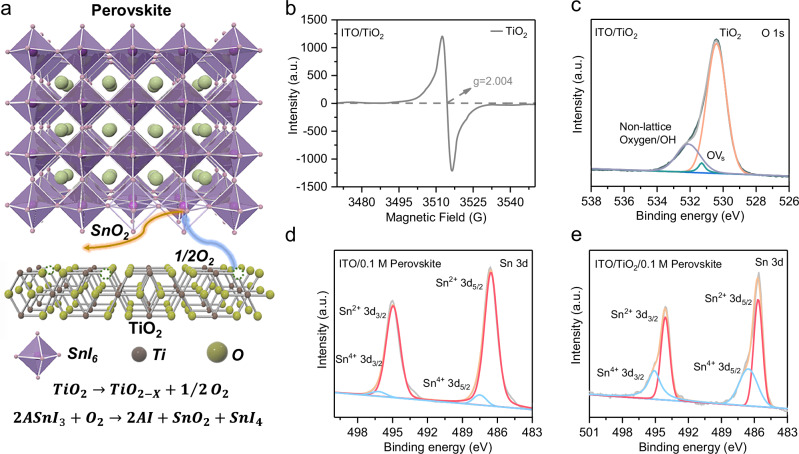
TiO2 is known to contain numerous oxygen vacancies (OVs) and trivalent titanium ions (Ti3+), particularly at its surface58–61. These OVs have the propensity to absorb molecular oxygen from the atmosphere, forming a charge transfer complex (O2– – Ti4+). Under the external stimuli, such as light or electrons, electron-hole pairs are generated, and the resulting holes in the valence band tend to recombine with O2–, leading to the desorption of oxygen. For the Sn-based perovskite, as the two 5 s electrons of the Sn elements without lanthanide shrinkage are highly active and easy to lose, it is very sensitive to the oxygen33. The desorbed oxygen accelerates the oxidation of Sn2+ to Sn4+ within the Sn-based perovskite. Therefore, this process can be expressed by two sequential steps, the desorption of oxygen from OVs in TiO2, followed by the oxidation of Sn-based perovskites by the released oxygen, as schematically illustrated in Fig. 1a64,65.
Fig. 1. Oxygen vacancies in TiO2 ETLs.
a Schematic diagram of the buried interface between the TiO2 ETL and Sn-based perovskite layer. Oxygen desorption from OVs in the TiO2 ETL accelerates the oxidation of Sn2+ to Sn4+ within the Sn-based perovskite. b EPR spectra of the TiO2 ETL, confirming the existence of OVs. c High-resolution XPS spectra in the O 1 s region of the TiO2 ETL, further supporting the existence of OVs. High-resolution XPS spectra of Sn-based perovskite films on (d) FTO and (e) FTO/TiO2 substrates after aging for 14 days, respectively. This distinct difference observed suggests that desorbed oxygen from TiO2 films leads to severe oxidation in Sn-based perovskites.
To validate this hyprothesis, the electron paramagnetic resonance (EPR) measurement was conducted on TiO2 films (Fig. 1b), which is able to evidence the OVs directly. An intense unpaired electron signal at g = 2.004 was recorded, suggesting the existence of OVs in TiO2 films61,66. Further analysis of the chemical valence states of Ti and O elements in TiO2 films was performed using the high-resolution X-ray photoelectron spectroscopy (XPS). Fig. 1c shows the O 1 s core-level specturm of the TiO2 film, revealing three distinct peaks. Peaks at 530.5 and 532.1 eV are attributed to lattice oxygen and non-lattice oxygen, respectively, while the peak at 531.3 eV is indexed to OVs, corroborating the presence of OVs in TiO2 films. Concurrently, the formation of Ti3+ ions was also observed (Supplementary Fig. 2). Peaks located at binding energies of 458.9 and 464.7 eV can be attributed to Ti4+ 2p3/2 and Ti4+ 2p1/2, respectively, consistent with rutile TiO2. In addition, Ti3+ 2p3/2 and Ti3+ 2p1/2 XPS peaks at binding energies of 457.7 and 463.8 eV can be detected as well, which is the by-product of the OVs.
To confirm the negative effects of OVs in TiO2 films on Sn-based perovskite films, XPS spectra were conducted for the Sn-based perovskite films deposited on both FTO substrates and FTO/TiO2 films, respectively. Since the XPS measurement is surface sensitive, a low concentration (0.1 M) of precursor solution of Sn-based perovskite was used to detect the chemical state of the buried interface between the TiO2 film and the Sn-based perovskite film. The presence of Ti 2p XPS peaks (Supplementary Fig. 3) confirms the validity of the XPS measurement for the buried interface. Supplementary Fig. 4 displays XPS spectra of fresh Sn-based perovskite films on FTO and FTO/TiO2 substrates, which suggests a similar Sn4+/Sn2+ ratio in both samples. To eliminate effects of other factors on Sn-based perovskite films, both samples were aged in the glovebox under low light conditions. Fig. 1d, e reveal XPS spectra of Sn-based perovskite films on FTO and FTO/TiO2 substrates after aging for 14 days, respectively. Notably, the Sn4+/Sn2+ ratio in Sn-based perovskite films on FTO substrates remained largely unchanged, while the Sn4+/Sn2+ ratio in Sn-based perovskite films on FTO/TiO2 films show a significant alternation. This distinct difference suggests that desorbed oxygen from TiO2 films result in severe oxidation in Sn-based perovskites, consequently compromising the stability of nip-type TPSCs over time.
Band structure
In additon to OVs, the mismatched band structure between TiO2 and the Sn-based perovskite is another contributing factor to the low performance of nip-type TPSCs, especially with regard to low VOC. Supplementary Fig. 5 presents the ultraviolet photoelectron spectroscopy (UPS) data, illustrating the valence band maximum (VBM) onset and the secondary electron cutoff energy boundary of TiO2 and Sn-based perovksite films. Combining their optical bandgaps derived from adsorption spectra (Supplementary Fig. 6a–d), the VBM and conduction band minimum (CBM) energies of TiO2 and Sn-based perovksite films were calculated, as listed in Supplementary Table 2. Notably, owing to the shallow CBM of the Sn-based perovksite, the conventional TiO2 ETL shows a substantial energy level offset due to its significantly deeper CBM position. This disparity in energy levels is crucial, as the maximum attainable VOC is determined by the quasi-Fermi level splitting (Supplementary Fig. 6e). Consequently, the deeper energy level of the TiO2 ETL results in a diminished VOC value in nip-type TPSCs, aligning with previously reported findings (Supplementary Table 1), thus rendering it disadvantageous for nip-type TPSCs.
Metal mixed-chalcogenide ETL
Having identified that the root cause of the low performance of nip-type TPSCs lies in the presence of OVs and deeper band positions within TiO2 ETLs, we recognize two potential strategies to address this issue. Firstly, we can aim to reduce the OVs in TiO2 by interface engineering and simultaneously adjust the band positions of TiO2 through composition engineering. Alternatively, we can explore the substitution of TiO2 with other n-type semiconductors. However, despite attempts to employ individual engineering approaches in nip-type TPSCs, notable performance enhancements have not been consistently achieved44,52. Moreover, the simultaneous application of both engineering strategies to TiO2 ETLs may yield opposite effects. Therefore, the pursuit of alternative ETLs for nip-type TPSCs to replace TiO2 becomes imperative. The ideal ETL for nip-type TPSCs should feature several key attributes, including a favorable band structure that aligns well with Sn-based perovskites, optical transparency, a compact morphology without pinholes, and high conductivity and mobility67–70. Following this line of thought, we have synthesized a metal mixed-chalcogenide, Sn(SxSey)2, as a promising candidate for ETLs in nip-type TPSCs. This material was synthesized by a hydrothermal method, as depicted in Supplementary Fig. 7, with detailed synthesis procedures provided in Methods. The composition distribution and crystallization of the typical metal mixed-chalcogenide, Sn(S0.92Se0.08)2, used in this study were verified through XPS, transmission electron microscopy (TEM), and X-ray diffraction (XRD) techniques, as shown in Supplementary Figs. 8–9.
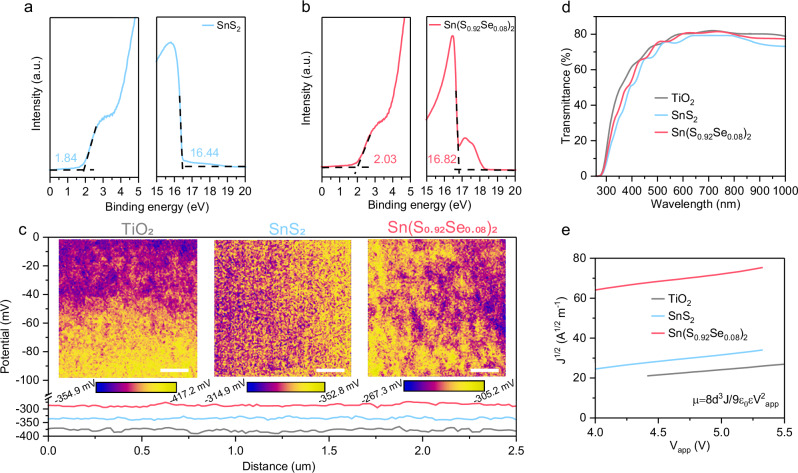
Figure 2a, b present UPS data of the VBM onset and the secondary electron cutoff energy boundary of SnS2 and Sn(S0.92Se0.08)2 ETLs, respectively. Similar to TiO2 ETLs, their VBM and CBM energies were computed by incorporating their optical bandgaps (Supplementary Fig. 10), as listed in Supplementary Table 2. Evidently, both SnS2 and Sn(S0.92Se0.08)2 ETLs reveal shallower CBM positions than TiO2 ETLs, indicating a smaller energy level offset with Sn-based perovskites and a higher VOC in the corresponding nip-type TPSCs. Furthermore, the Sn(S0.92Se0.08)2 ETL demonstrates the shallowest CBM position, suggesting its potential as the preferred candidate for nip-type TPSCs as the ETL. This is the main reason that we introduced the metal mixed-chalcogenide to act as the ETL in the nip-type TPSC in this study. To further validate the favorable band alignment of the Sn(S0.92Se0.08)2 ETL, the Kelvin probe force microscopy (KPFM) was employed to provide reliable local surface potentials, as shown in Fig. 2c. Obviously, the surface potentials of TiO2, SnS2 and Sn(S0.92Se0.08)2 ETLs exhibit a consistent trend with the UPS measurements, reaffirming the suitability of the Sn(S0.92Se0.08)2 film as the optimal candidate for nip-type TPSCs as the ETL.
Fig. 2. Metal chalcogenide ETLs.
UPS spectra of VBM onset and photoemission cutoff energy boundary of (a) SnS2 and (b) Sn(S0.92Se0.08)2 ETLs. The Sn(S0.92Se0.08)2 ETL shows the shallowest CBM position, highlighting its potential as the preferred ETL candidate for nip-type TPSCs. c KPFM curves and images of the TiO2, SnS2 and Sn(S0.92Se0.08)2 ETLs. The scalebars are 1 um. d UV-vis optical transmission spectra of TiO2, SnS2 and Sn(S0.92Se0.08)2 ETLs, revealing similar optical transparency between the Sn(S0.92Se0.08)2 ETL and the TiO2 ETL throughout the spectrum. e J-V curves at the trap-free SCLC regime of TiO2, SnS2 and Sn(S0.92Se0.08)2 ETLs, fitted with the Mott-Gurney law. The results suggest that the mobility of the Sn(S0.92Se0.08)2 ETL is an order of magnitude higher than that of the TiO2 ETL.
In addition to favorable band structure alignment with Sn-based perovskites, as an ideal ETL, the Sn(S0.92Se0.08)2 ETL should also possess optical transparency and a pinhole-free compact morphology. Figure 2d displays the UV-vis optical transmission spectra of the TiO2, SnS2 and Sn(S0.92Se0.08)2 ETLs, revealing the similar optical transparency of the Sn(S0.92Se0.08)2 ETL in the whole region. Supplementary Fig. 11a–c show top-view scanning electron microscopy (SEM) images of TiO2, SnS2, and Sn(S0.92Se0.08)2 ETLs. Similar to the commonly used TiO2 ETL, SnS2 and Sn(S0.92Se0.08)2 ETLs exhibit conformal and entire coverage on the FTO substrate, devoid of apparent pinholes. This observation is further corroborated by atomic force microscopy (AFM) images of TiO2, SnS2, and Sn(S0.92Se0.08)2 films, as show in Supplementary Fig. 11d–f. Moreover, TiO2, SnS2, and Sn(S0.92Se0.08)2 films reveal consecutive decreases in root-mean-square roughness (RMS) from 20.7 to12.8–8.37 nm, suggesting that the Sn(S0.92Se0.08)2 film possesses the most compact morphology, enhancing its applicability in nip-type TPSCs.
Finally, the conductivity and electron mobility of the Sn(S0.92Se0.08)2 film were investigated. Supplementary Fig. 12 illustrates the current-voltage (I-V) curves of the devices with the FTO/ETL/Ag structure, where the ETLs are TiO2, SnS2, and Sn(S0.92Se0.08)2 films respectively. Corresponding conductivities were listed in Supplementary Table 3. Clearly, the Sn(S0.92Se0.08)2 ETL reveals a higher conductivity of 13.8 × 10−3 S cm−1 than the TiO2 ETL (8.4 × 10−3 S cm−1) and the SnS2 ETL (12.7 × 10−3 S cm−1). To quantitatively assess electron mobility, the space-charge-limited current (SCLC) measurements were performed on devices with the FTO/Ag/ETL/Ag architecture. Supplementary Fig. S13 shows the current density-voltage (J-V) curves of the device, which generally exhibit three regions, including an Ohmic region, a trap-filling limited region, and a trap-free SCLC regime. Figure 2e zooms in on the J-V curves at the trap-free SCLC regime, which represents the Mott-Gurney law and the current density (J) can be written by Eq. (1)35:
| 1 |
where Vapp and μ denote the voltage and mobility at the trap-free region. Accordingly, the μ for the TiO2, SnS2, and Sn(S0.92Se0.08)2 ETLs were listed in Supplementary Table 3. It is evident that mobilities of SnS2 and Sn(S0.92Se0.08)2 ETLs are an order of magnitude higher than that of the TiO2 ETL, with the Sn(S0.92Se0.08)2 ETL exhibiting the highest mobility. In short, it is demonstrated that the Sn(S0.92Se0.08)2 ETL is indeed a promising candidate for the ETL in nip-type TPSCs, boasting a shallow CBM position, good optical transparency, compact morphology, and superior conductivity and mobility.
Interactions between metal mixed-chalcogenide ETLs and Sn-based perovskites
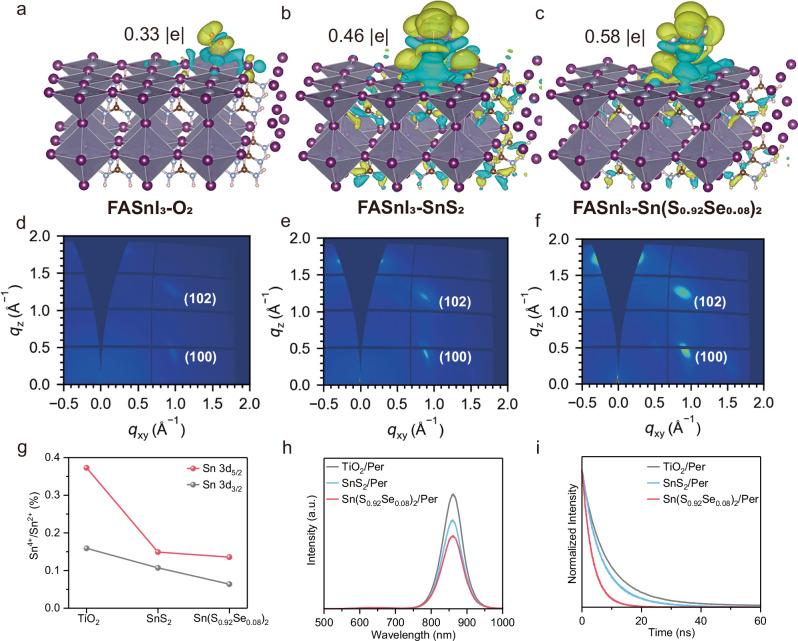
After establishing the Sn(S0.92Se0.08)2 film as a promising ETL in TPSCs, it is essential to elucidate the interaction between the Sn(S0.92Se0.08)2 ETL and the Sn-based perovskite. First-principles calculations were utilized to understand this interaction. As discussed above, oxygen (O2) molecules desorb from the OV sites in TiO2 ETLs under external stimuli. Supplementary Figure 14a shows that the adsorption energy (Eads) of O2 molecules on the surface of Sn-based perovskite is −0.28 eV. In contrast, the Eadss values of SnS2 and Sn(S0.92Se0.08)2 ETLs are −0.30 and −0.35 eV, respectively (Supplementary Fig. 14b, c). This result indicates that SnS2 and Sn(S0.92Se0.08)2 ETLs not only do not involve O2 molecules desorption, but also hinder the reaction between Sn2+ ions in Sn-based perovskites and O2 molecules present in air. Fig. 3a shows the calculated electron density distribution to describe the interaction between the O2 molecule and the Sn-based perovskite. Upon O2 adsorption on the Sn-based perovskite, the electron cloud density around Sn2+ noticeably transfers to the O2 molecules, facilitating the oxidation of Sn2+ by O2. However, for SnS2 and Sn(S0.92Se0.08)2 ETLs, the electron density around Sn2+ was significantly increased, effectively suppressing the oxidation of Sn2+ (Fig. 3b, c). To quantify this interaction, the Bader charge values of O2, SnS2, and Sn(S0.92Se0.08)2 adsorbed on Sn-based perovskites were computed, as shown in Fig. 3a–c. The Sn(S0.92Se0.08)2 ETL on the Sn-based perovskite reveals the highest Bader charge value, suggesting the strongest interaction between them.
Fig. 3. Strong interaction between metal chalcogenide ETLs and Sn-based perovskite layers.
The electron density distribution of Sn-based perovskites interacting with (a) O2, (b) SnS2 and (c) Sn(S0.92Se0.08)2 molecules, indicating that the Sn(S0.92Se0.08)2 ETL not only circumvents O2 molecules desorption, but also inhibits the reaction between Sn2+ ions in Sn-based perovskites and O2 molecules present in air. GIWAXS patterns of the Sn-based perovskite films grown on (d) TiO2, (e) SnS2, and (f) Sn(S0.92Se0.08)2 ETLs respectively. These results indicate that the Sn(S0.92Se0.08)2 ETL induces the highest crystalline phase purity at the buried interface due to the strong interaction. g The Sn4+/Sn2+ ratios in the Sn 3d5/2 and Sn 3d3/2 regions of the Sn-based perovskite films deposited on different ETLs, including TiO2, SnS2 and Sn(S0.92Se0.08)2 ETLs. The Sn-based perovskite with the Sn(S0.92Se0.08)2 ETL shows the lowest values, indicative of the strongest interaction between the two. h PL and (i) TRPL spectra of Sn-based perovskite films deposited on TiO2, SnS2, and Sn(S0.92Se0.08)2 ETLs, respectively. Both results suggest the fastest electron transfer in the structure of Sn-based perovskite films deposited on Sn(S0.92Se0.08)2 films.
To experimentally demonstrate the strong interaction between the Sn(S0.92Se0.08)2 ETL and the Sn-based perovskite, the interfacial effect of the Sn(S0.92Se0.08)2 ETL on the Sn-based perovskite was characterized using the grazing incidence wide-angle X-ray scattering (GIWAXS) technique. To collect buried interface information without any transfer and damage, the X-ray beam was illuminated from the back side of the Sn-based perovskite films deposited on PEN/ITO/ETL substrates, where ETLs are TiO2, SnS2, and Sn(S0.92Se0.08)2 films, as shown in Supplementary Fig. 15. Figure 3d–f exhibit the interface GIWAXS patterns of the Sn-based perovskite films grown on TiO2, SnS2, and Sn(S0.92Se0.08)2 ETLs respectively. The Sn-based perovskite films grown on SnS2 and Sn(S0.92Se0.08)2 ETLs reveal sharper and stronger Bragg spots compared to those grown on the TiO2 ETL, suggesting the incomplete structural transition of the Sn-based perovskite at the buried interface with the TiO2 ETL. Moreover, the Sn-based perovskite film grown on the Sn(S0.92Se0.08)2 ETL shows sharper and stronger Bragg spots compared to those grown on the SnS2 ETL, indicative of a higher crystalline phase purity at the corresponidng buried interface. Therefore, the use of the Sn(S0.92Se0.08)2 ETL enhances its interaction with the Sn-based perovskite, inhibits degradation of the Sn-based perovskites, and promotes the formation of a significant amount of crystallized perovskite.
The strong interaction suppresses the oxidation of Sn2+ in Sn-based perovskites, as demonstrated by XPS spectra after aging 14 days under low light illumination, as shown in Supplementary Fig. 16 and Fig. 3g. Compared to the Sn-based perovskite with the TiO2 ETL, Sn-based perovskites with metal chalcogenides show significantly reduced Sn4+/Sn2+ ratios for both Sn 3d5/2 and 3d3/2, with the Sn-based perovskite with the Sn(S0.92Se0.08)2 ETL reveals the lowest value, indicative of the strongest interaction between the two. Furthermore, the strong interaction not only suppresses the oxidation of Sn-based perovskites, but also facilitates fast electron transfer dynamics from the Sn(S0.92Se0.08)2 ETL to the Sn-based perovskite layer. Fig. 3h presents the photoluminescence (PL) spectra of the Sn-based perovskite films deposited on the TiO2, SnS2, and Sn(S0.92Se0.08)2 ETLs resepctively. Both the Sn-based perovskites deposited on the SnS2 and Sn(S0.92Se0.08)2 films display lower intensities than that deposited on the TiO2 films, indicating more efficient electron transfer from the Sn-based perovskite film to the metal chalcogenide. Meanwhile, the Sn-based perovskite film deposited on the Sn(S0.92Se0.08)2 film exhibits the lowest intensity among the three samples, which is consistent with the result that the Sn(S0.92Se0.08)2 film possesses the strongest interaction with the Sn-based perovskite. To gain more insights into effective electron transfer dynamics, time-resolved PL (TRPL) measurements were also performed to test the decay lifetime for the three samples (Fig. 3i). The corresponding TRPL lifetime was calculated by single-exponential fits to the TRPL decays, as listed in Supplementary Table 4. Clearly, the Sn-based perovskite film deposited on the Sn(S0.92Se0.08)2 film shows the shortest PL decay lifetime among the three samples. Such a short lifetime further indicates the fastest electron transfer in the structure of Sn-based perovskite films deposited on Sn(S0.92Se0.08)2 films.
Device performance
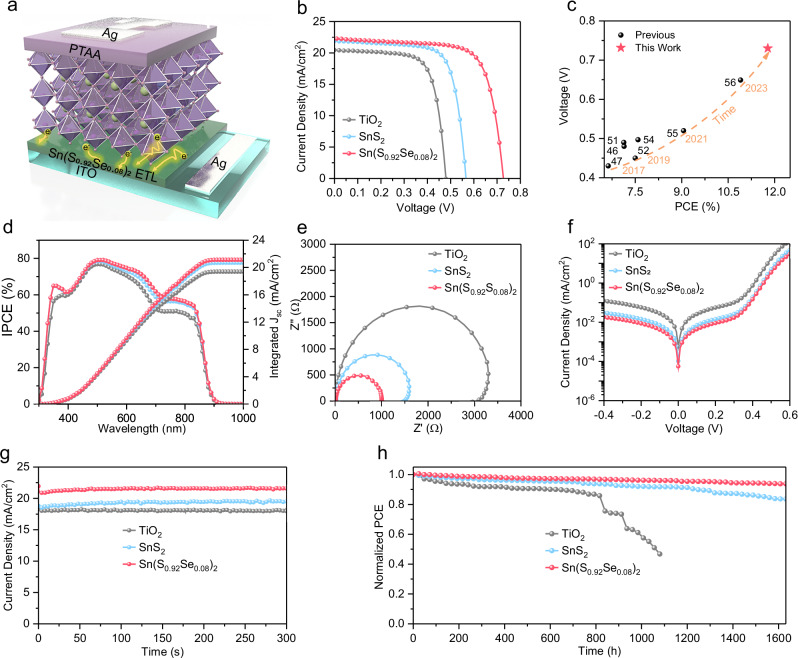
Next, we fabricated nip-type TPSCs with the structure of FTO/ETL/Sn-based perovskite/PTAA/Ag using TiO2, SnS2, and Sn(S0.92Se0.08)2 films as ETLs (Fig. 4a). Detailed information on the synthesis procedure can be found in the Method Section. Fig. 4b shows the current density-voltage (J–V) curves of the best-performing nip-type TPSCs with TiO2, SnS2, and Sn(S0.92Se0.08)2 ETLs. The corresponding photovoltaic parameters were listed in Supplementary Table 5. The nip-type TPSC with the TiO2 ETL exhibits a PCE of 6.98%, with a VOC of 0.48 V, a short circuit current density (JSC) of 20.47 mA/cm2, and a fill factor (FF) of 71.11%, comparable with previous reports. As anticipated, using metal chalcogenide as ETLs, all the photovoltaic parameters of nip-type TPSCs were significant increased. Specifically, the nip-type TPSC with the SnS2 ETL shows a PCE of 9.03%, with VOC = 0.57 V, JSC = 21.89 mA/cm2, and FF = 72.88%, and the nip-type TPSC with the Sn(S0.92Se0.08)2 ETL shows a PCE 11.78%, with VOC = 0.73 V, JSC = 22.28 mA/cm2, and FF = 72.68%. An enhancement of over 65% in PCE was achieved when the commonly used TiO2 ETL was substituted by the Sn(S0.92Se0.08)2 ETL in nip-type TPSCs. Notably, we also obtained a certified PCE of 10.57% for the nip-type TPSC first time from Shanghai Institute of Microsystem and Information Technology (Supplementary Fig. 17). As shown in Fig. 4b, c, this substantial enhancement can primarily be ascribed to the improved VOC, a consequence of the shallow CBM position in the Sn(S0.92Se0.08)2 ETL (Supplementary Table 2). Moreover, Sn(S0.92Se0.08)2 ETL also facilitates the growth of highly crystalline, vertically oriented and grain-through structures in the Sn-based perovskite film, as shown in Supplementary Figs. 18–19, which leads to a higher JSC for the corresponding nip-type TPSC. Fig. 4c further demonstrates that the impressive PCE of the nip-type TPSC with the Sn(S0.92Se0.08)2 film, which significantly surpasses those of previously reported nip-type TPSCs with TiO2 films. Despite this significant advance, it is imperative to further enhance the photovoltaic performance of nip-type TPSCs by several strategies in the future. For instance, introducing additives into the Sn(S0.92Se0.08)2 ETL could furture elevate its CBM. Additionally, applying an ultratin layer on the Sn(S0.92Se0.08)2 ETL surface may help to suppress the recombination and improve the morphology of the Sn-based perovskite layer. These approaches are crucial for further optimizing the performance of nip-type TPSCs.
Fig. 4. Photovoltaic performance of TPSCs with metal chalcogenide ETLs.
a Schematic diagram of nip-type TPSCs with the structure of FTO/ETL/Sn-based perovskite/PTAA/Ag, utilizing TiO2, SnS2, and Sn(S0.92Se0.08)2 films as ETLs. b J–V curves of nip-type TPSCs with TiO2, SnS2 and Sn(S0.92Se0.08)2 ETLs, respectively. c A comparison of PCE between this work and other reported PCEs (over 6.5%) of nip-type TPSCs. The impressive PCE of the nip-type TPSC with the Sn(S0.92Se0.08)2 film significantly surpasses those of previously reported nip-type TPSCs with TiO2 films. d EQE spectra and integrated Jsc values of the nip-type TPSCs with TiO2, SnS2 and Sn(S0.92Se0.08)2 ETLs, respectively. e Nyquist plots. f dark J–V curves, and (g) stabilized power output of nip-type TPSCs with TiO2, SnS2 and Sn(S0.92Se0.08)2 ETLs respectively. These result indicate that the nip-type TPSC with the Sn(S0.92Se0.08)2 ETL showcases the reduced charge transfer resistance, fastest electron transport, and lowest defect density among the three types ETLs. h Normalized PCE of unencapsulated nip-type TPSCs with TiO2, SnS2 and Sn(S0.92Se0.08)2 ETLs for over 1600 h in an N2 glovebox. These findings collectively support the potential of metal chalcogenide ETLs, particularly Sn(S0.92Se0.08)2, for advancing the performance and stability of nip-type TPSCs.
The other ratios of Se/S in the metal chalcogenide of Sn(SxSey)2 ETLs were also investigated in nip-type TPSCs, as shown in Supplementary Fig. 20 and Supplementary Table 5. Increasing the Se ratio results in a higher VOC due to the shallower CBM of the corresponding ETL, as show in Supplementary Fig. 21. However, this increase in Se ratio also leads to more severe nonradiative interfacial recombination within certain limits (Supplementary Fig. 22), Consequently, the TPSC with the Sn(S0.92Se0.08)2 ETL shows the highest PCE. Fig. 4d presents the incident photo-to-electric current conversion efficiency (IPCE) spectra and integrated JSCs of nip-type TPSCs with TiO2, SnS2, and Sn(S0.92Se0.08)2 ETLs. The margin of error for JSCs is around 5%, consistent with previous literature71. Such minor discrepancy can be ascribed to differences in measurement conditions (such as, the solar simulator and IPCE setup), preconditioning of samples for IPCE measurements, and variations in ion migration dynamics72. To verify reproducibility, 24 individual nip-type TPSCs with TiO2, SnS2, and Sn(SxSey)2 ETLs for each were fabricated, as shown in Supplementary Fig. 23. This result demonstrates that the PCEs of nip-type TPSCs with metal chalcogenide ETLs distribute over narrower ranges than that of nip-type TPSCs with TiO2 ETLs, indicating high reproducibility of formers.
To delve deeper into the superior performance of the nip-type TPSC with the Sn(S0.92Se0.08)2 ETL, we conducted futher analysis of photovoltaic parameters. Electrochemical impedance spectroscopy (EIS) spectra were employed to probe the interface properties of nip-type TPSCs with TiO2, SnS2, and Sn(S0.92Se0.08)2 ETLs, as shown in Fig. 4e. Comparatively, the nip-type TPSCs with SnS2 and Sn(S0.92Se0.08)2 ETLs reveal reduced series resistances and charge transfer resistances compared to those with TiO2 ETLs, implying significantly enhanced electron transport between the metal chalcogenide layer and the Sn-based perovskite layer. This observation is consistent with Fig. 3h, i and the nip-type TPSC with the Sn(S0.92Se0.08)2 ETL reveal the lowest resistance and charge transfer resistance, and thus the fastest electron transport. Additionally, the nip-type TPSC with the Sn(S0.92Se0.08)2 ETL reveals the highest carrier recombination resistance, suggesting effective suppression of carrier recombination at the interface between the Sn-based perovskite layer and the Sn(S0.92Se0.08)2 ETL. This suppression can be attributed to the reduced defect density in the Sn-based perovskite film and at the interface with the Sn(S0.92Se0.08)2 ETL. To explore these defects further, we analyzed the dark J-V curves, as shown in Fig. 4f. Clearly, the dark JSC of the nip-type TPSC with the Sn(S0.92Se0.08)2 ETL is one order of magnitude lower than that of the nip-type TPSC with the TiO2 ETL, indicating a reduced defect density upon substituting TiO2 with Sn(S0.92Se0.08)2 in nip-type TPSCs. Additionally, the plots of the VOC versus incident light intensity further supported these findings (Supplementary Fig. 24). The ideality factor (n = 1.28 kT/q) for the nip-type TPSC with the Sn(S0.92Se0.08)2 ETL is closer to 1 kT/q and much smaller than those of nip-type TPSCs with the c-TiO2 ETL (n = 1.64 kT/q) and the SnS2 ETL (n = 1.51 kT/q), signifying improved device characteristics.
Finally, the long-term stability of nip-type TPSCs with TiO2, SnS2, and Sn(S0.92Se0.08)2 ETLs was examined under ambient conditions. Fig. 4g shows the stable power outputs at the maximum power point for the nip-type TPSC with the Sn(S0.92Se0.08)2 ETL, indicating its robust stability under constant illumination. Fig. 4h reveals the normalized PCE of encapsulated nip-type TPSCs with TiO2, SnS2, and Sn(S0.92Se0.08)2 ETLs as a function of aging time under ambient atmosphere. Obviously, the degradation of the three devices is significantly different. The PCE of the nip-type TPSCs with the TiO2 ETL shows a rapid degradation after 912 h, which retains only 40% of its initial efficiency after 1080 h. The nip-type TPSCs with the SnS2 ETL still retains >80% of its initial efficiency after 1632 h. Notably, the nip-type TPSC with the Sn(S0.92Se0.08)2 ETL still retains over 95% of its initial efficiency after 1632 h, which is of particular interest for practical applications. This underscores the potential of metal chalcogenide ETLs, particularly Sn(S0.92Se0.08)2, as a top candidate for achieving long-term stability in nip-type TPSCs, as summarized in recent stability studies (Supplementary Table 1). To comprehend this excellent stability, XRD patterns for nip-type TPSCs with TiO2, SnS2, and Sn(S0.92Se0.08)2 ETLs were tested at different aging time, as shown in Supplementary Fig. 25. The XRD patterns of Sn-based perovskites on TiO2 ETLs exhibit an additional peak indexing to SnI4 (PDF#06-0232) after aging for 24 days64,65,73. In contrast, the XRD patterns of Sn-based perovksites on the metal chalcogenide ETLs (SnS2 and Sn(S0.92Se0.08)2) reveal the same peak even aging for 24 days. This result strongly verified that the Sn-based perovskites on TiO2 ETLs did react with the oxygen molecules desorbed from the OV sites in TiO2 ETL, as discussed in Fig. 3, suggesting that metal chalcogenides, especially the Sn(S0.92Se0.08)2, are promising ETL candidates for nip-type TPSCs with high PCE and stability.
Discussion
In conclusion, our investigation has unveiled the fundamental causes and underlying mechanisms behind the detrimental impact of metal oxide ETLs on the performance of nip-type TPSCs. This is primarily due to two factors: the oxygen molecules desorption from oxygen vacancies, which oxidizes Sn2+ to Sn4+ in Sn-based perovskites, and the mismatched energy levels of TiO2 ETLs, which reduce the VOC. To address these issues, we introduced an ETL, a metal mixed-chalcogenide of Sn(S0.92Se0.08)2, into the nip-type TPSC. Both experimental and theoretical findings demonstrate that the Sn(S0.92Se0.08)2 ETL circumvents O2 desorption, hinders the reaction between Sn2+ ions and O2 present in air, and offers a shallower CBM position, improved morphology, heightened conductivity, and increased electron mobility. Consequently, nip-type TPSCs with Sn(S0.92Se0.08)2 ETLs achieve a significantly elevated VOC, increasing from 0.48 to 0.73 V, and an enhanced PCE, rising from 6.98 – 11.78%, representing a >65% improvement. Furthermore, the Sn(S0.92Se0.08)2 ETL substantially enhances the operational stability of nip-type TPSCs, retaining over 95% of their initial efficiency after 1632 h, compared to repid degradation after 912 h with TiO2 ETLs. This study highlights the substantial potential of metal chalcogenide ETLs in advancing nip-type TPSC performance.
Methods
Materials
Tin (IV) chloride pentahydrate (SnCl4·5H2O, 98%), Selenourea (CH4N2Se, 98%), Thioacetamide (ACS, ≥99.0%) and 2-(2-Aminoethyl) isothiourea dihydrobromide (AET, 99%) were purchased from Aladdin. Formamidinium Iodide (FAI) was purchased from TCI. Phenethylammonium bromide (PEABr, 97%), Tin iodide (SnI2, AnhydroBeads, 99.99%), tin fluoride (SnF2, 99%), N, N-dimethylformamide (DMF, anhydrous, 99.8%), dimethyl sulfoxide (DMSO, anhydrous, 99.9%) and chlorobenzene (CB, anhydrous, 99.8%), were obtained from Sigma-Aldrich and used as received with no further purification. a poly (triaryl amine) semiconductor (PTAA) was purchased from Xi’an Baolaite Optoelectronics Technology Co.
Device fabrication
Synthesis of metal chalcogenide nanocrystalline (Sn(SxSey)2)
In a typical synthesis, SnCl4·5H2O (1 mmol) and thioacetamide (4 mmol) were dissolved in deionized water (50 mL) under vigorously magnetic stirring. The mixed solution was stirred for 1 h until a transparent solution was formed. The as-obtained transparent solution was transferred to a Teflon-lined stainless-steel autoclave and a quartz vessel was put into Teflon-lined autoclave to contain the HCl aqueous solution (0.5 ml HCl 37 wt% aqueous solution in 5 ml DI). Then heated in an electric oven at 160 °C for 6 h. After the hydrothermal synthesis, the obtained SnS2 powder was cleaned with distilled water and ethanol, twice. Eventually, the SnS2 precipitation was collected and redispersed in ethanol. For the synthesis of Sn(SxSey)2 (x + y = 1), Selenourea (CH4N2Se) was added to the mixing solution of SnCl4·5H2O and thioacetamide under vigorously magnetic stirring, and the reaction steps were the same as before.
Fabrication of ETL layers
The patterned ITO glass substrates were cleaned in an ultrasonic bath for 15 min with detergent, deionized water three times, and anhydrous ethanol, sequentially. Then, the ITO glass substrates were dried and cleaned with UV-Ozone for 20 min. After that, the synthesized SnS2 or Sn(SxSey)2 were spin-coated on the ITO at 2000 rpm for 30 s. The substrates heated in a tube furnace under the protection of a nitrogen atmosphere at 150°C for 30 min.
Fabrication of perovskite layers
The deposition of the Sn perovskite layers prepared in a glove box with oxygen content of 0.1 ppm nitrogen. For the perovskite precursor solution, a 0.8 M perovskite precursor comprised of PEABr, FAI, SnI2, SnF2 and AET with the molar ratio of 0.15:0.85:1:0.075:0.05 was added in a mixed solvent (DMSO: DMF = 1:4 v/v) heated at 70 °C for 1 h. The perovskite precursors were spin-coated on the surfaces of the ETL films at 1000 and 5000 rpm for 10 s and 30 s respectively. During the second stage, 320 μL Chlorobenzene was dropped onto the substrates. Finally, the substrates were annealed at temperature of 60 °C for 5 min and 80 °C for 10 min.
Fabrication of HTL layers
The PTAA solution (20 mg PTAA and 2.25 mg DPI-TPFB dissolved in 1 ml chlorobenzene) was spin coated on the surfaces of the perovskite layers at 2000 rpm for 30 s and then annealed at 70 °C for 10 min. Finally, a 120 nm Ag layer was deposited using the thermal evaporation system.
Characterizations
The morphologies of the films (Morphologies of ETLs) and devices were characterized by a field-emission scanning electron microscope (FESEM) (SU8010, Hitachi). X-ray diffraction pattern data for 2θ values were collected using a Bruker AXD8 Advance diffractometer with nickel filtered Cu Kα radiation (λ = 1.5406 Å). X-ray photoelectron spectroscopy (XPS) was collected using an Escalab 250Xi XPS system (Thermo Scientific). The ex(in)-situ GIWAXS experiments were carried out at beamline BL14B1 in Shanghai Synchrotron Radiation Facility (SSRF). Time-resolved photoluminescence (TRPL) spectra were achieved by a streak camera (Hamamatsu, C6860). The laser source is an amplified titanium/sapphire laser providing 800 nm 35 fs pulses at 2 kHz which is then frequency doubled for 400 nm excitation. Steady-state Photo-Luminescence (PL) spectra were measured by a FLS920 transient optical spectrometer (Edinburgh Instruments). The space-charge-limited current (SCLC) results were obtained using a Keithley 2400 source-meter unit. AFM images of perovskite films were obtained using AFM5500.
The J–V curves were performed using a Keithley 2400 source-meter unit under AM 1.5 G light illumination at 100 mW cm−2 (1 sun). (ENLITECH SS-X100R) with forward/reverse scanning modes (rate 0.01 V s−1). Before testing, the light intensity was calibrated using a standard Si reference cell (ENLITECH SRC-2020-KG1-RTD). The active area is 0.04-1 cm2. The IPCE measurement was carried out by the QE-R system (Enlitech). Impedance spectroscopic measurements were recorded by an alternating bias of 50 mV by Zennium-pro (Zahner). The corresponding spectra were fitted by Z-View software.
Computational details
Spin-polarized electronic structure calculations were performed using the plane-wave basis set approach as implemented in the Vienna ab initio simulation package (VASP)74. The projector augmented wave (PAW) method was used to represent the ion–core electron interactions75. The valence electrons were represented with a plane wave basis set with an energy cutoff of 450 eV. Electronic exchange and correlation were described with the Perdew–Burke–Ernzerhof (PBE) functional76. DFT-D3 method was used to treat the van der Waals interaction77. A 15 Å vacuum space was used to avoid interactions between surface slabs. A 2 × 2 × 1 Monkhorst–Pack scheme was used to generate the k-point grid for the modeled Pt materials78. The convergence criteria for the self-consistent electronic structure and geometry were set to 10−5 eV and 0.05 eV/Å, respectively.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Supplementary information
Source data
Acknowledgements
J.L. acknowledges the funding support from the National Natural Science Foundation of China (52102219 and 52471197) and the Natural Science Foundation of Shanghai (21ZR1404900).
Author contributions
T.L. and B.L. contributed equally to this work. J.L. conceived the idea, designed the experiments, and supervised the research. T.L. fabricated and characterized the devices. B.L. carried out the synthesis experiment. Y.Y. contributed to the GIWAXS test. Z.Z. contributed to the EPR spectra. P.W. and Z.J. contributed to IPCE spectra. L.D. carried out the EIS experiment. Y.Z. and Q.Z. supported this work. All authors analyzed and discussed the results. T.L. wrote the draft and J.L. reviewed and edited the manuscript.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewers for their contribution to the peer review of this work. A peer review file is available.
Data availability
The data supporting the findings of this study are provided in the main text and the Supplementary Information. Source data are provided with this paper.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Tianpeng Li, Bin Li.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-024-53713-4.
References
- 1.Chen, H. et al. Improved charge extraction in inverted perovskite solar cells with dual-site-binding. Science384, 189–193 (2024). [DOI] [PubMed] [Google Scholar]
- 2.Zhang, S. et al. Minimizing buried interfacial defects for efficient inverted perovskite solar cells. Science380, 404–409 (2023). [DOI] [PubMed] [Google Scholar]
- 3.Park, J. et al. Controlled growth of perovskite layers with volatile alkylammonium chlorides. Nature616, 724–730 (2023). [DOI] [PubMed] [Google Scholar]
- 4.Liang, Z. et al. Homogenizing out-of-plane cation composition in perovskite solar cells. Nature624, 560–563 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Min, H. et al. Perovskite solar cells with atomically coherent interlayers on SnO2 electrodes. Nature598, 444–450 (2021). [DOI] [PubMed] [Google Scholar]
- 6.Jiang, Q. et al. Surface passivation of perovskite film for efficient solar cells. Nat. Photon.13, 460–466 (2019). [Google Scholar]
- 7.Jung, E. H. et al. Efficient, stable and scalable perovskite solar cells using poly(3-hexylthiophene). Nature567, 511–515 (2019). [DOI] [PubMed] [Google Scholar]
- 8.Yoo, J. J. et al. Efficient perovskite solar cells via improved carrier management. Nature590, 587–593 (2021). [DOI] [PubMed] [Google Scholar]
- 9.Zhou, H. et al. Interface engineering of highly efficient perovskite solar cells. Science345, 542–546 (2014). [DOI] [PubMed] [Google Scholar]
- 10.Yang, T. et al. One-stone-for-two-birds strategy to attain beyond 25% perovskite solar cells. Nat. Commun.14, 839 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Warby, J. et al. Understanding performance limiting interfacial recombination in pin perovskite solar cells. Adv. Energy Mater.12, 2103567 (2022). [Google Scholar]
- 12.Stolterfoht, M. et al. Visualization and suppression of interfacial recombination for high-efficiency large-area pin perovskite solar cells. Nat. Energy3, 847–854 (2018). [Google Scholar]
- 13.Chen, B. et al. Passivation of the buried interface via preferential crystallization of 2D perovskite on metal oxide transport layers. Adv. Mater.33, e2103394 (2021). [DOI] [PubMed] [Google Scholar]
- 14.Stolterfoht, M. et al. The impact of energy alignment and interfacial recombination on the internal and external open-circuit voltage of perovskite solar cells. Energy Environ. Sci.12, 2778–2788 (2019). [Google Scholar]
- 15.Futscher, M. H. et al. Defect spectroscopy in halide perovskites is dominated by ionic rather than electronic defects. ACS Energy Lett.7, 140–144 (2022). [Google Scholar]
- 16.McGott, D. L. et al. 3D/2D passivation as a secret to success for polycrystalline thin-film solar cells. Joule5, 1057–1073 (2021). [Google Scholar]
- 17.Tao, S. et al. Absolute energy level positions in tin- and lead-based halide perovskites. Nat. Commun.10, 2560 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li, X. et al. On-device lead sequestration for perovskite solar cells. Nature578, 555–558 (2020). [DOI] [PubMed] [Google Scholar]
- 19.Wu, T. et al. Graphene-like conjugated molecule as hole-selective contact for operationally stable inverted perovskite solar cells and modules. Adv. Mater.35, 2300169 (2023). [DOI] [PubMed] [Google Scholar]
- 20.Bu, T. et al. Modulating crystal growth of formamidinium–caesium perovskites for over 200 cm2 photovoltaic sub-modules. Nat. Energy7, 528 (2022). [Google Scholar]
- 21.Tong, G. et al. Scalable fabrication of >90 cm2 perovskite solar modules with >1000 h operational stability based on the intermediate phase strategy. Adv. Energy Mater.11, 2003712 (2021). [Google Scholar]
- 22.Li, G. et al. Biotoxicity of halide perovskites in mice. Adv. Mater.36, 2306860 (2024). [DOI] [PubMed] [Google Scholar]
- 23.Jiang, X. et al. One-step synthesis of SnI2·(DMSO)x adducts for high-performance tin perovskite solar cells. J. Am. Chem. Soc.143, 10970–10976 (2021). [DOI] [PubMed] [Google Scholar]
- 24.Liu, J. et al. Lead-free solar cells based on tin halide perovskite films with high coverage and improved aggregation. Angew. Chem. Int. Ed.130, 13405–13409 (2018). [DOI] [PubMed] [Google Scholar]
- 25.Jiang, X. et al. Ultra-high open-circuit voltage of tin perovskite solar cells via an electron transporting layer design. Nat. Commun.11, 1245 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang, M. et al. Lead-free perovskite materials for solar cells. Nano Micro Lett.13, 1–36 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yue, S. et al. Synthesis, characterization, and stability studies of Ge-based perovskites of controllable mixed cation composition, produced with an ambient surfactant-free approach. ACS Omega4, 18219–18233 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jiang, F. et al. Chlorine-incorporation-induced formation of the layered phase for antimony-based Lead-free perovskite solar cells. J. Am. Chem. Soc.140, 1019–1027 (2018). [DOI] [PubMed] [Google Scholar]
- 29.Park, B. W. et al. Bismuth based hybrid perovskites A3Bi2I9 (a: methylammonium or cesium) for solar cell application. Adv. Mater.27, 6806–6813 (2015). [DOI] [PubMed] [Google Scholar]
- 30.Nie, R. et al. Mixed sulfur and iodide-based lead-free perovskite solar cells. J. Am. Chem. Soc.140, 872–875 (2018). [DOI] [PubMed] [Google Scholar]
- 31.Nie, R. et al. Efficient and stable antimony selenoiodide solar cells. Adv. Sci.8, 2003172 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen, J. et al. Efficient tin-based perovskite solar cells with trans-isomeric fulleropyrrolidine additives. Nat. Photon. 18, 464–470 (2024).
- 33.Li, T. et al. Functional layers in efficient and stable inverted tin-based perovskite solar cells. Joule7, 1966–1991 (2023). [Google Scholar]
- 34.Zhou, X. et al. Additive engineering with 2, 8-dibromo-dibenzothiophene-S, S-dioxide enabled tin-based perovskite solar cells with 14.98% power conversion efficiency. Energy Environ. Sci. 17, 2837–2844 (2024).
- 35.Li, T. et al. Alleviating the crystallization dynamics and suppressing the oxidation process for tin-based perovskite solar cells with fill factors exceeding 80 percent. Adv. Funct. Mater.33, 2308457 (2023). [Google Scholar]
- 36.Shi, Y. et al. Interfacial dipoles boost open-circuit voltage of tin halide perovskite solar cells. ACS Energy Lett.9, 1895–1897 (2024). [Google Scholar]
- 37.Noel, N. K. et al. Lead-free organic-inorganic tin halide perovskites for photovoltaic applications. Energy Environ. Sci.7, 3061–3068 (2014). [Google Scholar]
- 38.Hao, F. et al. Lead-free solid-state organic-inorganic halide perovskite solar cells. Nat. Photon.8, 489–494 (2014). [Google Scholar]
- 39.Islam, B. et al. Simulating the effect of inserting Sb2S3 as hole transport layer on SnS‑based thin‑film solar cells. J. Electron53, 4726–4739 (2024). [Google Scholar]
- 40.Kumar, M. H. et al. Lead-free halide perovskite solar cells with high photocurrents realized through vacancy modulation. Adv. Mater.26, 7122–7127 (2014). [DOI] [PubMed] [Google Scholar]
- 41.Koh, T. M. et al. Formamidinium tin-based perovskite with low Eg for photovoltaic applications. J. Mater. Chem. A.3, 14996–15000 (2015). [Google Scholar]
- 42.Hao, F. et al. Solvent-mediated crystallization of CH3NH3SnI3 films for heterojunction depleted perovskite solar cells. J. Am. Chem. Soc.137, 11445–11452 (2015). [DOI] [PubMed] [Google Scholar]
- 43.Lee, S. J. et al. Fabrication of efficient formamidinium tin iodide perovskite solar cells through SnF2–pyrazine complex. J. Am. Chem. Soc.138, 3974–3977 (2016). [DOI] [PubMed] [Google Scholar]
- 44.Li, W. et al. Addictive-assisted construction of all-inorganic CsSnIBr2 mesoscopic perovskite solar cells with superior thermal stability up to 473 K. J. Mater. Chem. A.4, 17104–17110 (2016). [Google Scholar]
- 45.Mahmud, J. et al. Design and analysis of a SnS2/WS2/V2O5 double-heterojunction toward high-performance photovoltaics. Energy Adv.2, 1843–1858 (2023). [Google Scholar]
- 46.Song, T.-B. et al. Importance of reducing vapor atmosphere in the fabrication of tin-based perovskite solar cells. J. Am. Chem. Soc.139, 836–842 (2017). [DOI] [PubMed] [Google Scholar]
- 47.Cao, D. H. et al. Thin films and solar cells based on semiconducting two-dimensional Ruddlesden–Popper (CH3(CH2)3NH3)2(CH3NH3)n−1SnnI3n+1 perovskites. ACS Energy Lett.2, 982–990 (2017). [Google Scholar]
- 48.Ke, W. et al. Enhanced photovoltaic performance and stability with a new type of hollow 3D perovskite {en}FASnI3. Sci. Adv.3, e1701293 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ke, W. et al. Efficient lead-free solar cells based on hollow {en}MASnI3 perovskites. J. Am. Chem. Soc.139, 14800–14806 (2017). [DOI] [PubMed] [Google Scholar]
- 50.Xiao, M. et al. Tin-based perovskite with improved coverage and crystallinity through tin-fluoride-assisted heterogeneous nucleation. Adv. Opt. Mater.6, 1700615 (2018). [Google Scholar]
- 51.Tsai, C.-M. et al. Control of crystal structures and optical properties with hybrid formamidinium and 2-hydroxyethylammonium cations for mesoscopic carbon-electrode tin-based perovskite solar cells. ACS Energy Lett.3, 2077–2085 (2018). [Google Scholar]
- 52.Chen, M. et al. Lead-free Dion–Jacobson tin halide perovskites for photovoltaics. ACS Energy Lett.4, 276–277 (2019). [Google Scholar]
- 53.Li, F. et al. A cation-exchange approach for the fabrication of efficient methylammonium tin iodide perovskite solar cells. Angew. Chem. Int. Ed.58, 6688–6692 (2019). [DOI] [PubMed] [Google Scholar]
- 54.Vegiraju, S. et al. Benzodithiophene hole-transporting materials for efficient tin-based perovskite solar cells. Adv. Funct. Mater.29, 1905393 (2019). [Google Scholar]
- 55.Li, F. et al. Effects of alkyl chain length on crystal growth and oxidation process of two-dimensional tin halide perovskites. ACS Energy Lett.5, 1422–1429 (2020). [Google Scholar]
- 56.Tao, Y. et al. Ambient-air-stable lead-free CsSnI3 solar cells with greater than 7.5% efficiency. J. Am. Chem. Soc.143, 4319–4328 (2021). [DOI] [PubMed] [Google Scholar]
- 57.Hu, M. et al. Regulating the surface passivation and residual strain in pure tin perovskite films. ACS Energy Lett.6, 3555–3562 (2021). [Google Scholar]
- 58.Hu, M. et al. Highly stable n–i–p structured formamidinium tin triiodide solar cells through the stabilization of surface Sn2+ cations. Adv. Funct. Mater.33, 2300693 (2023). [Google Scholar]
- 59.NREL Transforming Energy. Best Rsearch-Cell Efficiency Chart.https://www.nrel.gov/pv/cell-efficiency.html (2024).
- 60.Luo, M. Q. et al. Precisely dominating oxygen vacancy of Magneli phase titanium suboxides to efficiently remove quinolone antibiotics under visible light. Sep. Purif.341, 126626 (2024). [Google Scholar]
- 61.Feng, N. et al. Efficient and selective photocatalytic CH4 conversion to CH3OH with O2 by controlling overoxidation on TiO2. Nat. Commun.12, 4652 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang, Y. et al. Fluorine-induced oxygen vacancies on TiO2 nanosheets for photocatalytic indoor VOCs degradation. Appl. Catal. B. Environ.316, 121610 (2022). [Google Scholar]
- 63.Wei, Z.-W. et al. Reversed charge transfer and enhanced hydrogen spill over in platinum nanoclusters anchored on titanium oxide with rich oxygen vacancies boost hydrogen evolution reaction. Angew. Chem. Int. Ed.60, 16622–16627 (2021). [DOI] [PubMed] [Google Scholar]
- 64.Leijtens, T. et al. Mechanism of tin oxidation and stabilization by lead substitution in tin halide perovskites. ACS Energy Lett.2, 2159–2165 (2017). [Google Scholar]
- 65.Zhang, Z. et al. Mechanistic understanding of oxidation of tin-based perovskite solar cells and mitigation strategies. Angew. Chem. Int. Ed.62, e202308093 (2023). [DOI] [PubMed] [Google Scholar]
- 66.Gan, Q. et al. Defect-assisted selective surface phosphorus doping to enhance rate capability of titanium dioxide for sodium ion batteries. ACS Nano.13, 9247–9258 (2019). [DOI] [PubMed] [Google Scholar]
- 67.Park, S. et al. Advances in SnO2 for efficient and stable n–i–p perovskite solar cells. Adv. Mater.34, 2110438 (2022). [DOI] [PubMed] [Google Scholar]
- 68.Jiang, Q. et al. Enhanced electron extraction using SnO2 for high-efficiency planar-structure HC(NH2)2PbI3-based perovskite solar cells. Nat. Energy2, 16177 (2017). [Google Scholar]
- 69.Wang, L. et al. Strain modulation for light-stable n–i–p perovskite/silicon tandem solar cells. Adv. Mater.34, 2201315 (2022). [DOI] [PubMed] [Google Scholar]
- 70.Kumar, N. et al. A review on perovskite solar cells (PSCs), materials and applications. J. Mater.7, 940–956 (2021). [Google Scholar]
- 71.Liu, C. et al. Two-dimensional perovskitoids enhance stability in perovskite solar cells. Nature10.1038/s41586-024-07764-8 (2024). [DOI] [PubMed] [Google Scholar]
- 72.Michael, S. et al. Current density mismatch in perovskite solar cells. ACS Energy Lett.5, 2886–2888 (2020). [Google Scholar]
- 73.Liang, H. et al. High color purity lead-free perovskite light-emitting diodes via Sn stabilization. Adv. Sci.7, 1903213 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kresse, G. et al. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci.6, 15–50 (1996). [Google Scholar]
- 75.Kresse, G. et al. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B.59, 1758–1775 (1999). [Google Scholar]
- 76.Perdew, J. P. et al. Generalized gradient approximation made simple. Phys. Rev. Lett.77, 3865–3868 (1996). 1996. [DOI] [PubMed] [Google Scholar]
- 77.Grimme, S. et al. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys.132, 154104 (2010). [DOI] [PubMed] [Google Scholar]
- 78.Chadi, D. J. et al. Special points for brillouin-zone integrations. Phys. Rev. B.16, 1746–1747 (1977). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data supporting the findings of this study are provided in the main text and the Supplementary Information. Source data are provided with this paper.