Abstract
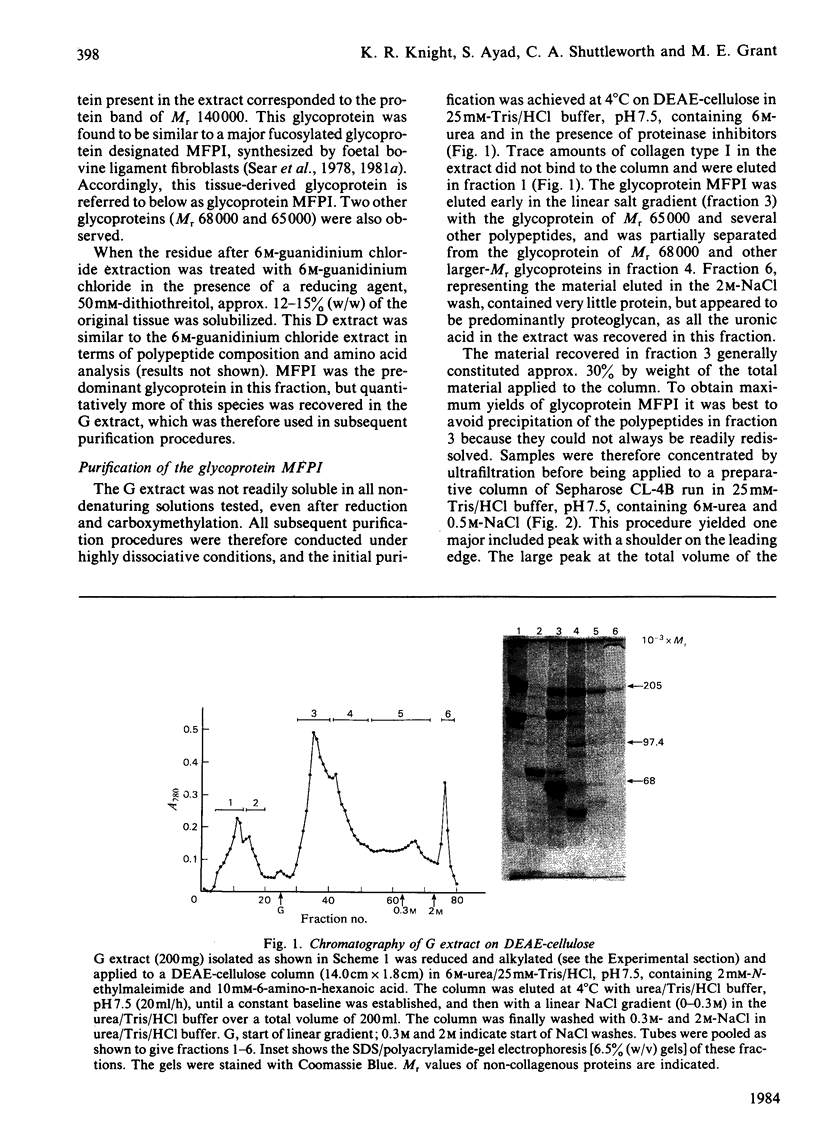
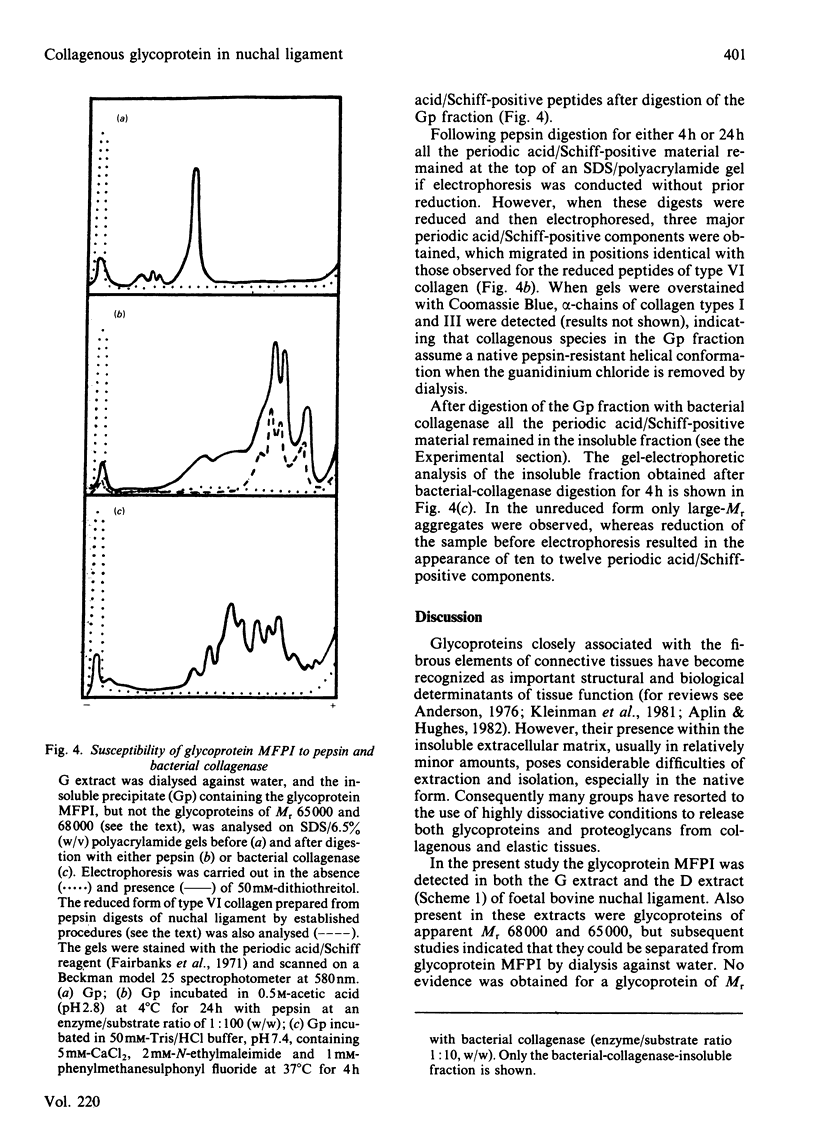
A collagenous glycoprotein (Mr 140000) was isolated from dissociative extracts of foetal bovine nuchal ligament and purified by a combination of ion-exchange and gel-filtration chromatography. This glycoprotein (designated MFPI) exists as a large-Mr disulphide-bonded aggregate in the absence of a reducing agent. The purified glycoprotein was shown to contain about 6% (w/w) carbohydrate, mostly as galactose, glucose and mannose. Amino acid analysis showed the presence of hydroxyproline and hydroxylysine, indicative of its collagenous nature. The collagenous nature of this glycoprotein was further investigated by enzyme digestion. Pepsin digestion produced three major fragments, which were identical with peptides of type VI collagen. Bacterial-collagenase digestion of the unreduced glycoprotein also produced several discrete peptides. However, reduction of the glycoprotein before bacterial-collagenase digestion resulted in the degradation of these discrete peptides. Glycoprotein MFPI extracted in dissociative conditions appears to be a larger-Mr form of type VI collagen, believed to originate from microfibrillar components in the intact tissue.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abedin M. Z., Ayad S., Weiss J. B. Isolation and native characterization of cysteine-rich collagens from bovine placental tissues and uterus and their relationship to types IV and V collagens. Biosci Rep. 1982 Jul;2(7):493–502. doi: 10.1007/BF01115247. [DOI] [PubMed] [Google Scholar]
- Alper R. Isolation and preliminary characterization of a structural glycoprotein complex from bovine corneal stroma. Curr Eye Res. 1982;2(7):479–487. doi: 10.3109/02713688208996352. [DOI] [PubMed] [Google Scholar]
- Anderson J. C. Glycoproteins of the connective tissue matrix. Int Rev Connect Tissue Res. 1976;7:251–322. doi: 10.1016/b978-0-12-363707-9.50012-5. [DOI] [PubMed] [Google Scholar]
- Aplin J. D., Hughes R. C. Complex carbohydrates of the extracellular matrix structures, interactions and biological roles. Biochim Biophys Acta. 1982 Dec;694(4):375–418. doi: 10.1016/0304-4157(82)90003-x. [DOI] [PubMed] [Google Scholar]
- Carter W. G. Transformation-dependent alterations is glycoproteins of extracellular matrix of human fibroblasts. Characterization of GP250 and the collagen-like GP140. J Biol Chem. 1982 Nov 25;257(22):13805–13815. [PubMed] [Google Scholar]
- Chambers C. A., Shuttleworth C. A., Ayad S., Grant M. E. Collagen heterogeneity and quantification in developing bovine nuchal ligament. Biochem J. 1984 Jun 1;220(2):385–394. doi: 10.1042/bj2200385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clamp J. R., Bhatti T., Chambers R. E. The determination of carbohydrate in biological materials by gas-liquid chromatography. Methods Biochem Anal. 1971;19:229–344. doi: 10.1002/9780470110386.ch3. [DOI] [PubMed] [Google Scholar]
- Cleary E. G., Gibson M. A. Elastin-associated microfibrils and microfibrillar proteins. Int Rev Connect Tissue Res. 1983;10:97–209. doi: 10.1016/b978-0-12-363710-9.50009-5. [DOI] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Furthmayr H., Wiedemann H., Timpl R., Odermatt E., Engel J. Electron-microscopical approach to a structural model of intima collagen. Biochem J. 1983 May 1;211(2):303–311. doi: 10.1042/bj2110303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuto D. K., Miller E. J. Characterization of a unique collagenous fraction from limited pepsin digests of human placental tissue: molecular organization of the native aggregate. Biochemistry. 1981 Mar 17;20(6):1635–1640. doi: 10.1021/bi00509a035. [DOI] [PubMed] [Google Scholar]
- Furuto D. K., Miller E. J. Isolation of a unique collagenous fraction from limited pepsin digests of human placental tissue. Characterization of one of the constituent polypeptide chains. J Biol Chem. 1980 Jan 10;255(1):290–295. [PubMed] [Google Scholar]
- Gibson M. A., Cleary E. G. A collagen-like glycoprotein from elastin-rich tissues. Biochem Biophys Res Commun. 1982 Apr 29;105(4):1288–1295. doi: 10.1016/0006-291x(82)90926-3. [DOI] [PubMed] [Google Scholar]
- Jander R., Rauterberg J., Glanville R. W. Further characterization of the three polypeptide chains of bovine and human short-chain collagen (intima collagen). Eur J Biochem. 1983 Jun 1;133(1):39–46. doi: 10.1111/j.1432-1033.1983.tb07427.x. [DOI] [PubMed] [Google Scholar]
- Jander R., Rauterberg J., Voss B., von Bassewitz D. B. A cysteine-rich collagenous protein from bovine placenta. Isolation of its constituent polypeptide chains and some properties of the non-denatured protein. Eur J Biochem. 1981;114(1):17–25. [PubMed] [Google Scholar]
- Kawaguchi T. Isolation of glycoproteins from bovine nuchal ligament. Connect Tissue Res. 1982;9(4):241–245. doi: 10.3109/03008208209160268. [DOI] [PubMed] [Google Scholar]
- Kleinman H. K., Klebe R. J., Martin G. R. Role of collagenous matrices in the adhesion and growth of cells. J Cell Biol. 1981 Mar;88(3):473–485. doi: 10.1083/jcb.88.3.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafuma C., Moczar M., Robert L. Isolation and characterization of lung connective-tissue glycoproteins. Biochem J. 1982 Jun 1;203(3):593–601. doi: 10.1042/bj2030593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurain G., Delvincourt T., Szymanowicz A. G. Isolation of a macromolecular collagenous fraction and AB2 collagen from calf skin. FEBS Lett. 1980 Oct 20;120(1):44–48. doi: 10.1016/0014-5793(80)81042-8. [DOI] [PubMed] [Google Scholar]
- Marton L. S., Arnason B. G. A basement membrane-associated glycoprotein from skeletal muscle. J Cell Biochem. 1982;19(4):363–381. doi: 10.1002/jcb.240190406. [DOI] [PubMed] [Google Scholar]
- Odermatt E., Risteli J., van Delden V., Timpl R. Structural diversity and domain composition of a unique collagenous fragment (intima collagen) obtained from human placenta. Biochem J. 1983 May 1;211(2):295–302. doi: 10.1042/bj2110295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross R., Bornstein P. The elastic fiber. I. The separation and partial characterization of its macromolecular components. J Cell Biol. 1969 Feb;40(2):366–381. doi: 10.1083/jcb.40.2.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sear C. H., Grant M. E., Jackson D. S. Biosynthesis and release of glycoproteins by human skin fibroblasts in culture. Biochem J. 1977 Oct 15;168(1):91–103. doi: 10.1042/bj1680091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sear C. H., Grant M. E., Jackson D. S. The nature of the microfibrillar glycoproteins of elastic fibres. A biosynthetic study. Biochem J. 1981 Feb 15;194(2):587–598. doi: 10.1042/bj1940587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sear C. H., Jones C. J., Knight K. R., Grant M. E. Elastogenesis and microfibrillar glycoprotein synthesis by bovine ligamentum nuchae cells in culture. Connect Tissue Res. 1981;8(3-4):167–170. doi: 10.3109/03008208109152368. [DOI] [PubMed] [Google Scholar]
- Sear C. H., Kewley M. A., Jones C. J., Grant M. E., Jackson D. S. The identification of glycoproteins associated with elastic-tissue microfibrils. Biochem J. 1978 Mar 15;170(3):715–718. doi: 10.1042/bj1700715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serafini-Fracassini A., Ventrella G., Field M. J., Hinnie J., Onyezili N. I., Griffiths R. Characterization of a structural glycoprotein from bovine ligamentum nuchae exhibiting dual amine oxidase activity. Biochemistry. 1981 Sep 15;20(19):5424–5429. doi: 10.1021/bi00522a011. [DOI] [PubMed] [Google Scholar]
- Shuttleworth C. A., Berry L., Wilson N. H. Biosynthesis of glycoproteins by rabbit dental pulp fibroblasts in culture. Arch Oral Biol. 1982;27(8):645–650. doi: 10.1016/0003-9969(82)90187-x. [DOI] [PubMed] [Google Scholar]



