Abstract
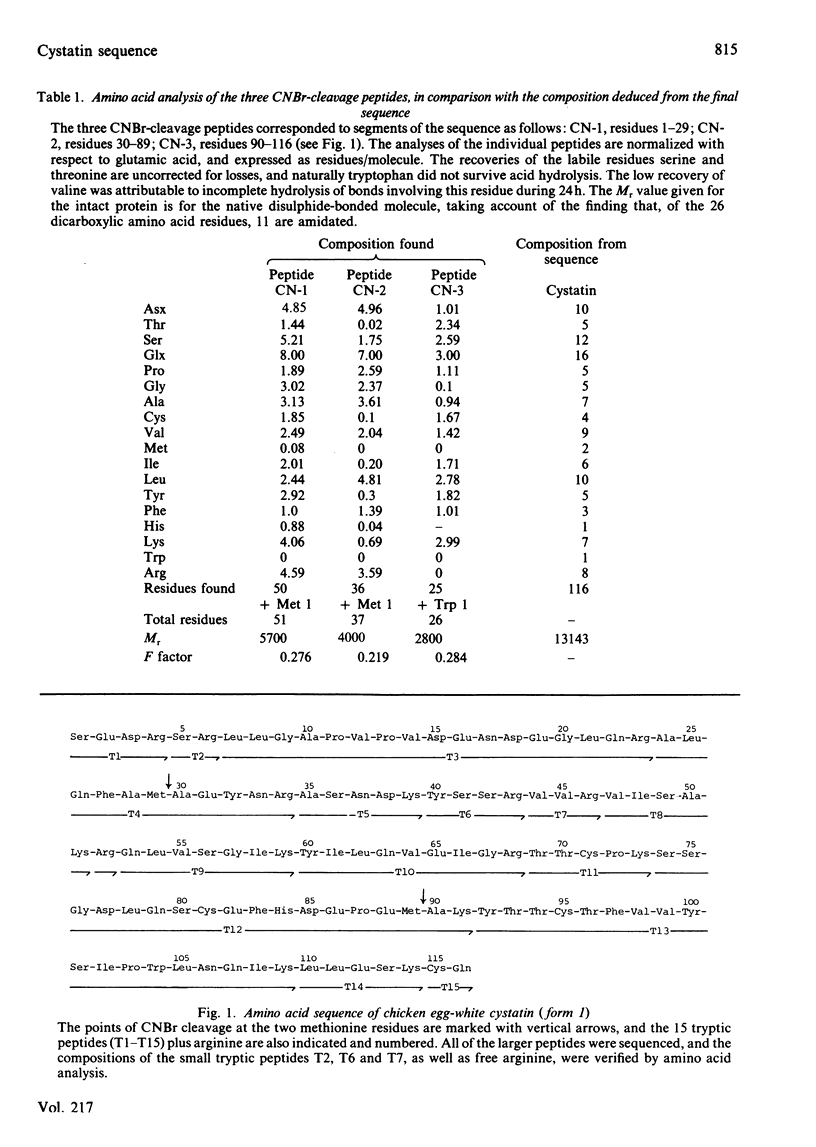
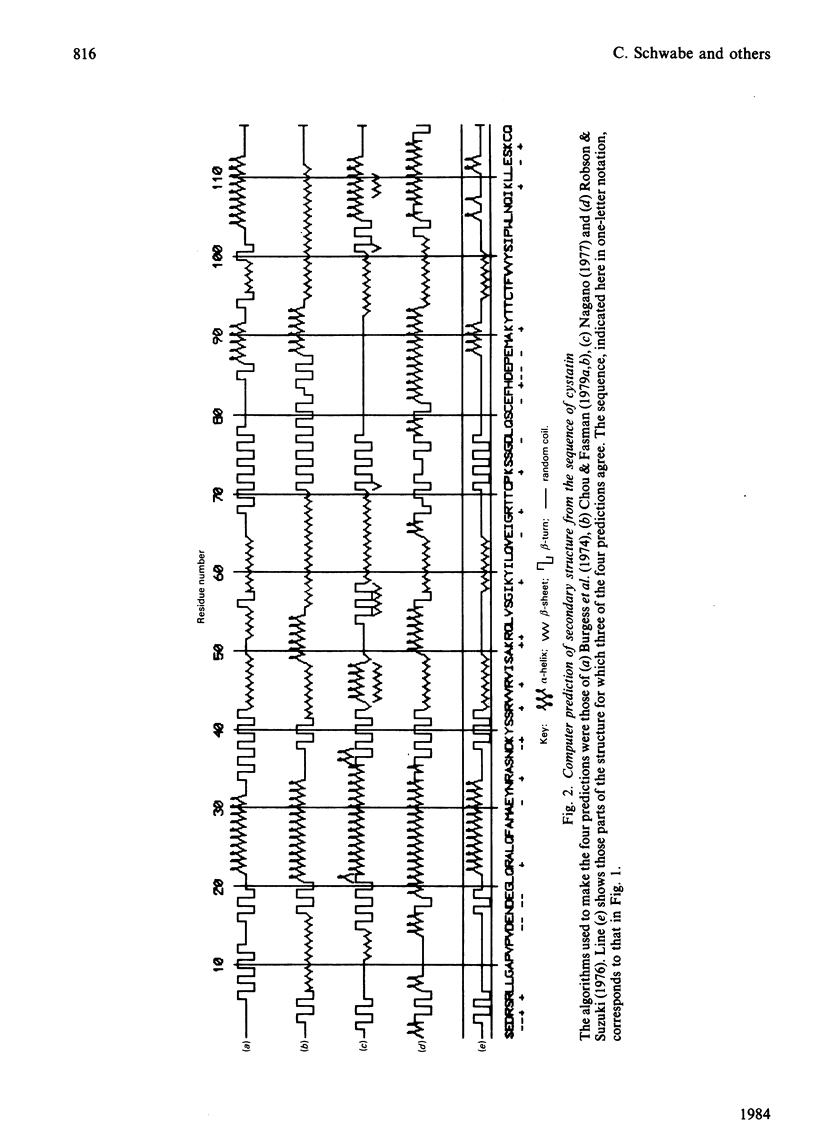
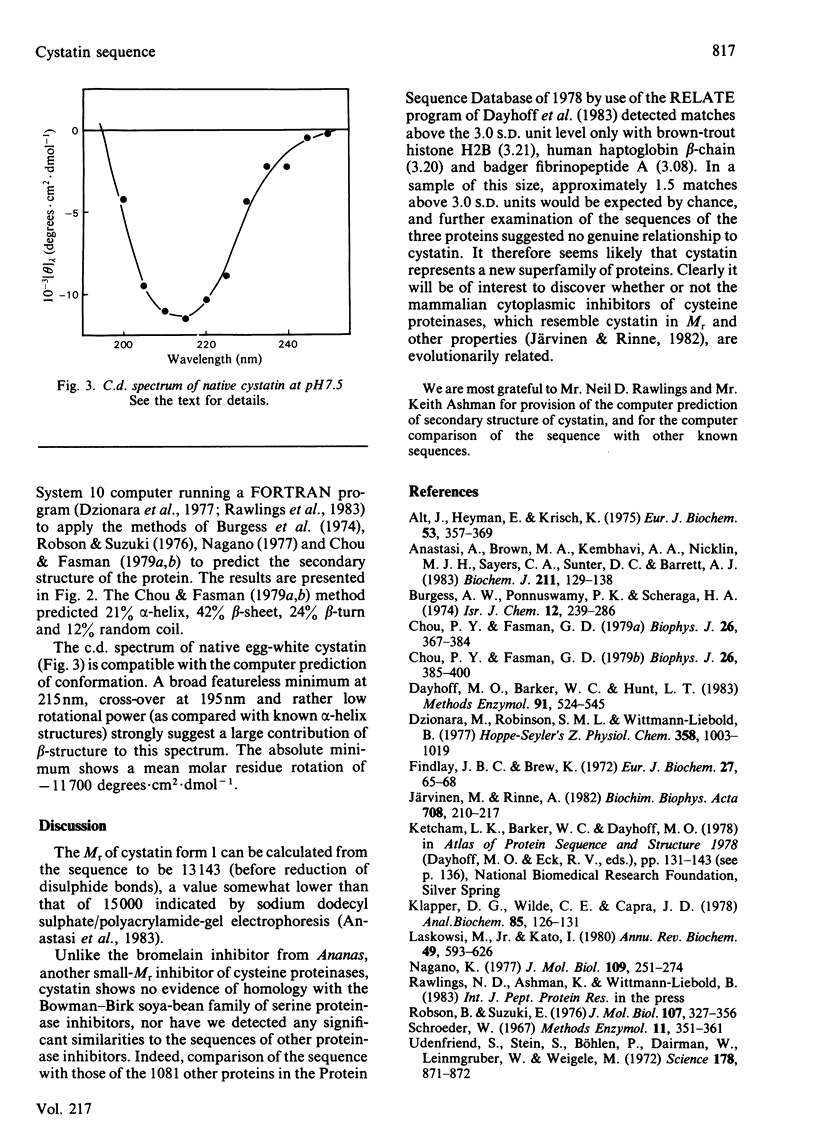
The amino acid sequence of cystatin, the protein from chicken egg-white that is a tight-binding inhibitor of many cysteine proteinases, is reported. Cystatin is composed of 116 amino acid residues, and the Mr is calculated to be 13 143. No striking similarity to any other known sequence has been detected. The results of computer analysis of the sequence and c.d. spectrometry indicate that the secondary structure includes relatively little alpha-helix (about 20%) and that the remainder is mainly beta-structure.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alt J., Heymann E., Krisch K. Characterization of an inducible amidase from Pseudomonas acidovorans AE1. Eur J Biochem. 1975 May 6;53(2):357–369. doi: 10.1111/j.1432-1033.1975.tb04076.x. [DOI] [PubMed] [Google Scholar]
- Anastasi A., Brown M. A., Kembhavi A. A., Nicklin M. J., Sayers C. A., Sunter D. C., Barrett A. J. Cystatin, a protein inhibitor of cysteine proteinases. Improved purification from egg white, characterization, and detection in chicken serum. Biochem J. 1983 Apr 1;211(1):129–138. doi: 10.1042/bj2110129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Conservation of chain reversal regions in proteins. Biophys J. 1979 Jun;26(3):385–399. doi: 10.1016/S0006-3495(79)85260-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Prediction of beta-turns. Biophys J. 1979 Jun;26(3):367–383. doi: 10.1016/S0006-3495(79)85259-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayhoff M. O., Barker W. C., Hunt L. T. Establishing homologies in protein sequences. Methods Enzymol. 1983;91:524–545. doi: 10.1016/s0076-6879(83)91049-2. [DOI] [PubMed] [Google Scholar]
- Dzionara M., Robinson S. M., Wittmann-Liebold B. Secondary structures of proteins from the 30S subunit of the Escherichia coli ribosome. Hoppe Seylers Z Physiol Chem. 1977 Aug;358(8):1003–1019. doi: 10.1515/bchm2.1977.358.2.1003. [DOI] [PubMed] [Google Scholar]
- Findlay J. B., Brew K. The complete amino-acid sequence of human -lactalbumin. Eur J Biochem. 1972 May;27(1):65–86. doi: 10.1111/j.1432-1033.1972.tb01812.x. [DOI] [PubMed] [Google Scholar]
- Järvinen M., Rinne A. Human spleen cysteineproteinase inhibitor. Purification, fractionation into isoelectric variants and some properties of the variants. Biochim Biophys Acta. 1982 Nov 9;708(2):210–217. [PubMed] [Google Scholar]
- Klapper D. G., Wilde C. E., 3rd, Capra J. D. Automated amino acid sequence of small peptides utilizing Polybrene. Anal Biochem. 1978 Mar;85(1):126–131. doi: 10.1016/0003-2697(78)90282-8. [DOI] [PubMed] [Google Scholar]
- Laskowski M., Jr, Kato I. Protein inhibitors of proteinases. Annu Rev Biochem. 1980;49:593–626. doi: 10.1146/annurev.bi.49.070180.003113. [DOI] [PubMed] [Google Scholar]
- Nagano K. Triplet information in helix prediction applied to the analysis of super-secondary structures. J Mol Biol. 1977 Jan 15;109(2):251–274. doi: 10.1016/s0022-2836(77)80033-8. [DOI] [PubMed] [Google Scholar]
- Robson B., Suzuki E. Conformational properties of amino acid residues in globular proteins. J Mol Biol. 1976 Nov 5;107(3):327–356. doi: 10.1016/s0022-2836(76)80008-3. [DOI] [PubMed] [Google Scholar]
- Udenfriend S., Stein S., Böhlen P., Dairman W., Leimgruber W., Weigele M. Fluorescamine: a reagent for assay of amino acids, peptides, proteins, and primary amines in the picomole range. Science. 1972 Nov 24;178(4063):871–872. doi: 10.1126/science.178.4063.871. [DOI] [PubMed] [Google Scholar]


