Abstract
Protein-protein interactions between SH2 domains and segments of proteins that include a post-translationally phosphorylated tyrosine residue (pY) underpin numerous signal transduction cascades that allow cells to respond to their environment. Dysregulation of the writing, erasing, and reading of these posttranslational modifications is a hallmark of human disease, notably cancer. Elucidating the precise role of the SH2 domain-containing adaptor proteins Crk and CrkL in tumor cell migration and invasion is challenging because there are no specific and potent antagonists available. Crk and CrkL SH2s interact with a region of the docking protein p130Cas containing 15 potential pY-containing tetrapeptide motifs. This chapter summarizes recent efforts toward peptide antagonists for this Crk/CrkL-p130Cas interaction. We describe our protocol for recombinant expression and purification of Crk and CrkL SH2s for functional assays and our procedure to determine the consensus binding motif from the p130Cas sequence. To develop a more potent antagonist, we employ methods often associated with structure-based drug design. Computational docking using Rosetta FlexPepDock, which accounts for peptides having a greater number of conformational degrees of freedom than small organic molecules that typically constitute libraries, provides quantitative docking metrics to prioritize candidate peptides for experimental testing. A battery of biophysical assays, including fluorescence polarization, differential scanning fluorimetry and saturation transfer difference nuclear magnetic resonance spectroscopy, were employed to assess the candidates. In parallel, GST pulldown competition assays characterized protein-protein binding in vitro. Taken together, our methodology yields peptide antagonists of the Crk/CrkL-p130Cas axis that will be used to validate targets, assess druggability, foster in vitro assay development, and potentially serve as lead compounds for therapeutic intervention.
Keywords: Crk, CrkL, p130Cas, SH2 domains, phosphotyrosine, peptide ligands, computational docking
1. Introduction
1.1. SH2 domains
Protein-protein interactions (PPI) are essential to signal transduction cascades that allow cells to respond to their environment. A key regulatory mechanism of these signaling cascades is the post-translational modification of tyrosine residues in which the side chain hydroxyl is replaced with a phosphate (Hunter, 2012). This phosphorylation process requires “writers,” kinase enzymes that catalyze phosphoryl transfer (Z. Wang & Cole, 2014), “erasers,” phosphatase enzymes that catalyze the reverse process (Chen et al., 2017), and “readers” that evaluate the phosphorylation state of a given substrate and transmit that information downstream (Diop et al., 2022). PPIs underpin each of these steps, “reading,” “writing,” and “erasing.” Taken together, tyrosine phosphorylation represents a key regulatory mechanism of numerous biological processes, including cell signaling, differentiation, and growth. Dysregulation of these posttranslational modifications is a hallmark of human disease, notably cancer (Hunter, 2009).
A modular protein domain called the src homology 2 or SH2 domains is the most abundant and best-characterized tyrosine phosphate “reader.” The name SH2 stems from the presence of this domain in the cellular proto-oncogene tyrosine kinase analog of a gene of the Rous sarcoma virus (abbreviated src) (Sadowski et al., 1986). This protein also contains a kinase domain (technically the src homology 1 or SH1 domain) and a src homology 3 or SH3 domain that serves as a “reader” domain for PPIs with proline-rich protein segments. According to the SH2 database (Bajusz et al., 2023), a total of 110 human proteins contain one or more SH2 domains. SH2 domains consist of ~100 amino acids. The highly conserved three-dimensional structure consists of a central antiparallel beta sheet sandwiched by alpha helices on both sides. The CATH domain structure database (Greene et al., 2007) classifies this fold in the “Alpha Beta” class, “2-layer Sandwich” architecture, “SHC Adaptor Protein” topology and “SH2 domain” homology (superfamily 3.30.505.10). An array of basic amino acids in a pocket on the edge of the central sheet are key to ability of this domain to recognize a phosphorylated tyrosine residue (Waksman et al., 1993). If this binding site were the only locus of interaction driving this PPI, then SH2 domains would bind indiscriminately to any accessible phosphorylated tyrosine residues. Specificities for certain amino acid sequences adjacent to the phosphotyrosine (pY) are dictated by a second shallow binding pocket on the other edge of the central sheet (Bradshaw & Waksman, 2002). This binding motif, in which the two binding pockets flanking the central antiparallel beta sheets interact with two points of the peptide, one pY and the other downstream one to five residues (pY+1 to pY+5) is referred to as the “two-pronged plug two-holed socket” binding model and rationalizes the specificities of different members of the SH2 domain superfamily (Bradshaw et al., 1998; Kimber et al., 2000; B. A. Liu et al., 2010; Machida & Mayer, 2005; Marasco & Carlomagno, 2020; Marengere et al., 1994; Marengere & Pawson, 1994; Songyang et al., 1995; Tinti et al., 2013).
Figure 1 shows an example of the “two-pronged plug two-holed socket” binding model for the SH2 domain (yellow ribbons) of the protein Crk (which will be discussed in more detail in section 1.2.1 below) bound to a phosphopeptide (blue sticks) drawn from PDB 1JU5 (Donaldson et al., 2002). The pY binding pocket is lined with basic residues (for Crk SH2 R20 and R38 – red sticks) and hydrogen bond acceptors (for Crk SH2 S40 and S41) that are arranged in three-dimensional configuration optimized to interact with the negatively charged phosphate group of the pY sidechain. Additionally, the aromatic phenyl ring rests against an aliphatic residue (for Crk SH2 I61). Selectivity toward specific peptides stems from the other face of the central sheet. For Crk SH2, this pocket is lined with hydrophobic residues (Y60, I89 and L109) that evolved to fit proline residues. The backbone nitrogen in the pY+1 position makes a hydrogen bond with the backbone carbonyl of H59, while its side chain and the glutamine in the pY+2 position do not clash with the protein, allowing the proline in the pY+3 position to fit into the specificity pocket. Hence, Crk SH2 preferentially binds protein segments with sequence pYXXP.
Fig. 1.
Structure of Crk SH2 domain (yellow ribbons) bound to a peptide containing the residues pYAQP (blue sticks). Critical Crk SH2 residues involved in the interaction are shown as red sticks. Structures are drawn from PDB 1JU5.
Dysregulation of SH2-mediated PPIs have been implicated in human disease, including cancers (Park, 2021; Smithgall, 1995; Vidal et al., 2001), leading academic and non-academics efforts to develop peptide, peptidomimetic and small molecule SH2 antagonists (Cody et al., 2000; Kraskouskaya et al., 2013) targeting specific proteins. For example, the oncogenic transcription factor signal transducer and activator of transcription 3 (STAT3) undergoes Jak-mediated phosphorylation, resulting in dimerization via intermolecular pY-SH2 interactions, which activates STAT3 thereby upregulating target genes resulting in oncogenesis (Al Zaid Siddiquee & Turkson, 2008). Starting from its SH2-domain binding motif peptide, Turkson and co-workers used alanine scanning and chemical synthesis to develop a smaller peptidomimetic lead with four-fold greater affinity for STAT3 in in vitro assays (Turkson et al., 2004). The review by Kraskouskaya et al. details antagonist development for SH2 domains from seven targets (Stat3, Stat5, c-Src, Lck, Syk, Grb2, Grb7) (Kraskouskaya et al., 2013). In all cases, peptide are used to validate targets and assess druggability. Additionally, optimized peptide inhibitors provide invaluable positive controls for in vitro assay development. Hence, even though they may or may not serve as leads themselves, SH2-binding peptides remain an important chemical tool in rational design efforts of SH2 antagonists, particulary for understudied adaptor molecules, such as Crk and CrkL (B. A. Liu et al., 2010; Posern et al., 1998).
Though the idea of employing peptides to study proteins with SH2s is well-established, the specific tools used to design and validate the peptide-protein interaction continue to evolve. We focus on applications of FlexPepDock (London et al., 2011; Raveh, London, & Schueler-Furman, 2010), a computational tool within the Rosetta framework (Rohl et al., 2004) that enable high-resolution modeling of peptide-protein complexes, to the design of peptide inhibitors of the Crk and CrkL SH2 domain (see section 1.2 below for more details on these proteins). Biophysical screening techniques employed in structure-based discovery (Hubbard & Murray, 2011) are employed to evaluate and refine the modeling. In vitro assays provide a final link joining the computational model and biophysical screens with the actual cellular proteins on the Crk/CrkL-p130Cas axis.
1.2. Crk/CrkL-p130Cas axis
Our interest in SH2 domain antagonists stems from our efforts in elucidating the precise role of the proteins Crk and CrkL in tumor cell migration and invasion. We have recently demonstrated that glioma cell motility is a robust readout of Crk and CrkL activity (Park et al., 2021) and that overexpression of these proteins contributes to poor prognosis in several types of human cancers, including glioblastoma (Park, 2021). Hence, Crk and CrkL have been proposed as therapeutic targets for cancer treatment. Unfortunately, to our knowledge, no specific Crk and CrkL antagonists have been disclosed. Prior to describing our methodology for developing peptide antagonists for the Crk/CrkL SH2 domains, we will describe these proteins along with p130Cas, the canonical binding partner, in greater detail in sections 1.2.1, 1.2.2, and 1.2.3 below.
1.2.1. Crk
The gene c-crk is the cellular homolog (Reichman et al., 1992) of the transforming viral oncogene v-crk (chicken tumor virus 10 regulator of kinases), originally identified from chicken tumor virus no. 10 (CT10) and avian sarcoma virus (B. J. Mayer et al., 1988; Tsuchie et al., 1989). There are two isoforms of the corresponding cellular protein, called CrkI and CrkII (Reichman et al., 1992). The latter protein, CrkII, consists of 304 amino acids divided into three modular domains: an N-terminal SH2 domain (Figure 1) and two SH3 domains separated by a linker that includes a structured region called the inter-SH3 core (Kobashigawa et al., 2007). The linker between the SH3 domains includes Tyr221. Upon phosphorylation of this residue by the kinase Abl (Feller et al., 1994), CrkII undergoes domain reorientation mediated by intramolecular interactions between the phosphorylated Tyr221 and the SH2 domain that induce a closed autoinhibitory conformation (Donaldson et al., 2002; Kobashigawa et al., 2007). The other isoform, CrkI, lacks the Y221 phosphorylation site and C-terminal SH3 domain. As would be predicted from a construct lacking regulatory elements, the CrkI is significantly more transforming and oncogenic (D. Liu, 2014; Matsuda et al., 1992; Ogawa et al., 1994). Note that the transforming viral oncoprotein v-Crk also lacks these regulatory elements.
1.2.2. CrkL
In addition to CrkI and CrkII, there is another member of this family of proteins, called Crk-like or CrkL. This protein is expressed by a distinct gene (ten Hoeve et al., 1993) and consists of 303 amino acids with 52% sequence identity with CrkII. Like CrkII, CrkL contains an N-terminal SH2 domain and two SH3 domains, separated by a linker that includes the tyrosine residue (at position 207 in CrkL) that is phosphorylated by Abl kinase, resulting in intramolecular binding with the CrkL SH2 that prevents this domain from interacting with other potential ligands (Jankowski et al., 2012). The SH2 and SH3 domains for CrkII and CrkL have 72% sequence identity and bind the same consensus peptide epitopes, albeit with different affinities (Posern et al., 1998). Although initial studies suggested that Crk (CrkI and CrkII) and CrkL play different functional roles in signal transduction partly due to differences in interdomain interactions (J. Wang et al., 2011), individual and combined genetic ablations indicated that Crk and CrkL play essential overlapping roles in various biological processes (Park, 2021). Hence, designing specific antagonists for Crk and CrkL as chemical biology tools would be invaluable for unraveling the distinct and overlapping functions of these two similar proteins.
1.2.3. p130Cas
The protein p130Cas, which is also called breast cancer anti-estrogen resistance protein 1 (BCAR1), is a docking protein that interacts with a number of binding partners, including Crk and CrkL (the acronym Cas stands for Crk-associated substrate) (Bouton et al., 2001; Defilippi et al., 2006; Sakai et al., 1994). In the cell, p130Cas is an important node in signaling networks that regulate both normal and pathological cell growth (Barrett et al., 2013; Tikhmyanova et al., 2010). There are 8 isoforms in humans with the canonical isoform consisting of 870 amino acids (Camacho Leal et al., 2015). This protein is divided into six modular domains: an N-terminal SH3 domain, the proline-rich segment, the substrate domain, the serine-rich domain, a src-binding domain with epitopes that bind both SH2 and SH3 domains and a C-terminal FAT domain (Briknarová et al., 2005; Gemperle et al., 2017; Mace et al., 2011; Sakai et al., 1994; Wisniewska et al., 2005). The substrate domain contains 15 repeats of the motif YXXP which is the consensus sequence for phosphorylation by the “writers” in the Src family kinases. Upon phosphorylation, these sites are the consensus motif for “readers” from the proteins Crk and CrkL. This canonical interaction represents the starting point for our efforts to design peptide Crk/CrkL SH2 antagonists as described in Section 2.
2. Peptide design and synthesis
2.1. Introduction
The substrate domain of p130Cas has 15 YXXP motifs in which the tyrosine is phosphorylated by Src family kinases. Motifs 6–15 and 6–14 have been identified as the major binding sites for Crk SH2 (Shin et al., 2004) and v-Crk SH2 (Kirsch et al., 2002), respectively. Based on these references, we designed a consensus pY-containing peptide to serve as a positive control for antagonist development.
2.2. Procedure
Identify the YXXP motifs in the substrate domain of mouse p130Cas (https://www.ncbi.nlm.nih.gov/protein/NP_001185768.1) (Shin et al., 2004) and align them. Set the position of tyrosine as 0 and mark the relative positions of other amino acid residues. Then, calculate how many motifs have conserved amino acid residues for each position. As shown in Figure 2A, both tyrosine (position 0) and proline (position +3) were conserved in all 15 YXXP motifs. The two amino acids between tyrosine and proline, aspartate (D) and valine (V), were conserved in at least 9 motifs (Figure 2B). The positions for −2, −1, and +4 outside of the YXXP motif had the amino acid residues that were conserved in at least 6 motifs (Figure 2B, C and D). In contrast, conservation dropped beyond −2 and +4, where amino acids were conserved in less than 6 motifs. Therefore, we selected DVYDVPP as the conserved peptide sequence (see note 1).
A similar analysis of the YXXP motifs in the substrate domain of human p130Cas also led to the selection of DVYDVPP. Analyses of the YDXP motifs 6–14 in the substrate domain of mouse and human p130Cas (Shin et al., 2004) resulted in the same sequence (data not shown).
Tyrosine at 0 was replaced with pY (DVpYDVPP), generating our “positive control” peptide.
Phosphotyrosine was replaced with phenylalanine (DVFDVPP), generating our “negative control” peptide.
Peptides can be ordered from a vendor or synthesized in-house. The addition of 5-carboxyfluorescein (FAM) to the N-terminus is required for fluorescence assays (Sections 5.1 below).
Fig. 2. Designing a pY-peptide from the p130Cas substrate domain (SD).
(A) 15 YXXP motifs from the substrate domain of mouse and human p130Cas were aligned. Conserved tyrosine and proline residues are indicated in red. (B) The position of tyrosine was set as 0, and the relative positions of other amino acid residues were marked. The number of motifs for each amino acid residue was counted. (C) The top 3 conserved amino acids were plotted for each position. The amino acids that are conserved in at least 6 motifs were selected. (D) The sequence logo is based on the multiple sequence alignment in panel A and is generated by WebLogo (https://weblogo.threeplusone.com).
2.3. Notes
A longer peptide can be designed by accommodating less conserved amino acid residues at the N- and C-termini. While this may increase the specificities for some YXXP motifs, this may also decrease the affinities of other YXXP motifs. Since Schust et al. (Schust et al., 2006) used a heptapeptide and were able to identify a compound that selectively inhibited STAT3 compared to other STAT proteins, we hypothesized that a heptapeptide containing the YXXP motif would be an optimal length for the starting peptide sequence.
3. Protein expression and purification
3.1. Introduction
Recombinant protein expression in Escherichia coli is a ubiquitous technique in biochemistry and biotechnology that has been reviewed extensively (Baeshen et al., 2015; Gileadi, 2017; Jia & Jeon, 2016; Rosano et al., 2019; Structural Genomics Consortium et al., 2008). We will limit our discussion in this section to the strategy we employed to express SH2 domains from Crk and CrkL. Plasmid DNAs for expressing the SH2 domains in E. coli can be generated by the conventional molecular cloning techniques (Berger & Kimmel, 1987) or the whole plasmids can be synthesized by a vendor. An important strategic decision to consider is whether or not to include glutathione S-transferases (GST), the polyhistidine tag, or other affinity tags (Lichty et al., 2005; Schäfer et al., 2015) to the construct. These tags simplify purification from bacterial cell lysates, add a supplementary epitope tag to facilitate detection in various biochemical assays without requiring a protein-specific antibody, and may stabilize the construct, which could increase the yield. On the other hand, affinity tags may complicate the screening of small molecules. These tags can be removed chemically or enzymatically, although this step necessitates additional purification. Our protocol below includes options for expression and purification with or without the GST tag. Depending on which assay the protein is to be used for, we expressed and purified proteins with and without the affinity tag. Note that counter-screens against the GST protein alone may be required if the GST-tagged protein is used for small molecule screening.
3.2. Equipment and supplies
Excella E24 incubator shaker (New Brunswick Scientific) for bacterial culture with shaking
Infinite M200 Pro NanoQuant microplate reader (Tecan) for absorbance measurement
Sorvall Legend XFR centrifuge and Sorvall Legend Micro 21R centrifuge (Thermo Scientific) for high-speed centrifugation
Sonic Dismembrator ultrasound processor (Fisher Scientific) for bacterial cell lysis
iBright FL1000 imaging system (Thermo Fisher Scientific) for imaging of GelCode Blue-stained gels
Disposable plastic scintillation vials, 20 mL for sonicating bacterial cells
Gel loading tips (Thermo Fisher Scientific) for washing glutathione agarose beads and retrieving protein samples from dialysis cassettes
Slide-A-Lyzer G2 dialysis cassette, 10K MWCO, 0.5–3 mL (Thermo Scientific)
3.3. Reagents
pFLAG-CMV-5a vector (Sigma Aldrich)
pGEX-4T-3 and other pGEX vectors (GE Healthcare)
BL21(DE3) competent E. coli (New England Biolabs)
Isopropyl β-D-1-thiogalactopyranoside (IPTG) (Fisher Scientific)
Phosphate-buffered saline (PBS), 10X, pH 7.4 (Gibco, Life Technologies)
Glutathione agarose beads (Pierce, Fisher Scientific)
Complete Mini (EDTA-free) (Roche, Sigma Aldrich)
Glutathione, reduced (Fisher Scientific)
Thrombin (GE Healthcare, Fisher Scientific)
Phenylmethanesulfonyl fluoride (PMSF) (Fisher Scientific)
NuPAGE Bis-Tris 4–12% precast gels (1.0 mm, 15-well), NuPAGE LDS sample buffer (4X), NuPAGE sample reducing agent (10X), NuPAGE MES SDS running buffer (20X), and NuPAGE antioxidant (all from Invitrogen, Thermo Fisher Scientific)
SeeBlue Plus2 (Invitrogen, Thermo Fisher Scientific) or Chameleon Duo (Li-COR) pre-stained protein ladder
GelCode Blue stain reagent (ThermoFisher Scientific)
3.4. Procedure
Construct plasmid DNAs for GST- and FLAG-tagged Crk and CrkL SH2. We cloned mouse Crk cDNA 1 – 354, which encodes mouse Crk protein 1 – 118 identical to human CRK protein. We cloned mouse CrkL cDNA 1 – 306, which encodes mouse CrkL protein 1 – 102 with one amino acid difference from human CRKL protein.
Re-transform BL21(DE3) competent E. coli with plasmids for GST-CrkSH2-FLAG or GST-CrkLSH2-FLAG for the production of GST-tagged proteins in bacteria, pick a single colony for each construct, transfer it to a sterile plastic tube containing 5 mL LB broth plus ampicillin, and culture overnight at 37°C in a bacterial shaker.
Next morning, transfer the bacterial cells to large flasks containing 225 mL LB broth plus ampicillin and measure the absorbance at 600 nm. Culture the bacterial cells in the large flasks for longer (approximately 2–3 hours) at 37°C in a bacterial shaker until the net absorbance by bacteria reaches 0.6–0.8.
Remove the flasks containing the bacterial cells and place them at room temperature. Aliquot 1 mL bacterial suspension to microcentrifuge tubes, centrifuge at 9,600 x g for 4 min, remove the supernatants, and store the bacterial cell pellets at −80°C (“Pre-IPTG”). Cool down the bacterial shaker to room temperature (RT).
Add IPTG to bacterial cells to the final concentration of 1 mM and culture at RT in a bacterial shaker overnight to induce expression of GST-tagged proteins (see note 1).
Next morning, determine the net absorbance value. Aliquot 1 mL bacterial suspension to microcentrifuge tubes, centrifuge at 9,600 x g for 4 min, remove the supernatants, and store the bacterial cell pellets at −80°C (“Post-IPTG”).
Transfer the bacterial cells to 250 mL conical centrifuge tubes and centrifuge cells at 4,000 x g for 20 min at 4°C. Place the conical centrifuge tubes containing bacterial cell pellets on ice, add 10 mL of ice-cold 1X PBS plus Complete Mini, and resuspend the bacterial cells.
Transfer the bacterial cell suspensions to 20 mL plastic scintillation vials on ice and sonicate them using a 1/2-inch probe tip for 2 min with 50% amplitude, alternating between 10-second bursts and 10-second pauses. Clean the sonicator probe before and after sample sonication with 70% ethanol and 1X PBS.
Transfer the bacterial cell lysates to 50 mL conical centrifuge tubes and centrifuge them at 500 x g for 5 min at 4°C. The supernatants have cleared bacterial cell lysates that are ready for protein purification. Transfer 50 μL of the supernatants to microcentrifuge tubes and store at −80°C (“Cleared lysate”).
Gently invert the bottle containing glutathione agarose slurry to make it homogenous and transfer 3 mL of glutathione agarose slurry to 15 mL centrifuge tubes on ice. Add 8 mL of ice-cold PBS containing Complete Mini, mix by tapping/inverting, and centrifuge at 500 x g for 5 min at 4°C. Then, remove the supernatants. Repeat the wash one more time.
Transfer the cleared bacterial cell lysates (from 9) to the 15 mL tubes on ice containing washed glutathione agarose beads (from 10). Rotate the 15 mL tubes in the cold room overnight to precipitate GST-tagged proteins with glutathione agarose beads.
Next day, centrifuge the 15 mL tubes containing glutathione agarose beads at 500 x g for 5 min at 4°C. Transfer 50 μL of the supernatants to microcentrifuge tubes and store at −80°C (“GluAgarose-Sup”). Discard the rest of the supernatants.
Wash the remaining glutathione agarose beads by adding 8 mL of ice-cold PBS plus Complete Mini, gently resuspending by tapping/inverting, and centrifuging at 500 x g for 5 min at 4°C. Remove the supernatants with 10 mL pipettes. Centrifuge again at 500 x g for 1 min at 4°C and remove the supernatants using a P1000 pipette tip with a gel loading tip to remove the residual supernatants. Repeat the wash two more times. The pellets contain the glutathione agarose beads to which GST-tagged proteins are bound and are ready for the next step, Option 1 or 2.
Prepare reduced glutathione for Option 1 as follows. Prepare 50 mL of 50 mM Tris-HCl and adjust the pH to 8.0. Transfer 20 mL of 50 mM Tris-HCl to a new 50 mL tube and add 61.6 mg of reduced glutathione to make 10 mM reduced glutathione. Adjust the pH to 9.0. Add Complete Mini and mix by vortexing.
Option 1: purify proteins with the GST tag using reduced glutathione as follows. Add 3 mL of the reduced glutathione solution (from 14) to the glutathione agarose beads in 15 mL tubes and rotate in the cold room for 3 hours. Centrifuge at 500 x g for 5 min at 4°C and transfer the supernatants to new 15 mL tubes on ice using a P1000 pipette tip with a gel loading tip to minimize taking the beads. The samples are ready for dialysis. Transfer 50 uL of the supernatants to microcentrifuge tubes and store them at −80°C (“GluAgarose-Glu-Sup”). Add 3 mL of reduced glutathione solution to the remaining glutathione agarose beads, mix by vortexing, take 0.2 mL of slurry to microcentrifuge tubes, and store the samples at −80°C (“GluAgarose-Glu-Beads”). The supernatant and bead samples will be compared to analyze the efficiency of the elution using reduced glutathione.
Option 2: purify proteins without the GST tag using thrombin digestion as follows. Add 2.95 mL of PBS and 50 μL of thrombin (containing 50 U) to the glutathione agarose beads (from 13) and rotate at RT for 3 hours. At the end of the rotation, add PMSF to the final concentration of 1 mM to stop thrombin activity. Centrifuge at 500 x g for 5 min at 4°C and transfer the supernatants to new 15 mL tubes on ice using a P1000 pipette tip with a gel loading tip to minimize taking the beads. The samples are ready for dialysis. Transfer 50 μL of the supernatants to microcentrifuge tubes and store the samples at −80°C for SDS-PAGE and GelCode Blue staining (“GluAgarose-Thr-Sup”). Add 3 mL of reduced glutathione solution to the remaining glutathione agarose beads, mix by vortexing, take 0.2 mL of slurry to microcentrifuge tubes, and store the samples at −80°C (“GluAgarose-Thr-Beads”). The supernatant and bead samples will be compared to analyze the efficiency of the thrombin digestion.
Prepare dialysis buffer containing 25 mM HEPES (pH 7.5) and 50 mM NaCl. In the cold room, transfer 500 mL of dialysis buffer to 500 mL beakers with magnetic stirrers and soak the dialysis cassettes in the dialysis buffer to hydrate the dialysis membranes. Open the dialysis cassettes and transfer 3 mL of protein samples from Options 1 (from 15) or 2 (from 16) to the dialysis cassettes using P1000 tips with gel loading tips, remove the air by pressing the dialysis membranes on both sides, and close the cassettes. Gently mix the dialysis buffer in beakers overnight by stirring with a magnetic bar. Next morning, change with fresh dialysis buffer (500 mL/beaker) and continue the dialysis for 2 more hours.
In the cold room, transfer dialyzed protein samples from the dialysis cassettes to 50 mL tubes on ice using a P1000 tip with a gel loading tip. Aliquot the purified protein samples and store at −80°C for future experimental use. Transfer 50 μL of the samples to microcentrifuge tubes and store at −80°C (“GluAgarose-Glu-Sup-Dial” or “GluAgarose-Thr-Sup-Dial”).
Lyse the bacterial cells obtained before and after the IPTG induction as follows. Recover the bacterial cell pellets from −80°C and thaw them on ice. Add 0.25 mL and 0.5 mL of ice-cold PBS plus Complete Mini to “Pre-IPTG” samples (from 4) and “Post-IPTG” samples (from 6), respectively. Sonicate the bacterial cells in 1.5 mL microcentrifuge tubes using a 1/16-inch probe tip with 20% amplitude and 3 cycles of 10-second bursts and 20-second pauses. Clean the sonicator probe before and after sample sonication with 70% ethanol and 1X PBS. Centrifuge the bacterial cell lysates at 13,800 x g for 20 min at 4°C and transfer the supernatants to new microcentrifuge tubes for protein quantification, SDS-PAGE, and GelCode Blue staining.
After quantifying protein samples, mix protein samples with NuPAGE LDS sample buffer and NuPAGE sample reducing agent, and heat the samples at 85°C for 5 min for denaturation. Perform SDS-PAGE with NuPAGE Bis-Tris 4–12% precast gels (1.0 mm, 15-well), NuPAGE MES SDS running buffer, and NuPAGE antioxidant.
Remove the polyacrylamide gels, place them in square petri dishes, rinse them with pure H2O several times, stain with GelCode Blue (20 mL/gel/tray), and destain with pure H2O overnight. Acquire images of GelCode Blue staining of protein samples and quantify the bands using the iBright FL1000 imaging system or other imaging systems.
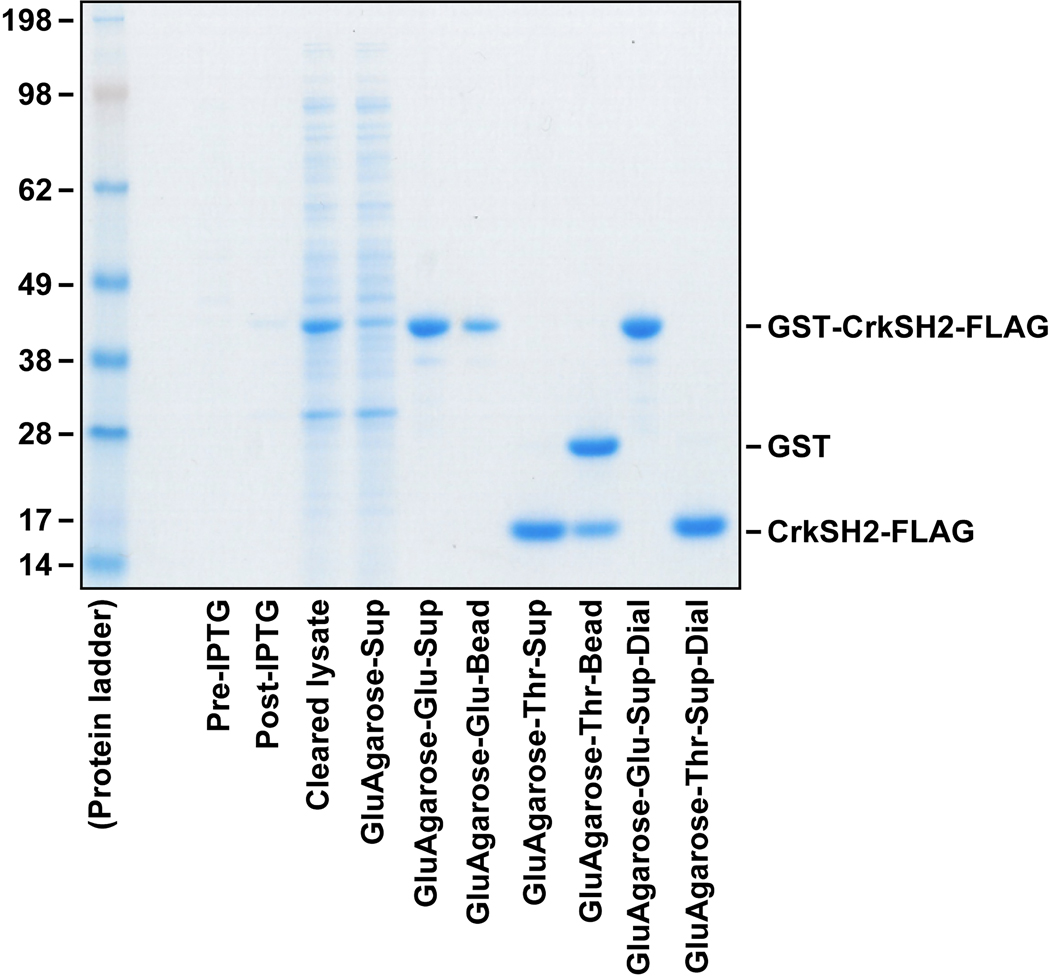
Finally, analyze the efficiencies of the purification steps and determine the purities and quantities of the purified proteins (Figure 3).
Fig. 3. GelCode Blue staining of proteins at various protein purification steps.
Two micrograms of protein samples collected from each purification step were subjected to SDS-PAGE and GelCode Blue staining.
3.5. Notes
Optimal temperatures and incubation times for IPTG induction can be varied depending on the proteins. Regarding the SH2 domains of Crk and CrkL, IPTG induction at RT overnight produced a large quantity of proteins. When we tested different temperatures (20°C, 25°C, 30°C, and 37°C), the yields for Crk SH2 were similar. When we compared induction time between 3 h and overnight, an overnight induction produced a more protein amount for Crk SH2, although a 3-hour induction also produced a significant amount of protein. However, for the substrate domain of p130Cas SD, the overnight induction produced a much lower yield than the 3-hour induction. Therefore, the optimal induction time and temperature should be determined for each protein.
Initially, we added glycerol and Complete Mini to the dialysis buffer to stabilize purified proteins and prevent protein aggregation (Vagenende et al., 2009), and we did not have any problems in using the purified proteins for some biochemical assays (see section 5 and 6 below). However, glycerol and Complete Mini would produce significant background signals in other assays. We purified Crk SH2 and CrkL SH2 proteins without adding glycerol or Complete Mini after dialysis for these assays.
4. Modeling of peptide–protein complexes
4.1. Introduction
Computational docking to model protein-ligand interactions plays a central role in modern medicinal chemistry and chemical biology research (Cosconati et al., 2010; Stanzione et al., 2021). Many software packages for computational docking are available (Forli et al., 2016; Sousa et al., 2013). At the core, these platforms provide predictions of the conformation and binding free energy of a molecule to its target, thereby (hopefully) directing the discovery process toward favorable ligands. Peptide ligands present additional computational challenges compared with small molecule ligands, primarily due to the need to account for the greater number of degrees of conformational freedom expected for peptide ligands (Ciemny et al., 2018). The program Rosetta FlexPepDock Ab initio accounts for both protein and peptide ligand flexibility using Monte-Carlo refinement and minimization of the peptide backbone along with side chain optimization (Raveh et al., 2011; Raveh, London, & Schueler-Furman, 2010). Though more computationally intensive than alternatives that treat the receptor as rigid, FlexPepDock generates more accurate predicted structures (Marcu et al., 2017; Tubert-Brohman et al., 2013), hence we employ FlexPepDock to model peptide-SH2 interactions.
Peptides enjoy at least one computational advantage over small molecules. Due to their modular nature, structure-activity relationships can be quantified easily using changes in binding free energy upon mutation (ddG; predicted ΔΔG). This metric assesses whether changing a particular side chain would be predicted to be thermodynamically advantageous or deleterious. We employed two ddG algorithms in Rosetta, Cartesian and Flex ddG. Cartesian ddG iteratively repacks and minimizes residues within 6 Å of the mutated residue (Frenz et al., 2020). Flex ddG generates an ensemble of backbone conformations to find an average ddG value across the ensemble, which facilitates sampling of side chains that are significantly different than the starting sequence (Barlow et al., 2018). Taken together, computational docking of peptide-receptor interactions using FlexPepDock along with Flex and Cartesian ddG plays a central role in our research protocol.
4.2. Software and Computational Resources
Rosetta commit 63c0207e7234c2f2a0922eb7a295603d4ad8c0b6 was used for this work. The following individual programs were employed for the following tasks:
RosettaCM – Homology modeling
Relax – Iterative repacking and refinement to sample 1,000 low energy protein conformations
Minimize – Gradient-based minimization of a protein structure
Cartesian_ddg – Prediction of ΔΔG
Flex ddG – Prediction of ΔΔG, incorporating increased receptor flexibility
FlexPepDock – Protein-peptide docking to generate 1,000 docked models
Hardware:
Rosetta requires a Unix-like operating system, such as Linux or MacOS X. For this project, calculations were performed using the hardware listed below. We would expect analogous results with analogous hardware configurations.
Most tasks were performed on a MacBook Pro laptop, 16 GB RAM, 10 compute cores
More computationally intensive tasks were performed on Linux nodes in the High Performing Computer facility operated by the Center for Research Computing at the University of Kansas. The exact configurations vary.
4.3. Procedure
4.3.1. Approach
Our iterative approach of incorporating knowledge from experimental data to improve the reliability of the computational docking is summarized in Figure 4. Computational docking is possible because Crk and CrkL SH2 structures in complex with phosphopeptides are deposited in the PDB. For Crk, the SH2 domain structure is included in PDB ID 1JU5 (bound) and 2EYV (unbound) (Donaldson et al., 2002; Kobashigawa et al., 2007). For CrkL, the SH2 domain structure is included in PDB ID 2LQN (unbound) and 2LQW (bound) (Jankowski et al., 2012). All structures for Crk and CrkL SH2 domains were solved using Nuclear Magnetic Resonance (NMR) spectroscopy data. Prior to using FlexPepDock, these structures required remodeling and refinement, as described below. These receptors, along with peptide ligands, are the input to the computational docking methods used. We initially benchmarked docking protocols (the set of flags, constraints, and post-docking analysis strategies) using the positive and negative control peptides, as described below. Once initial docking protocols were set, we performed computational screening on a series of “mutant” peptides by swapping a given amino acid for another of the canonical amino acids to determine candidates for binding experiments as described in sections 5 and 6 below. It is important to appreciate that this iterative process evolves as new information is uncovered and the protocols are refined to improve performance.
Fig. 4. Schema representing the general approach for the use of molecular modeling for peptide design.
4.3.2. Remodeling the Crk SH2-peptide complex and benchmarking FlexPepDock
The first benchmark of FlexPepDock is the trivial case of docking the native peptide sequence to Crk SH2, with success measured by the recapitulation of the experimentally-determined bound structure. Curiously, the initial FlexPepDock output structures did not match the structure in the PDB. To understand this result, we reexamined the experimentally-determined Crk SH2 structure. This structure (PDB entry 1JU5) is a ternary structure of Crk SH2 bound to both the phosphopeptide and Abl SH3 domain that was published more than 20 years ago (Donaldson et al., 2002). Using Rosetta’s force field to evaluate the energetics of this structure reveals an overall unfavorable energy due to the number of steric clashes, unfavored side chain rotamers, and backbone phi and psi angles in Ramachandran unfavored regions. Moreover, Molprobity (Williams et al., 2018), an online structure validation tool, scored this structure in the 3rd percentile for both the Clash and Molprobity scores. Hence, we determined that the experimentally-determined bound conformation would be unlikely to be sampled in our simulations because of its backbone torsion angles and side chain rotamers. To address this issue, in the absence of NMR data, we re-modeled the Crk SH2-peptide interaction using RosettaCM with the NMR model as the template (Song et al., 2013). To simplify the calculation, we removed the so-called “DE loop,” a proline-rich loop located between β-strand D and E (residues S65-S85) from the SH2 domain. This region contains the binding epitope to the Abl SH3 domain and is not involved in the interactions between phosphopeptides and Crk SH2. The RosettaCM structure with the best packing of the pYXXP motif, as determined by visual inspection, was relaxed using Rosetta, then FlexPepDock was run using the top-scoring model as input. The resulting structure recapitulated the input structure, thereby validating our computational docking protocol on the trivial case.
The second benchmark was docking the positive control (DVpYDVPP), negative control (DVFDVPP), and a comparable portion of the native (GPpYAQPS) peptide using FlexPepDock, with success measured by a strong preference for the positive control over the negative control. The peptide sequences were threaded onto the new, re-modeled complex, removing extra residues not found in the heptapeptides. Figure 5 shows the calculated structures of the docked positive and negative control peptides. Though the calculated structures of the bound positive and negative control peptides seem similar at first glance, there are notable differences. In addition to the lack of the electrostatic and hydrogen bond interactions between the phosphate group and R38, R20, S40, and S41 there is significantly less engagement of the proline into the specificity pocket. FlexPepDock outputs several quantitative metrics: the score (e.g., the energy of the entire complex in Rosetta Energy Units), the energy of the interfacial residues, the energy of the peptide (accounting for strain), a reweighed score that combines the scores of the complex, interface, and peptide, a count of hydrogen bonds and a count of interfacial buried unsatisfied polar groups. In all metrics, the positive control peptide docking outperformed the negative control, as summarized in Table 1. Furthermore, the positive control performed comparably with the native peptide in all metrics, which could be expected due to the input structure being optimized for the native peptide. In fact, the positive control had fewer buried unsaturated polar groups and lower interface energy, leading to the hypothesis that the positive control (DVpYDVPP) would bind with comparable affinity at the native peptide (GPpYAQPS). Later, modeling substituted peptides identified that minimizing the complex before FlexPepDock, rather than prepacking, led to more consistent results. This, and any other potential change in the protocol, was benchmark tested with the controls to ensure that results remained consistent with experimental data.
Fig. 5. Comparison of positive and negative controlled docking.
Positive control complex (green spheres and cyan sticks) with the negative control peptide conformation (violet sticks) are overlaid.
Table 1:
FlexPepDock metrics from native, positive, and negative control benchmarking.
| Peptide | Score | Reweigh score | #HB | #BUns | Interface score | Peptide score |
|---|---|---|---|---|---|---|
| Native: GPpYAQPS | −323.3 | −381.2 | 9 | 8 | −35.7 | −22.2 |
| Pos: DVpYDVPP | −320.7 | −378.9 | 10 | 3 | −38.1 | −20.1 |
| Neg: DVFDVPP | −303.6 | −340.1 | 2 | 1 | −25.7 | −10.5 |
Scoring units are in Rosetta energy units (REU).
4.3.3. Design and modeling of substituted peptides
The long-term goal of this project is the development of optimized Crk and CrkL SH2 antagonist peptides. This section will outline our general computational strategy. Given that there are millions of heptapeptide sequences with a pY in the third position, we employ a two-step strategy for rapid screening to select peptides most likely to bind tightly. In the first step, we triage unfavorable mutations based on Cartesian ddG, which mutates the residue and locally refines the complex, and Flex ddG, which samples backbone conformations before mutating the residue(s) and locally refining the complex for each backbone. Top-scoring peptides in step one are docked using FlexPepDock in the second step. Top-scoring peptides from FlexPepDock are evaluated and specific constructs are tested experimentally using techniques described in Sections 5 and 6. The results from these experiments provide information to improve the docking protocols and our selection criteria.
5. Characterization of peptide-protein interaction
5.1. Fluorescence polarization (FP) assay
5.1.1. Introduction
The fluorescence polarization (FP) assays are based on the differential mobility of free peptide compared to a protein-bound probe. A fluorescent dye, in our case 5-carboxyfluorescein (FAM), is covalently attached to the peptide for this assay. Upon excitation by linearly polarized light, this fluorophore on protein-bound peptides rotates slowly, emits polarized light, and has a high intrinsic polarization value within its fluorescence lifetime. On the other hand, for unbound FAM-conjugated peptides, the fast-rotating fluorophore depolarizes and has low intrinsic polarization values. The FP assay is used widely in high-throughput screening for small molecule drug discovery (Du, 2015; Hendrickson et al., 2020).
5.1.2. Equipment, reagents, and supplies
We employed an Agilent Synergy NEO HTS Multi-mode Microplate reader fluorescence spectrometer and black 384-well microplates (Corning, Cat No 3575) with 20 μL total volume per well. FP filter cubes corresponding to 480-nm excitation and 535-nm emissions were used for the measurements. We would expect analogous results using equivalent instrumentation and equipment.
The FP values were calculated according to Equation 1,
| (eq. 1) |
where S is the parallel emission intensity, P is the perpendicular emission intensity, and G is the grating factor. The value of the G factor generally ranges from 0.9 to 1.2, depending on the instrument. The units of FP are milliP (or mP), which measure the fraction of the probe bound to the protein. All of the experiments were independently repeated at least three times.
5.1.3. Procedure
The first step is to determine the optimal buffer and receptor/ligand concentrations at which to perform this assay in 384-well format. N-terminal labeled phosphorylated peptide, FAM-DVpYDVPP (2 nM, 4 nM, and 8 nM), as well as negative control peptide (FAM-DVYDVP), were titrated against increasing concentrations of Crk SH2 protein (10 nM to 20 μM) in 10 different buffers with FP read at 30-min intervals until 3 h after incubation at 30°C (data not shown). The maximum FP signal was observed with 150 nM Crk SH2 and 4 nM phosphorylated peptide in 20 mM HEPES pH 7.5. Next, the impact of additives was assessed. DTT, NaCl, Triton X-100, CHAPS, DMSO, and glycerol did not affect polarization values significantly (data not shown). Next, a time course experiment was performed to identify the optimal time of incubation and the stability of components (data not shown).
In principle, the FAM moiety could perturb the thermodynamics and/or kinetics of the peptide-protein interaction relative to the nonconjugated peptide. To verify that the activities of the FAM-conjugated peptides are not compromised by the fluorophore, we employ an FP competition assay as a final control. In this assay, a mixture containing 4 nM of FAM-labeled phosphorylated peptide and 150 nM Crk SH2 was incubated with unlabeled competitor peptides. The binding of Crk SH2 with FAM-DVpYDVPP was effectively competed out in the presence of unlabeled DVpYDVPP and much less significantly with the unphosphorylated DVYDVPP or DVFDVPP peptide (data not shown). The mP values were determined, and the concentrations required for 50% displacement of the probe peptide were calculated using non-linear regression analysis in GraphPad Prism 9.
The results of the FP specificity assay are shown in Figure 6A. In this assay various concentrations of FAM-conjugated peptides are titrated into 4 nM Crk SH2 in 20 mM HEPES pH 7.5 and 0.025% Triton X-100. For the FAM-conjugated positive control peptide (FAM-DVpYDVPP, black), a significant increase in FP is measured, whereas for the FAM-conjugated negative control (FAM-DVYDVPP) no significant increase in FP is observed.
Fig. 6. Biophysical screens of interactions between peptide ligands and Crk and Crk SH2 domains.
(A) Specificity of Crk SH2 FP assay for phosphorylated peptide (FAM-DVpYDVPP, black). No polarization is detected with unphosphorylated peptide (FAM-DVYDVPP, gray). (B) Crk SH2 thermal unfolding curve (dashed line) was found to shift to the right in the presence of the phosphorylated peptide (DVpYDVPP, black), whereas no significant shift in Tm was observed with the unphosphorylated peptide (DVYDVPP, gray). (C) Stack plot of NMR spectra showing aromatic moieties for peptides DVpYDVPP (left) and DVFDVPP (right). The off-resonance saturation spectrum is shown on the bottom and used as a control. Stacked above this control are STD spectra of samples containing 2000 μM peptide and 20 μM Crk SH2 (2nd from bottom), 20 μM CrkL SH2 (3rd from bottom), and protein-free control (top).
5.2. Differential scanning fluorimetry (DSF) assay
5.2.1. Introduction
The differential scanning fluorimetry (DSF) assay (also known as the thermal shift assay (TSA)) relies on an increase in protein thermal stability upon ligand binding (Matulis et al., 2005) and is widely used in drug discovery efforts (Gao et al., 2020). A target protein with and without ligand is melted in the presence of SYPRO Orange dye, which fluoresces upon binding to hydrophobic regions of protein exposed during thermal denaturation. Plotting fluorescence or the first derivative of fluorescence versus temperature produces a melting curve from which the melting temperature (Tm), defined as the temperature at which half the population is unfolded or denatured, is determined. The thermal stabilization (ΔTm), which is defined as the change in protein Tm upon ligand binding, is proportional to the concentration and affinity of the ligands.
5.2.2. Equipment, reagents, and supplies
We employed a Lightcycler Roche 480, SYPRO Orange (Invitrogen, S6650, 5000X), and Roche LightCycler PCR plates (catalog No. 04729749001) for this assay. We would expect equivalent results with equivalent instrumentation.
5.2.3. Procedure
The assay is performed using 384-well PCR plates. Peptides in DMSO or buffer were dispensed acoustically using the Echo 655 (Beckman, Brea, CA). Crk proteins (final concentration of 10 μM) were added using Multidrop Combi (ThermoFisher). The plates were centrifuged at 1000 rpm for 1 min and sealed with Top Seal (Revvity Inc.). The protein was preincubated with peptides for 30 min at room temperature, followed by the addition of SYPRO orange. Roche LightCycler 480 instrument was set to run the temperature scan from 25 to 95°C at a rate of 0.2°C/s. The fluorescence reading for each well (excitation window, 470–505 nm; absorption window, 540–700 nm) was analyzed using the Roche LightCycler 480 Protein Melting Analysis Tool and used for Tm calling to identify the temperature at which the first derivative of the unfolding curve is maximal (see note 1).
5.2.4. Optimization
The DSF assay also requires optimization of buffer conditions. Twenty-two buffers ranging in pH from 5 to 10 were tested using Crk SH2 thermal melting. The buffer (in this case, 50 mM HEPES, pH 7.5) in which the melting curves had the highest fluorescence with a single peak/valley, Tm of 50.7°C, was selected for further optimization. Titration of both Crk SH2 protein (5, 7.5, and 10 μM) and SYPRO Orange dye (5X, 8X, and 10X) was performed, and the conditions with single peaked curves of high fluorescence were selected for further experiments. To study the effect of DMSO/peptides, 8 μL samples containing purified Crk SH2 protein (10 μM) were incubated with 10X SYPRO Orange in the presence of various concentrations of peptides (300, 200, 100, 75, 50, 25, 12.5, 6.25, and 0 μM) or various concentrations of DMSO (0, 0.25, 0.5, 1, and 2%) in 50 mM HEPES, pH 7.5, and 150 mM NaCl. As shown in Figure 6B, unfolding curves shifted with the binding of Crk SH2 to phosphorylated peptide (DVpYDVPP). There was no significant binding to the unphosphorylated DVYDVPP peptide.
5.2.5. Notes
In general, the reactions can be set up in any available qPCR machine with its own compatible protein melt analysis software. A number of open analysis tools are also available (Lee et al., 2019; Rosa et al., 2015; C. K. Wang et al., 2012; T. Wu et al., 2020).
5.3. Saturation transfer difference (STD) nuclear magnetic resonance (NMR) spectroscopy
5.3.1. Introduction
Saturation transfer difference (STD) nuclear magnetic resonance (NMR) probes interactions between small-molecule ligands and macromolecular receptors, such as a protein or a protein domain. In its standard form, this experiment requires no isotopic enrichment of the ligand or macromolecule. Concentrations of the ligand are usually in the range of 10−3 moles/L. Concentrations of the protein are usually in the range of 10−5 moles/L. Given these sample demands, it is not surprising that STD NMR is widely used in screens of libraries of 102-104 compounds in drug discovery efforts in academic and non-academic settings (Begley et al., 2013; Harner et al., 2013; Hubbard & Murray, 2011). STD can be used to provide a yes-no answer to the question of whether a given ligand binds to a protein with Kd in the 10−3-10−8 M range (Viegas et al., 2011). Additionally, STD can be used to assess the binding epitope of a ligand (Cala & Krimm, 2015; M. Mayer & Meyer, 2001) and to quantify binding constants (Angulo et al., 2010; Y. Wang et al., 2004) in some cases. Peptide-protein interactions have been studied using STD-NMR, though careful attention to acquisition and processing parameters is required to avoid false positives (Rauber & Berger, 2012), as discussed below.
The spin physics that underpins the STD experiment has been described in detail elsewhere (Angulo & Nieto, 2011; M. Mayer & Meyer, 1999). Briefly, the STD experiment relies on equalizing the population of lower energy “spin-up” and higher energy “spin-down” angular momentum quantum states of protons in the macromolecule. This process is called “saturation”. Signals from saturated protons vanish from the NMR spectrum in the best-case scenario, hence this technique is widely used for water suppression (Hoult, 1976). By analogy to optical spectroscopy, saturation can be thought of as temporary, reversible photobleaching. In the STD experiment, this saturation is transferred intramolecularly and, potentially, intermolecularly by the Nuclear Overhauser Effect (Neuhaus & Williamson, 2000), resulting in a reduction in signal intensity by a few percent. In practice, this effect is exploited using difference spectroscopy. In other words, the experiment is performed twice in an interleaved manner to avoid systematic errors. In one experiment, the macromolecule is saturated, whereas in the other, the saturation frequency is set off-resonance. Signals from ligands interacting with the saturated macromolecule will be partially suppressed in the former experiment but not in the latter. Hence, the presence of signals in the difference spectrum (off-resonance minus on-resonance saturation) is evidence of binding.
For the experiments described below, we prepared 3 samples for each peptide study: the STD samples with Crk and CrkL SH2 proteins (2 mM peptide, 20 μM protein), and the protein-free control (2 mM peptide, 0 μM protein). These concentrations represent a compromise to conserve protein and peptide sample while collecting data with sufficient signal-to-noise ratio in the difference spectra, given our instrumentation (see below), to discern the binding epitope. For visualization, we stack the off-resonance saturation spectrum with the difference spectra (referred to as the STD spectra) for samples with Crk SH2, CrkL SH2, and the protein-free control. Figure 6C shows the aromatic region of these spectra, focused on the para-substituted phenyl moiety of the pY in the positive control peptide (DVpYDVPP) and the phenylalanine sidechain of the F in the negative control sample (DVFDVPP) (Figure 6A bottom row). The presence of peaks in the STD spectra of the Crk SH2 and CrkL SH2 (Figure 6C middle two rows) indicates non-covalent interactions (e.g., binding) between these protein domains and this peptide ligand. Roughly speaking, the strength of this interaction is proportional to the relative intensities, which can be quantified using the STD amplification factor (M. Mayer & Meyer, 2001). The absence of peaks in the protein-free control provides an important check on the experiment. Because there is no macromolecule to saturate, no saturation transfer to the ligand is expected, and no peaks are observed (Figure 6C top row).
5.3.2. Equipment
A Bruker AVIII 600 MHz NMR spectrometer equipped with a room-temperature (e.g. not cryogenically-cooled) triple resonance inverse probe and a 24-position NMRCase autosampler was used for data acquisition and processing. This spectrometer runs Topspin software (version 3). We used the software MestreNova (version 14) for data visualization and analysis. Instruments with different configurations from other vendors and running different software should possess equivalent functionality, and we would expect equivalent results.
5.3.3. Procedure
5.3.3.1. Sample preparation
We prepare 10 mM stock solutions of the peptides DVpYDVPP-OH (positive control) and DVFDVPP-OH (negative control) in a buffer (see note 1) and check carefully for solubility by eye. If the peptides are not soluble in the buffer at this concentration, DMSO-d6 can be used, though caution should be taken to keep the final percentage of DMSO-d6 in the NMR samples (step 3) less than or equal to 5%. To avoid freeze-thaw cycles we store these stocks in the freezer until use.
Prepare Crk and CrkL SH2 samples. To avoid multiple freeze-thaw cycles of the protein stocks, we prepared an aliquot of protein for each peptide in 2 mL centrifuge tubes that were frozen and then immediately prior to data acquisition. The final volume of the frozen aliquot equal 400 μL so that a 1-to-5 dilution of the 10 mM peptide stocks (prepared in step 1) result in 500 μL NMR samples. The concentration of protein in these frozen aliquots should equal 25 μM so that when 100 μL peptide is added, the protein concentration equals 20 μM and the peptide: protein ratio is 100:1. One would need to determine the dilution factor for the protein stocks based on the concentration of protein samples provided for the expression and purification (as described in Section 3 above). It is critical to ensure the final NMR samples contain at least 10% D2O for the field-frequency lock. We either prepare our buffer with 10% D2O or simply add ~50 μL D2O to the NMR tube.
Using peptide samples prepared in step 1 and protein samples prepared in step 2, prepare NMR samples by transferring 100 μL of the peptide into each of the centrifuge tubes. Then, transfer to an NMR tube.
5.3.3.2. NMR spectroscopy
Run a 1D 1H NMR spectrum with water suppression on the buffer and/or the protein-free control sample. Although, in principle, any water suppression technique is acceptable, we use the excitation sculpting method (Hwang & Shaka, 1995) because the STD pulse sequence (‘stddiffesgp.3’) that will be employed in step 2 uses this method. In our experience, the 1D 1H NMR spectrum with water suppression is an excellent test of the status of the spectrometer, particularly when run in automation. If this experiment is acceptable (e.g., peptide signals are evident, the residual water signal intensity is on the order of the intensity of the ligand peaks, and the baseline is not distorted), then the magnetic field homogeneity (shimming), probe tuning and matching, and radiofrequency pulse calibrations are acceptable as well. If this experiment is not acceptable, calibration/optimization of the instrument should be performed. This process has been described elsewhere (Cavanagh et al., 1995; Giraudeau et al., 2015; Keifer, 1999; P. S. C. Wu & Otting, 2005; Zheng & Price, 2010)
Run the STD experiment on the positive control sample, preferably using automation. This experiment is a pseudo-2D experiment, running alternating off- and on-resonance saturation in an interleaved manner. Many, but not all, parameters from the 1D 1H NMR spectrum with water suppression can be used for this experiment. In our experience, the critical parameters to adjust for the STD experiment are the following: interscan delay, saturation frequencies, the saturation pulse train, saturation time, spinlock power, and spin lock mixing time. To set these parameters, we were guided by previous work examining relatively weak interactions between peptides and nanoparticles, which required long saturation pulse trains (up to 15 s) and an interscan delay of 15 s (Zhang & Casabianca, 2018). The saturation frequency is set to 30 ppm for off-resonance saturation. The on-resonance saturation pulse must be optimized for the specific peptide. Because the protein macromolecule contains all or many of the same chemical moieties as the peptide ligands, it can be difficult to selectively saturate only the receptor (Rauber & Berger, 2012). Presumably, the higher molecular weight protein would have broader line widths than the low molecular weight ligands. These broader lines would include tails to the region upfield from 0 ppm (i.e., chemical shifts less than or equal to 0 ppm), which would enable us to saturate signals from the receptor only. On the other hand, the further the on-resonance saturation pulse train is from the peak maximum, the less efficiently this pulse train saturates the macromolecular signals. As a compromise, the on-resonance saturation pulse is set to 0.25 ppm. In our protein-free control samples, we see some selective saturation of peptide ligand signals, specifically methyl groups from valine residues. Hence, the binding of these epitopes cannot be discerned in our final analyses. Because computational docking indicates that these moieties will not interact with the SH2 domain (see Section 4 above), this compromise was deemed acceptable. The saturation pulse train is a series of 50 ms Gaussian pulses applied at a power of 50 Hz (e.g., power level set to the nutation frequency of 50 Hz). Total saturation time can be arrayed from 500 ms to 15 s (interscan delay) to see the saturation profile, which can be useful to discern modest differences in weak binding fragments. We use a saturation time of 7.5 s as a compromise to visualize the interaction. The pulse program we use includes a spinlock period to suppress signals from intramolecular saturation transfer within the protein (Xia et al., 2010). We set this spin lock time to 70 ms and power to 9.6 kHz. The total experiment time is 6 hours using the loop parameter, l1, which determines the number of interleaved blocks of 8 scans alternating between off- and on-resonance saturation.
Process the STD experiment. The STD experiment is run as an interleaved pseudo-2D alternating between off- and on-resonance saturation. We employ the Bruker processing script ‘stdsplit’, which separates the pseudo-2D into an off-resonance saturation reference spectrum and difference spectrum. These spectra are then visualized in Topspin and/or 3rd-party software, namely MestreNova. The STD amplification factor (M. Mayer & Meyer, 2001) for each peak can be measured as a ratio of the peak integral of the difference spectrum and reference spectrum multiplied by the peptide excess. The STD amplification factor is the relative intensity of the difference signal from the ligand compared to the signal of the protein and is used to quantify the interaction to estimate binding constants and map the binding epitope.
For the protein-free control sample, we also record homonuclear and heteronuclear 2D experiments, namely the 1H-1H COSY, TOCSY and NOESY/ROESY, and 1H-13C HSQC and HMBC experiments for resonance assignment and structure elucidation. The details of these experiments have been described in detail elsewhere (Braun et al., 1998; Wuthrich, 1986).
5.3.4. Notes
During the expression and purification steps of the protein (see section 3 above), an acceptable pH, ionic strength, and buffer have been chosen for the protein. These conditions may not be optimal for NMR spectroscopy. High ionic strength, for instance, will reduce the efficiency of NMR signal detection, result in a lower signal-to-noise ratio, and should be avoided, if possible. Likewise, Good’s Buffers, EDTA, and other protonated additives have NMR signals that may interfere with the measurement of signals in the STD spectrum, even if there is no direct overlap between ligand and additive signals. Various vendors offer deuterated analogs of these additive compounds. A better solution is to use a non-protonated buffer, if possible. Of course, the protein must be stable and well-behaved at the chosen concentration in the chosen buffer for the duration of the NMR experiment. For Crk SH2 and CrkL SH2, we used phosphate buffered saline (e.g., 10 mM Na2HPO4, 1.8 mM KH2PO4, 2137 mM NaCl, 2.7 mM KCl, pH 7.4).
6. Modulation of protein-protein interactions by peptides (GST pulldown)
6.1. Introduction
To complement the biophysical assays (section 5), we assess the activity of the candidate peptides using an in vitro protein-protein binding competition assay. As the binding partner of Crk and CrkL SH2, we prepared tyrosine phosphorylated p130Cas (pY-p130Cas) from 293T cells. While the endogenous level of pY-p130Cas is low in 293T cells, transfection of the cells with cDNAs for the full-length CrkII, CrkI, or CrkL proteins significantly elevated the pY-p130Cas level. Activities of peptides as inhibitors (or activators) of the SH2-mediated PPI can be tested in vitro using the GST pulldown assay in which GST-tagged SH2 domains and their binding proteins are precipitated with glutathione agarose beads (Figure 7A). After inducing tyrosine phosphorylation of p130Cas in 293T cells, the total cell lysates were prepared, and an excessive amount of purified GST-tagged Crk SH2 or CrkL SH2 was mixed with the cell lysates to out-compete the endogenous and overexpressed Crk and CrkL and precipitate pY-p130Cas. Peptides were added as competitive inhibitors of the interaction between GST-SH2 and pY-p130Cas. As shown in Figure 7B, FAM-DVpYDVPP modestly inhibited co-precipitation of Crk SH2 and pY-p130Cas, whereas FAM-DVFDVPP did not.
Fig. 7. GST pulldown of GST-CrkSH2 and pY-p130Cas using glutathione agarose beads.
(A) A schematic diagram of the GST pulldown assay. A pY-containing peptide was added as a competitive inhibitor. (B) Effects of FAM-conjugated peptides on GST pulldown of GST-CrkSH2 and pY-p130Cas. Proteins precipitated by glutathione agarose beads were analyzed with Western blot analyses using anti-pY-p130Cas and anti-FLAG antibodies. The pY-containing peptide, but not the peptide containing phenylalanine, inhibited the GST pulldown of pY-p130Cas.
6.2. Equipment and supplies
Sorvall Legend Micro 21R centrifuge (Thermo Scientific) for high-speed centrifugation of microcentrifuge tubes at 4°C.
Tube revolver rotator (ThermoFisher Scientific)
NanoQuant (Tecan) for protein and nucleic acid quantification
Odyssey CLx imaging system (LI-COR) for quantitative Western blots
Gel loading tips, standard, round (Thermo Fisher Scientific)
6.3. Reagents
pcDNA3.1/myc-His vector (Invitrogen) for mammalian cell expression of Crk and CrkL
293T cells (American Type Culture Collection, ATCC)
Dulbecco’s Modified Eagle Medium (DMEM) (Gibco, Fisher Scientific)
X-tremeGENE 9 (Roche, Sigma Aldrich) for mammalian cell transfection
Dulbecco’s phosphate buffered saline (DPBS) with calcium and magnesium (Corning, Fisher Scientific)
1% NP-40 lysis buffer containing 50 mM Tris-HCl adjusted to pH 7.5, 150 mM NaCl, 5 mM EDTA, 25 mM NaF, 2 mM Na3VO4, 0.1% sodium deoxycholate, Complete Mini (protease inhibitor cocktail; Roche, Sigma Aldrich), and PhosSTOP (phosphatase inhibitor cocktail; Roche, Sigma Aldrich)
GST pulldown buffer containing 20 mM HEPES adjusted to pH 7.5, 0.5 mM dithiothreitol (DTT), 0.5% Triton X-100, Complete Mini, and PhosSTOP.
Glutathione agarose beads (Pierce, Fisher Scientific)
NuPAGE SDS-PAGE and transfer system: NuPAGE Bis-Tris 4–12% precast gels (1.0 mm, 15-well), NuPAGE lithium dodecyl sulfate (LDS) sample buffer (4X), NuPAGE sample reducing agent (10X), NuPAGE MES SDS running buffer (20X), NuPAGE transfer buffer (10X), NuPAGE antioxidant, Nitrocellulose/filter paper sandwich (0.45 μm, 8.3 × 7.3 cm) (all from Invitrogen)
Chameleon Duo pre-stained protein ladder (LI-COR)
Intercept TBS blocking buffer (LI-COR)
Mouse anti-FLAG M2 antibody (Sigma)
Rabbit anti-pY410-p130Cas or anti-p130Cas antibodies (Cell Signaling Technology)
IRDye 800CW goat anti-mouse IgG secondary antibody (LI-COR)
IRDye 800CW goat anti-rabbit IgG secondary antibody (LI-COR)
6.4. Procedure
Subclone the mouse full-length cDNAs of CrkII, CrkI, and CrkL into the pcDNA3.1/myc-His B vector to express Myc/His-tagged CrkII, CrkI, and CrkL (see note 1).
Culture 293T cells in Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 10% FBS and 1% penicillin/streptomycin in a 37°C CO2 incubator. When cells reach 70–80% confluency, transfect 293T cells with plasmids for Myc/His-tagged CrkII, CrkI, and CrkL expression using X-tremeGENE 9. Overexpression of CrkII, CrkI, or CrkL in 293T cells induces tyrosine phosphorylation of p130Cas (see note 2).
One day after transfection, wash the 293T cells twice with ice-cold Dulbecco’s phosphate buffered saline (DPBS) with calcium and magnesium and add ice-cold 0.5 mL/60 mm dish of 1% NP-40 lysis buffer containing 50 mM Tris-HCl adjusted to pH 7.5, 150 mM NaCl, 5 mM EDTA, 25 mM NaF, 2 mM Na3VO4, 0.1% sodium deoxycholate, the protease inhibitor cocktail Complete Mini, and the phosphatase inhibitor cocktail PhosSTOP (Park & Curran, 2014). Collect the cell lysates using cell scrapers into microcentrifuge tubes on ice. Dissociate cell debris by vortexing, centrifuge at 20,000 x g for 10 min at 4°C, and transfer the supernatants to new microcentrifuge tubes on ice.
After quantifying the protein concentration, aliquot the 293T cell lysates and store them at −80°C until use.
Prepare the GST pulldown buffer, derived from the FP assay buffer, containing 20 mM HEPES adjusted to pH 7.5, 0.5 mM dithiothreitol (DTT), 0.5% Triton X-100, Complete Mini, and PhosSTOP.
Transfer 2 sets of glutathione agarose slurry to 2 microcentrifuge tubes and wash with the GST pulldown buffer by tapping/inverting and centrifuging at 3,500 x g for 15 sec at 4°C. Remove the supernatants, add the GST pulldown buffer, resuspend, and aliquot to new microcentrifuge tubes to the number of samples.
Recover 293T cell lysates from −80°C and thaw them on ice. Add 90 μg of CrkI-expressing 293T cell lysates for Crk SH2 pulldown to each tube of the first set of microcentrifuge tubes containing glutathione agarose beads. For CrkL SH2 pulldown, add 360 μg of CrkL-overexpressing 293T cell lysate. Add 400 μL of the GST pulldown buffer to each tube and rotate in the cold room for 1 hour for pre-clearing. This pre-clearing step will remove sticky proteins in the 293T cell lysate that nonspecifically bind to glutathione agarose beads.
In a separate set of microcentrifuge tubes, add 100 μL of the GST pulldown buffer, aliquot 2 μg/tube of GST-tagged Crk SH2 or CrkL SH2. Then, add peptides or chemicals to mix with GST-tagged protein. Rotate in the cold room for 1 hour for mixing.
Centrifuge both sets of tubes at 3,500 x g for 15 sec at 4°C and transfer the pre-cleared 293T cell lysates to the tubes containing GST-tagged protein and peptides/chemicals. Mix by tapping/inverting, and rotate in the cold room for 1 hour to allow GST-tagged protein to bind to glutathione agarose beads.
Centrifuge the tubes containing cell lysates, GST-tagged SH2 protein, and peptides/chemicals at 3,500 x g for 15 sec at 4°C. Transfer the reaction mixtures to the second set of microcentrifuge tubes containing washed glutathione agarose beads and rotate them in the cold room overnight. This step will precipitate GST-tagged SH2 protein and its binding partner protein pY-p130Cas.
Next day, centrifuge at 3,500 x g for 15 sec at 4°C, place the tubes on ice, and remove the supernatants first using a P1000 pipette tip and second using a gel loading tip to remove the supernatants as much as possible without removing agarose beads. Add the 600 μL/tube of GST pulldown buffer, mix by tapping/inverting, centrifuge, and remove the supernatants in the same way. Repeat this wash 4 more times to remove unbound proteins.
Add SDS sample buffer containing NuPAGE lithium dodecyl sulfate (LDS) sample buffer and NuPAGE sample reducing agent to the washed glutathione agarose beads. Heat at 85°C for 5 min and vortex for denaturation and centrifuge at 20,000 x g for 2 min at 4°C to cool down the samples.
Perform SDS-PAGE with NuPAGE Bis-Tris 4–12% pre-cast gels, MES buffer, and NuPAGE antioxidants and transfer proteins to nitrocellulose membranes.
Perform Western blot analyses using the primary antibodies for the FLAG epitope tag and pY-p130Cas to detect GST- and FLAG-tagged SH2 protein and pY-p130Cas. Use IR dye-conjugated secondary antibodies, wash the nitrocellulose membrane thoroughly, and let the membranes dry overnight in the dark. Scan the membranes using the Odyssey CLx imaging system and quantify the intensities of bands.
6.5. Notes
Since GST-tagged Crk SH2 and CrkL SH2 are also tagged with the FLAG epitope tag for detection with an anti-FLAG antibody, full-length cDNA constructs for CrkII, CrkI, and CrkL expression in 293T cells were designed to have the Myc/His tag for detection by anti-Myc or anti-His antibody. The calculated protein sizes (http://web.expasy.org/protparam/) of GST-CrkSH2 (41.8 kDa) and GST-CrkLSH2 (40.4 kDa) are similar to Myc/His-tagged full-length CrkII (39.3 kDa) and CrkL (38.1 kDa). However, the use of the anti-FLAG antibody will clearly distinguish GST-tagged Crk SH2 and CrkL SH2 from CrkII and CrkL.
Crk SH2 showed a higher affinity to FAM-DVpYDVPP derived from p130Cas than CrkL SH2. Therefore, it is likely that p130Cas has a higher affinity to CrkI and CrkII than CrkL. To avoid potential complications, we used the lysates of CrkI- or CrkII-overexpressing 293T cells for the pulldown of GST-CrkSH2 and pY-p130Cas and the lysates of CrkL-overexpressing 293T cells for the pulldown of GST-CrkLSH2 and pY-p130Cas.
7. Outlook
This chapter summarizes recent efforts toward peptide antagonists of the Crk/CrkL-p130Cas axis. The project was motivated by unanswered questions regarding the precise biochemical role of Crk and CrkL in tumor cell migration and invasion. At present, there are no specific and potent inhibitors of these proteins. Crk/CrkL antagonists would be invaluable tools to validate targets and assess druggability, as well as, to assist in vitro assay development. In addition, such compounds could be leads for the development of novel therapeutic interventions for cancer and other human diseases. We elected to focus on inhibitors of the SH2 domains of Crk and CrkL. Our iterative strategy for antagonist development employs computational docking to prioritize candidate peptides for experimental testing using a battery of biophysical and in vitro assays, which in turn are used to improve computational docking. Taken together, these tools form a strategic pipeline for the early-stage development of therapeutic polypeptides to address protein-protein interactions and other challenging drug targets. Improved delivery techniques, such as nanosponges, have helped renew interest in the development of peptide-based therapeutics and challenge preconceived notions of the drawbacks of this class of molecules (Tiwari & Bhattacharya, 2022; L. Wang et al., 2022). Tools to design and test peptides, such as those described in this chapter, will play an increasingly critical role in medicinal chemistry moving forward.
Acknowledgments
This work was supported by an MCA Partners Advisory Board grant from Children’s Mercy Hospital (CMH) and the University of Kansas Cancer Center (KUCC) (TP), and the NIH Center of Biomedical Research Excellence for Chemical Biology of Infectious Disease (award number 5P20GM113117) (DJ & AR). Support for NMR instrumentation and software was provided by NSF Major Research Instrumentation Grant (1625923), NIH Shared Instrumentation Grants (S10RR024664 and S10OD016360), and an Institutional Development Award (IDeA) from the National Institute of General Medical Sciences of the National Institutes of Health (award number P20GM103418).
References
- Al Zaid Siddiquee K, & Turkson J. (2008). STAT3 as a target for inducing apoptosis in solid and hematological tumors. Cell Research, 18(2), 254–267. 10.1038/cr.2008.18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angulo J, Enríquez-Navas PM, & Nieto PM (2010). Ligand–Receptor Binding Affinities from Saturation Transfer Difference (STD) NMR Spectroscopy: The Binding Isotherm of STD Initial Growth Rates. Chemistry – A European Journal, 16(26), 7803–7812. 10.1002/chem.200903528 [DOI] [PubMed] [Google Scholar]
- Angulo J, & Nieto PM (2011). STD-NMR: Application to transient interactions between biomolecules—a quantitative approach. European Biophysics Journal, 40(12), 1357–1369. 10.1007/s00249-011-0749-5 [DOI] [PubMed] [Google Scholar]
- Baeshen MN, Al-Hejin AM, Bora RS, Ahmed MMM, Ramadan HAI, Saini KS, Baeshen NA, & Redwan EM (2015). Production of Biopharmaceuticals in E. coli: Current Scenario and Future Perspectives. Journal of Microbiology and Biotechnology, 25(7), 953–962. 10.4014/jmb.1412.12079 [DOI] [PubMed] [Google Scholar]
- Bajusz D, Pándy-Szekeres G, Takács Á, de Araujo ED, & Keserű GM (2023). SH2db, an information system for the SH2 domain. Nucleic Acids Research, 51(W1), W542–W552. 10.1093/nar/gkad420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow KA, Ó Conchúir S, Thompson S, Suresh P, Lucas JE, Heinonen M, & Kortemme T. (2018). Flex ddG: Rosetta Ensemble-Based Estimation of Changes in Protein-Protein Binding Affinity upon Mutation. The Journal of Physical Chemistry. B, 122(21), 5389–5399. 10.1021/acs.jpcb.7b11367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett A, Pellet-Many C, Zachary IC, Evans IM, & Frankel P. (2013). p130Cas: A key signalling node in health and disease. Cellular Signalling, 25(4), 766–777. 10.1016/j.cellsig.2012.12.019 [DOI] [PubMed] [Google Scholar]
- Begley DW, Moen SO, Pierce PG, & Zartler ER (2013). Saturation Transfer Difference NMR for Fragment Screening. Current Protocols in Chemical Biology, 5(2), 251–268. 10.1002/9780470559277.ch130118 [DOI] [PubMed] [Google Scholar]
- Berger SL, & Kimmel AR (Eds.). (1987). Guide to molecular cloning techniques. Methods in Enzymology (Vol. 152). Academic Press. [PubMed] [Google Scholar]
- Bouton AH, Riggins RB, & Bruce-Staskal PJ (2001). Functions of the adapter protein Cas: Signal convergence and the determination of cellular responses. Oncogene, 20(44), 6448–6458. 10.1038/sj.onc.1204785 [DOI] [PubMed] [Google Scholar]
- Bradshaw JM, Grucza RA, Ladbury JE, & Waksman G. (1998). Probing the “Two-Pronged Plug Two-Holed Socket” Model for the Mechanism of Binding of the Src SH2 Domain to Phosphotyrosyl Peptides: A Thermodynamic Study. Biochemistry, 37(25), 9083–9090. 10.1021/bi973147k [DOI] [PubMed] [Google Scholar]
- Bradshaw JM, & Waksman G. (2002). Molecular recognition by SH2 domains. In Janin J. & Wodak SJ (Eds.), Protein Modules and Protein-Protein Interactions. Advances in Protein Chemistry (Vol. 61, pp. 161–210). Elsevier. 10.1016/S0065-3233(02)61005-8 [DOI] [PubMed] [Google Scholar]
- Braun S, Kalinowski H-O, & Berger S. (1998). 150 and more basic NMR experiments: A practical course (2nd expanded ed.). Wiley-VCH. [Google Scholar]
- Briknarová K, Nasertorabi F, Havert ML, Eggleston E, Hoyt DW, Li C, Olson AJ, Vuori K, & Ely KR (2005). The Serine-rich Domain from Crk-associated Substrate (p130cas) Is a Four-helix Bundle. Journal of Biological Chemistry, 280(23), 21908–21914. 10.1074/jbc.M501258200 [DOI] [PubMed] [Google Scholar]
- Cala O, & Krimm I. (2015). Ligand-Orientation Based Fragment Selection in STD NMR Screening. Journal of Medicinal Chemistry, 58(21), 8739–8742. 10.1021/acs.jmedchem.5b01114 [DOI] [PubMed] [Google Scholar]
- Camacho Leal M. del P, Sciortino M, Tornillo G, Colombo S, Defilippi P, & Cabodi S. (2015). p130Cas/BCAR1 scaffold protein in tissue homeostasis and pathogenesis. Gene, 562(1), 1–7. 10.1016/j.gene.2015.02.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh J, Fairbrother WJ, Palmer AG, & Skelton NJ (1995). Protein NMR Spectroscopy: Principles and Practice. Academic Press. [Google Scholar]
- Chen MJ, Dixon JE, & Manning G. (2017). Genomics and evolution of protein phosphatases. Science Signaling, 10(474), eaag1796. 10.1126/scisignal.aag1796 [DOI] [PubMed] [Google Scholar]
- Ciemny M, Kurcinski M, Kamel K, Kolinski A, Alam N, Schueler-Furman O, & Kmiecik S. (2018). Protein-peptide docking: Opportunities and challenges. Drug Discovery Today, 23(8), 1530–1537. 10.1016/j.drudis.2018.05.006 [DOI] [PubMed] [Google Scholar]
- Cody W, Lin Z, Panek R, Rose D, & Rubin J. (2000). Progress in the Development of Inhibitors of SH2 Domains. Current Pharmaceutical Design, 6(1), 59–98. 10.2174/1381612003401532 [DOI] [PubMed] [Google Scholar]
- Cosconati S, Forli S, Perryman AL, Harris R, Goodsell DS, & Olson AJ (2010). Virtual screening with AutoDock: Theory and practice. Expert Opinion on Drug Discovery, 5(6), 597–607. 10.1517/17460441.2010.484460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Defilippi P, Di Stefano P, & Cabodi S. (2006). p130Cas: A versatile scaffold in signaling networks. Trends in Cell Biology, 16(5), 257–263. 10.1016/j.tcb.2006.03.003 [DOI] [PubMed] [Google Scholar]
- Diop A, Santorelli D, Malagrinò F, Nardella C, Pennacchietti V, Pagano L, Marcocci L, Pietrangeli P, Gianni S, & Toto A. (2022). SH2 Domains: Folding, Binding and Therapeutical Approaches. International Journal of Molecular Sciences, 23(24), 15944. 10.3390/ijms232415944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson LW, Gish G, Pawson T, Kay LE, & Forman-Kay JD (2002). Structure of a regulatory complex involving the Abl SH3 domain, the Crk SH2 domain, and a Crk-derived phosphopeptide. Proceedings of the National Academy of Sciences, 99(22), 14053–14058. 10.1073/pnas.212518799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Y. (2015). Fluorescence polarization assay to quantify protein-protein interactions in an HTS format. In Meyerkord C. & Fu H. (Eds.), Protein-Protein Interactions. Methods in Molecular Biology (Vol. 1278, pp. 529–544). Humana Press. 10.1007/978-1-4939-2425-7_35 [DOI] [PubMed] [Google Scholar]
- Feller SM, Knudsen B, & Hanafusa H. (1994). C-Abl kinase regulates the protein binding activity of c-Crk. The EMBO Journal, 13(10), 2341–2351. 10.1002/j.1460-2075.1994.tb06518.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forli S, Huey R, Pique ME, Sanner MF, Goodsell DS, & Olson AJ (2016). Computational protein–ligand docking and virtual drug screening with the AutoDock suite. Nature Protocols, 11(5), 905–919. 10.1038/nprot.2016.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frenz B, Lewis SM, King I, DiMaio F, Park H, & Song Y. (2020). Prediction of Protein Mutational Free Energy: Benchmark and Sampling Improvements Increase Classification Accuracy. Frontiers in Bioengineering and Biotechnology, 8, 558247. 10.3389/fbioe.2020.558247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao K, Oerlemans R, & Groves MR (2020). Theory and applications of differential scanning fluorimetry in early-stage drug discovery. Biophysical Reviews, 12(1), 85–104. 10.1007/s12551-020-00619-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gemperle J, Hexnerová R, Lepšík M, Tesina P, Dibus M, Novotný M, Brábek J, Veverka V, & Rosel D. (2017). Structural characterization of CAS SH3 domain selectivity and regulation reveals new CAS interaction partners. Scientific Reports, 7(1), 8057. 10.1038/s41598-017-08303-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gileadi O. (2017). Recombinant Protein Expression in E. coli: A Historical Perspective. In Burgess-Brown N. (Ed.), Heterologous Gene Expression in E.coli. Methods in Molecular Biology (Vol. 1586, pp. 3–10). 10.1007/978-1-4939-6887-9_1 [DOI] [PubMed] [Google Scholar]
- Giraudeau P, Silvestre V, & Akoka S. (2015). Optimizing water suppression for quantitative NMR-based metabolomics: A tutorial review. Metabolomics, 11(5), 1041–1055. 10.1007/s11306-015-0794-7 [DOI] [Google Scholar]
- Greene LH, Lewis TE, Addou S, Cuff A, Dallman T, Dibley M, Redfern O, Pearl F, Nambudiry R, Reid A, Sillitoe I, Yeats C, Thornton JM, & Orengo CA (2007). The CATH domain structure database: New protocols and classification levels give a more comprehensive resource for exploring evolution. Nucleic Acids Research, 35(suppl_1), D291–297. 10.1093/nar/gkl959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harner MJ, Frank AO, & Fesik SW (2013). Fragment-based drug discovery using NMR spectroscopy. Journal of Biomolecular NMR, 56(2), 65–75. 10.1007/s10858-013-9740-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrickson OD, Taranova NA, Zherdev AV, Dzantiev BB, & Eremin SA (2020). Fluorescence Polarization-Based Bioassays: New Horizons. Sensors, 20(24), 7132. 10.3390/s20247132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoult D. (1976). Solvent peak saturation with single phase and quadrature Fourier transformation. Journal of Magnetic Resonance, 21(2), 337–347. 10.1016/0022-2364(76)90081-0 [DOI] [Google Scholar]
- Hubbard RE, & Murray JB (2011). Experiences in Fragment-Based Lead Discovery. In Kuo LC (Ed.), Fragment-Based Drug Design: Tools, Practical Approaches, and Examples. Methods in Enzymology (Vol. 493, pp. 509–531). Elsevier. 10.1016/B978-0-12-381274-2.00020-0 [DOI] [PubMed] [Google Scholar]
- Hunter T. (2009). Tyrosine phosphorylation: Thirty years and counting. Current Opinion in Cell Biology, 21(2), 140–146. 10.1016/j.ceb.2009.01.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter T. (2012). Why nature chose phosphate to modify proteins. Philosophical Transactions of the Royal Society B: Biological Sciences, 367(1602), 2513–2516. 10.1098/rstb.2012.0013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang T, & Shaka A. (1995). Water Suppression That Works—Excitation Sculpting Using Arbitrary Wave-Forms and Pulsed-Field Gradients. Journal of Magnetic Resonance Series A, 112(2), 275–279. 10.1006/jmra.1995.1047 [DOI] [Google Scholar]
- Jankowski W, Saleh T, Pai M-T, Sriram G, Birge RB, & Kalodimos CG (2012). Domain organization differences explain Bcr-Abl’s preference for CrkL over CrkII. Nature Chemical Biology, 8(6), 590–596. 10.1038/nchembio.954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia B, & Jeon CO (2016). High-throughput recombinant protein expression in Escherichia coli: Current status and future perspectives. Open Biology, 6(8). 10.1098/rsob.160196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keifer PA (1999). 90° pulse width calibrations: How to read a pulse width array. Concepts in Magnetic Resonance: An Educational Journal, 11(3), 165–180. [DOI] [Google Scholar]
- Kimber MS, Nachman J, Cunningham AM, Gish GD, Pawson T, & Pai EF (2000). Structural Basis for Specificity Switching of the Src SH2 Domain. Molecular Cell, 5(6), 1043–1049. 10.1016/S1097-2765(00)80269-5 [DOI] [PubMed] [Google Scholar]
- Kirsch KH, Kensinger M, Hanafusa H, & August A. (2002). A p130Castyrosine phosphorylated substrate domain decoy disrupts v-Crk signaling. BMC Cell Biology, 3(1), 18. 10.1186/1471-2121-3-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobashigawa Y, Sakai M, Naito M, Yokochi M, Kumeta H, Makino Y, Ogura K, Tanaka S, & Inagaki F. (2007). Structural basis for the transforming activity of human cancer-related signaling adaptor protein CRK. Nature Structural & Molecular Biology, 14(6), 503–510. 10.1038/nsmb1241 [DOI] [PubMed] [Google Scholar]
- Kraskouskaya D, Duodu E, Arpin CC, & Gunning PT (2013). Progress towards the development of SH2 domain inhibitors. Chemical Society Reviews, 42(8), 3337. 10.1039/c3cs35449k [DOI] [PubMed] [Google Scholar]
- Lee P-H, Huang XX, Teh BT, & Ng L-M (2019). TSA-CRAFT: A Free Software for Automatic and Robust Thermal Shift Assay Data Analysis. SLAS Discovery : Advancing Life Sciences R & D, 24(5), 606–612. 10.1177/2472555218823547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichty JJ, Malecki JL, Agnew HD, Michelson-Horowitz DJ, & Tan S. (2005). Comparison of affinity tags for protein purification. Protein Expression and Purification, 41(1), 98–105. 10.1016/j.pep.2005.01.019 [DOI] [PubMed] [Google Scholar]
- Liu BA, Jablonowski K, Shah EE, Engelmann BW, Jones RB, & Nash PD (2010). SH2 Domains Recognize Contextual Peptide Sequence Information to Determine Selectivity. Molecular & Cellular Proteomics, 9(11), 2391–2404. 10.1074/mcp.M110.001586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D. (2014). The adaptor protein Crk in immune response. Immunology & Cell Biology, 92(1), 80–89. 10.1038/icb.2013.64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- London N, Raveh B, Cohen E, Fathi G, & Schueler-Furman O. (2011). Rosetta FlexPepDock web server—High resolution modeling of peptide-protein interactions. Nucleic Acids Research, 39(suppl_2), W249–253. 10.1093/nar/gkr431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mace PD, Wallez Y, Dobaczewska MK, Lee JJ, Robinson H, Pasquale EB, & Riedl SJ (2011). NSP-Cas protein structures reveal a promiscuous interaction module in cell signaling. Nature Structural & Molecular Biology, 18(12), 1381–1387. 10.1038/nsmb.2152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machida K, & Mayer BJ (2005). The SH2 domain: Versatile signaling module and pharmaceutical target. Biochimica et Biophysica Acta (BBA) - Proteins and Proteomics, 1747(1), 1–25. 10.1016/j.bbapap.2004.10.005 [DOI] [PubMed] [Google Scholar]
- Marasco M, & Carlomagno T. (2020). Specificity and regulation of phosphotyrosine signaling through SH2 domains. Journal of Structural Biology: X, 4, 100026. 10.1016/j.yjsbx.2020.100026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcu O, Dodson E-J, Alam N, Sperber M, Kozakov D, Lensink MF, & Schueler-Furman O. (2017). FlexPepDock lessons from CAPRI peptide-protein rounds and suggested new criteria for assessment of model quality and utility. Proteins, 85(3), 445–462. 10.1002/prot.25230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marengere LEM, & Pawson T. (1994). Structure and function of SH2 domains. Journal of Cell Science, 1994(Supplement 18), 97–104. 10.1242/jcs.1994.Supplement_18.14 [DOI] [PubMed] [Google Scholar]
- Marengere LEM, Songyang Z, Gish GD, Schaller MD, Parsons JT, Stern MJ, Cantley LC, & Pawson T. (1994). SH2 domain specificity and activity modified by a single residue. Nature, 369(6480), 502–505. 10.1038/369502a0 [DOI] [PubMed] [Google Scholar]
- Matsuda M, Tanaka S, Nagata S, Kojima A, Kurata T, & Shibuya M. (1992). Two species of human CRK cDNA encode proteins with distinct biological activities. Molecular and Cellular Biology, 12(8), 3482–3489. 10.1128/mcb.12.8.3482-3489.1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matulis D, Kranz JK, Salemme FR, & Todd MJ (2005). Thermodynamic Stability of Carbonic Anhydrase: Measurements of Binding Affinity and Stoichiometry Using ThermoFluor. Biochemistry, 44(13), 5258–5266. 10.1021/bi048135v [DOI] [PubMed] [Google Scholar]
- Mayer BJ, Hamaguchi M, & Hanafusa H. (1988). A novel viral oncogene with structural similarity to phospholipase C. Nature, 332(6161), 272–275. 10.1038/332272a0 [DOI] [PubMed] [Google Scholar]
- Mayer M, & Meyer B. (1999). Characterization of Ligand Binding by Saturation Transfer Difference NMR Spectroscopy. Angewandte Chemie International Edition, 38(12), 1784–1788. [DOI] [PubMed] [Google Scholar]
- Mayer M, & Meyer B. (2001). Group Epitope Mapping by Saturation Transfer Difference NMR To Identify Segments of a Ligand in Direct Contact with a Protein Receptor. Journal of the American Chemical Society, 123(25), 6108–6117. 10.1021/ja0100120 [DOI] [PubMed] [Google Scholar]
- Neuhaus D, & Williamson MP (2000). The nuclear Overhauser effect in structural and conformational analysis (2nd ed.). Wiley-VCH. [Google Scholar]
- Ogawa S, Toyoshima H, Kozutsumi H, Hagiwara K, Sakai R, Tanaka T, Hirano N, Mano H, Yazaki Y, & Hirai H. (1994). The C-terminal SH3 domain of the mouse c-Crk protein negatively regulates tyrosine-phosphorylation of Crk associated p130 in rat 3Y1 cells. Oncogene, 9(6), 1669–1678. [PubMed] [Google Scholar]
- Park T. (2021). Crk and CrkL as Therapeutic Targets for Cancer Treatment. Cells, 10(4), 739. 10.3390/cells10040739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park T, & Curran T. (2014). Essential roles of Crk and CrkL in fibroblast structure and motility. Oncogene, 33(43), 5121–5132. 10.1038/onc.2013.453 [DOI] [PubMed] [Google Scholar]
- Park T, Large N, & Curran T. (2021). Quantitative assessment of glioblastoma phenotypes in vitro establishes cell migration as a robust readout of Crk and CrkL activity. Journal of Biological Chemistry, 296, 100390. 10.1016/j.jbc.2021.100390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posern G, Zheng J, Knudsen BS, Kardinal C, Müller KB, Voss J, Shishido T, Cowburn D, Cheng G, Wang B, Kruh GD, Burrell SK, Jacobson CA, Lenz DM, Zamborelli TJ, Adermann K, Hanafusa H, & Feller SM (1998). Development of highly selective SH3 binding peptides for Crk and CRKL which disrupt Crk-complexes with DOCK180, SoS and C3G. Oncogene, 16(15), 1903–1912. 10.1038/sj.onc.1201714 [DOI] [PubMed] [Google Scholar]
- Rauber C, & Berger S. (2012). Saturation Transfer Difference NMR Studies of the Interaction of the Proteinkinase CK2 with Peptides. Protein & Peptide Letters, 19(9), 934–939. 10.2174/092986612802084447 [DOI] [PubMed] [Google Scholar]
- Raveh B, London N, & Schueler-Furman O. (2010). Sub-angstrom modeling of complexes between flexible peptides and globular proteins. Proteins, 78(9), 2029–2040. 10.1002/prot.22716 [DOI] [PubMed] [Google Scholar]
- Raveh B, London N, & Schueler-Furman O. (2010). Sub-angstrom modeling of complexes between flexible peptides and globular proteins. Proteins: Structure, Function, and Bioinformatics, 78(9), 2029–2040. 10.1002/prot.22716 [DOI] [PubMed] [Google Scholar]
- Raveh B, London N, Zimmerman L, & Schueler-Furman O. (2011). Rosetta FlexPepDock ab-initio: Simultaneous folding, docking and refinement of peptides onto their receptors. PloS One, 6(4), e18934. 10.1371/journal.pone.0018934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichman CT, Mayer BJ, Keshav S, & Hanafusa H. (1992). The product of the cellular crk gene consists primarily of SH2 and SH3 regions. Cell Growth & Differentiation : The Molecular Biology Journal of the American Association for Cancer Research, 3(7), 451–460. [PubMed] [Google Scholar]
- Rohl CA, Strauss CEM, Misura KMS, & Baker D. (2004). Protein structure prediction using Rosetta. In Brand L. & Johnson ML (Eds.), Numerical Computer Methods, Part D. Methods in Enzymology (Vol. 383, pp. 66–93). 10.1016/S0076-6879(04)83004-0 [DOI] [PubMed] [Google Scholar]
- Rosa N, Ristic M, Seabrook SA, Lovell D, Lucent D, & Newman J. (2015). Meltdown: A Tool to Help in the Interpretation of Thermal Melt Curves Acquired by Differential Scanning Fluorimetry. Journal of Biomolecular Screening, 20(7), 898–905. 10.1177/1087057115584059 [DOI] [PubMed] [Google Scholar]
- Rosano GL, Morales ES, & Ceccarelli EA (2019). New tools for recombinant protein production in Escherichia coli: A 5-year update. Protein Science, 28(8), 1412–1422. 10.1002/pro.3668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadowski I, Stone JC, & Pawson T. (1986). A noncatalytic domain conserved among cytoplasmic protein-tyrosine kinases modifies the kinase function and transforming activity of Fujinami sarcoma virus P130gag-fps. Molecular and Cellular Biology, 6(12), 4396–4408. 10.1128/mcb.6.12.4396-4408.1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai R, Iwamatsu A, Hirano N, Ogawa S, Tanaka T, Mano H, Yazaki Y, & Hirai H. (1994). A novel signaling molecule, p130, forms stable complexes in vivo with v-Crk and v-Src in a tyrosine phosphorylation-dependent manner. The EMBO Journal, 13(16), 3748–3756. 10.1002/j.1460-2075.1994.tb06684.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schäfer F, Seip N, Maertens B, Block H, & Kubicek J. (2015). Purification of GST-Tagged Proteins. In Lorsch JR (Ed.), Laboratory Methods in Enzymology: Protein Part D. Methods in enzymology (Vol. 559, pp. 127–139). 10.1016/bs.mie.2014.11.005 [DOI] [PubMed] [Google Scholar]
- Schust J, Sperl B, Hollis A, Mayer TU, & Berg T. (2006). Stattic: A Small-Molecule Inhibitor of STAT3 Activation and Dimerization. Chemistry & Biology, 13(11), 1235–1242. 10.1016/j.chembiol.2006.09.018 [DOI] [PubMed] [Google Scholar]
- Shin N-Y, Dise RS, Schneider-Mergener J, Ritchie MD, Kilkenny DM, & Hanks SK (2004). Subsets of the Major Tyrosine Phosphorylation Sites in Crk-associated Substrate (CAS) Are Sufficient to Promote Cell Migration. Journal of Biological Chemistry, 279(37), 38331–38337. 10.1074/jbc.M404675200 [DOI] [PubMed] [Google Scholar]
- Smithgall TE (1995). SH2 and SH3 domains: Potential targets for anti-cancer drug design. Journal of Pharmacological and Toxicological Methods, 34(3), 125–132. 10.1016/1056-8719(95)00082-7 [DOI] [PubMed] [Google Scholar]
- Song Y, DiMaio F, Wang RY-R, Kim D, Miles C, Brunette T, Thompson J, & Baker D. (2013). High-resolution comparative modeling with RosettaCM. Structure, 21(10), 1735–1742. 10.1016/j.str.2013.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Songyang Z, Gish G, Mbamalu G, Pawson T, & Cantley LC (1995). A Single Point Mutation Switches the Specificity of Group III Src Homology (SH) 2 Domains to That of Group I SH2 Domains. Journal of Biological Chemistry, 270(44), 26029–26032. 10.1074/jbc.270.44.26029 [DOI] [PubMed] [Google Scholar]
- Sousa SF, Ribeiro AJM, Coimbra JTS, Neves RPP, Martins SA, Moorthy NSHN, Fernandes PA, & Ramos MJ (2013). Protein-ligand docking in the new millennium—A retrospective of 10 years in the field. Current Medicinal Chemistry, 20(18), 2296–2314. 10.2174/0929867311320180002 [DOI] [PubMed] [Google Scholar]
- Stanzione F, Giangreco I, & Cole JC (2021). Chapter Four—Use of molecular docking computational tools in drug discovery. In Witty DR & Cox B. (Eds.), Progress in Medicinal Chemistry (Vol. 60, pp. 273–343). Elsevier. 10.1016/bs.pmch.2021.01.004 [DOI] [PubMed] [Google Scholar]
- Structural Genomics Consortium, Architecture et Fonction des Macromolécules Biologiques, Berkeley Structural Genomics Center, China Structural Genomics Consortium, Integrated Center for Structure and Function Innovation, Israel Structural Proteomics Center, Joint Center for Structural Genomics, Midwest Center for Structural Genomics, New York Structural GenomiX Research Center for Structural Genomics, Northeast Structural Genomics Consortium, Oxford Protein Production Facility, Protein Sample Production Facility, Max Delbrück Center for Molecular Medicine, RIKEN Structural Genomics/Proteomics Initiative, & SPINE2-Complexes. (2008). Protein production and purification. Nature Methods, 5(2), 135–146. 10.1038/nmeth.f.202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- ten Hoeve J, Morris C, Heisterkamp N, & Groffen J. (1993). Isolation and chromosomal localization of CRKL, a human crk-like gene. Oncogene, 8(9), 2469–2474. [PubMed] [Google Scholar]
- Tikhmyanova N, Little JL, & Golemis EA (2010). CAS proteins in normal and pathological cell growth control. Cellular and Molecular Life Sciences, 67(7), 1025–1048. 10.1007/s00018-009-0213-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinti M, Kiemer L, Costa S, Miller ML, Sacco F, Olsen JV, Carducci M, Paoluzi S, Langone F, Workman CT, Blom N, Machida K, Thompson CM, Schutkowski M, Brunak S, Mann M, Mayer BJ, Castagnoli L, & Cesareni G. (2013). The SH2 Domain Interaction Landscape. Cell Reports, 3(4), 1293–1305. 10.1016/j.celrep.2013.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiwari K, & Bhattacharya S. (2022). The ascension of nanosponges as a drug delivery carrier: Preparation, characterization, and applications. Journal of Materials Science: Materials in Medicine, 33(3), 28. 10.1007/s10856-022-06652-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchie H, Chang CH, Yoshida M, & Vogt PK (1989). A newly isolated avian sarcoma virus, ASV-1, carries the crk oncogene. Oncogene, 4(11), 1281–1284. [PubMed] [Google Scholar]
- Tubert-Brohman I, Sherman W, Repasky M, & Beuming T. (2013). Improved Docking of Polypeptides with Glide. Journal of Chemical Information and Modeling, 53(7), 1689–1699. 10.1021/ci400128m [DOI] [PubMed] [Google Scholar]
- Turkson J, Kim JS, Zhang S, Yuan J, Huang M, Glenn M, Haura E, Sebti S, Hamilton AD, & Jove R. (2004). Novel peptidomimetic inhibitors of signal transducer and activator of transcription 3 dimerization and biological activity. Molecular Cancer Therapeutics, 3(3), 261–269. 10.1158/1535-7163.261.3.3 [DOI] [PubMed] [Google Scholar]
- Vagenende V, Yap MGS, & Trout BL (2009). Mechanisms of protein stabilization and prevention of protein aggregation by glycerol. Biochemistry, 48(46), 11084–11096. 10.1021/bi900649t [DOI] [PubMed] [Google Scholar]
- Vidal M, Gigoux V, & Garbay C. (2001). SH2 and SH3 domains as targets for anti-proliferative agents. Critical Reviews in Oncology/Hematology, 40(2), 175–186. 10.1016/S1040-8428(01)00142-1 [DOI] [PubMed] [Google Scholar]
- Viegas A, Manso J, Nobrega FL, & Cabrita EJ (2011). Saturation-Transfer Difference (STD) NMR: A Simple and Fast Method for Ligand Screening and Characterization of Protein Binding. Journal of Chemical Education, 88(7), 990–994. 10.1021/ed101169t [DOI] [Google Scholar]
- Waksman G, Shoelson SE, Pant N, Cowburn D, & Kuriyan J. (1993). Binding of a high affinity phosphotyrosyl peptide to the Src SH2 domain: Crystal structures of the complexed and peptide-free forms. Cell, 72(5), 779–790. 10.1016/0092-8674(93)90405-f [DOI] [PubMed] [Google Scholar]
- Wang CK, Weeratunga SK, Pacheco CM, & Hofmann A. (2012). DMAN: a Java tool for analysis of multi-well differential scanning fluorimetry experiments. Bioinformatics, 28(3), 439–440. 10.1093/bioinformatics/btr664 [DOI] [PubMed] [Google Scholar]
- Wang J, Che Y, Li G, Liu B, Shen T, Wang H, & Linghu H. (2011). Crk and CrkL present with different expression and significance in epithelial ovarian carcinoma. Molecular Carcinogenesis, 50(7), 506–515. 10.1002/mc.20745 [DOI] [PubMed] [Google Scholar]
- Wang L, Wang N, Zhang W, Cheng X, Yan Z, Shao G, Wang X, Wang R, & Fu C. (2022). Therapeutic peptides: Current applications and future directions. Signal Transduction and Targeted Therapy, 7(1), 48. 10.1038/s41392-022-00904-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Liu D, & Wyss DF (2004). Competition STD NMR for the detection of high-affinity ligands and NMR-based screening. Magnetic Resonance in Chemistry, 42(6), 485–489. 10.1002/mrc.1381 [DOI] [PubMed] [Google Scholar]
- Wang Z, & Cole PA (2014). Catalytic mechanisms and regulation of protein kinases. In Shokat KM (Ed.), Protein Kinase Inhibitors in Research and Medicine. Methods in Enzymology (Vol. 548, pp. 1–21). 10.1016/B978-0-12-397918-6.00001-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams CJ, Headd JJ, Moriarty NW, Prisant MG, Videau LL, Deis LN, Verma V, Keedy DA, Hintze BJ, Chen VB, Jain S, Lewis SM, Arendall WB 3rd, Snoeyink J, Adams PD, Lovell SC, Richardson JS, & Richardson DC (2018). MolProbity: More and better reference data for improved all-atom structure validation. Protein Science : A Publication of the Protein Society, 27(1), 293–315. 10.1002/pro.3330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisniewska M, Bossenmaier B, Georges G, Hesse F, Dangl M, Künkele K-P, Ioannidis I, Huber R, & Engh RA (2005). The 1.1Å Resolution Crystal Structure of the p130cas SH3 Domain and Ramifications for Ligand Selectivity. Journal of Molecular Biology, 347(5), 1005–1014. 10.1016/j.jmb.2005.02.017 [DOI] [PubMed] [Google Scholar]
- Wu PSC, & Otting G. (2005). Rapid pulse length determination in high-resolution NMR. Journal of Magnetic Resonance, 176(1), 115–119. 10.1016/j.jmr.2005.05.018 [DOI] [PubMed] [Google Scholar]
- Wu T, Yu J, Gale-Day Z, Woo A, Suresh A, Hornsby M, & Gestwicki JE (2020). Three Essential Resources to Improve Differential Scanning Fluorimetry (DSF) Experiments. bioRxiv. 10.1101/2020.03.22.002543 [DOI] [Google Scholar]
- Wuthrich K. (1986). NMR of proteins and nucleic acids. Wiley. [Google Scholar]
- Xia Y, Zhu Q, Jun K, Wang J, & Gao X. (2010). Clean STD-NMR spectrum for improved detection of ligand-protein interactions at low concentration of protein. Magnetic Resonance in Chemistry, 48(12), 918–924. 10.1002/mrc.2687 [DOI] [PubMed] [Google Scholar]
- Zhang Y, & Casabianca LB (2018). Probing Amino Acid Interaction with a Polystyrene Nanoparticle Surface Using Saturation-Transfer Difference (STD)-NMR. The Journal of Physical Chemistry Letters, 9(23), 6921–6925. 10.1021/acs.jpclett.8b02785 [DOI] [PubMed] [Google Scholar]
- Zheng G, & Price WS (2010). Solvent signal suppression in NMR. Progress in Nuclear Magnetic Resonance Spectroscopy, 56(3), 267–288. 10.1016/j.pnmrs.2010.01.001 [DOI] [PubMed] [Google Scholar]