Abstract
Background/Objectives: The human gut microbiome is a complex ecosystem of microorganisms that can influence our health and exercise habits. On the other hand, physical exercise can also impact our microbiome, affecting our health. Our narrative review examines the bidirectional relationship between physical activity and the gut microbiome, as well as the potential for targeted probiotic regimens to enhance sports performance. Methods: We conducted a comprehensive literature review to select articles published up till January 2024 on the topics of physical exercise, sports, probiotics, and gut microbiota from major scientific databases, incorporating over 100 studies. Results: We found that the impact of physical activity on the gut microbiome varies with the type and intensity of exercise. Moderate exercise promotes a healthy immune system, while high-intensity exercise for a long duration can cause a leaky gut and consequent systemic inflammation, which may disrupt the microbial balance. Combining aerobic and resistance training significantly affects bacterial diversity, linked to a lower prevalence of chronic metabolic disorders. Furthermore, exercise enhances gut microbiome diversity, increases SCFA production, improves nutrient utilization, and modulates neural and hormonal pathways, improving gut barrier integrity. Our findings also showed probiotic supplementation is associated with decreased inflammation, enhanced sports performance, and fewer gastrointestinal disturbances, suggesting that the relationship between the gut microbiome and physical activity is mutually influential. Conclusions: The bidirectional relationship between physical activity and the gut microbiome is exemplified by how exercise can promote beneficial bacteria while a healthy gut microbiome can potentially enhance exercise ability through various mechanisms. These findings underscore the importance of adding potential tailored exercise regimens and probiotic supplementation that consider individual microbiome profiles into exercise programs.
Keywords: physical activity, exercise, training, athletes, gut microbiota, probiotics
1. Introduction
The human gut microbiome is a complex community of microorganisms, including bacteria, archaea, fungi, viruses, and protozoa. It comprises over a thousand bacterial species that can significantly influence the host’s overall well-being [1,2]. In a healthy gut, anaerobic species are typically responsible for producing anti-inflammatory short-chain fatty acids (SCFAs) through the fermentation of complex dietary sugars [3]. SCFAs play crucial roles in maintaining gut integrity and modulating the immune system. For example, SCFA butyrate serves as the primary energy source for colonocytes, enhancing epithelial barrier function and preventing the translocation of pathogens and toxins into the bloodstream [4]. Additionally, SCFAs can influence the immune system by promoting the differentiation of regulatory T cells, which help to maintain immune tolerance and reduce inflammation [5]. However, the beneficial metabolic activities of these anaerobic species are often compromised in conditions of gut dysbiosis, leading to a reduced production of SCFAs and other byproducts [3].
The delicate balance of microbial species and their byproducts is intricately linked to overall health, influencing a range of diseases. Extensive research has revealed significant associations between microbial imbalances and chronic conditions such as metabolic syndrome, obesity, antibiotic use, and depression [6,7]. These conditions are often characterized by a shift from a dominance of anaerobes to facultative species, which can disrupt normal metabolic processes and lead to systemic inflammation [3]. Such microbial imbalances reduce microbial diversity, which can adversely affect the gut’s functionality in multiple ways. For instance, a less diverse microbiome diminishes the gut’s efficiency in metabolizing nutrients, which can contribute to the energy imbalance observed in obesity and metabolic syndrome. A diverse microbiome enhances the capacity for polysaccharide fermentation, leading to increased SCFA production, which not only provides energy to the host but also supports glucose homeostasis [8]. A decreased gut microbiota diversity has been linked to a higher prevalence of coronary vascular disease and nonalcoholic fatty liver disease [9]. Moreover, reduced microbial diversity also weakens the gut’s defensive mechanisms against pathogenic bacteria such as Proteobacteria, further exacerbating the risk of dysbiosis and its associated health conditions [10,11].
This interplay between microbial diversity and metabolic health is intricately connected to physical activity. Regular exercise has been shown to promote microbial diversity, facilitating a more favorable environment for beneficial microbial populations that enhance SCFA production. The effects of physical activity on overall well-being include improvements in physical, mental, and gut health, achieved through complex biological mechanisms and interactions. Regular engagement in physical exercises not only helps to manage and reduce the risk of developing metabolic syndrome but also improves mental health, anxiety, and depression [12,13]. Exercise regulates the immune system by prompting an acute rise in interleukin-6 (IL-6), which helps to reduce inflammation [14]. However, extended durations of strenuous exercise can lead to a transient increase in inflammation due to muscle tissue damage [14]. These interactions also extend to gut health, where regular exercise is correlated with the mitigation of disease states associated with gut dysbiosis [15,16,17]. The exact causal mechanisms behind these correlations are still under investigation. Recent studies reveal that while high-intensity, long-duration exercise can cause a leaky gut and dysbiosis, low-to-moderate exercise maintains microbiome health [18]. The effects of physical activity on the microbiome vary significantly with the type of exercise, such as endurance training, resistance training, and different sports [19].
Research demonstrates that athletes often develop distinct microbiome profiles after exercise, marked by increased beneficial microbial species and SCFAs [20]. Additionally, higher muscle strength in older adults is associated with specific microbiome compositions, indicating that a healthy microbiome can contribute to maintaining physical strength and functionality as we age [21]. Conversely, a compromised gut barrier can lead to joint inflammation, adversely affecting movement and physical performance [22].
Despite the emerging evidence of the relationship between physical activity and gut microbiome health, several gaps remain in the current literature. While the fact that exercise influences microbial diversity and SCFA production is documented, understanding specific bidirectional relationships between gut microbiome profiles and exercises of various intensities requires a comprehensive exploration. Our review aims to integrate findings from animal and human studies to consolidate current knowledge, identify gaps, and direct future research toward the relationship between physical activity and gut microbiota.
2. Methods: Search Strategy and Selection Criteria
We conducted a comprehensive literature review in January 2024 to select articles on physical exercise, sports, and gut microbiota from PubMed, Google Scholar, Scopus, and NCBI. The search included keywords such as “physical activity”, “physical training”, “aerobic exercise”, “high-intensity interval training (HIIT)”, “resistance training”, “athletic performance”, “elite athletes”, “exercise intensity”, “gut microbiota”, “microbiota diversity”, “probiotics”, “SCFA”, and “metabolic health”. We screened article abstracts to assess whether they should be included in the full-text screening. We included over 100 animal and human original research studies that specifically examined the relationship between gut microbiota and physical exercise. Non-peer-reviewed articles and those on relevant topics that do not directly address the review’s focus were excluded. Our narrative review underscores the importance of physical exercise, its connection with gut microbiota, and its beneficial effects on chronic illnesses and athletic performance. The collected data encompass various parameters, including the type of exercise, shifts in bacterial species composition, dosage and strains of administered probiotics, research design, study duration, geographic location, sample size, participants’ age, and participants’ Body Mass Index (BMI).
3. Gut Microbiome Effects of Exercise in Animals
Exercise is increasingly recognized as influencing the gut microbiome, impacting its composition and function [23]. Various types of physical activity, such as aerobic exercise and resistance training, can alter the diversity and metabolic activity of gut microbes. This chapter explores the relationship between physical activity and the gut microbiome in animal models (Table S1), providing a comprehensive overview of how various forms of exercise influence the microbial communities within the gut.
3.1. Voluntary Aerobic Exercise
Voluntary aerobic exercise is increasingly recognized for its influence on gut health. In a laboratory study by Mika et al., rats housed in cages equipped with running wheels were allowed voluntary wheel access to perform voluntary aerobic exercise during the experimental period [24,25]. Compared to rats who were assigned to the sedentary (control) group, the voluntary aerobic exercise group showed an increased abundance of beneficial bacterial genera like Lactobacillus and Bifidobacterium [25]. Lactobacillus is known for its potential to enhance human intestinal barrier integrity. It plays a crucial role in preventing and treating gastrointestinal infections, reducing intestinal inflammation, improving digestive processes, and enhancing nutrient absorption [26]. On the other hand, Bifidobacteria has anti-inflammatory and antiviral properties. It aids in regulating the immune system and improves gut health by assisting in the assimilation of dietary fibers and regulating fat storage [27]. The impact of voluntary aerobic exercise can extend to increasing the abundance of other beneficial bacterial families such as Ruminococcaceae and Lachnospiraceae [24]. These changes in the microbiome also lead to a higher overall concentration of n-butyrate, an SCFA that confers many benefits for gastrointestinal health and immune system health [28]. Overall, the evidence suggests that voluntary aerobic exercise is related to an increase in the abundance of numerous genera and families that have beneficial effects in enhancing gut health and promoting the growth of healthy bacteria in the gut.
3.2. Moderate Aerobic Exercise
Building on the effects observed with voluntary aerobic exercise, moderate aerobic exercise seems to have discernible effects on the gut microbiome of mice. In Lamoureux, Grandy, and Langille’s controlled laboratory study, 42 mice were split into three groups: a voluntary exercise group (10 mice), a forced exercise group (11 mice), and a sedentary control group (21 mice). The intervention (exercise) was implemented over 8 weeks, and the gut microbial diversity, changes in inflammatory markers, and lean body mass were measured. The authors had not observed any significant changes in the overall gut microbial diversity or inflammatory markers after mice underwent moderate aerobic exercise; however, advanced computational methods such as random forest machine learning were able to discern notable differences between the gut microbiome of mice that moderately exercised and mice that did not [29]. The study also found increases in the Bacteroides and Lactobacillus genus. Both voluntary and moderate aerobic exercise increased certain useful genera in the gut microbiome, showing its beneficial effects on the gut microbiome; however, moderate aerobic exercise did not see as much change in gut microbial diversity.
3.3. Intense Aerobic Exercise
In contrast to moderate aerobic exercise, intense aerobic exercise enhances the growth of additional genera such as Firmicutes and Bacteroidetes, which in turn promote positive health output. Research on thoroughbred racehorses has shown that intense aerobic exercise was associated with significant shifts in the levels of the Bacteroidetes and Firmicutes phyla [30]. Both of the phyla increased in abundance. It is noteworthy as both of these phyla are beneficial individually, but an imbalance in their ratios (more Firmicutes as compared to Bacteroidetes) can cause significant physiological harm [31]. The Firmicutes phylum plays an important role in digestive health and energy harvesting [32]. Additionally, the Bacteroidetes phylum is involved in the breakdown of complex carbohydrates, a process that requires significant aid from bacteria [33], helping in better absorption and utilization. A recurring theme across all forms of aerobic exercise is the increase in beneficial bacterial strains aiding in better gut health. Voluntary aerobic exercise saw an increase in the abundance of numerous genera and families. Moderate aerobic exercise did not see as much change in gut microbial diversity but did see a change in some gut microbial species. Intense exercise resulted in alterations in the two most populous good genera of the gut microbiome, Bacteroidetes and Firmicutes.
3.4. Resistance Training
Resistance training is a type of exercise that involves performing physical activities to increase strength. Research has shown that resistance training may confer benefits to the gut microbiome in animal models. Research on rats has shown that resistance training altered the gut microbiota composition, with an increase in Lactobacillus and Bifidobacterium [34]. Resistance training protects against autoimmune diseases. This protection is achieved by changing the composition or function of the gut microbiota, particularly Akkermansia muciniphila, which in turn leads to a reduction in the activity or presence of T helper 17 (Th17) cells [35]. Similar to voluntary aerobic exercise, studies on resistance training suggest that it is good for the gut microbiota and overall gut health by conferring an increase in the abundance of the Lactobacillus and Bifidobacterium genera.
4. Effects of Different Types of Exercise on the Human Gut Microbiome
Research consistently supports that those various types of exercise influence human gut microbiome composition in ways similar to those observed in animal models (Tables S2–S6, Figure 1). Interventions utilizing multiple exercise routines (aerobic and resistance) had more of a significant influence on bacterial diversity than, for instance, resistance training alone. Studies indicate that low-intensity exercise programs may have a limited positive impact on gut microbiota diversity compared to high-intensity exercise. However, research shows that intense, prolonged exercise could lead to an increase in inflammation [18,36]. Regardless of the type of exercise intervention, most studies in the tables reveal a similar trend of observations concerning the effects on the major phyla of the gut microbiome as explored in this chapter.
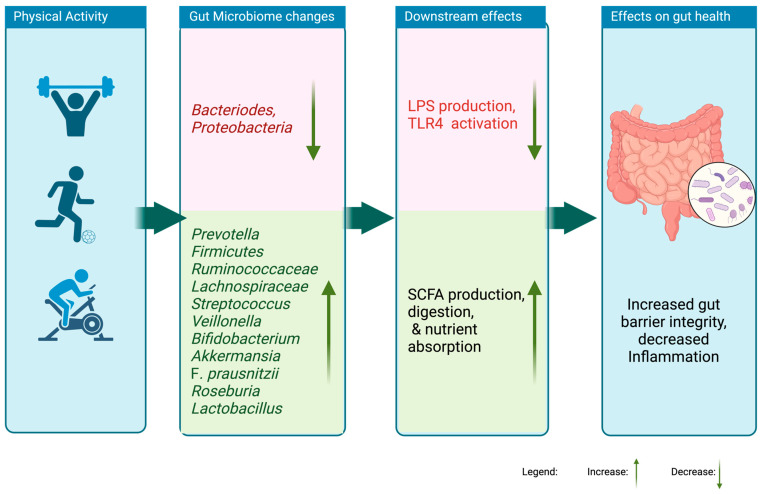
Figure 1.
Effects of physical-activity-induced changes in the gastrointestinal microbiome.
4.1. Overall Gut Microbiota Diversity
Exercise has repeatedly been demonstrated to positively influence the alpha diversity of the gut microbiota, contributing to overall gut health. For instance, swimmers who participated in a 7-week high-intensity interval training (HIIT) program exhibited a significant increase in alpha diversity within their fecal microbiota [37]. Similarly, a 6-week endurance training was associated with increased beta diversity in a cohort of previously sedentary overweight women [38]. Notably, combining multiple exercise modalities, such as different aerobic exercises and resistance training, appears to exert a more pronounced effect on bacterial diversity compared to employing just one type of exercise. However, intense, prolonged physical activities could lead to an increase in inflammation, suggesting a delicate balance between exercise intensity and its health benefits [18,36]. Intense physical activity can also increase appetite and lead to higher energy intake, often driving athletes to consume energy-dense diets. These diets, particularly those high in fat and refined carbohydrates, can negatively alter the gut microbiome by promoting the growth of pro-inflammatory bacteria and reducing microbial diversity [39]. Thus, it can be concluded that exercise is associated with human gut microbiome diversity, which can be beneficial for overall health.
4.2. The Role of the Firmicutes Phylum in Gut Health
Engaging in regular physical activity is correlated with an increase in the bacteria of the dominant phylum Firmicutes, which plays a vital role in various gut functions and overall intestinal health [40]. Notably, individuals who engage in regular physical exercise show a marked increase in the abundance of SCFAs produced by Firmicutes, particularly those from the Ruminococcaceae, Lachnospiraceae, and Erysipelotrichaceae families [41,42,43]. Ruminococcus is a diverse genus of bacteria within the gut microbiome, encompassing both beneficial and potentially harmful species [44]. These bacteria have been found to respond positively to increases in physical activity, with their populations rising as a person becomes more physically active [43,45,46,47,48,49,50,51,52]. Ruminococcus includes two species, Ruminococcus gauvreaui and Faecalibacterium prausnitzii, both of which are associated with enhanced health outcomes. Higher levels of Ruminococcus gauvreaui correlate with improved cardiorespiratory fitness and greater insulin sensitivity [44]. Faecalibacterium prausnitzii produces the SCFA succinate, which is a substrate for intestinal gluconeogenesis, a process that improves glucose homeostasis [53]. Research has found that Faecalibacterium prausnitzii levels are significantly higher in women who engaged in high physical activity in the previous week [54]. Additionally, endurance runners were found to have higher levels of Faecalibacterium prausnitzii and SCFA succinate compared to healthy non-athletes [51].
The Lachnospiraceae family includes SCFA-producing bacteria such as Dorea, Coprococcus, and Roseburia [55]. Dorea produces SCFA acetate, a compound directly linked to glucose levels and known for its positive influence on glucose metabolism [56]. Research has shown that the abundance of Dorea is higher in individuals who engage in physical activity, suggesting that regular exercise may amplify its beneficial effects [38,49,57]. Coprococcus produces SCFA butyrate, which reduces the severity of atopic conditions such as atopic dermatitis [58]. Coprococcus is significantly enriched in individuals who engage in regular physical activity [42,47,49,59,60]. Roseburia is another genus of bacteria that produces SCFA butyrate. The abundance of Roseburia is significantly higher in actively exercising adults, demonstrating a beneficial impact on gut metabolism and immunity through butyrate production. Notably, Dorea, Coprococcus, and Roseburia have also been found to increase following a 21.1 km athletic marathon or a 3748.91 km rowing event lasting 33 days and 22 h [47,57].
Other Firmicutes have been associated with enhanced gut health and improved metabolic responses, including species like Streptococcus, Romboutsia, Holdemanella, Ruminococcaceae, Blautia, Ruminiclostridium, Clostridium phoceensis, Streptococcus suis, Clostridium bolteae, Lactobacillus, Anaerostipes hadrus, and Veillonella. Studies have shown that Streptococcus and Romboutsia are more abundant in physically active adults, suggesting a link between these bacteria and regular physical activity [61,62,63]. Notably, specific Streptococcus species have demonstrated anti-inflammatory properties in vitro by significantly reducing cytokines such as TNF-α, IL-1β, and IL-6 [47,50,52].
Holdemanella levels have been observed to increase dramatically following a weeks-long exercise intervention [64]. Research has shown that Holdemanella can mitigate hyperglycemia by restoring levels of the hormone GLP-1, which is crucial for blood sugar regulation [65]. Studies involving athletes have revealed increases in Ruminococcaceae, Blautia, Ruminiclostridium, and Clostridium phoceensis after moderate-intensity exercises, such as treadmill workouts [50].
Further studies focusing on elite athletes, including those from 16 different sports, have indicated a considerable predominance of Firmicutes members like Streptococcus suis, Clostridium bolteae, Lactobacillus, and Anaerostipes hadrus in those participating in moderately active sports, such as fencing [62]. High-intensity sports participants, such as field hockey and rowing athletes, show higher levels of Lactobacillus acidophilus and Faecalibacterium prausnitzii [62].
Observational and interventional studies have consistently shown that physical activity increases SCFA-producing bacteria like Veillonella [45,63,66,67,68]. This is particularly noteworthy as Veillonella, a lactate-utilizing species when colonized in mice, has been shown to improve exercise performance. Additionally, Veillonella is abundantly found in the gut microbiomes of elite athletes post-marathon, highlighting its potential benefits to athletic performance [69].
These findings underscore the profound impact of physical activity on the gut microbiome. Maintaining an active lifestyle can significantly alter and potentially enhance the growth of Firmicutes, which are associated with promoting better health and athletic performance.
4.3. The Role of the Bacteroidetes Phylum in Gut Health
Bacteroidetes are a group of gut commensals that play a crucial role in protecting against pathogens and supplying nutrients to other microbial residents within the gut ecosystem [70]. Recent studies have explored the relationship between physical activity and the presence of specific Bacteroidetes species, including Prevotella intermedia, Bacteroides caccae, and Parabacteroides. For instance, individuals engaging in high-intensity sports exhibit significantly higher levels of Prevotella intermedia and Bacteroides caccae [37]. Additionally, Parabacteroides are found in higher abundance in physically active adults [71,72]. A recent systematic review also highlighted that sustained exercise routines are associated with notable increases in Prevotella levels [73]. This relationship underscores the dynamic nature of Bacteroidetes, which can adapt and shift in response to exercise duration and intensity.
4.4. The Role of the Verrucomicrobia Phylum in Gut Health
Several studies reported a specific increase in the species Akkermansia muciniphila of the Verrucomicrobia phylum [54,61,67,74]. Akkermansia muciniphila has been shown to play a protective role in preventing various metabolic disorders, including diabetes. [61]. Zhong et al. studied the effects of aerobic and resistance exercise, finding a rise in SCFA-producing bacteria linked with anti-inflammatory effects such as Verrucomicrobia [75].
4.5. The Role of the Actinobacteria Phylum in Gut Health
Actinobacteria are one of the four main phyla of the human gut microbiome, and, despite their tiny number, they play an important role in gut homeostasis [76] Bifidobacterium, a genus of the Actinobacteria phylum, stands out due to its prevalence in physically active adults [54,62,64,74,77,78,79]. Studies have shown that engaging in high-intensity activity can lead to significantly higher levels of Bifidobacterium [48].
Bifidobacterium produces acetate, a short-chain fatty acid (SCFA) that protects intestinal epithelial cells from apoptosis triggered by the O157 toxin of Escherichia coli [80]. Acetate also plays a crucial role in inducing goblet cell differentiation, mucin secretion, and the sialylation process, all of which are essential for maintaining a healthy and functional gut barrier [80,81]. Bifidobacterium also produces lactate, which further stimulates through cross-feeding the production of other beneficial SCFAs such as butyrates by Lactobacilli, Bacteroides, or certain Enterobacteria [82].
4.6. The Role of the Proteobacteria Phylum in Gut Health
Proteobacteria, which include species such as Escherichia coli, play a critical role in indicating disturbances within the gut microbiome [83]. An imbalance characterized by elevated levels of Proteobacteria is often linked to poorer gut health and can exacerbate conditions such as chronic intestinal inflammation in mice [84]. Many studies have shown that the abundance of Proteobacteria decreases with moderate physical activity [38,48,75,78,85,86]. Zhong et al., who studied the effects of aerobic and resistance exercise, also found a decrease in pro-inflammatory bacteria such as Proteobacteria [75]. However, elite athletes participating in intense exercise showed a modest rise in Proteobacteria, which might be attributed to adverse effects on the gut microbiota caused by overloaded training [52,54,87]. Tabone et al., who studied fecal samples from athletes who completed a treadmill exercise, found an increase in Proteobacteria’s Escherichia coli species. [50].
5. Probiotic Supplementation in Sports Performance
Probiotic supplementation has garnered significant attention in sports science for its potential to enhance athletic performance through improved gut health and various physical parameters (Table S7; Figure 2). Certain sports supplements like whey protein, polyphenols, and other compounds have also been shown to interact with the gut microbiota by promoting the growth of healthy species and limiting species producing toxic metabolites [88]. Creatine, a popular dietary supplement among athletes, has been shown to increase Bacteroides and Firmicutes species while decreasing Proteobacteria, Fusobacteriota, Crenobacter, and Shewanella species in animal models [89]. Intense exercise can lead to injuries, often requiring antibiotic treatment. Antibiotics, while necessary for treating infections, can disrupt the gut microbiome [90], potentially counteracting the benefits of probiotic supplementation. This dual impact on the gut highlights the need for a more comprehensive approach to managing gut health in athletes, particularly those undergoing bacterial infection treatment.
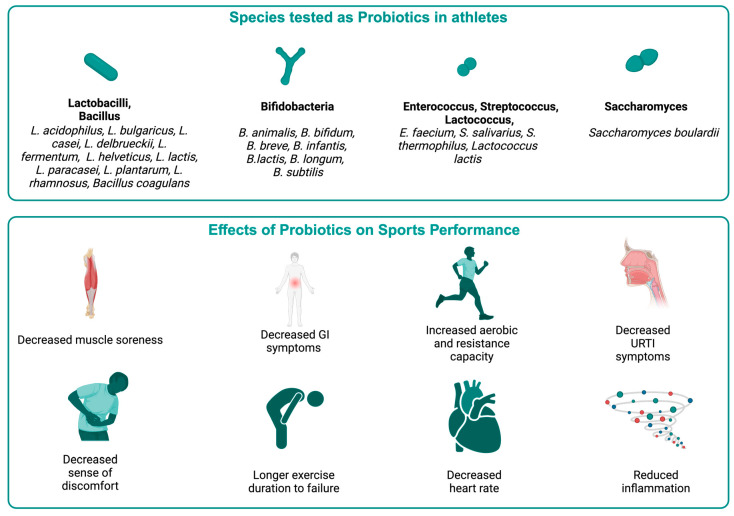
Figure 2.
Effects of probiotics on athletic health and performance.
A blend of Bifidobacterium and Lactobacillus strains at high doses experienced increased VO2 max and extended exercise durations before failure, alongside reduced heart rates during activity [91]. Elite cyclists taking a probiotic mixture of Bifidobacterium animalis and Lactobacillus helveticus experienced significantly less nausea, vomiting, and inflammation during training [92]. Male runners on a 45 billion CFU multi-strain probiotic regimen for four weeks demonstrated extended running times before fatigue under heat conditions [93]. Elite rugby players consuming a diverse probiotic regimen (including Lactobacillus acidophilus, Bifidobacterium animalis, Bifidobacterium bifidum, Bifidobacterium lactis, Streptococcus thermophilus, and Saccharomyces boulardii) reported alleviated leg heaviness and muscle pain, which likely contributed to improved performance and recovery [94].
A study was conducted on trained volunteer athletes who were given a combination of probiotics including Bifidobacterium bifidum, Bifidobacterium lactis, Enterococcus faecium, Lactobacillus acidophilus, Lactobacillus brevis, and Lactococcus lactis at a dose of 1 × 10¹⁰ CFU/day for 12 weeks. The researchers found an increase in the ratio of subjects in the control group who had one or more upper respiratory tract infection (URTI) symptoms, suggesting that the intervention group had a better immune response and potentially better overall health and endurance [95]. Elite athletes administered Lactobacillus helveticus, which significantly reduced the duration and severity of upper respiratory tract infection (URTI) symptoms and led to them experiencing an increased sense of energy [96]. Division I female athletes supplementing with Bacillus subtilis reported improvements in strength measures and reductions in body fat percentage, indicating enhanced physical performance and recovery capabilities [97]. Badminton players consuming Lactobacillus casei with orange juice daily showed improvements in aerobic capacity [98]. Athletes supplemented with Lactococcus lactis exhibited increased CD86 expression and decreased cumulative days of fatigue, indicating improved immune function and reduced fatigue, which is particularly beneficial for endurance sports [99].
Marathon runners taking probiotics containing B. animalis, B. bifidum, and L. acidophilus for 28 days reported fewer gastrointestinal symptoms and enhanced gut health during strenuous exercise [100]. Probiotics may mitigate exercise-induced oxidative damage and inflammation, which are integral to maintaining athletic performance. Endurance-trained men taking multiple probiotics containing B. bifidum, B. lactis, E. faecium, L. acidophilus, L. brevis, and Lactococcus lactis exhibited reduced markers of inflammation and oxidative stress, along with decreased fecal zonulin levels, suggesting improved intestinal barrier function [101]. Athletes consuming Lactobacillus paracasei and Lactobacillus rhamnosus showed increased plasma antioxidant levels and passive reactive oxygen species (ROS) [102]. Triathletes administered Lactobacillus plantarum demonstrated significant reductions in inflammatory markers such as TNF-α, interleukin-6 (IL-6), and interleukin-8, along with increased levels of anti-inflammatory IL-10 and plasma branched-chain amino acids [103]. By minimizing inflammation, probiotics enable athletes to maintain higher levels of performance and comfort during training and competitions.
Probiotic supplementation can alter the intestinal flora, alleviating gastrointestinal issues and enhancing athletic performance as discussed in an earlier section. It also reduces fatigue signs and muscular soreness, which indirectly contributes to athletic performance. It also reduces the immune-suppressive effects and upper respiratory tract infections induced by vigorous physical activity as discussed. Probiotics can decrease the expression of nuclear factor kappa β (NF-κB) and pro-inflammatory cytokines while increasing levels of anti-inflammatory cytokines [104]. Anti-inflammatory cytokines help to reduce muscle inflammation and degeneration. Probiotics also influence the function of macrophages, which are crucial for muscle healing [105]. During physical exercise, muscle fibers generate myokines such as anti-inflammatory IL-10 and IL-6, which also possess systemic anti-inflammatory properties [106].
Oxidative stress and exercise-induced hyperthermia can lead to gastrointestinal issues during endurance exercise. Probiotics can help maintain intestinal integrity by enhancing the uptake of glucose and amino acids during extended physical activity. After physical activity, probiotics that include species such as L. paracasei can enhance the absorption of branched-chain amino acids, which are essential for muscle growth and repair [107]. Probiotics containing L. plantarum may improve iron utilization, which boosts aerobic capacity and endurance by accelerating erythropoiesis [108]. Additionally, L. plantarum supplementation can enhance skeletal muscle glycogen storage, providing a readily available energy source during physical activity and increasing endurance [109]. However, research on the potential of probiotics in nutritional metabolism related to exercise is still limited.
6. Potential Mechanisms of Action
The bidirectional association between physical activity and gut microbiota presents several potential implications for general health and sports nutrition. Exercise can improve intestinal health and metabolism, while probiotics can impact muscle function and athletic performance (Figure 3). This section delves into the latest research to explore two main areas: the potential mechanisms by which physical activity influences gut health and how the gut microbiome affects muscle metabolism and overall health.
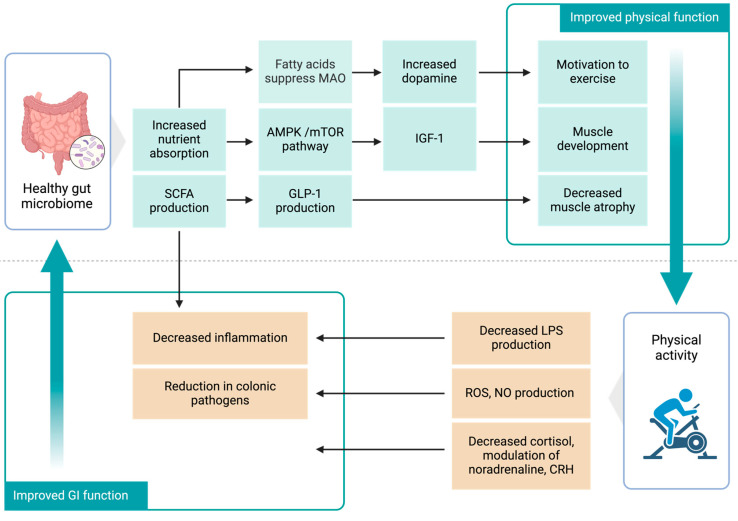
Figure 3.
Mechanisms underlying the mutual relationship between physical activity and the gut microbiome.
6.1. Potential Mechanisms of Physical Activity in Gut Health
The link between exercise and the gut microbiota is multifaceted and bidirectional, with considerable effects on metabolic and muscle functioning, supporting the idea of a gut–muscle axis. This section will explore the mechanisms of how physical activity can influence the gut microbiota (Figure 3).
Exercise can impact gut health through several interconnected mechanisms. During exercise, mitochondria in skeletal muscles produce reactive oxygen and nitrogen species (RONS) [110]. RONS can trigger immune responses in the cells lining the colon through serotonin signaling pathways [111]. The effectiveness of the immune response is closely linked with the development of the gut microbiota [112]. Moderate aerobic exercise boosts the production of immunoglobulin A in the gastrointestinal tract, enhancing the gut microbiota’s ability to prevent intestinal pathogen colonization [113]. Regular moderate-intensity exercise, particularly resistance training, reduces circulating levels of lipopolysaccharides (LPS) and decreases the expression of Toll-like receptors [114]. This reduction is associated with decreased inflammation and improved gut barrier function [115].
Exercise has been shown in studies to have an essential role in controlling bile acid pools, which promotes the health of the host gut microbiota [116]. Bile salt metabolism is an essential component of the gut microbiota. The secondary bile acids generated by the microbiota are detected by the tissues via the activation of the Farnesoid X receptor (FXR) and the G-protein-coupled bile acid receptor, which play critical roles in energy metabolism. The gut microbiota can efficiently control the body’s metabolic capacity and muscle anabolism by inhibiting FXR [117]. Hydrogen sulfide is another messenger generated by cysteine breakdown by gut microbiota that controls the metabolism of intestinal epithelial and immunological cells [118]. Hydrogen sulfide may be a possible target for enhancing myogenesis and improving muscle function. Supplementation with sodium hydrosulfide stimulates myogenic gene expression and improves the regeneration of muscles in mice [119].
6.2. Potential Mechanisms of the Gut Microbiome in Muscle Metabolism and Health
The gut microbiome regulates muscle metabolism via pathways involving AMP-activated protein kinase (AMPK) and downstream signaling pathways such as the mammalian target of rapamycin (mTOR). These pathways control a variety of physiological activities like glucose, protein, and lipid synthesis [120]. Studies suggest that SCFAs can activate muscle AMPK directly by raising the adenosine monophosphate/triphosphate (AMP/ATP) ratio [121].
The gut microbiome significantly influences muscle metabolism through critical pathways such as AMP-activated protein kinase (AMPK) and the mammalian target of rapamycin (mTOR). Insulin Growth Factor (IGF-1), a primary modulator of bone and muscle development, has a considerable impact on the mTOR pathway, which is critical for muscle synthesis [122]. These pathways thus are pivotal in regulating essential physiological functions, including glucose, protein, and lipid synthesis [120]. Physical exercise benefits from this synthesis as it enhances energy production and metabolic efficiency.
A strong correlation exists between SCFAs, produced by gut bacteria, and muscular strength [121,123]. SCFAs can directly activate AMPK in muscles [121]. This activation is beneficial during physical exercise as it plays an important role in muscle development and growth [124]. Mice treated with antibiotics to disturb the gut microbiome demonstrated reduced soleus and plantaris hypertrophy after wheel running as compared to those not treated [125]. One notable SCFA, butyrate, promotes the release of glucagon-like peptide-1 (GLP-1) from colonic cultures, suggesting that it could play a role in reducing muscle atrophy [126]. Butyrate specifically supports aerobic metabolism in skeletal muscles by boosting mitochondrial activities such as increasing oxidative phosphorylation, further underscoring the importance of SCFAs in muscle health and performance [127].
The gut microbiota plays a significant role in protein metabolism. In the small intestine, bacteria such as Clostridium, Bacillus, Streptococcus, and Proteobacteria are among the most abundant species involved in amino acid fermentation, while Clostridia is particularly prevalent in the large intestine [128]. Additionally, these bacteria can enhance the host’s ability to absorb amino acids. For instance, Bacillus coagulans can increase amino acid levels in the serum following milk protein consumption [129].
The gut microbiome can affect the production of hormones such as insulin and androgens, which are essential for protein synthesis and muscle development [130]. Minimal or excessive physical activity can lead to a disturbed gut microbiome, which can have profound effects on these processes. For instance, the bacterium Bacteroides, known for its pro-inflammatory properties, promotes insulin resistance by enhancing polysaccharide fermentation, lipogenesis, and the development of host adipose tissue [40]. Elevated levels of Bacteroides are commonly observed in individuals with hyperandrogenic conditions, such as Polycystic Ovary Syndrome [131]. Recent studies have shown that androgen insufficiency, induced by castration, not only alters the gut flora but also increases the risk of obesity and the loss of thigh muscle in mice [132]. Interestingly, the use of antibiotics has been found to minimize these alterations [132].
Recent research has emphasized the gut–brain–muscle axis, which connects a healthy gut with athletic performance. Research in animal models suggests that the gut microbiota may influence mood and stress by interacting with the central nervous system. In mice, it has been found that intestinal microbiota’s fatty acids stimulate afferent sensory neurons, which signal the brain to suppress monoamine oxidase (MAO) expression during exercise. This suppression leads to increased dopamine signaling, which in turn motivates exercise behaviors and enhances performance. [133].
Stress initiated by exercise can stimulate the hypothalamic–pituitary–adrenal axis, resulting in the production of cortisol and noradrenaline, both of which affect the gut microbiota [134]. The production of corticotropin-releasing factor also alters gastrointestinal function, influencing inflammatory processes, colonic transit duration, mucosal secretory functions, barrier functions, and the growth of intestinal tract bacteria [135]. Additionally, stress can increase plasma noradrenaline levels, affecting the gut microbiota and increasing the aggressiveness of infectious enteric bacteria [136]. Excessive physical activity can raise body temperature and cause extreme heat stress, negatively impacting the gastrointestinal microbiome. It reduces gut blood flow, resulting in relative ischemia and increased intestinal permeability, which may allow bacteria to migrate from the intestines and cause gastrointestinal issues [137].
7. Future Research and Perspectives
While probiotics have shown beneficial effects for athletes, our analysis identified several limitations in the studies that could potentially bias our analyses. Research into the modulation of the gut microbiome through probiotics has utilized a variety of strains, dosages, and supplementation durations, complicating direct comparisons and the ability to draw definitive conclusions about the most effective strains or dosages. Furthermore, the lack of standardized outcomes across these studies adds another layer of complexity. While some investigations focus on aerobic capacity and VO2 max, others might assess muscle soreness or gastrointestinal symptoms. This inconsistency in measured outcomes hampers the establishment of a consistent set of metrics for evaluating the efficacy of probiotic supplementation. Additionally, variations in demographic characteristics, such as age, sex, and baseline health status, across studies could influence outcomes and their applicability. Notably, most studies administering probiotics have been conducted with male athletes, with fewer focusing on female athletes, potentially affecting the interpretations due to physiological differences between genders.
Current research predominantly focuses on bacteria within the gut microbiota, yet this complex ecosystem comprises a broader array of microbial taxa, including fungi and archaea. To fully understand the interactions between physical activity and gut health, future research should expand its scope to assess these diverse microbial communities. This approach will help to identify the most beneficial types of exercise prescriptions tailored to individual microbiome profiles. Additionally, reporting microbiome data at the species level, rather than at the broader phylum level, would provide more precise insights that are crucial for developing targeted clinical interventions. This level of detail is essential for translating microbiome research into practical health solutions.
The impact of physical activity on the gut microbiome remains uncertain and varies substantially across studies. To enhance reliability and precision, standardized study designs such as RCTs with larger sample sizes are necessary. Many current cross-sectional studies rely on self-reported questionnaires that might include biases and inaccuracies that affect the research results. More comparable results regarding the effects of physical activity on the gut microbiome could be achieved by employing more objective measurement techniques like wearable fitness trackers. Rather than measuring vastly different outcomes, there should be a core set of metrics that all studies should include, such as microbial diversity changes and the intensity of exercise, to allow for better cross-study comparisons. These studies should also consider potential confounding factors such as BMI, nutrition, and biotic consumption to better isolate the specific effects of physical activity. A broader range of environmental factors, such as geographic location, pollution, and antibiotic use can also profoundly impact gut health. By controlling for these variables, researchers can better isolate the effects of probiotics or physical activity on the microbiome. Future multi-omics studies could provide a holistic view of the biological effects of physical activity, which will help to unravel the numerous genes and molecular pathways that are altered.
8. Conclusions
Research suggests that the impact of physical activity on gut microbiota differs based on the type, intensity, and duration of exercise. Regular exercise enhances the growth of beneficial bacteria and short-chain fatty acids (SCFAs), improving gut health and potentially warding off metabolic diseases. Furthermore, exercise boosts microbial diversity, which is vital for both metabolic and immune health, and enhances an anti-inflammatory state throughout the body. The alteration of the beneficial gut microbiota with exercise and probiotics increases compounds including SCFAs, bile acids, and hydrogen sulfide, which can improve muscle mass and exercise endurance. Furthermore, research indicates that gut flora may play a crucial role in macronutrient absorption and modulate neuroendocrine pathways, resulting in enhanced gut health, increased athletic ability, and fewer health issues. More research on metabolic and physiologic pathways is needed to better describe the relationship between physical activity and gut microbiota.
Acknowledgments
We thank Philippe Piccardi from the WCM-Q Health Sciences Library for his invaluable contributions towards editing and improving the manuscript. Hidenori Miyagawa at WCM-Q edited the manuscript’s graphical assets. A few figures were created using BioRender.com.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/nu16213663/s1. Table S1: Studies investigating effects on gut microbiome following intervention with exercise in animals; Table S2: Studies investigating effects on gut microbiome based on general physical activity (PA) and cardiorespiratory fitness (CRF); Table S3: Studies investigating effects on gut microbiome based on resistance exercise; Table S4: Studies investigating effects on gut microbiome based on aerobic exercise (AE); Table S5: Studies investigating effects on gut microbiome based on combined resistance and aerobic exercise; Table S6: Studies investigating gut microbiome in professional sportspersons; Table S7. Studies investigating effects of probiotic supplementation in sports performance. References [138,139,140,141,142,143,144,145,146,147,148,149,150,151,152,153,154,155,156,157,158,159,160,161,162,163,164,165,166,167,168,169,170,171,172,173,174,175,176,177,178,179,180,181,182,183,184,185,186,187,188] are cited in the supplementary materials.
Author Contributions
S.V.: writing—original draft idea, writing, review, editing, methodology, investigation, formal analysis, visualization, and data curation. S.R.: writing—original draft idea, writing, review, editing, investigation, and visualization. A.K.: writing—review, editing, investigation, and data curation. F.Z.: Writing—review, editing, and investigation. A.C.: writing—original draft idea, review, and editing, visualization, validation, supervision, resources, project administration, methodology, investigation, funding acquisition, formal analysis, and data curation. All authors have read and agreed to the published version of the manuscript.
Data Availability Statement
No new data were created in this study. The data that support the findings of this study are included within the article and Supplementary Materials.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Mondot S., Lepage P. The human gut microbiome and its dysfunctions through the meta-omics prism. Ann. N. Y. Acad. Sci. 2016;1372:9–19. doi: 10.1111/nyas.13033. [DOI] [PubMed] [Google Scholar]
- 2.Sankar S.A., Lagier J.-C., Pontarotti P., Raoult D., Fournier P.-E. The human gut microbiome, a taxonomic conundrum. Syst. Appl. Microbiol. 2015;38:276–286. doi: 10.1016/j.syapm.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 3.Kriss M., Hazleton K.Z., Nusbacher N.M., Martin C.G., Lozupone C.A. Low diversity gut microbiota dysbiosis: Drivers, functional implications and recovery. Curr. Opin. Microbiol. 2018;44:34–40. doi: 10.1016/j.mib.2018.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shin Y., Han S., Kwon J., Ju S., Choi T.G., Kang I., Kim S.S. Roles of Short-Chain Fatty Acids in Inflammatory Bowel Disease. Nutrients. 2023;15:4466. doi: 10.3390/nu15204466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu X.F., Shao J.H., Liao Y.T., Wang L.N., Jia Y., Dong P.J., Liu Z.Z., He D.D., Li C., Zhang X. Regulation of short-chain fatty acids in the immune system. Front. Immunol. 2023;14:1186892. doi: 10.3389/fimmu.2023.1186892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Walker R.L., Vlamakis H., Lee J.W.J., Besse L.A., Xanthakis V., Vasan R.S., Shaw S.Y., Xavier R.J. Population study of the gut microbiome: Associations with diet, lifestyle, and cardiometabolic disease. Genome Med. 2021;13:188. doi: 10.1186/s13073-021-01007-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu L., Wang H., Chen X., Zhang Y., Zhang H., Xie P. Gut microbiota and its metabolites in depression: From pathogenesis to treatment. EBioMedicine. 2023;90:104527. doi: 10.1016/j.ebiom.2023.104527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Portincasa P., Bonfrate L., Vacca M., De Angelis M., Farella I., Lanza E., Khalil M., Wang D.Q.H., Sperandio M., Di Ciaula A. Gut Microbiota and Short Chain Fatty Acids: Implications in Glucose Homeostasis. Int. J. Mol. Sci. 2022;23:1105. doi: 10.3390/ijms23031105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang Y., Xu J., Wang X., Ren X., Liu Y. Changes of intestinal bacterial microbiota in coronary heart disease complicated with nonalcoholic fatty liver disease. BMC Genom. 2019;20:862. doi: 10.1186/s12864-019-6251-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Horrocks V., King O.G., Yip A.Y.G., Marques I.M., McDonald J.A.K. Role of the gut microbiota in nutrient competition and protection against intestinal pathogen colonization. Microbiology. 2023;169:001377. doi: 10.1099/mic.0.001377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rashidi A., Koyama M., Dey N., McLean J.S., Hill G.R. Colonization resistance is dispensable for segregation of oral and gut microbiota. BMC Med. Genom. 2023;16:31. doi: 10.1186/s12920-023-01449-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee J., Kim Y., Jeon J.Y. Association between physical activity and the prevalence of metabolic syndrome: From the Korean National Health and Nutrition Examination Survey, 1999–2012. SpringerPlus. 2016;5:1870. doi: 10.1186/s40064-016-3514-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Noetel M., Sanders T., Gallardo-Gómez D., Taylor P., del Pozo Cruz B., van den Hoek D., Smith J.J., Mahoney J., Spathis J., Moresi M., et al. Effect of exercise for depression: Systematic review and network meta-analysis of randomised controlled trials. BMJ. 2024;384:e075847. doi: 10.1136/bmj-2023-075847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nieman D.C., Wentz L.M. The compelling link between physical activity and the body’s defense system. J. Sport Health Sci. 2019;8:201–217. doi: 10.1016/j.jshs.2018.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pearce M., Garcia L., Abbas A., Strain T., Schuch F.B., Golubic R., Kelly P., Khan S., Utukuri M., Laird Y., et al. Association Between Physical Activity and Risk of Depression: A Systematic Review and Meta-analysis. JAMA Psychiatry. 2022;79:550–559. doi: 10.1001/jamapsychiatry.2022.0609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pojednic R., D’Arpino E., Halliday I., Bantham A. The Benefits of Physical Activity for People with Obesity, Independent of Weight Loss: A Systematic Review. Int. J. Environ. Res. Public Health. 2022;19:4981. doi: 10.3390/ijerph19094981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baptista L.C., Sun Y., Carter C.S., Buford T.W. Crosstalk Between the Gut Microbiome and Bioactive Lipids: Therapeutic Targets in Cognitive Frailty. Front. Nutr. 2020;7:17. doi: 10.3389/fnut.2020.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Clauss M., Gérard P., Mosca A., Leclerc M. Interplay Between Exercise and Gut Microbiome in the Context of Human Health and Performance. Front. Nutr. 2021;8:637010. doi: 10.3389/fnut.2021.637010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wegierska A.E., Charitos I.A., Topi S., Potenza M.A., Montagnani M., Santacroce L. The Connection Between Physical Exercise and Gut Microbiota: Implications for Competitive Sports Athletes. Sports Med. 2022;52:2355–2369. doi: 10.1007/s40279-022-01696-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O’Brien M.T., O’Sullivan O., Claesson M.J., Cotter P.D. The Athlete Gut Microbiome and its Relevance to Health and Performance: A Review. Sports Med. 2022;52:119–128. doi: 10.1007/s40279-022-01785-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fielding R.A., Reeves A.R., Jasuja R., Liu C., Barrett B.B., Lustgarten M.S. Muscle strength is increased in mice that are colonized with microbiota from high-functioning older adults. Exp. Gerontol. 2019;127:110722. doi: 10.1016/j.exger.2019.110722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zaiss M.M., Joyce Wu H.-J., Mauro D., Schett G., Ciccia F. The gut-joint axis in rheumatoid arthritis. Nat. Rev. Rheumatol. 2021;17:224–237. doi: 10.1038/s41584-021-00585-3. [DOI] [PubMed] [Google Scholar]
- 23.Cullen J.M.A., Shahzad S., Dhillon J. A systematic review on the effects of exercise on gut microbial diversity, taxonomic composition, and microbial metabolites: Identifying research gaps and future directions. Front. Physiol. 2023;14:1292673. doi: 10.3389/fphys.2023.1292673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matsumoto M., Inoue R., Tsukahara T., Ushida K., Chiji H., Matsubara N., Hara H. Voluntary Running Exercise Alters Microbiota Composition and Increases n-Butyrate Concentration in the Rat Cecum. Biosci. Biotechnol. Biochem. 2008;72:572–576. doi: 10.1271/bbb.70474. [DOI] [PubMed] [Google Scholar]
- 25.Mika A., Van Treuren W., González A., Herrera J.J., Knight R., Fleshner M. Exercise is More Effective at Altering Gut Microbial Composition and Producing Stable Changes in Lean Mass in Juvenile versus Adult Male F344 Rats. PLoS ONE. 2015;10:e0125889. doi: 10.1371/journal.pone.0125889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dempsey E., Corr S.C. Lactobacillus spp. for Gastrointestinal Health: Current and Future Perspectives. Front. Immunol. 2022;13:840245. doi: 10.3389/fimmu.2022.840245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen J., Chen X., Ho C.L. Recent Development of Probiotic Bifidobacteria for Treating Human Diseases. Front. Bioeng. Biotechnol. 2021;9:770248. doi: 10.3389/fbioe.2021.770248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu H., Wang J., He T., Becker S., Zhang G., Li D., Ma X. Butyrate: A Double-Edged Sword for Health? Adv. Nutr. 2018;9:21–29. doi: 10.1093/advances/nmx009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lamoureux E.V., Grandy S.A., Langille M.G.I. Moderate Exercise Has Limited but Distinguishable Effects on the Mouse Microbiome. mSystems. 2017;2:10-1128. doi: 10.1128/mSystems.00006-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Górniak W., Cholewińska P., Szeligowska N., Wołoszyńska M., Soroko M., Czyż K. Effect of Intense Exercise on the Level of Bacteroidetes and Firmicutes Phyla in the Digestive System of Thoroughbred Racehorses. Animals. 2021;11:290. doi: 10.3390/ani11020290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Magne F., Gotteland M., Gauthier L., Zazueta A., Pesoa S., Navarrete P., Balamurugan R. The Firmicutes/Bacteroidetes Ratio: A Relevant Marker of Gut Dysbiosis in Obese Patients? Nutrients. 2020;12:1474. doi: 10.3390/nu12051474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rawat P.S., Li Y., Zhang W., Meng X., Liu W. Hungatella hathewayi, an Efficient Glycosaminoglycan-Degrading Firmicutes from Human Gut and Its Chondroitin ABC Exolyase with High Activity and Broad Substrate Specificity. Appl. Environ. Microbiol. 2022;88:e0154622. doi: 10.1128/aem.01546-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Flint H.J., Scott K.P., Duncan S.H., Louis P., Forano E. Microbial degradation of complex carbohydrates in the gut. Gut Microbes. 2012;3:289–306. doi: 10.4161/gmic.19897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Castro A.P., Silva K.K.S., Medeiros C.S.A., Alves F., Araujo R.C., Almeida J.A. Effects of 12 weeks of resistance training on rat gut microbiota composition. J. Exp. Biol. 2021;224:242543. doi: 10.1242/jeb.242543. [DOI] [PubMed] [Google Scholar]
- 35.Chen H., Shen L., Liu Y., Ma X., Long L., Ma X., Ma L., Chen Z., Lin X., Si L., et al. Strength Exercise Confers Protection in Central Nervous System Autoimmunity by Altering the Gut Microbiota. Front. Immunol. 2021;12:628629. doi: 10.3389/fimmu.2021.628629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Deng R., Wang M., Song Y., Shi Y. A Bibliometric Analysis on the Research Trend of Exercise and the Gut Microbiome. Microorganisms. 2023;11:903. doi: 10.3390/microorganisms11040903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bielik V., Hric I., Ugrayová S., Kubáňová L., Putala M., Grznár Ľ., Penesová A., Havranová A., Šardzíková S., Grendar M., et al. Effect of High-intensity Training and Probiotics on Gut Microbiota Diversity in Competitive Swimmers: Randomized Controlled Trial. Sports Med.-Open. 2022;8:64. doi: 10.1186/s40798-022-00453-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Munukka E., Ahtiainen J.P., Puigbó P., Jalkanen S., Pahkala K., Keskitalo A., Kujala U.M., Pietilä S., Hollmén M., Elo L., et al. Six-Week Endurance Exercise Alters Gut Metagenome That Is not Reflected in Systemic Metabolism in Over-weight Women. Front. Microbiol. 2018;9:2323. doi: 10.3389/fmicb.2018.02323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Minaya D.M., Turlej A., Joshi A., Nagy T., Weinstein N., DiLorenzo P., Hajnal A., Czaja K. Consumption of a high energy density diet triggers microbiota dysbiosis, hepatic lipidosis, and microglia activation in the nucleus of the solitary tract in rats. Nutr. Diabetes. 2020;10:20. doi: 10.1038/s41387-020-0119-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Samuel B.S., Gordon J.I. A Humanized Gnotobiotic Mouse Model of Host-Archaeal-Bacterial Mutualism. Proc. Natl. Acad. Sci. USA. 2006;103:10011–10016. doi: 10.1073/pnas.0602187103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cheng R., Wang L., Le S., Yang Y., Zhao C., Zhang X., Yang X., Xu T., Xu L., Wiklund P., et al. A randomized controlled trial for response of microbiome network to exercise and diet intervention in patients with nonalcoholic fatty liver disease. Nat. Commun. 2022;13:2555. doi: 10.1038/s41467-022-29968-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Estaki M., Pither J., Baumeister P., Little J.P., Gill S.K., Ghosh S., Ahmadi-Vand Z., Marsden K.R., Gibson D.L. Cardiorespiratory fitness as a predictor of intestinal microbial diversity and distinct metagenomic functions. Microbiome. 2016;4:42. doi: 10.1186/s40168-016-0189-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Verheggen R.J.H.M., Konstanti P., Smidt H., Hermus A.R.M.M., Thijssen D.H.J., Hopman M.T.E. Eight-week exercise training in humans with obesity: Marked improvements in insulin sensitivity and modest changes in gut microbiome. Obesity. 2021;29:1615–1624. doi: 10.1002/oby.23252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Crost E.H., Coletto E., Bell A., Juge N. Ruminococcus gnavus: Friend or foe for human health. FEMS Microbiol. Rev. 2023;47:fuad014. doi: 10.1093/femsre/fuad014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Manor O., Dai C.L., Kornilov S.A., Smith B., Price N.D., Lovejoy J.C., Gibbons S.M., Magis A.T. Health and disease markers correlate with gut microbiome composition across thousands of people. Nat. Commun. 2020;11:5206. doi: 10.1038/s41467-020-18871-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bycura D., Santos A.C., Shiffer A., Kyman S., Winfree K., Sutliffe J., Pearson T., Sonderegger D., Cope E., Caporaso J.G. Impact of Different Exercise Modalities on the Human Gut Microbiome. Sports. 2021;9:14. doi: 10.3390/sports9020014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhao X., Zhang Z., Hu B., Huang W., Yuan C., Zou L. Response of Gut Microbiota to Metabolite Changes Induced by Endurance Exercise. Front. Microbiol. 2018;9:765. doi: 10.3389/fmicb.2018.00765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zeppa S.D., Amatori S., Sisti D., Gervasi M., Agostini D., Piccoli G., Pazienza V., Gobbi P., Rocchi M.B.L., Sestili P., et al. Nine weeks of high-intensity indoor cycling training induced changes in the microbiota composition in non-athlete healthy male college students. J. Int. Soc. Sports Nutr. 2021;18:74. doi: 10.1186/s12970-021-00471-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang R., Cai Y., Lu W., Zhang R., Shao R., Yau S.-Y., Stubbs B., McIntyre R.S., Su K.-P., Xu G., et al. Exercise effect on the gut microbiota in young adolescents with subthreshold depression: A randomized psychoeducation-controlled Trial. Psychiatry Res. 2023;319:115005. doi: 10.1016/j.psychres.2022.115005. [DOI] [PubMed] [Google Scholar]
- 50.Tabone M., Bressa C., García-Merino J.A., Moreno-Pérez D., Van E.C., Castelli F.A., Fenaille F., Larrosa M. The effect of acute moderate-intensity exercise on the serum and fecal metabolomes and the gut microbiota of cross-country endurance athletes. Sci. Rep. 2021;11:3558. doi: 10.1038/s41598-021-82947-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Morishima S., Aoi W., Kawamura A., Kawase T., Takagi T., Naito Y., Tsukahara T., Inoue R. Intensive, prolonged exercise seemingly causes gut dysbiosis in female endurance runners. J. Clin. Biochem. Nutr. 2021;68:253–258. doi: 10.3164/jcbn.20-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Han M., Yang K., Yang P., Zhong C., Chen C., Wang S., Lu Q., Ning K. Stratification of athletes’ gut microbiota: The multifaceted hubs associated with dietary factors, physical characteristics and performance. Gut Microbes. 2020;12:1842991. doi: 10.1080/19490976.2020.1842991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.De Vadder F., Kovatcheva-Datchary P., Zitoun C., Duchampt A., Bäckhed F., Mithieux G. Microbiota-Produced Succinate Improves Glucose Homeostasis via Intestinal Gluconeogenesis. Cell Metab. 2016;24:151–157. doi: 10.1016/j.cmet.2016.06.013. [DOI] [PubMed] [Google Scholar]
- 54.Bressa C., Bailén-Andrino M., Pérez-Santiago J., González-Soltero R., Pérez M., Montalvo-Lominchar M.G., Maté-Muñoz J.L., Domínguez R., Moreno D., Larrosa M. Differences in gut microbiota profile between women with active lifestyle and sedentary women. PLoS ONE. 2017;12:e0171352. doi: 10.1371/journal.pone.0171352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vacca M., Celano G., Calabrese F.M., Portincasa P., Gobbetti M., De Angelis M. The Controversial Role of Human Gut Lachnospiraceae. Microorganisms. 2020;8:573. doi: 10.3390/microorganisms8040573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Palmnäs-Bédard M.S.A., Costabile G., Vetrani C., Åberg S., Hjalmarsson Y., Dicksved J., Riccardi G., Landberg R. The human gut microbiota and glucose metabolism: A scoping review of key bacteria and the potential role of SCFAs. Am. J. Clin. Nutr. 2022;116:862–874. doi: 10.1093/ajcn/nqac217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Keohane D.M., Woods T., O’Connor P., Underwood S., Cronin O., Whiston R., O’Sullivan O., Cotter P., Shanahan F., Molloy M.G.M. Four men in a boat: Ultra-endurance exercise alters the gut microbiome. J. Sci. Med. Sport. 2019;22:1059–1064. doi: 10.1016/j.jsams.2019.04.004. [DOI] [PubMed] [Google Scholar]
- 58.Alam M.J., Xie L., Yap Y.-A., Marques F.Z., Robert R. Manipulating Microbiota to Treat Atopic Dermatitis: Functions and Therapies. Pathogens. 2022;11:642. doi: 10.3390/pathogens11060642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lin T.-Y., Wu P.-H., Lin Y.-T., Hung S.-C. Characterization of Gut Microbiota Composition in Hemodialysis Patients With Normal Weight Obesity. J. Clin. Endocrinol. Metab. 2020;105:2006–2014. doi: 10.1210/clinem/dgaa166. [DOI] [PubMed] [Google Scholar]
- 60.Nylund L., Nermes M., Isolauri E., Salminen S., Vos W.M., Satokari R. Severity of atopic disease inversely correlates with intestinal microbiota diversity and butyrate-producing bacteria. Allergy. 2015;70:241–244. doi: 10.1111/all.12549. [DOI] [PubMed] [Google Scholar]
- 61.Liu Y., Wang Y., Ni Y., Cheung C.K.Y., Lam K.S.L., Wang Y., Xia Z., Ye D., Guo J., Tse M.A., et al. Gut Microbiome Fermentation Determines the Efficacy of Exercise for Diabetes Prevention. Cell Metab. 2020;31:77–91.e75. doi: 10.1016/j.cmet.2019.11.001. [DOI] [PubMed] [Google Scholar]
- 62.O’Donovan C.M., Madigan S.M., Garcia-Perez I., Rankin A., O’ Sullivan O., Cotter P.D. Distinct microbiome composition and metabolome exists across subgroups of elite Irish athletes. J. Sci. Med. Sport. 2020;23:63–68. doi: 10.1016/j.jsams.2019.08.290. [DOI] [PubMed] [Google Scholar]
- 63.Grosicki G.J., Durk R.P., Bagley J.R. Rapid gut microbiome changes in a world-class ultramarathon runner. Physiol. Rep. 2019;7:e14313. doi: 10.14814/phy2.14313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Magzal F., Shochat T., Haimov I., Tamir S., Asraf K., Tuchner-Arieli M., Even C., Agmon M. Increased physical activity improves gut microbiota composition and reduces short-chain fatty acid concentrations in older adults with insomnia. Sci. Rep. 2022;12:2265. doi: 10.1038/s41598-022-05099-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Romaní-Pérez M., López-Almela I., Bullich-Vilarrubias C., Rueda-Ruzafa L., Gómez Del Pulgar E.M., Benítez-Páez A., Liebisch G., Lamas J.A., Sanz Y. Holdemanella biformis improves glucose tolerance and regulates GLP-1 signaling in obese mice. FASEB J. 2021;35:e21734. doi: 10.1096/fj.202100126R. [DOI] [PubMed] [Google Scholar]
- 66.Houttu V., Boulund U., Nicolaou M., Holleboom A.G., Grefhorst A., Galenkamp H., Born B.-J.v.d., Zwinderman K., Nieuwdorp M. Physical Activity and Dietary Composition Relate to Differences in Gut Microbial Patterns in a Multi-Ethnic Cohort—The HELIUS Study. Metabolites. 2021;11:858. doi: 10.3390/metabo11120858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Barton W., Cronin O., Garcia-Perez I., Whiston R., Holmes E., Woods T., Molloy C.B., Molloy M.G., Shanahan F., Cotter P.D., et al. The effects of sustained fitness improvement on the gut microbiome: A longitudinal, repeated measures case-study approach. Transl. Sports Med. 2021;4:174–192. doi: 10.1002/tsm2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hintikka J.E., Munukka E., Valtonen M., Luoto R., Ihalainen J.K., Kallonen T., Waris M., Heinonen O.J., Ruuskanen O., Pekkala S. Gut Microbiota and Serum Metabolome in Elite Cross-Country Skiers: A Controlled Study. Metabolites. 2022;12:335. doi: 10.3390/metabo12040335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gross K., Santiago M., Krieger J.M., Hagele A.M., Zielinska K., Scheiman J., Jäger R., Kostic A., Kerksick C.M. Impact of probiotic Veillonella atypica FB0054 supplementation on anaerobic capacity and lactate. iScience. 2024;27:108643. doi: 10.1016/j.isci.2023.108643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zafar H., Milton H., Saier J. Gut Bacteroides species in health and disease. Gut Microbes. 2021;13:1848158. doi: 10.1080/19490976.2020.1848158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Warbeck C., Dowd A.J., Kronlund L., Parmar C., Daun J.T., Wytsma-Fisher K., Millet G.Y., Schick A., Reimer R.A., Fung T., et al. Feasibility and effects on the gut microbiota of a 12-week high-intensity interval training plus lifestyle education intervention on inactive adults with celiac disease. Appl. Physiol. Nutr. Metab. 2020;46:325–336. doi: 10.1139/apnm-2020-0459. [DOI] [PubMed] [Google Scholar]
- 72.Liang R., Zhang S., Peng X., Yang W., Xu Y., Wu P., Chen J., Cai Y., Zhou J. Characteristics of the gut microbiota in professional martial arts athletes: A comparison between different competition levels. PLoS ONE. 2019;14:e0226240. doi: 10.1371/journal.pone.0226240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cataldi S., Bonavolontà V., Poli L., Clemente F.M., De Candia M., Carvutto R., Silva A.F., Badicu G., Greco G., Fischetti F. The Relationship between Physical Activity, Physical Exercise, and Human Gut Microbiota in Healthy and Unhealthy Subjects: A Systematic Review. Biology. 2022;11:479. doi: 10.3390/biology11030479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Torquati L., Gajanand T., Cox E.R., Willis C.R.G., Zaugg J., Keating S.E., Coombes J.S. Effects of exercise intensity on gut microbiome composition and function in people with type 2 diabetes. Eur. J. Sport Sci. 2023;23:530–541. doi: 10.1080/17461391.2022.2035436. [DOI] [PubMed] [Google Scholar]
- 75.Zhong F., Wen X., Yang M., Lai H.-Y., Momma H., Cheng L., Sun X., Nagatomi R., Huang C. Effect of an 8-week Exercise Training on Gut Microbiota in Physically Inactive Older Women. Int. J. Sports Med. 2021;42:610–623. doi: 10.1055/a-1301-7011. [DOI] [PubMed] [Google Scholar]
- 76.Binda C., Lopetuso L.R., Rizzatti G., Gibiino G., Cennamo V., Gasbarrini A. Actinobacteria: A relevant minority for the maintenance of gut homeostasis. Dig. Liver Dis. 2018;50:421–428. doi: 10.1016/j.dld.2018.02.012. [DOI] [PubMed] [Google Scholar]
- 77.Castro-Mejía J.L., Khakimov B., Krych Ł., Bülow J., Bechshøft R.L., Højfeldt G., Mertz K.H., Garne E.S., Schacht S.R., Ahmad H.F., et al. Physical fitness in community-dwelling older adults is linked to dietary intake, gut microbiota, and metabolomic signatures. Aging Cell. 2020;19:e13105. doi: 10.1111/acel.13105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhu Q., Jiang S., Du G. Effects of exercise frequency on the gut microbiota in elderly individuals. MicrobiologyOpen. 2020;9:e1053. doi: 10.1002/mbo3.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Erlandson K.M., Liu J., Johnson R., Dillon S., Jankowski C.M., Kroehl M., Robertson C.E., Frank D.N., Tuncil Y., Higgins J., et al. An exercise intervention alters stool microbiota and metabolites among older, sedentary adults. Ther. Adv. Infect. Dis. 2021;8:204993612110270. doi: 10.1177/20499361211027067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Goto Y. Epithelial Cells as a Transmitter of Signals from Commensal Bacteria and Host Immune Cells. Front. Immunol. 2019;10:2057. doi: 10.3389/fimmu.2019.02057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fusco W., Lorenzo M.B., Cintoni M., Porcari S., Rinninella E., Kaitsas F., Lener E., Mele M.C., Gasbarrini A., Collado M.C., et al. Short-Chain Fatty-Acid-Producing Bacteria: Key Components of the Human Gut Microbiota. Nutrients. 2023;15:2211. doi: 10.3390/nu15092211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhao S., Lau R., Zhong Y., Chen M.-H. Lactate cross-feeding between Bifidobacterium species and Megasphaera indica contributes to butyrate formation in the human colonic environment. Appl. Environ. Microbiol. 2024;90:e0101923. doi: 10.1128/aem.01019-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Litvak Y., Byndloss M.X., Tsolis R.M., Bäumler A.J. Dysbiotic Proteobacteria expansion: A microbial signature of epithelial dysfunction. Curr. Opin. Microbiol. 2017;39:1–6. doi: 10.1016/j.mib.2017.07.003. [DOI] [PubMed] [Google Scholar]
- 84.Carvalho F.A., Koren O., Goodrich J.K., Johansson M.E., Nalbantoglu I., Aitken J.D., Su Y., Chassaing B., Walters W.A., González A., et al. Transient Inability to Manage Proteobacteria Promotes Chronic Gut Inflammation in TLR5-Deficient Mice. Cell Host Microbe. 2012;12:139–152. doi: 10.1016/j.chom.2012.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lkhagva E., Chung H.-J., Ahn J.-S., Hong S.-T. Host Factors Affect the Gut Microbiome More Significantly than Diet Shift. Microorganisms. 2021;9:2520. doi: 10.3390/microorganisms9122520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Quiroga R., Nistal E., Estébanez B., Porras D., Juárez-Fernández M., Martínez-Flórez S., García-Mediavilla M.V., de Paz J.A., González-Gallego J., Sánchez-Campos S., et al. Exercise training modulates the gut microbiota profile and impairs inflammatory signaling pathways in obese children. Exp. Mol. Med. 2020;52:1048–1061. doi: 10.1038/s12276-020-0459-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Jang L.-G., Choi G., Kim S.-W., Kim B.-Y., Lee S., Park H. The combination of sport and sport-specific diet is associated with characteristics of gut microbiota: An observational study. J. Int. Soc. Sports Nutr. 2019;16:21. doi: 10.1186/s12970-019-0290-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Donati Zeppa S., Agostini D., Gervasi M., Annibalini G., Amatori S., Ferrini F., Sisti D., Piccoli G., Barbieri E., Sestili P., et al. Mutual Interactions among Exercise, Sport Supplements and Microbiota. Nutrients. 2019;12:17. doi: 10.3390/nu12010017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hua Y., Huang W., Wang F., Jing Z., Li J., Wang Q., Zhao Y. Metabolites, gene expression, and gut microbiota profiles suggest the putative mechanisms via which dietary creatine increases the serum taurine and g-ABA contents in Megalobrama amblycephala. Fish Physiol. Biochem. 2023;49:253–274. doi: 10.1007/s10695-023-01177-6. [DOI] [PubMed] [Google Scholar]
- 90.Jaroszewski J., Mamun N., Czaja K. Bidirectional Interaction between Tetracyclines and Gut Microbiome. Antibiotics. 2023;12:1438. doi: 10.3390/antibiotics12091438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mazur-Kurach P., Frączek B., Klimek A.T. Does Multi-Strain Probiotic Supplementation Impact the Effort Capacity of Competitive Road Cyclists? Int. J. Environ. Res. Public Health. 2022;19:12205. doi: 10.3390/ijerph191912205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Schreiber C., Tamir S., Golan R., Weinstein A., Weinstein Y. The effect of probiotic supplementation on performance, inflammatory markers and gastro-intestinal symptoms in elite road cyclists. J. Int. Soc. Sports Nutr. 2021;18:36. doi: 10.1186/s12970-021-00432-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Shing C.M., Peake J.M., Lim C.L., Briskey D., Walsh N.P., Fortes M.B., Ahuja K.D.K., Vitetta L. Effects of probiotics supplementation on gastrointestinal permeability, inflammation and exercise performance in the heat. Eur. J. Appl. Physiol. 2014;114:93–103. doi: 10.1007/s00421-013-2748-y. [DOI] [PubMed] [Google Scholar]
- 94.Harnett J.E., Pyne D.B., McKune A.J., Penm J., Pumpa K.L. Probiotic supplementation elicits favourable changes in muscle soreness and sleep quality in rugby players. J. Sci. Med. Sport. 2021;24:195–199. doi: 10.1016/j.jsams.2020.08.005. [DOI] [PubMed] [Google Scholar]
- 95.Strasser B., Geiger D., Schauer M., Gostner J.M., Gatterer H., Burtscher M., Fuchs D. Probiotic Supplements Beneficially Affect Tryptophan-Kynurenine Metabolism and Reduce the Incidence of Upper Respiratory Tract Infections in Trained Athletes: A Randomized, Double-Blinded, Placebo-Controlled Trial. Nutrients. 2016;8:752. doi: 10.3390/nu8110752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Michalickova D., Minic R., Dikic N., Andjelkovic M., Kostic-Vucicevic M., Stojmenovic T., Nikolic I., Djordjevic B. Lactobacillus helveticus Lafti L10 supplementation reduces respiratory infection duration in a cohort of elite athletes: A randomized, double-blind, placebo-controlled trial. Appl. Physiol. Nutr. Metab. 2016;41:782–789. doi: 10.1139/apnm-2015-0541. [DOI] [PubMed] [Google Scholar]
- 97.Toohey J.C., Townsend J.R., Johnson S.B., Toy A.M., Vantrease W.C., Bender D., Crimi C.C., Stowers K.L., Ruiz M.D., VanDusseldorp T.A., et al. Effects of Probiotic (Bacillus subtilis) Supplementation During Offseason Resistance Training in Female Division I Athletes. J. Strength Cond. Res. 2020;34:3173. doi: 10.1519/JSC.0000000000002675. [DOI] [PubMed] [Google Scholar]
- 98.Salleh R.M., Kuan G., Aziz M.N.A., Rahim M.R.A., Rahayu T., Sulaiman S., Kusuma D.W.Y., Adikari A.M.G.C.P., Razam M.S.M., Radhakrishnan A.K., et al. Effects of probiotics on anxiety, stress, mood and fitness of badminton players. Nutrients. 2021;13:1783. doi: 10.3390/nu13061783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Komano Y., Shimada K., Naito H., Fukao K., Ishihara Y., Fujii T., Kokubo T., Daida H. Efficacy of heat-killed Lactococcus lactis JCM 5805 on immunity and fatigue during consecutive high intensity exercise in male athletes: A randomized, placebo-controlled, double-blinded trial. J. Int. Soc. Sports Nutr. 2018;15:39. doi: 10.1186/s12970-018-0244-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Pugh J.N., Sparks A.S., Doran D.A., Fleming S.C., Langan-Evans C., Kirk B., Fearn R., Morton J.P., Close G.L. Four weeks of probiotic supplementation reduces GI symptoms during a marathon race. Eur. J. Appl. Physiol. 2019;119:1491–1501. doi: 10.1007/s00421-019-04136-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lamprecht M., Bogner S., Schippinger G., Steinbauer K., Fankhauser F., Hallstroem S., Schuetz B., Greilberger J.F. Probiotic supplementation affects markers of intestinal barrier, oxidation, and inflammation in trained men; a randomized, double-blinded, placebo-controlled trial. J. Int. Soc. Sports Nutr. 2012;9:45. doi: 10.1186/1550-2783-9-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Martarelli D., Verdenelli M.C., Scuri S., Cocchioni M., Silvi S., Cecchini C., Pompei P. Effect of a Probiotic Intake on Oxidant and Antioxidant Parameters in Plasma of Athletes During Intense Exercise Training. Curr. Microbiol. 2011;62:1689–1696. doi: 10.1007/s00284-011-9915-3. [DOI] [PubMed] [Google Scholar]
- 103.Huang W.C., Wei C.C., Huang C.C., Chen W.L., Huang H.Y. The beneficial effects of Lactobacillus plantarum PS128 on high-intensity, exercise-induced oxidative stress, inflammation, and performance in triathletes. Nutrients. 2019;11:353. doi: 10.3390/nu11020353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kwok K.O., Fries L.R., Silva-Zolezzi I., Thakkar S.K., Iroz A., Blanchard C. Effects of Probiotic Intervention on Markers of Inflammation and Health Outcomes in Women of Reproductive Age and Their Children. Front. Nutr. 2022;9:889040. doi: 10.3389/fnut.2022.889040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ziemkiewicz N., Hilliard G., Pullen N.A., Garg K. The Role of Innate and Adaptive Immune Cells in Skeletal Muscle Regeneration. Int. J. Mol. Sci. 2021;22:3265. doi: 10.3390/ijms22063265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ostrowski K., Rohde T., Asp S., Schjerling P., Pedersen B.K. Pro- and anti-inflammatory cytokine balance in strenuous exercise in humans. J. Physiol. 1999;515:287–291. doi: 10.1111/j.1469-7793.1999.287ad.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Jäger R., Zaragoza J., Purpura M., Iametti S., Marengo M., Tinsley G.M., Anzalone A.J., Oliver J.M., Fiore W., Biffi A., et al. Probiotic Administration Increases Amino Acid Absorption from Plant Protein: A Placebo-Controlled, Randomized, Double-Blind, Multicenter, Crossover Study. Probiotics Antimicrob. Proteins. 2020;12:1330–1339. doi: 10.1007/s12602-020-09656-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Vonderheid S.C., Tussing-Humphreys L., Park C., Pauls H., OjiNjideka Hemphill N., LaBomascus B., McLeod A., Koenig M.D. A Systematic Review and Meta-Analysis on the Effects of Probiotic Species on Iron Absorption and Iron Status. Nutrients. 2019;11:2938. doi: 10.3390/nu11122938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lee M.-C., Hsu Y.-J., Ho H.-H., Kuo Y.-W., Lin W.-Y., Tsai S.-Y., Chen W.-L., Lin C.-L., Huang C.-C. Effectiveness of human-origin Lactobacillus plantarum PL-02 in improving muscle mass, exercise performance and anti-fatigue. Sci. Rep. 2021;11:19469. doi: 10.1038/s41598-021-98958-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Chen M.-M., Li Y., Deng S.-L., Zhao Y., Lian Z.-X., Yu K. Mitochondrial Function and Reactive Oxygen/Nitrogen Species in Skeletal Muscle. Front. Cell Dev. Biol. 2022;10:826981. doi: 10.3389/fcell.2022.826981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Regmi S.C., Park S.-Y., Ku S.K., Kim J.-A. Serotonin regulates innate immune responses of colon epithelial cells through Nox2-derived reactive oxygen species. Free Radic. Biol. Med. 2014;69:377–389. doi: 10.1016/j.freeradbiomed.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 112.Wiertsema S.P., van Bergenhenegouwen J., Garssen J., Knippels L.M.J. The Interplay between the Gut Microbiome and the Immune System in the Context of Infectious Diseases throughout Life and the Role of Nutrition in Optimizing Treatment Strategies. Nutrients. 2021;13:886. doi: 10.3390/nu13030886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hernández-Urbán A.J., Drago-Serrano M.E., Cruz-Baquero A., García-Hernández A.L., Arciniega-Martínez I.M., Pacheco-Yépez J., Guzmán-Mejía F., Godínez-Victoria M. Exercise improves intestinal IgA production by T-dependent cell pathway in adults but not in aged mice. Front. Endocrinol. 2023;14:1190547. doi: 10.3389/fendo.2023.1190547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Khan M.S., Ikram M., Park J.S., Park T.J., Kim M.O. Gut Microbiota, Its Role in Induction of Alzheimer’s Disease Pathology, and Possible Therapeutic Interventions: Special Focus on Anthocyanins. Cells. 2020;9:853. doi: 10.3390/cells9040853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Cavalcante P.A.M., Gregnani M.F., Henrique J.S., Ornellas F.H., Araújo R.C. Aerobic but not Resistance Exercise Can Induce Inflammatory Pathways via Toll-Like 2 and 4: A Systematic Review. Sports Med. -Open. 2017;3:42. doi: 10.1186/s40798-017-0111-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zhang M., Xiao B., Chen X., Ou B., Wang S. Physical exercise plays a role in rebalancing the bile acids of enterohepatic axis in non-alcoholic fatty liver disease. Acta Physiol. 2024;240:e14065. doi: 10.1111/apha.14065. [DOI] [PubMed] [Google Scholar]
- 117.Tveter K.M., Villa-Rodriguez J.A., Cabales A.J., Zhang L., Bawagan F.G., Duran R.M., Roopchand D.E. Polyphenol-induced improvements in glucose metabolism are associated with bile acid signaling to intestinal farnesoid X receptor. BMJ Open Diabetes Res. Care. 2020;8:e001386. doi: 10.1136/bmjdrc-2020-001386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Buret A.G., Allain T., Motta J.-P., Wallace J.L. Effects of Hydrogen Sulfide on the Microbiome: From Toxicity to Therapy. Antioxid. Redox Signal. 2022;36:211–219. doi: 10.1089/ars.2021.0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zhang Y., Masters L., Wang Y., Wu L., Pei Y., Guo B., Parissenti A., Lees S.J., Wang R., Yang G. Cystathionine gamma-lyase/H 2 S signaling facilitates myogenesis under aging and injury condition. FASEB J. 2021;35:e21511. doi: 10.1096/fj.202002675R. [DOI] [PubMed] [Google Scholar]
- 120.Marcondes-de-Castro I.A., Reis-Barbosa P.H., Marinho T.S., Aguila M.B., Mandarim-de-Lacerda C.A. AMPK/mTOR pathway significance in healthy liver and non-alcoholic fatty liver disease and its progression. J. Gastroenterol. Hepatol. 2023;38:1868–1876. doi: 10.1111/jgh.16272. [DOI] [PubMed] [Google Scholar]
- 121.den Besten G., Gerding A., van Dijk T.H., Ciapaite J., Bleeker A., van Eunen K., Havinga R., Groen A.K., Reijngoud D.-J., Bakker B.M. Protection against the Metabolic Syndrome by Guar Gum-Derived Short-Chain Fatty Acids Depends on Peroxisome Proliferator-Activated Receptor γ and Glucagon-Like Peptide-1. PLoS ONE. 2015;10:e0136364. doi: 10.1371/journal.pone.0136364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Bakker A.D., Jaspers R.T. IL-6 and IGF-1 Signaling Within and Between Muscle and Bone: How Important is the mTOR Pathway for Bone Metabolism? Curr. Osteoporos. Rep. 2015;13:131–139. doi: 10.1007/s11914-015-0264-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Frampton J., Murphy K.G., Frost G., Chambers E.S. Short-chain fatty acids as potential regulators of skeletal muscle metabolism and function. Nat. Metab. 2020;2:840–848. doi: 10.1038/s42255-020-0188-7. [DOI] [PubMed] [Google Scholar]
- 124.Thomson D.M. The Role of AMPK in the Regulation of Skeletal Muscle Size, Hypertrophy, and Regeneration. Int. J. Mol. Sci. 2018;19:3125. doi: 10.3390/ijms19103125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Valentino T.R., Vechetti I.J., Mobley C.B., Dungan C.M., Golden L., Goh J., McCarthy J.J. Dysbiosis of the gut microbiome impairs mouse skeletal muscle adaptation to exercise. J. Physiol. 2021;599:4845–4863. doi: 10.1113/JP281788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Tolhurst G., Heffron H., Lam Y.S., Parker H.E., Habib A.M., Diakogiannaki E., Cameron J., Grosse J., Reimann F., Gribble F.M. Short-Chain Fatty Acids Stimulate Glucagon-Like Peptide-1 Secretion via the G-Protein―Coupled Receptor FFAR2. Diabetes. 2012;61:364–371. doi: 10.2337/db11-1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Rose S., Bennuri S.C., Davis J.E., Wynne R., Slattery J.C., Tippett M., Delhey L., Melnyk S., Kahler S.G., MacFabe D.F., et al. Butyrate enhances mitochondrial function during oxidative stress in cell lines from boys with autism. Transl. Psychiatry. 2018;8:42. doi: 10.1038/s41398-017-0089-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Dai Z., Wu Z., Hang S., Zhu W., Wu G. Amino acid metabolism in intestinal bacteria and its potential implications for mammalian reproduction. Mol. Hum. Reprod. 2015;21:389–409. doi: 10.1093/molehr/gav003. [DOI] [PubMed] [Google Scholar]
- 129.Stecker R.A., Moon J.M., Russo T.J., Ratliff K.M., Mumford P.W., Jäger R., Purpura M., Kerksick C.M. Bacillus coagulans GBI-30, 6086 improves amino acid absorption from milk protein. Nutr. Metab. 2020;17:93. doi: 10.1186/s12986-020-00515-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Qi X., Yun C., Pang Y., Qiao J. The impact of the gut microbiota on the reproductive and metabolic endocrine system. Gut Microbes. 2021;13 doi: 10.1080/19490976.2021.1894070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Lin W., Wen L., Wen J., Xiang G. Effects of Sleeve Gastrectomy on Fecal Gut Microbiota and Short-Chain Fatty Acid Content in a Rat Model of Polycystic Ovary Syndrome. Front. Endocrinol. 2021;12:747888. doi: 10.3389/fendo.2021.747888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Harada N., Hanaoka R., Horiuchi H., Kitakaze T., Mitani T., Inui H., Yamaji R. Castration influences intestinal microflora and induces abdominal obesity in high-fat diet-fed mice. Sci. Rep. 2016;6:23001. doi: 10.1038/srep23001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Dohnalová L., Lundgren P., Carty J.R.E., Goldstein N., Wenski S.L., Nanudorn P., Thiengmag S., Huang K.-P., Litichevskiy L., Descamps H.C., et al. A microbiome-dependent gut-brain pathway regulates motivation for exercise. Nature. 2022;612:739. doi: 10.1038/s41586-022-05525-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Rusch J.A., Layden B.T., Dugas L.R. Signalling cognition: The gut microbiota and hypothalamic-pituitary-adrenal axis. Front. Endocrinol. 2023;14:1130689. doi: 10.3389/fendo.2023.1130689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Chatoo M., Li Y., Ma Z., Coote J., Du J., Chen X. Involvement of Corticotropin-Releasing Factor and Receptors in Immune Cells in Irritable Bowel Syndrome. Front. Endocrinol. 2018;9:21. doi: 10.3389/fendo.2018.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Matijašić M., Meštrović T., Paljetak H.Č., Perić M., Barešić A., Verbanac D. Gut Microbiota beyond Bacteria-Mycobiome, Virome, Archaeome, and Eukaryotic Parasites in IBD. Int. J. Mol. Sci. 2020;21:2668. doi: 10.3390/ijms21082668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Walter E., Watt P., Gibson O.R., Wilmott A.G.B., Mitchell D., Moreton R., Maxwell N.S. Exercise hyperthermia induces greater changes in gastrointestinal permeability than equivalent passive hyperthermia. Physiol. Rep. 2021;9:e14945. doi: 10.14814/phy2.14945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Evans C.C., LePard K.J., Kwak J.W., Stancukas M.C., Laskowski S., Dougherty J., Moulton L., Glawe A., Wang Y., Leone V., et al. Exercise prevents weight gain and alters the gut microbiota in a mouse model of high fat diet-induced obesity. PLoS ONE. 2014;9:e92193. doi: 10.1371/journal.pone.0092193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Choi J.J., Eum S.Y., Rampersaud E., Daunert S., Abreu M.T., Toborek M. Exercise attenuates PCB-induced changes in the mouse gut microbiome. Environ. Health Perspect. 2013;121:725–730. doi: 10.1289/ehp.1306534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Campbell S.C., Wisniewski P.J., Noji M., McGuinness L.R., Häggblom M.M., Lightfoot S.A., Joseph L.B., Kerkhof L.J. The Effect of Diet and Exercise on Intestinal Integrity and Microbial Diversity in Mice. PLoS ONE. 2016;11:e0150502. doi: 10.1371/journal.pone.0150502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Giacco A., delli Paoli G., Simiele R., Caterino M., Ruoppolo M., Bloch W., Kraaij R., Uitterlinden A.G., Santillo A., Senese R., et al. Exercise with food withdrawal at thermoneutrality impacts fuel use, the microbiome, AMPK phosphorylation, muscle fibers, and thyroid hormone levels in rats. Physiol. Rep. 2020;8:e14354. doi: 10.14814/phy2.14354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Meng Y., Chen L., Lin W., Wang H., Xu G., Weng X. Exercise Reverses the Alterations in Gut Microbiota Upon Cold Exposure and Promotes Cold-Induced Weight Loss. Front. Physiol. 2020;11:311. doi: 10.3389/fphys.2020.00311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Ribeiro F.M., Ribeiro C.F.A., Garcia A.C.M., Castro A.P., Almeida J.A., Franco O.L., Petriz B.A. Limited Effects of Low-to-Moderate Aerobic Exercise on the Gut Microbiota of Mice Subjected to a High-Fat Diet. Nutrients. 2019;11:149. doi: 10.3390/nu11010149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Almeida M.L.M.d., Feringer J.W.H., Carvalho J.R.G., Rodrigues I.M., Jordão L.R., Fonseca M.G., Carneiro de Rezende A.S., de Queiroz Neto A., Weese J.S., Costa M.C.d., et al. Intense Exercise and Aerobic Conditioning Associated with Chromium or L-Carnitine Supplementation Modified the Fecal Microbiota of Fillies. PLoS ONE. 2016;11:e0167108. doi: 10.1371/journal.pone.0167108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Houghton D., Stewart C.J., Stamp C., Nelson A., Aj Ami N.J., Petrosino J.F., Wipat A., Trenell M.I., Turnbull D.M., Greaves L.C. Impact of Age-Related Mitochondrial Dysfunction and Exercise on Intestinal Microbiota Composition. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2018;73:571–578. doi: 10.1093/gerona/glx197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Walshe N., Cabrera-Rubio R., Collins R., Puggioni A., Gath V., Crispie F., Cotter P.D., Brennan L., Mulcahy G., Duggan V. A Multiomic Approach to Investigate the Effects of a Weight Loss Program on the Intestinal Health of Overweight Horses. Front. Vet. Sci. 2021;8:668120. doi: 10.3389/fvets.2021.668120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Lambert J.E., Myslicki J.P., Bomhof M.R., Belke D.D., Shearer J., Reimer R.A. Exercise training modifies gut microbiota in normal and diabetic mice. Appl. Physiol. Nutr. Metab. 2015;40:749–752. doi: 10.1139/apnm-2014-0452. [DOI] [PubMed] [Google Scholar]
- 148.Langsetmo L., Johnson A., Demmer R., Fino N., Orwoll E., Ensrud K., Hoffman A.R., Cauley J.A., Shmagel A., Meyer K., et al. The Association between Objectively Measured Physical Activity and the Gut Microbiome among Older Community Dwelling Men. J. Nutr. Health Aging. 2019;23 doi: 10.1007/s12603-019-1194-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Castellanos N., Diez G.G., Antúnez-Almagro C., Bailén M., Bressa C., Soltero R.G., Pérez M., Larrosa M. A Critical Mutualism—Competition Interplay Underlies the Loss of Microbial Diversity in Sedentary Lifestyle. Front. Microbiol. 2019;10:3142. doi: 10.3389/fmicb.2019.03142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Gallè F., Valeriani F., Cattaruzza M.S., Gianfranceschi G., Liguori R., Antinozzi M., Mederer B., Liguori G., Spica V.R. Mediterranean Diet, Physical Activity and Gut Microbiome Composition: A Cross-Sectional Study among Healthy Young Italian Adults. Nutrients. 2020;12:2164. doi: 10.3390/nu12072164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Whisner C.M., Maldonado J., Dente B., Krajmalnik-Brown R., Bruening M. Diet, physical activity and screen time but not body mass index are associated with the gut microbiome of a diverse cohort of college students living in university housing: A cross-sectional study. BMC Microbiol. 2018;18:210. doi: 10.1186/s12866-018-1362-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Bai J., Hu Y., Bruner D.W. Composition of gut microbiota and its association with body mass index and lifestyle factors in a cohort of 7–18 years old children from the American Gut Project. Pediatr. Obes. 2019;14:e12480. doi: 10.1111/ijpo.12480. [DOI] [PubMed] [Google Scholar]
- 153.Zhang W., Li J., Lu S., Han N., Miao J., Zhang T., Qiang Y., Kong Y., Wang H., Gao T., et al. Gut microbiota community characteristics and disease-related microorganism pattern in a population of healthy Chinese people. Sci. Rep. 2019;9:1594. doi: 10.1038/s41598-018-36318-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Carter S.J., Hunter G.R., Blackston J.W., Liu N., Lefkowitz E.J., Pol W.J.V.D., Morrow C.D., Paulsen J.A., Rogers L.Q. Gut microbiota diversity associates with cardiorespiratory fitness in post-primary treatment breast cancer survivors. Exp. Physiol. 2019;104:529–539. doi: 10.1113/EP087404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Palmas V., Pisanu S., Madau V., Casula E., Deledda A., Cusano R., Uva P., Vascellari S., Loviselli A., Manzin A., et al. Gut microbiota markers associated with obesity and overweight in Italian adults. Sci. Rep. 2021;11:5532. doi: 10.1038/s41598-021-84928-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Santarossa S., Sitarik A.R., Johnson C.C., Li J., Lynch S.V., Ownby D.R., Ramirez A., Yong G.L., Cassidy-Bushrow A.E. Associations of physical activity with gut microbiota in pre-adolescent children. Phys. Act. Nutr. 2021;25:24–37. doi: 10.20463/pan.2021.0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Shivani S., Kao C.-Y., Chattopadhyay A., Chen J.-W., Lai L.-C., Lin W.-H., Lu T.-P., Huang I.-H., Tsai M.-H., Teng C.-H., et al. Uremic Toxin-Producing Bacteroides Species Prevail in the Gut Microbiota of Taiwanese CKD Patients: An Analysis Using the New Taiwan Microbiome Baseline. Front. Cell. Infect. Microbiol. 2022;12:726256. doi: 10.3389/fcimb.2022.726256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Frugé A.D., Smith K.S., Bail J.R., Rais-Bahrami S., Demark-Wahnefried W. Biomarkers Associated With Tumor Ki67 and Cathepsin L Gene Expression in Prostate Cancer Patients Participating in a Presurgical Weight Loss Trial. Front. Oncol. 2020;10:544201. doi: 10.3389/fonc.2020.544201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Kern T., Blond M.B., Hansen T.H., Rosenkilde M., Quist J.S., Gram A.S., Ekstrøm C.T., Hansen T., Stallknecht B., Kern T., et al. Structured exercise alters the gut microbiota in humans with overweight and obesity—A randomized controlled trial. Int. J. Obes. 2019;44:125–135. doi: 10.1038/s41366-019-0440-y. [DOI] [PubMed] [Google Scholar]
- 160.Yang Y., Shi Y., Wiklund P., Tan X., Wu N., Zhang X., Tikkanen O., Zhang C., Munukka E., Cheng S. The Association between Cardiorespiratory Fitness and Gut Microbiota Composition in Premenopausal Women. Nutrients. 2017;9:792. doi: 10.3390/nu9080792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Durk R.P., Castillo E., Márquez-Magaña L., Grosicki G.J., Bolter N.D., Lee C.M., Bagley J.R. Gut Microbiota Composition is Related to Cardiorespiratory Fitness in Healthy Young Adults. Int. J. Sport Nutr. Exerc. Metab. 2019;29:249–253. doi: 10.1123/ijsnem.2018-0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Morita E., Yokoyama H., Imai D., Takeda R., Ota A., Kawai E., Hisada T., Emoto M., Suzuki Y., Okazaki K. Aerobic Exercise Training with Brisk Walking Increases Intestinal Bacteroides in Healthy Elderly Women. Nutrients. 2019;11:868. doi: 10.3390/nu11040868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Allen J.M., Mailing L.J., Niemiro G.M., Moore R., Cook M.D., White B.A., Holscher H.D., Woods J.A. Exercise Alters Gut Microbiota Composition and Function in Lean and Obese Humans. Med. Sci. Sports Exerc. 2018;50:747–757. doi: 10.1249/MSS.0000000000001495. [DOI] [PubMed] [Google Scholar]
- 164.Taniguchi H., Tanisawa K., Sun X., Kubo T., Hoshino Y., Hosokawa M., Takeyama H., Higuchi M. Effects of short-term endurance exercise on gut microbiota in elderly men. Physiol. Rep. 2018;6:e13935. doi: 10.14814/phy2.13935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Resende A.S., Leite G.S.F., Junior A.H.L. Changes in the Gut Bacteria Composition of Healthy Men with the Same Nutritional Profile Undergoing 10-Week Aerobic Exercise Training: A Randomized Controlled Trial. Nutrients. 2021;13:2839. doi: 10.3390/nu13082839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Shukla S.K., Cook D., Meyer J., Vernon S.D., Le T., Clevidence D., Robertson C.E., Schrodi S.J., Yale S., Frank D.N. Changes in Gut and Plasma Microbiome following Exercise Challenge in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS) PLoS ONE. 2015;10:e0145453. doi: 10.1371/journal.pone.0145453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Motiani K.K., Collado M.C., Eskelinen J.-J., Virtanen K.A., Löyttyniemi E., Salminen S., Nuutila P., Kalliokoski K.K., Hannukainen J.C. Exercise Training Modulates Gut Microbiota Profile and Improves Endotoxemia. Med. Sci. Sports Exerc. 2020;52:94–104. doi: 10.1249/MSS.0000000000002112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Mahdieh M.S., Maryam J., Bita B., Neda F., Motahare M., Mahboobeh B., S Q.L., Behrooz S.K. A pilot study on the relationship between Lactobacillus, Bifidibactrium counts and inflammatory factors following exercise training. Arch. Physiol. Biochem. 2023;129:778–787. doi: 10.1080/13813455.2021.1871763. [DOI] [PubMed] [Google Scholar]
- 169.Sun S., Lei O.K., Nie J., Shi Q., Xu Y., Kong Z. Effects of Low-Carbohydrate Diet and Exercise Training on Gut Microbiota. Front. Nutr. 2022;9:884550. doi: 10.3389/fnut.2022.884550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Sato M., Suzuki Y. Alterations in intestinal microbiota in ultramarathon runners. Sci. Rep. 2022;12:6984. doi: 10.1038/s41598-022-10791-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Qiu L., Gong F., Wu J., You D., Zhao Y., Xu L., Cao X., Bao F. Exercise Interventions Improved Sleep Quality through Regulating Intestinal Microbiota Composition. Int. J. Environ. Res. Public Health. 2022;19:2385. doi: 10.3390/ijerph191912385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Fukuchi M., Sugita M., Banjo M., Yonekura K., Sasuga Y. The impact of a competitive event and the efficacy of a lactic acid bacteria-fermented soymilk extract on the gut microbiota and urinary metabolites of endurance athletes: An open-label pilot study. PLoS ONE. 2022;17:e0262906. doi: 10.1371/journal.pone.0262906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Karl J.P., Margolis L.M., Madslien E.H., Murphy N.E., Castellani J.W., Gundersen Y., Hoke A.V., Levangie M.W., Kumar R., Chakraborty N., et al. Changes in intestinal microbiota composition and metabolism coincide with increased intestinal permeability in young adults under prolonged physiological stress. Am. J. Physiol. Gastrointest. Liver Physiol. 2017;312:G559–G571. doi: 10.1152/ajpgi.00066.2017. [DOI] [PubMed] [Google Scholar]
- 174.Cronin O., Barton W., Skuse P., Penney N.C., Garcia-Perez I., Murphy E.F., Woods T., Nugent H., Fanning A., Melgar S., et al. A Prospective Metagenomic and Metabolomic Analysis of the Impact of Exercise and/or Whey Protein Supplementation on the Gut Microbiome of Sedentary Adults. mSystems. 2018;3 doi: 10.1128/mSystems.00044-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Huber Y., Pfirrmann D., Gebhardt I., Labenz C., Gehrke N., Straub B.K., Ruckes C., Bantel H., Belda E., Clément K., et al. Improvement of non-invasive markers of NAFLD from an individualised, web-based exercise program. Aliment. Pharmacol. Ther. 2019;50:930–939. doi: 10.1111/apt.15427. [DOI] [PubMed] [Google Scholar]
- 176.Mokhtarzade M., Molanouri Shamsi M., Abolhasani M., Bakhshi B., Sahraian M.A., Quinn L.S., Negaresh R. Home-based exercise training influences gut bacterial levels in multiple sclerosis. Complement. Ther. Clin. Pract. 2021;45:101463. doi: 10.1016/j.ctcp.2021.101463. [DOI] [PubMed] [Google Scholar]
- 177.Wei S., Brejnrod A.D., Trivedi U., Mortensen M.S., Johansen M.Y., Karstoft K., Vaag A.A., Ried-Larsen M., Sørensen S.J. Impact of intensive lifestyle intervention on gut microbiota composition in type 2 diabetes: A post-hoc analysis of a randomized clinical trial. Gut Microbes. 2022;14 doi: 10.1080/19490976.2021.2005407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Fart F., Rajan S.K., Wall R., Rangel I., Ganda-Mall J.P., Tingö L., Brummer R.J., Repsilber D., Schoultz I., Lindqvist C.M. Differences in Gut Microbiome Composition between Senior Orienteering Athletes and Community-Dwelling Older Adults. Nutrients. 2020;12:2610. doi: 10.3390/nu12092610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Petersen L.M., Bautista E.J., Nguyen H., Hanson B.M., Chen L., Lek S.H., Sodergren E., Weinstock G.M. Community characteristics of the gut microbiomes of competitive cyclists. Microbiome. 2017;5:98. doi: 10.1186/s40168-017-0320-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Mörkl S., Lackner S., Müller W., Gorkiewicz G., Kashofer K., Oberascher A., Painold A., Holl A., Holzer P., Meinitzer A., et al. Gut microbiota and body composition in anorexia nervosa inpatients in comparison to athletes, overweight, obese, and normal weight controls. Int. J. Eat. Disord. 2017;50:1421–1431. doi: 10.1002/eat.22801. [DOI] [PubMed] [Google Scholar]
- 181.Šoltys K., Lendvorský L., Hric I., Baranovičová E., Penesová A., Mikula I., Bohmer M., Budiš J., Vávrová S., Grones J., et al. Strenuous Physical Training, Physical Fitness, Body Composition and Bacteroides to Prevotella Ratio in the Gut of Elderly Athletes. Front. Physiol. 2021;12:670989. doi: 10.3389/fphys.2021.670989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Kulecka M., Fraczek B., Mikula M., Zeber-Lubecka N., Karczmarski J., Paziewska A., Ambrozkiewicz F., Jagusztyn-Krynicka K., Cieszczyk P., Ostrowski J. The composition and richness of the gut microbiota differentiate the top Polish endurance athletes from sedentary controls. Gut Microbes. 2020;11:1374–1384. doi: 10.1080/19490976.2020.1758009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Xu Y., Zhong F., Zheng X., Lai H.-Y., Wu C., Huang C. Disparity of Gut Microbiota Composition Among Elite Athletes and Young Adults With Different Physical Activity Independent of Dietary Status: A Matching Study. Front. Nutr. 2022;9:843076. doi: 10.3389/fnut.2022.843076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Jäger R., Shields K.A., Lowery R.P., De Souza E.O., Partl J.M., Hollmer C., Purpura M., Wilson J.M. Probiotic Bacillus coagulans GBI-30, 6086 reduces exercise-induced muscle damage and increases recovery. PeerJ. 2016;4:e2276. doi: 10.7717/peerj.2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Roberts J.D., Suckling C.A., Peedle G.Y., Murphy J.A., Dawkins T.G., Roberts M.G. An Exploratory Investigation of Endotoxin Levels in Novice Long Distance Triathletes, and the Effects of a Multi-Strain Probiotic/Prebiotic, Antioxidant Intervention. Nutrients. 2016;8:733. doi: 10.3390/nu8110733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Salarkia N., Ghadamli L., Zaeri F., Sabaghian Rad L. Effects of probiotic yogurt on performance, respiratory and digestive systems of young adult female endurance swimmers: A randomized controlled trial. Med. J. Islam. Repub. Iran. 2013;27:141–146. [PMC free article] [PubMed] [Google Scholar]
- 187.West N.P., Pyne D.B., Cripps A.W., Hopkins W.G., Eskesen D.C., Jairath A., Christophersen C.T., Conlon M.A., Fricker P.A. Lactobacillus fermentum (PCC) supplementation and gastrointestinal and respiratory-tract illness symptoms: A randomised control trial in athletes. Nutr. J. 2011;10:30. doi: 10.1186/1475-2891-10-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Paulsen J.A., Ptacek T.S., Carter S.J., Liu N., Kumar R., Hyndman L., Lefkowitz E.J., Morrow C.D., Rogers L.Q. Gut microbiota composition associated with alterations in cardiorespiratory fitness and psychosocial outcomes among breast cancer survivors. Support. Care Cancer Off. J. Multinatl. Assoc. Support. Care Cancer. 2017;25:1563–1570. doi: 10.1007/s00520-016-3568-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No new data were created in this study. The data that support the findings of this study are included within the article and Supplementary Materials.