Abstract
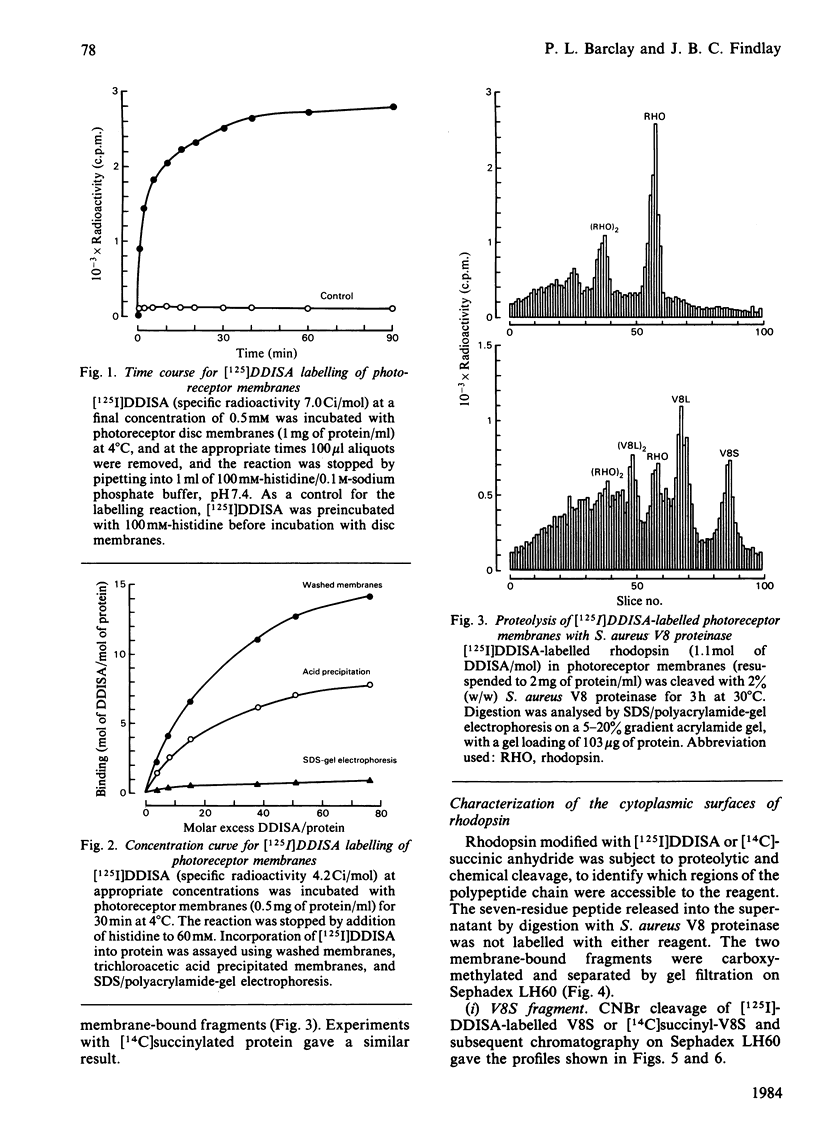
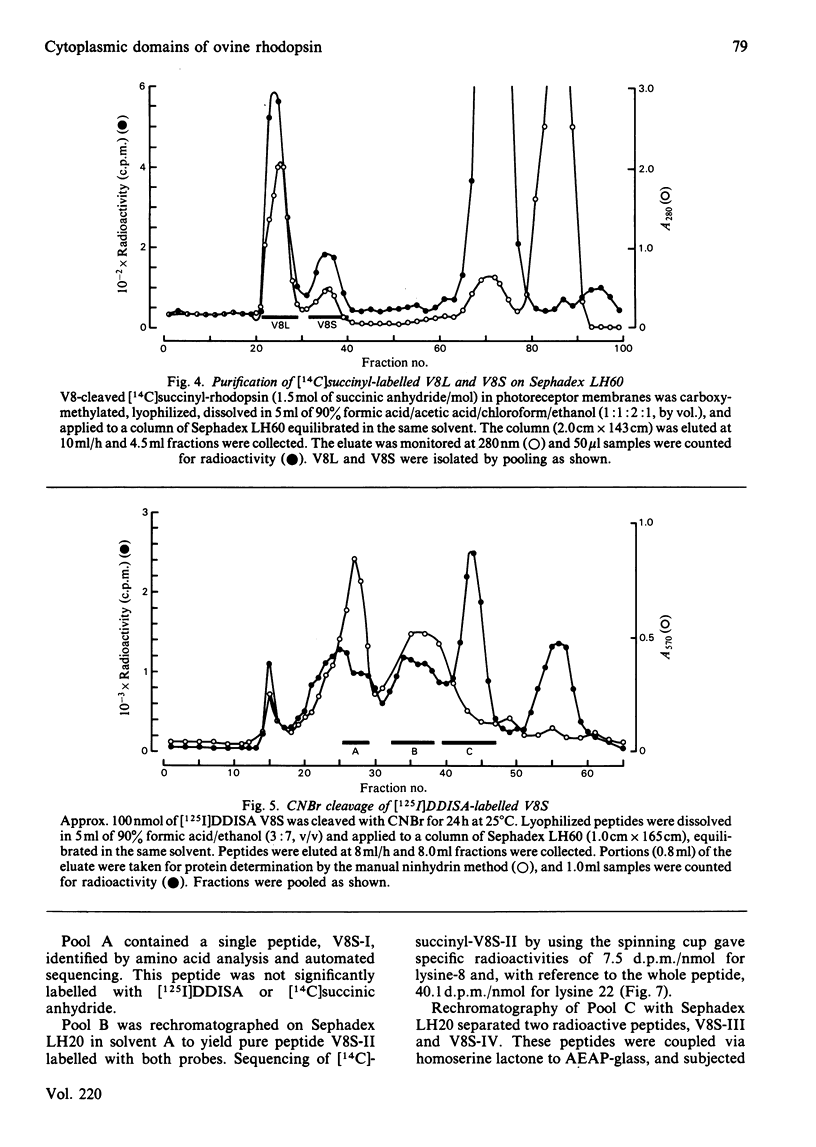
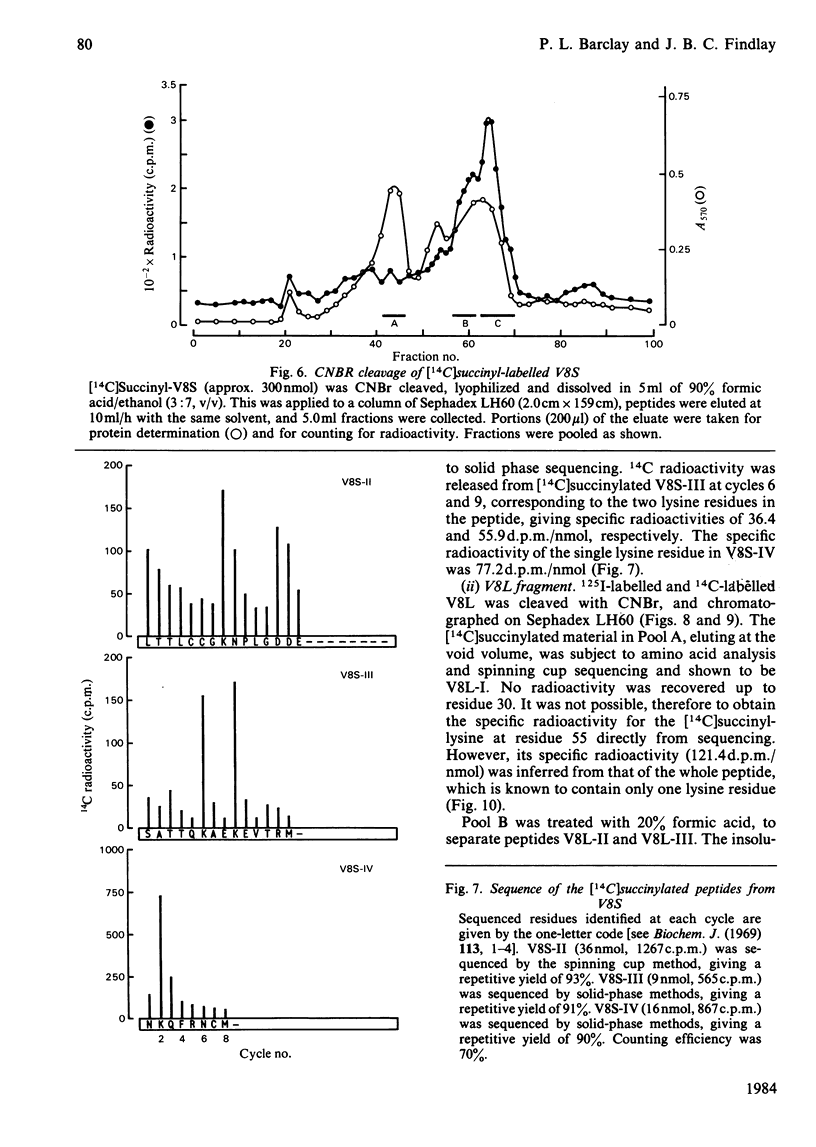
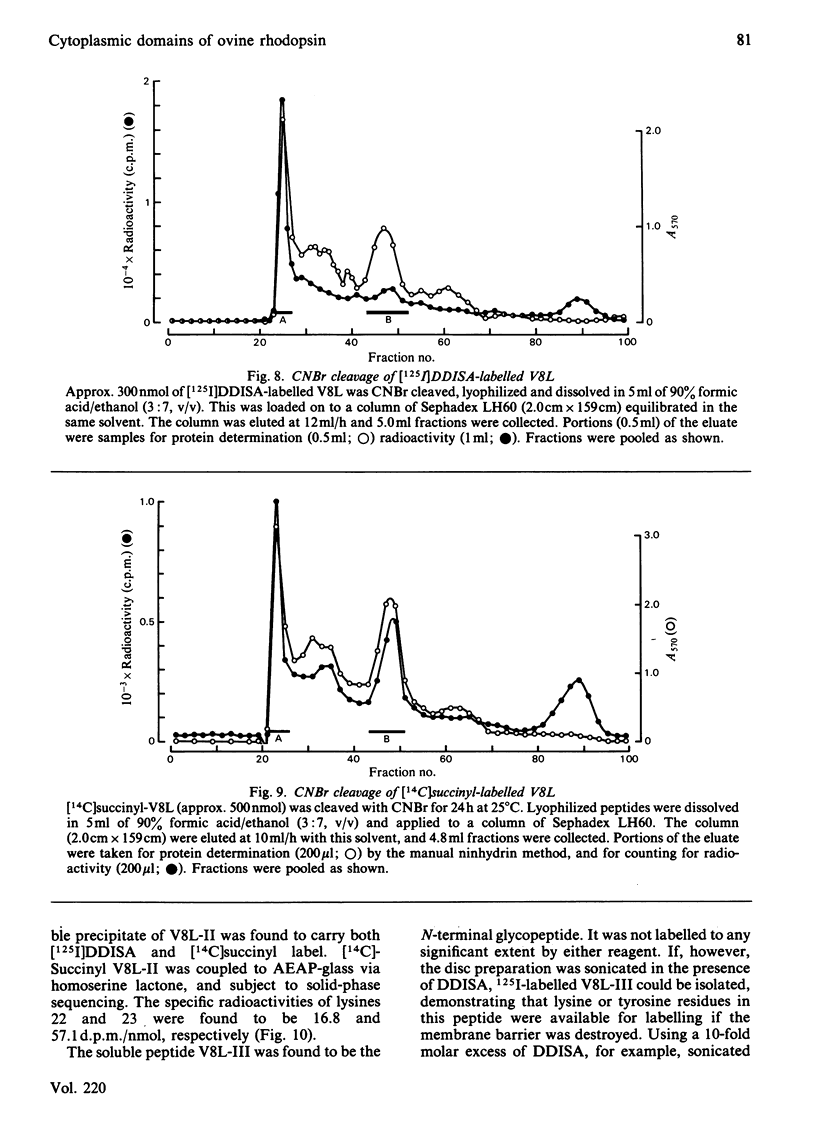
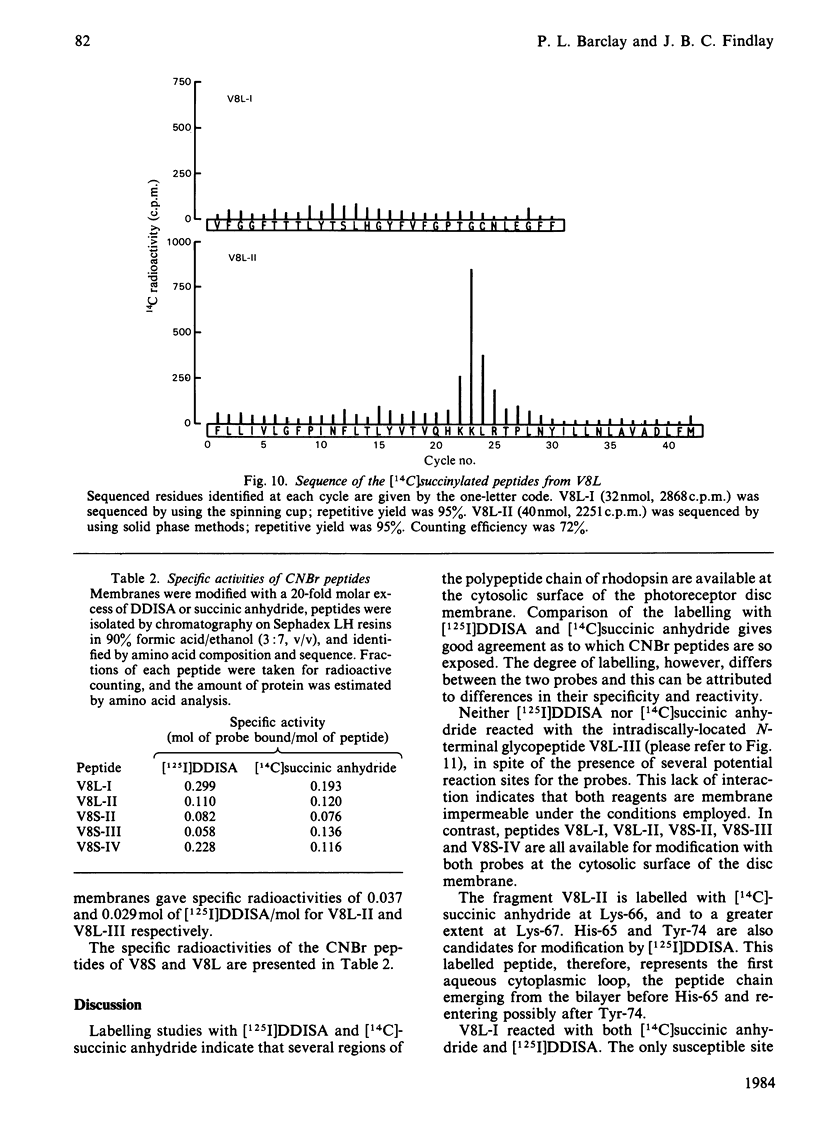
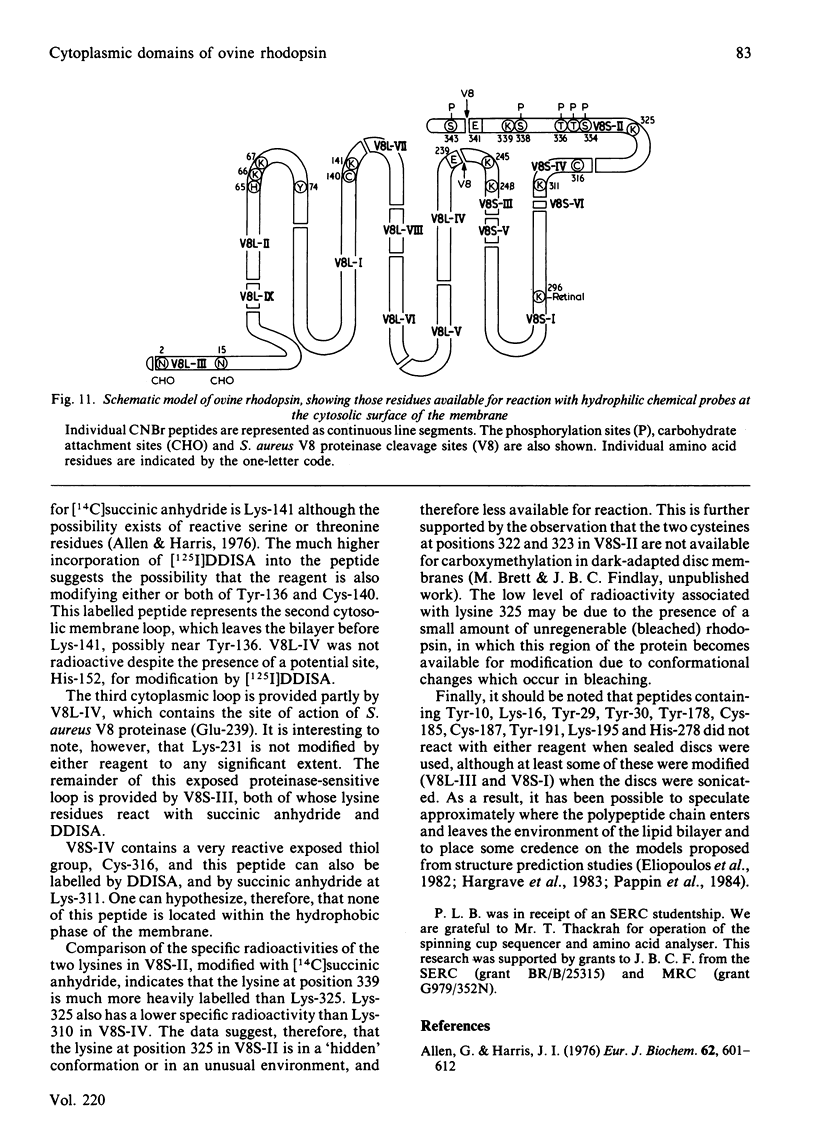
The disposition of polypeptide chain of ovine rhodopsin in the photoreceptor disc membrane was investigated by using two hydrophilic reagents, 3,5-di-[125I]iodo-4-diazobenzenesulphonate [( 125I]DDISA) and [14C]succinic anhydride. Both reagents were used to modify rhodopsin in intact disc membranes under conditions where no loss of A500 occurred. Reaction of [125I]DDISA with rhodopsin approached completion after 30 min. Binding was saturated at a 75-fold molar excess of reagent, which gave binding ratios of up to 2 mol/mol of rhodopsin. Proteolysis of rhodopsin, using Staphylococcus aureus V8 proteinase, yielded two membrane-bound fragments, both of which contained bound radioactive probe. Subsequent CNBr cleavage of these fragments produced five radiolabelled peptides which corresponded to the C-terminal region and cytoplasmic loops of rhodopsin. Similar studies with [14C]-succinic anhydride also gave binding ratios of up to 2 mol/mol of rhodopsin. Sequencing of the [14C]succinylated peptides identified the location of the reactive sites as lysine residues 66, 67, 141, 245, 248, 311, 325 and 339 in the polypeptide chain. Non-permeability of both probes was demonstrated by the absence of any radioactivity associated with the intradiscal N-terminal glycopeptide. Sonication of membranes in the presence of [125I]DDISA led to the incorporation of label in this peptide.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen G., Harris J. I. Succinylation of glyceraldehyde-3-phosphate dehydrogenase from Bacillus stearothermophilus. A reactive threonine residue in the apoenzyme. Eur J Biochem. 1976 Mar 1;62(3):601–612. doi: 10.1111/j.1432-1033.1976.tb10195.x. [DOI] [PubMed] [Google Scholar]
- Booth A. G., Kenny A. J. Proteins of the kidney microvillar membrane. Asymmetric labelling of the membrane by lactoperoxidase-catalysed radioiodination and by photolysis of 3,5-di[125I]iodo-4-azidobenzenesulphonate. Biochem J. 1980 Apr 1;187(1):31–44. doi: 10.1042/bj1870031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brett M., Findlay J. B. Investigation of the organization of rhodopsin in the sheep photoreceptor membrane by using cross-linking reagents. Biochem J. 1979 Jan 1;177(1):215–223. doi: 10.1042/bj1770215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brett M., Findlay J. B. Isolation and characterization of the CNBr peptides from the proteolytically derived N-terminal fragment of ovine opsin. Biochem J. 1983 Jun 1;211(3):661–670. doi: 10.1042/bj2110661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark S. P., Molday R. S. Orientation of membrane glycoproteins in sealed rod outer segment disks. Biochemistry. 1979 Dec 25;18(26):5868–5873. doi: 10.1021/bi00593a017. [DOI] [PubMed] [Google Scholar]
- Dratz E. A., Miljanich G. P., Nemes P. P., Gaw J. E., Schwartz S. The structure of rhodopsin and its disposition in the rod outer segment disk membrane. Photochem Photobiol. 1979 Apr;29(4):661–670. doi: 10.1111/j.1751-1097.1979.tb07746.x. [DOI] [PubMed] [Google Scholar]
- Edwards R. M., Kempson S. A., Carlson G. L., Dousa T. P. Diazontized (125I) diiodosulafanilic acid as a label for cell surface membranes. Studies on erythrocytes. Biochim Biophys Acta. 1979 May 3;553(1):54–65. doi: 10.1016/0005-2736(79)90030-0. [DOI] [PubMed] [Google Scholar]
- Findlay J. B., Brett M., Pappin D. J. Primary structure of C-terminal functional sites in ovine rhodopsin. Nature. 1981 Sep 24;293(5830):314–317. doi: 10.1038/293314a0. [DOI] [PubMed] [Google Scholar]
- Helmkamp R. W., Sears D. A. A label for the red cell membrane: diazotized diiodosulfanilic acid. Int J Appl Radiat Isot. 1970 Nov;21(11):683–685. doi: 10.1016/0020-708x(70)90127-4. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Law P. Y., Ostwald T. J., Way E. L., Loh H. H. Interaction of diazosulfanilic acid and the opiate receptor: inhibition of specific binding to synaptic membranes and labeling of a membrane lipid stereospecifically protected by opioids. Mol Pharmacol. 1981 May;19(3):355–366. [PubMed] [Google Scholar]
- Mas M. T., Wang J. K., Hargrave P. A. Topography of rhodopsin in rod outer segment disk membranes. Photochemical labeling with N-(4-azido-2-nitrophenyl)-2-aminoethanesulfonate. Biochemistry. 1980 Feb 19;19(4):684–691. doi: 10.1021/bi00545a012. [DOI] [PubMed] [Google Scholar]
- Ovchinnikov YuA Rhodopsin and bacteriorhodopsin: structure-function relationships. FEBS Lett. 1982 Nov 8;148(2):179–191. doi: 10.1016/0014-5793(82)80805-3. [DOI] [PubMed] [Google Scholar]
- Pappin D. J., Findlay J. B. Sequence variability in the retinal-attachment domain of mammalian rhodopsins. Biochem J. 1984 Feb 1;217(3):605–613. doi: 10.1042/bj2170605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pober J. S., Iwanij V., Reich E., Stryer L. Transglutaminase-catalyzed insertion of a fluorescent probe into the protease-sensitive region of rhodopsin. Biochemistry. 1978 May 30;17(11):2163–2168. doi: 10.1021/bi00604a021. [DOI] [PubMed] [Google Scholar]
- Raubach R. A., Nemes P. P., Dratz E. A. Chemical labeling and freeze-fracture studies on the localization of rhodopsin in the rod outer segment disk membrane. Exp Eye Res. 1974 Jan;18(1):1–12. doi: 10.1016/0014-4835(74)90038-4. [DOI] [PubMed] [Google Scholar]
- Röhlich P. Photoreceptor membrane carbohydrate on the intradiscal surface of retinal rod disks. Nature. 1976 Oct 28;263(5580):789–791. doi: 10.1038/263789a0. [DOI] [PubMed] [Google Scholar]
- Saari J. C. The accessibility of bovine rhodopsin in photoreceptor membranes. J Cell Biol. 1974 Nov;63(2 Pt 1):480–491. doi: 10.1083/jcb.63.2.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sale G. J., Towner P., Akhtar M. Functional rhodopsin complex consisting of three noncovalently linked fragments. Biochemistry. 1977 Dec 13;16(25):5641–5649. doi: 10.1021/bi00644a040. [DOI] [PubMed] [Google Scholar]
- Sale G. J., Towner P., Akhtar M. Topography of the rhodopsin molecule. Identification of the domain phosphorylated. Biochem J. 1978 Nov 1;175(2):421–430. doi: 10.1042/bj1750421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiva D. A., Sears D. A. Surface labeling of normal human peripheral blood lymphocytes with a nonpenetrating radioactive probe. J Reticuloendothel Soc. 1981 Aug;30(2):129–145. [PubMed] [Google Scholar]
- Tam S. W., Fenton J. W., 2nd, Detwiler T. C. Platelet thrombin receptors. Binding of alpha-thrombin is coupled to signal generation by a chymotrypsin-sensitive mechanism. J Biol Chem. 1980 Jul 25;255(14):6626–6632. [PubMed] [Google Scholar]
- Yu B. P., Masoro E. J., Morley T. F. Analysis of the arrangement of protein components in the sarcomplasmic reticulum of rat skeletal muscle. J Biol Chem. 1976 Apr 10;251(7):2037–2043. [PubMed] [Google Scholar]


