Abstract
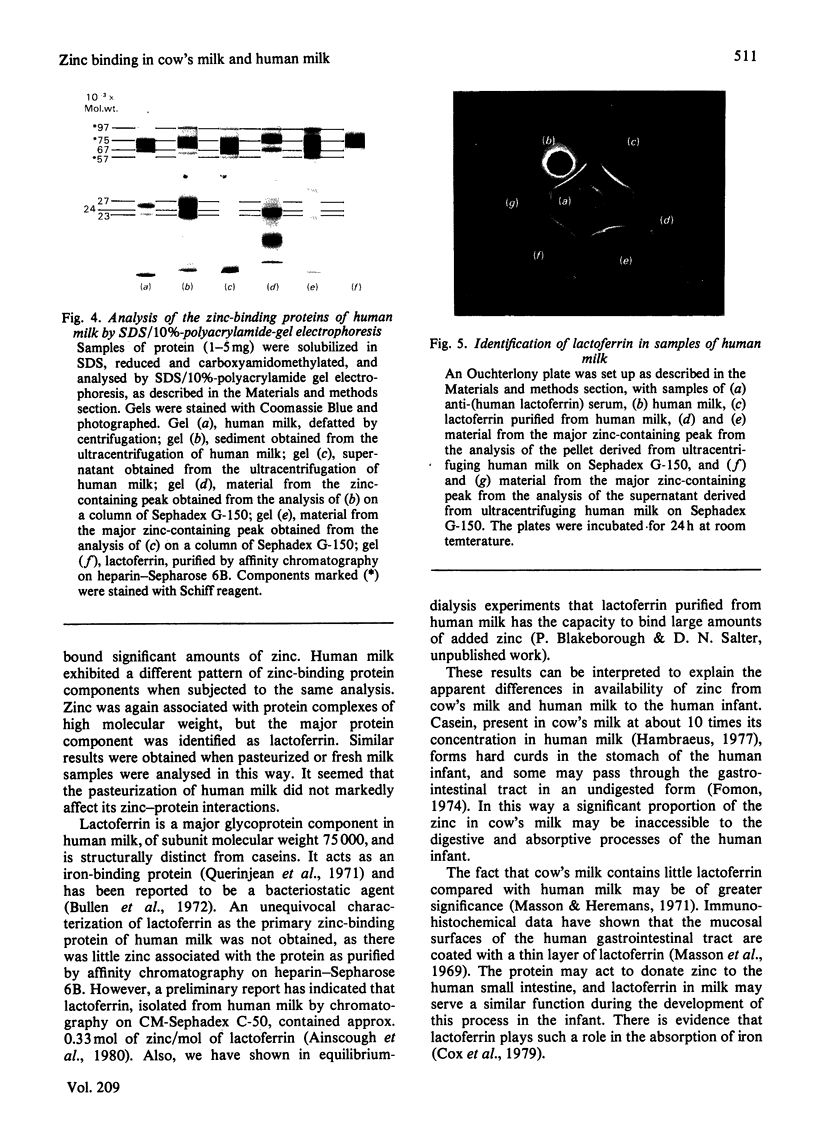
In both cow's milk and human milk, zinc was associated with proteins of high molecular weight (greater than 100 000), as judged by analysis with Sephadex G-75. Precipitation of the casein at pH 4.6 and filtration of the resultant acid whey on Sephadex G-25 led, however, to the recovery of about 90% of the zinc as a compound of low molecular weight, which was tentatively identified as zinc citrate. Over 95% of the zinc of cow's milk was sedimented with the casein micelles on ultracentrifugation. Filtration of these micellar caseins on Sephadex G-150 gave two peaks containing zinc, which corresponded to aggregates of alpha-casein-kappa-casein and of alpha-casein-beta-casein. Ultracentrifugation of human milk sedimented only approx. 40% of total zinc. Analysis of sediment and supernatant on Sephadex G-150, however, indicated that about 85% of the zinc was associated with a protein complex of molecular weight greater than 150 000. The major protein of this complex was identified as lactoferrin. A minor zinc-binding component of average molecular weight 30 000 was also observed in the supernatant. The results indicated that zinc is bound to different macromolecules in cow's and human milk. This may be a factor affecting the bioavailability to the human infant of zinc from the two milks, and it is suggested that in human milk lactoferrin may be involved in the uptake of zinc.
Full text
PDF


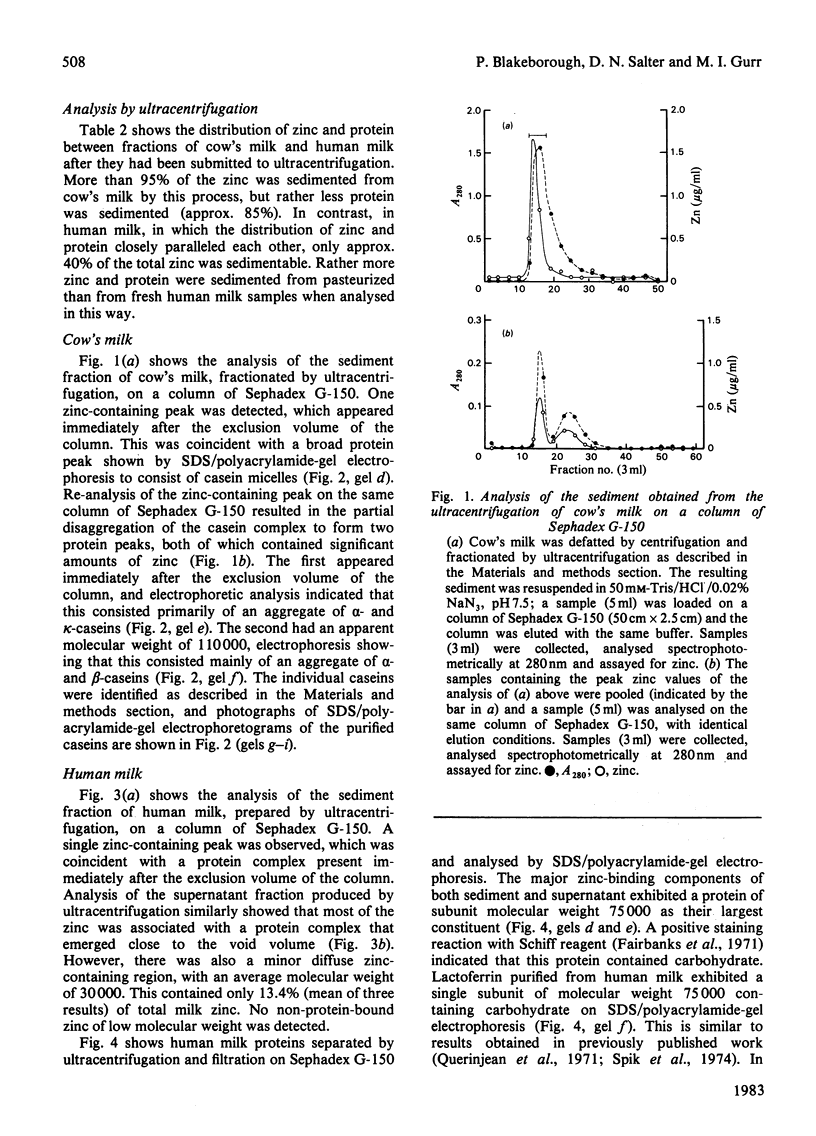

Images in this article
Selected References
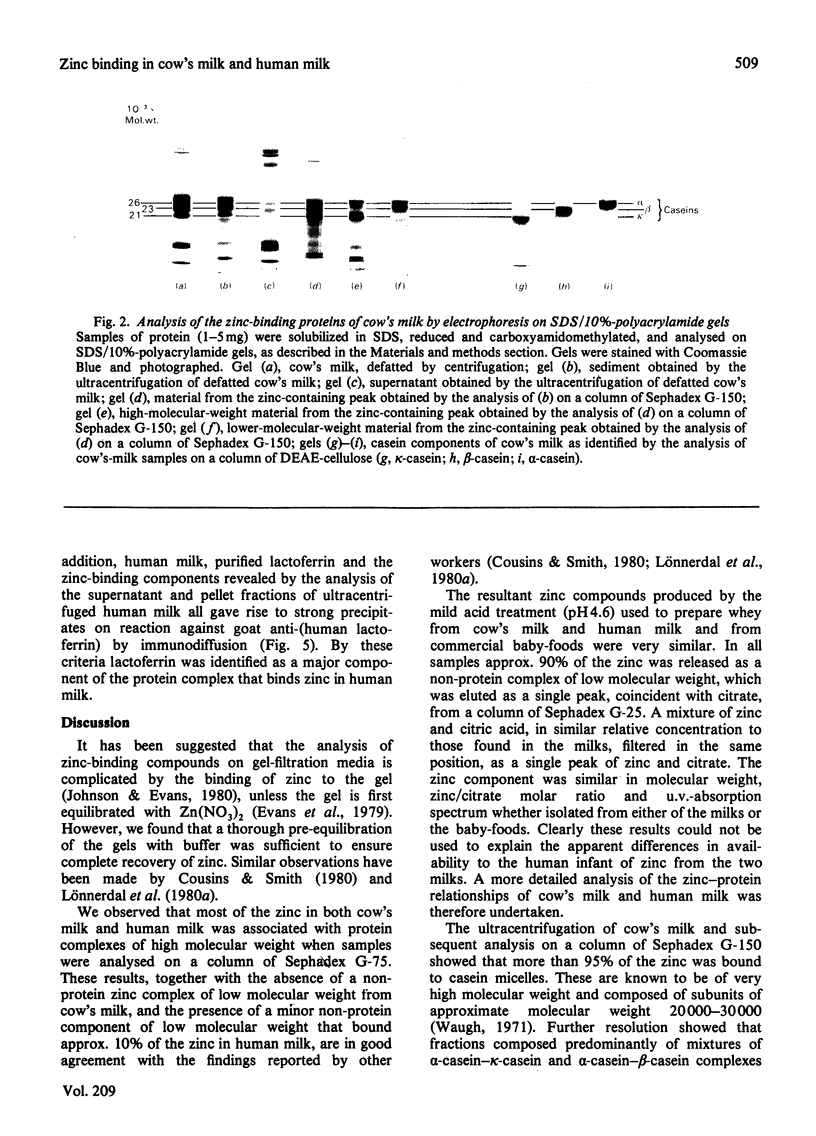
These references are in PubMed. This may not be the complete list of references from this article.
- Ainscough E. W., Brodie A. M., Plowman J. E. Zinc transport by lactoferrin in human milk. Am J Clin Nutr. 1980 Jun;33(6):1314–1315. doi: 10.1093/ajcn/33.6.1314. [DOI] [PubMed] [Google Scholar]
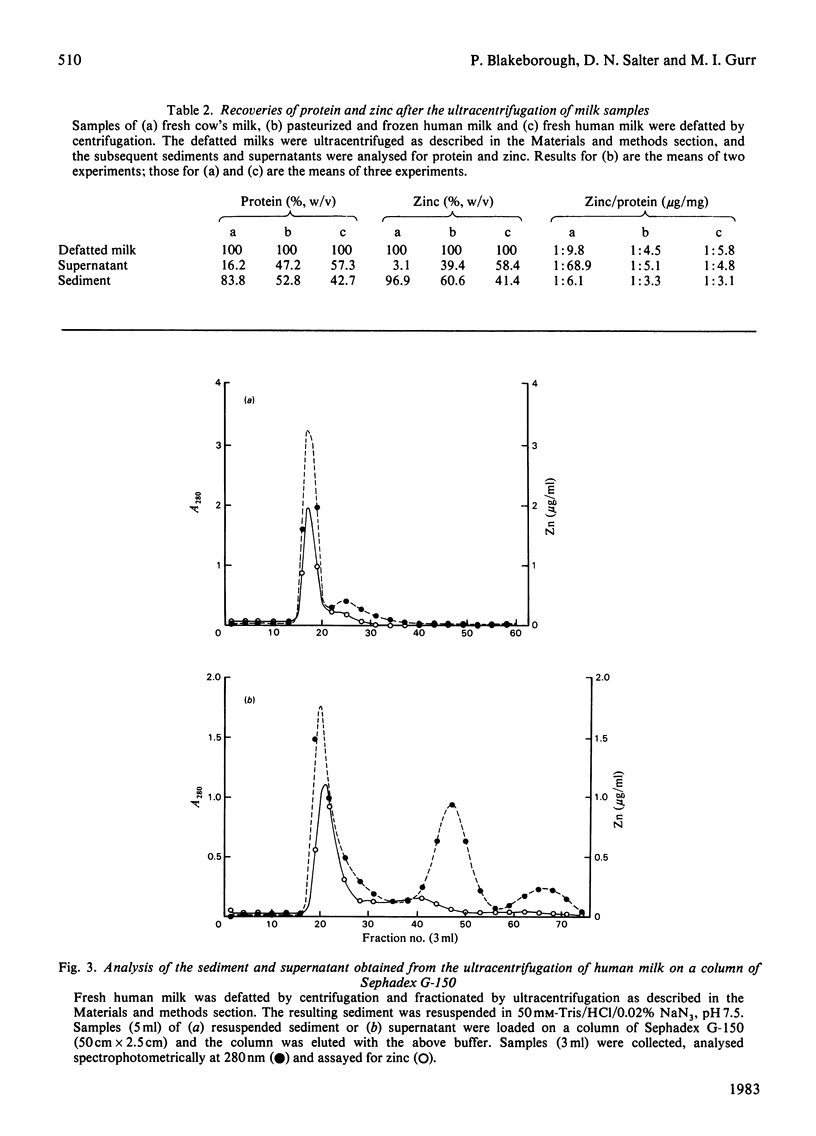
- Bläckberg L., Hernell O. Isolation of lactoferrin from human whey by a single chromatographic step. FEBS Lett. 1980 Jan 14;109(2):180–183. doi: 10.1016/0014-5793(80)81081-7. [DOI] [PubMed] [Google Scholar]
- Bullen J. J., Rogers H. J., Leigh L. Iron-binding proteins in milk and resistance to Escherichia coli infection in infants. Br Med J. 1972 Jan 8;1(5792):69–75. doi: 10.1136/bmj.1.5792.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousins R. J., Smith K. T., Failla M. L., Markowitz L. A. Origin of low molecular weight zinc-binding complexes from rat intestine. Life Sci. 1978 Oct 30;23(17-18):1819–1826. doi: 10.1016/0024-3205(78)90114-5. [DOI] [PubMed] [Google Scholar]
- Cousins R. J., Smith K. T. Zinc-binding properties of bovine and human milk in vitro: influence of changes in zinc content. Am J Clin Nutr. 1980 May;33(5):1083–1087. doi: 10.1093/ajcn/33.5.1083. [DOI] [PubMed] [Google Scholar]
- Cox T. M., Mazurier J., Spik G., Montreuil J., Peters T. J. Iron binding proteins and influx of iron across the duodenal brush border. Evidence for specific lactotransferrin receptors in the human intestine. Biochim Biophys Acta. 1979 Nov 15;588(1):120–128. doi: 10.1016/0304-4165(79)90377-5. [DOI] [PubMed] [Google Scholar]
- David G. S., Reisfeld R. A. Protein iodination with solid state lactoperoxidase. Biochemistry. 1974 Feb 26;13(5):1014–1021. doi: 10.1021/bi00702a028. [DOI] [PubMed] [Google Scholar]
- Eckhert C. D., Sloan M. V., Duncan J. R., Hurley L. S. Zinc binding: a difference between human and bovine milk. Science. 1977 Feb 25;195(4280):789–790. doi: 10.1126/science.836589. [DOI] [PubMed] [Google Scholar]
- Evans G. W., Johnson E. C. Growth stimulating effect of picolinic acid added to rat diets. Proc Soc Exp Biol Med. 1980 Dec;165(3):457–461. doi: 10.3181/00379727-165-41004. [DOI] [PubMed] [Google Scholar]
- Evans G. W., Johnson E. C. Zinc absorption in rats fed a low-protein diet and a low-protein diet supplemented with tryptophan or picolinic acid. J Nutr. 1980 May;110(5):1076–1080. doi: 10.1093/jn/110.5.1076. [DOI] [PubMed] [Google Scholar]
- Evans G. W., Johnson P. E., Brushmiller J. G., Ames R. W. Detection of labile zinc-binding ligands in biological fluids by modified gel filtration chromatography. Anal Chem. 1979 Jun;51(7):839–843. doi: 10.1021/ac50043a016. [DOI] [PubMed] [Google Scholar]
- Evans G. W., Johnson P. E. Characterization and quantitation of a zinc-binding ligand in human milk. Pediatr Res. 1980 Jul;14(7):876–880. doi: 10.1203/00006450-198007000-00007. [DOI] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Hahn C., Evans G. W., Sandstead H. H. Identification of a low molecular weight 65Zn complex in rat intestine. Proc Soc Exp Biol Med. 1973 Dec;144(3):793–795. doi: 10.3181/00379727-144-37684. [DOI] [PubMed] [Google Scholar]
- Hambidge K. M., Walravens P. A., Casey C. E., Brown R. M., Bender C. Plasma zinc concentrations of breast-fed infants. J Pediatr. 1979 Apr;94(4):607–608. doi: 10.1016/s0022-3476(79)80025-6. [DOI] [PubMed] [Google Scholar]
- Hambraeus L. Proprietary milk versus human breast milk in infant feeding. A critical appraisal from the nutritional point of view. Pediatr Clin North Am. 1977 Feb;24(1):17–36. doi: 10.1016/s0031-3955(16)33384-3. [DOI] [PubMed] [Google Scholar]
- Hurley L. S., Duncan J. R., Sloan M. V., Eckhert C. D. Zinc-binding ligands in milk and intestine: a role in neonatal nutrition? Proc Natl Acad Sci U S A. 1977 Aug;74(8):3547–3549. doi: 10.1073/pnas.74.8.3547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley L. S., Lonnerdal B., Stanislowski A. G. Zinc citrate, human milk, and acrodermatitis enteropathica. Lancet. 1979 Mar 24;1(8117):677–678. doi: 10.1016/s0140-6736(79)91132-2. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Louis C., Shooter E. M. The proteins of rabbit skeletal muscle sarcoplasmic reticulum. Arch Biochem Biophys. 1972 Dec;153(2):641–655. doi: 10.1016/0003-9861(72)90383-9. [DOI] [PubMed] [Google Scholar]
- Lönnerdal B., Keen C. L., Sloan M. V., Hurley L. S. Molecular localization of zinc in rat milk and neonatal intestine. J Nutr. 1980 Dec;110(12):2414–2419. doi: 10.1093/jn/110.12.2414. [DOI] [PubMed] [Google Scholar]
- Lönnerdal B., Stanislowski A. G., Hurley L. S. Isolation of a low molecular weight zinc binding ligand from human milk. J Inorg Biochem. 1980 Jan;12(1):71–78. doi: 10.1016/s0162-0134(00)80044-6. [DOI] [PubMed] [Google Scholar]
- Masson P. L., Heremans J. F. Lactoferrin in milk from different species. Comp Biochem Physiol B. 1971 May 15;39(1):119–129. doi: 10.1016/0305-0491(71)90258-6. [DOI] [PubMed] [Google Scholar]
- OUCHTERLONY O. Diffusion-in-gel methods for immunological analysis. Prog Allergy. 1958;5:1–78. [PubMed] [Google Scholar]
- Parkash S., Jenness R. Status of zinc in cow's milk. J Dairy Sci. 1967 Feb;50(2):127–134. doi: 10.3168/jds.S0022-0302(67)87376-4. [DOI] [PubMed] [Google Scholar]
- Querinjean P., Masson P. L., Heremans J. F. Molecular weight, single-chain structure and amino acid composition of human lactoferrin. Eur J Biochem. 1971 Jun 11;20(3):420–425. doi: 10.1111/j.1432-1033.1971.tb01408.x. [DOI] [PubMed] [Google Scholar]
- Rebello T., Lönnerdal B., Hurley L. S. Picolinic acid in milk, pancreatic juice, and intestine: inadequate for role in zinc absorption. Am J Clin Nutr. 1982 Jan;35(1):1–5. doi: 10.1093/ajcn/35.1.1. [DOI] [PubMed] [Google Scholar]
- Sandstead H. H. Zinc nutrition in the United States. Am J Clin Nutr. 1973 Nov;26(11):1251–1260. doi: 10.1093/ajcn/26.11.1251. [DOI] [PubMed] [Google Scholar]
- Smith K. T., Failla M. L., Cousins R. J. Identification of albumin as the plasma carrier for zinc absorption by perfused rat intestine. Biochem J. 1979 Dec 15;184(3):627–633. doi: 10.1042/bj1840627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song M. K., Adham N. F. Evidence for an important role of prostaglandins E2 and F2 in the regulation of zinc transport in the rat. J Nutr. 1979 Dec;109(12):2152–2159. doi: 10.1093/jn/109.12.2152. [DOI] [PubMed] [Google Scholar]
- Song M. K., Adham N. F. Role of prostaglandin E2 in zinc absorption in the rat. Am J Physiol. 1978 Feb;234(2):E99–105. doi: 10.1152/ajpendo.1978.234.2.E99. [DOI] [PubMed] [Google Scholar]
- Thompson M. P. DEAE-cellulose-urea chromatography of casein in the presence of 2-mercaptoethanol. J Dairy Sci. 1966 Jul;49(7):792–795. doi: 10.3168/jds.S0022-0302(66)87947-X. [DOI] [PubMed] [Google Scholar]
- Ugel A. R., Chrambach A., Rodbard D. Fractionation and characterization of an oligomeric series of bovine keratohyalin by polyacrylamide gel electrophoresis. Anal Biochem. 1971 Oct;43(2):410–426. doi: 10.1016/0003-2697(71)90271-5. [DOI] [PubMed] [Google Scholar]