Abstract
Helicobacter pylori infection causes chronic gastritis, which can progress to severe gastroduodenal pathologies, including peptic ulcer, gastric cancer and gastric mucosa-associated lymphoid tissue lymphoma. H. pylori is usually transmitted in childhood and persists for life if untreated. The infection affects around half of the population in the world but prevalence varies according to location and sanitation standards. H. pylori has unique properties to colonize gastric epithelium in an acidic environment. The pathophysiology of H. pylori infection is dependent on complex bacterial virulence mechanisms and their interaction with the host immune system and environmental factors, resulting in distinct gastritis phenotypes that determine possible progression to different gastroduodenal pathologies. The causative role of H. pylori infection in gastric cancer development presents the opportunity for preventive screen-and-treat strategies. Invasive, endoscopy-based and non-invasive methods, including breath, stool and serological tests, are used in the diagnosis of H. pylori infection. Their use depends on the specific individual patient history and local availability. H. pylori treatment consists of a strong acid suppressant in various combinations with antibiotics and/or bismuth. The dramatic increase in resistance to key antibiotics used in H. pylori eradication demands antibiotic susceptibility testing, surveillance of resistance and antibiotic stewardship.
Introduction
Helicobacter pylori is the most frequent cause of chronic gastritis and variably leads to severe gastroduodenal pathologies in some patients, including gastric and duodenal peptic ulcer disease (PUD), gastric cancer, and gastric mucosa-associated lymphoid tissue (MALT) lymphoma1–3. The diverse pathologies attributed to H. pylori infection are caused by complex interactions of bacterial virulence, host genetics and environmental factors4,5, which result in different phenotypes of chronic gastritis (Table 1). These phenotypes are defined as antral-predominant, corpus-predominant gastritis or pangastritis according to the highest gastritis severity within gastric anatomical compartments.
Table 1 |.
Disease phenotypes of H. pylori infection
| Phenotype | Frequency | Localization | Effect on secretory function | Possible outcomesa |
|---|---|---|---|---|
| Mild gastritis phenotype | Most patients | No specific gastric compartment predominantly affected | Normal acid secretion | Asymptomatic in most patients, no significant clinical outcome |
| Duodenal ulcer phenotype | 10–15% of patients | Antral-predominant gastritis | High gastrin and acid secretion and impaired inhibitory control of acid secretion | Dyspeptic symptoms, duodenal ulcer |
| Gastric cancer phenotype | ~1% of patients | Corpus-predominant gastritis | Low or absent acid secretion; variable gastrin secretion | Severe atrophic gastritis and intestinal metaplasia, gastric cancer |
Gastric ulcer and mucosa-associated lymphoid tissue lymphoma are not associated with a distinct gastritis phenotype. Gastric ulcer is associated more frequently with predominant corpus-type gastritis and low acid secretion. H. pylori, Helicobacter pylori.
The milestone discovery of H. pylori invalidated the dogmatic assumption of the acidic stomach as a sterile organ. This finding required a fundamental revision of gastric pathophysiology and gastroduodenal pathologies. Although spiral microorganisms in the stomach had been reported6, it was not until 1982 that Warren and Marshall identified a bacterial infection as the cause of chronic gastritis and succeeded in isolating the responsible microorganism7 (Fig. 1). The proof of concept that H. pylori infection causes gastritis was obtained by voluntary self-experiments with ingestion of a bacterial broth and cure of gastritis following H. pylori eradication (that is, fulfilment of the Koch’s postulates)8,9. The Koch’s postulates requires proof of causality for a pathogen to induce disease and cure of disease when the causal agent is removed — this finding was eventually confirmed in clinical trials10. The bacterium originally referred to as Campylobacter pylori (C. pyloridis) became reclassified as H. pylori in 1989 (ref. 11). Peptic ulcer, considered an acid-driven disease in the traditional pathophysiological concept, became an infection-driven disease12–14. The standard therapy with long-term acid suppression became short-term H. pylori eradication therapy14. For the discovery that eventually led to the permanent cure of peptic ulcers by H. pylori eradication, Marshall and Warren were awarded the Nobel prize in Physiology or Medicine in 2005 (ref. 15). To this day, continuous scientific progress and new clinical developments have led to frequent modifications and updates to the clinical management of H. pylori10.
Fig. 1 |. Key developments in H. pylori clinical research and management.
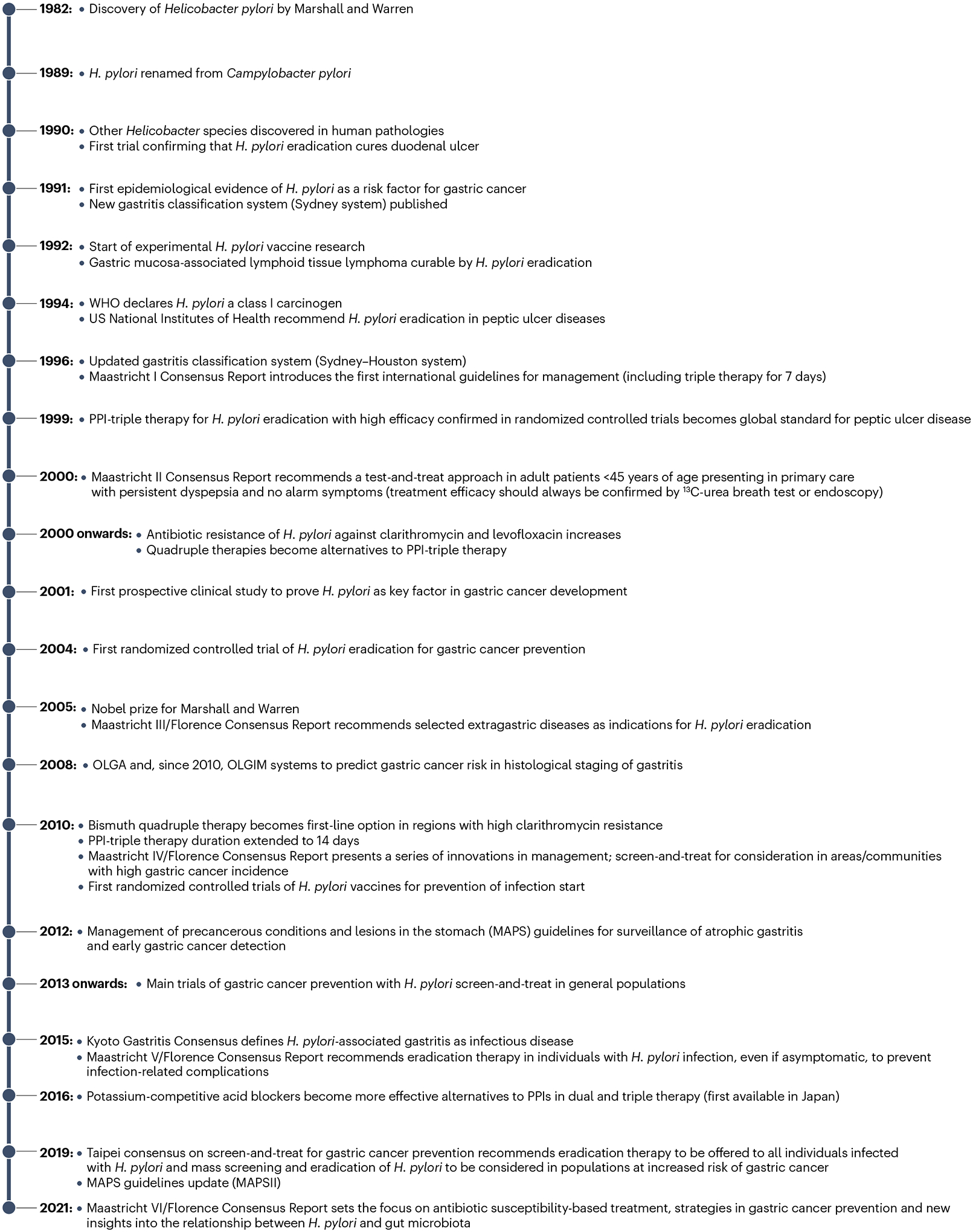
Helicobacter pylori was discovered and reported at a conference in 1982 but the finding was not further disseminated before the first publication in 1983 (ref. 7). The timeline highlights key developments in clinical research of H. pylori infection and its therapeutic management since 1982 (refs. 1,10–12,14,15,17,21,70,184,224,241,311,336,377–383). OLGA, Operative Link on Gastritis Assessment; OLGIM, Operative Link on Gastritis/Intestinal Metaplasia Assessment; PPI, proton pump inhibitor.
H. pylori infects nearly half of the population in the world, with strong differences between geographical areas but with consistent trends towards a decreasing incidence16. Around 80% of individuals with H. pylori infection remain asymptomatic, but gastritis develops in all individuals with the infection, with unpredictable and potentially severe individual outcomes as well as high morbidity and mortality17,18.
This Primer provides an update on current epidemiological trends of H. pylori infection, key aspects of its pathogenicity and its role in gastroduodenal pathologies. An important focus is also on gastric cancer prevention by H. pylori eradication. The diagnostic and therapeutic management of H. pylori infection is discussed according to current international guidelines. The dramatic increase in antibiotic resistance requires special measures, including the incorporation of new molecular methods for antibiotic susceptibility testing, the adaptation of individual treatment regimens and the implementation of antibiotic stewardship.
Epidemiology
H. pylori infection
Once individuals acquire H. pylori infection, the pathogen usually persists throughout their lifetime2. However, spontaneous clearance was reported in 9 of 58 (15.5%) children during the 20 years of follow-up of a retrospective cohort study from 2002 (ref. 19). Clearance of H. pylori does often occur in patients with advanced atrophic gastritis20. The global prevalence of H. pylori infection in adults has declined from 50–55% to 43% during 2014–2020 (refs. 16,17), mostly attributed to improvement of socioeconomic status, living standards and hygiene conditions16,21–23. The increased use of antibiotics, including eradication therapies, in individuals with the infection might be a further contributor.
Prevalence varies substantially with age, ethnicity, associated diseases, geographic regions, socioeconomic status and hygiene conditions16,21. For young age groups, the 2002 study showed that most newly acquired H. pylori infections occurred before the age of 10 years19. The overall crude incidence rate was 1.4% per year, ranging from 2.1% at 4–5 years, 1.5% at age 7–9 years, to 0.3% at 21–23 years of age19. During 2014–2020, the prevalence of infection in children and adults was higher in low-income and middle-income countries, including in Africa, the Eastern Mediterranean, Russia, and Middle America and South America, than in high-income countries but was reduced in Western Pacific regions17 (Fig. 2). The prevalence of infection is higher in adults than in children. It is also higher in rural developing areas than in urban developed regions2. Prevalence of H. pylori infection in children has been decreasing owing to improvements in socioeconomic status and hygiene conditions; however, the global prevalence in children remained as high as 34% during 2014–2020 (refs. 17,24). The higher prevalence in older individuals compared with children is explained by most (90%) of H. pylori infections being acquired in childhood and persisting throughout life rather than by a higher risk of infection at older age.
Fig. 2 |. Prevalence of H. pylori infection in adults and children.
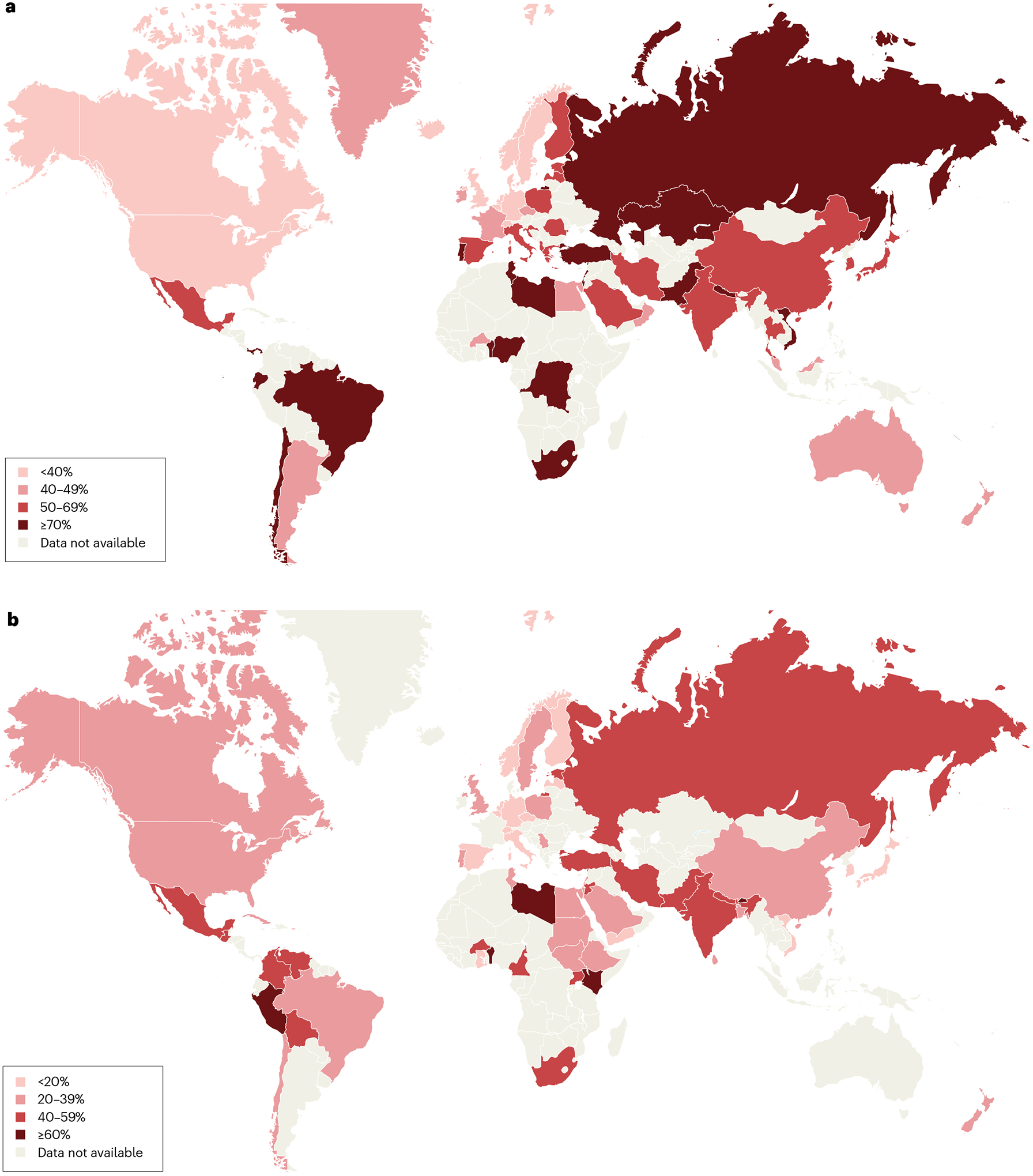
a,b, Global map of Helicobacter pylori infection prevalence in adults during 1970–2016 (part a) and in children and adolescents (<20 years) during 2000–2021 (part b). In adults, the prevalence was highest in Africa, Eastern Mediterranean regions, Russia, Middle America and South America. In children, the prevalence was lower than that in adults in Russia, Western Pacific regions and European regions. However, the prevalence of H. pylori infection was similarly high in children and adults in Africa, Eastern Mediterranean regions, and Middle America and South America16,24.
Some studies suggest increased susceptibilities to H. pylori infection in certain populations based on genetics and ethnicity; however, food sharing and housing habits may also have a role22–24. For example, in the Sumatra islands of Indonesia, the prevalence of H. pylori infection is very low in the Malay and Java populations, but is high in Batak populations, indicating that genetic factors may contribute to differential host susceptibility25. Gene and genome-wide association studies have identified that polymorphisms in IL-1B, Toll-like receptor 1 (TLR1) locus and the FCGR2A locus are associated with H. pylori seroprevalence26,27. However, a 2022 study has cast doubt on a role of the TLR1/6/10 locus in H. pylori seroprevalence28, and further studies are needed29.
Faecal–oral and oral–oral routes are considered the most likely routes of transmission30,31. Contaminated water may be a source of infection in developing countries32. H. pylori can be cultivated from the vomitus, stool and saliva of individuals with infection, indicating the potential transmissibility via these routes33. However, future studies about transmission pathways and their relative importance are urgently needed.
Person-to-person transmission within families, especially from mothers and siblings with the infection, is common in developing countries34. Genotyping studies have shown that strain concordance was detected in 10 of 18 (56%) mother–offspring and in 0 of 17 father–offspring relations35. Concordant strains in siblings were detected in 29 of 36 (81%) families35. Nevertheless, transmission within couples or spouses remains controversial35–37. In two studies, the ribopatterns of H. pylori strains were similar in 8 of 18 (44%) and 5 of 23 (22%) couples with H. pylori infection35,36. However, another study showed that, although restriction fragment length polymorphism patterns were similar in 5 of 13 couples, further restriction fragment length polymorphism using restriction endonucleases revealed distinct patterns in these 5 couples, indicating that transmission between spouses is infrequent37. Due to the extremely high genetic diversity of H. pylori, even short nucleotide sequences can be highly informative about transmission pathways and the direction of transmission between two individuals. Seven-gene multilocus sequence typing38 and, more recently, whole-genome sequence analysis39 have enabled the reconstruction of the spread of H. pylori in families and have great potential to answer open questions in H. pylori epidemiology.
The annual reinfection or recrudescence rate after successful eradication is low (<2%) in adults in developed countries but is higher (5–10%) in adults in developing countries and in children17,40. Some randomized trials showed that a strategy of family-based H. pylori screening and treatment can reduce the recurrence rate more than a single-patient approach41. Further well-designed, large-scale randomized trials are warranted to validate whether family-based screening and eradication may reduce the transmission of H. pylori within families.
H. pylori infection-related diseases
H. pylori infection is an important causal factor of gastric cancer, duodenal ulcer and gastric ulcer42.
Peptic ulcer disease.
Lifetime prevalence of PUD in individuals with H. pylori infection is estimated at around 10%14,43,44. After 10 years, >11% of individuals with the infection develop PUD compared with 1% of individuals without the infection45. In a prospective study, the lifetime risk of developing duodenal ulcer and gastric ulcer was respectively increased by 18.4-fold and 2.9-fold in individuals with infection with cagA-positive H. pylori strains46.
Since the 2000s, the global prevalence of PUD is declining47–50 in parallel with a decreasing prevalence of H. pylori infection16 for various reasons47,51–54. The epidemiological trend indicates an increasing role of NSAIDs, including acetylsalicylic acid, which independently increase the risk of gastroduodenal ulcer and ulcer bleeding44,53. Of note, the risk of PUD when using these drugs is further increased in the presence of H. pylori infection55,56.
Despite changing trends in PUD worldwide, H. pylori remains the most prevalent cause of PUD. A study from Denmark showed an odds ratio of 4.3 (95% CI 2.2–8.3) for the association between H. pylori infection and PUD57. In a meta-analysis including endoscopic surveys and national screening programmes in unselected population samples from Europe and China, the pooled prevalence of PUD was 6.8% and PUD was associated with H. pylori infection in 91% of cases58. Around 3,000,000 diagnoses of PUD per year are estimated to relate to H. pylori infections and ~90% of patients with duodenal ulcers and 70–90% of patients with gastric ulcers have H. pylori infection with variation according to geographical areas52,53,58–60.
Gastric cancer.
Around 90% of gastric cancer cases can be attributed to H. pylori infection61. In 2018, 812,000 gastric cancers, including non-Hodgkin lymphoma of gastric location, were recorded, accounting for ~37% of all cancers driven by a chronic infection, which makes H. pylori the most frequent carcinogenic pathogen62. Gastric cancer incidence and mortality differ significantly between regions, with the highest rates in Asia and Eastern Europe. The lifetime risk of gastric cancer is 1–5% in individuals with H. pylori infection, depending on ethnicity and environmental factors2,17,63. Some populations are at an increased risk of gastric cancer following H. pylori infection, probably due to genetic factors, housing situation and dietary habits, for example, increased consumption of salted or pickled foods in East Asian populations. In addition, substantially higher gastric cancer incidence is found in indigenous populations worldwide64 and in ethnic groups in the USA, including Asian Americans65. Socioeconomic, dietary and lifestyle factors, such as smoking and extent of salt intake, are contributing factors to gastric cancer development, but they are all subordinate to the presence of H. pylori infection66,67.
Extra-gastric diseases.
Unexplained iron-deficiency anaemia, vitamin B12 deficiency and some cases of idiopathic thrombocytopenic purpura can be related to H. pylori infection68,69. Antigen mimicry-induced autoimmunity related to H. pylori has been suggested in idiopathic thrombocytopenic purpura70,71. Furthermore, other associations of H. pylori infection with diseases localized outside the stomach have been reported, including cardiovascular diseases, ischaemic heart disease, metabolic syndrome, diabetes mellitus, hepatobiliary diseases, non-alcohol fatty liver disease and neurodegenerative diseases, which have been attributed to persistent and low-grade systemic inflammation72–74. Most of these associations are based on limited and inconsistent data and remain inconclusive, and only a few, mostly observational, studies have documented a significant decrease in some of these manifestations when H. pylori is eradicated73.
In children, particularly in the USA and Europe, an inverse association between H. pylori infection and asthma and allergy has been reported75–77, although this link has not been unequivocally confirmed78. The often reported inverse association between H. pylori infection and the risk of gastro-oesophageal reflux disease (GERD), Barrett oesophagus and oesophageal adenocarcinoma remains highly controversial79,80, and evidence for positive and negative associations exists79–85. Explanations for the discrepancies might lie in differing study protocols and H. pylori testing methodologies as well as in heterogeneity in the selection of patient and control populations. At present, the controversial findings and debates about a potential benefit of H. pylori for specific clinical scenarios have no confirmation nor impact on the management of the infection86.
Mechanisms/pathophysiology
H. pylori microbiology
H. pylori is Gram-negative, microaerophilic curved or S-shaped bacteria that are highly motile due to a unipolar bundle of sheathed flagella. The cell envelope has a characteristic Gram-negative structure, but many other components have unique features adapted to the habitat of H. pylori in the human stomach2. In comparison with many other pathogenic bacteria, H. pylori has a small ~1.6-Mbp genome consisting of a single circular chromosome that encodes ~1,600 proteins87,88. The H. pylori core genome consists of ~1,100 genes present in all H. pylori strains, whereas the remaining accessory part of the genome comprises genes variably found in strain subsets89, for example, a large number of diverse restriction–modification systems (genetic elements that provide protection against foreign DNA), providing variable DNA methylation90. Extensive variation of genome content and gene sequences between strains, and even within the bacteria present in the stomach of one individual39,91,92, is a prominent characteristic of H. pylori and results from the unusual combination of very high mutation and recombination rates93. H. pylori has a high mutation frequency due to lack of a classical mismatch repair pathway in combination with the pro-mutagenic properties of its DNA polymerase I94,95. H. pylori is naturally competent and can take up DNA by means of the unique ComB DNA uptake system with similarities to a type IV secretion system (T4SS)96,97.
DNA sequence diversity can rapidly spread through H. pylori populations due to recombination between strains98,99. After import, DNA can be integrated into the chromosome based on homology, and such chromosomal imports have a unique bimodal length distribution, enabling H. pylori to adapt its genome to new environments in an extremely efficient way100.
H. pylori strains show a characteristic population structure that reflects their coevolution with their human hosts and has led to conclusions about the history of its association with humans101–103. H. pylori was acquired by modern humans in Africa at least ~100,000 years ago, possibly by a host jump from an unknown animal source. The most ancestral phylogeographic population of H. pylori is hpAfrica2, mostly found in Southern Africa. Further important, widespread and more recently evolved populations include hpAfrica1, hpNEAfrica, hpEurope, hpEastAsia, hpAsia2 and hpSahul104,105. A major step in the evolution of H. pylori from the ancestral hpAfrica2 population to the populations that have spread over the globe was the acquisition of the cag pathogenicity island (cagPAI) by ancestral H. pylori from an unknown source. cagPAI encodes components of the Cag T4SS106,107, which is a protein complex that spans the bacterial cell envelope and can directly deliver diverse effector molecules into host cells following adherence. Hence, whether strains possess an active Cag T4SS has substantial effects on their interaction with hosts. cagPAI-positive strains elicit far more inflammation than cagPAI-negative strains.
Bacterial factors involved in colonization and pathogenesis
H. pylori is highly adapted to the colonization of a unique ecological niche in the deep gastric mucus layer. Several mechanisms, including motility, urease production, adhesion and others, are important in H. pylori colonization (Box 1).
Box 1. Bacterial, environmental and host factors contributing to H. pylori-induced gastric cancer pathogenesis.
Bacterial virulence factors
Environmental factors
Host genetic factors
Single-nucleotide polymorphisms in cytokine and growth factor genes encoding proteins that have been implicated in pathogenesis (IL-1β, IL-2, IL-6, IL-8, IL-10, IL-13, IL-17A/B, IFNγ, TNF, TGFβ) and their receptors (IL-RN, TGFR), innate immune receptors shown to be activated by Helicobacter pylori (TLR2, TLR4, CD14, NOD1, NOD2), enzymes involved in signal transduction cascades (PLCE1, PKLR, PRKAA1), glycoproteins (MUC1, PSCA) and DNA repair enzymes (ERCC2, XRCC1, XRCC3)29,387
Gastric inflammatory phenotypes and associated gastric functions165–167
Corpus-predominant gastritis
Atrophic gastritis (Operative Link on Gastritis Assessment (OLGA) III–IV)
Hypochlorhydria
High gastrin levels
Low pepsinogen I levels and ratio of pepsinogen I to pepsinogen II
Gastric dysbiosis of microbes other than H. pylori160,364,384
Motility.
Flagella-driven motility is essential for the entry of H. pylori into the mucus layer and for maintaining a swimming reservoir in the mucus108 (Fig. 3). H. pylori has a unipolar bundle of rotating sheathed flagella, with filaments composed of two flagellin proteins109 that evade activating the innate immune system via TLR5 due to specific adaptation of their amino acid sequences110,111. The direction of movement is controlled by chemotaxis and energy taxis, enabling bacteria orientation through pH and bicarbonate (and possibly other) gradients in the gastric mucus112. Motility can be inhibited in vitro by small molecule compounds that reduce H. pylori colonization density, which may be a future treatment approach113.
Fig. 3 |. H. pylori infection and pathogenesis.
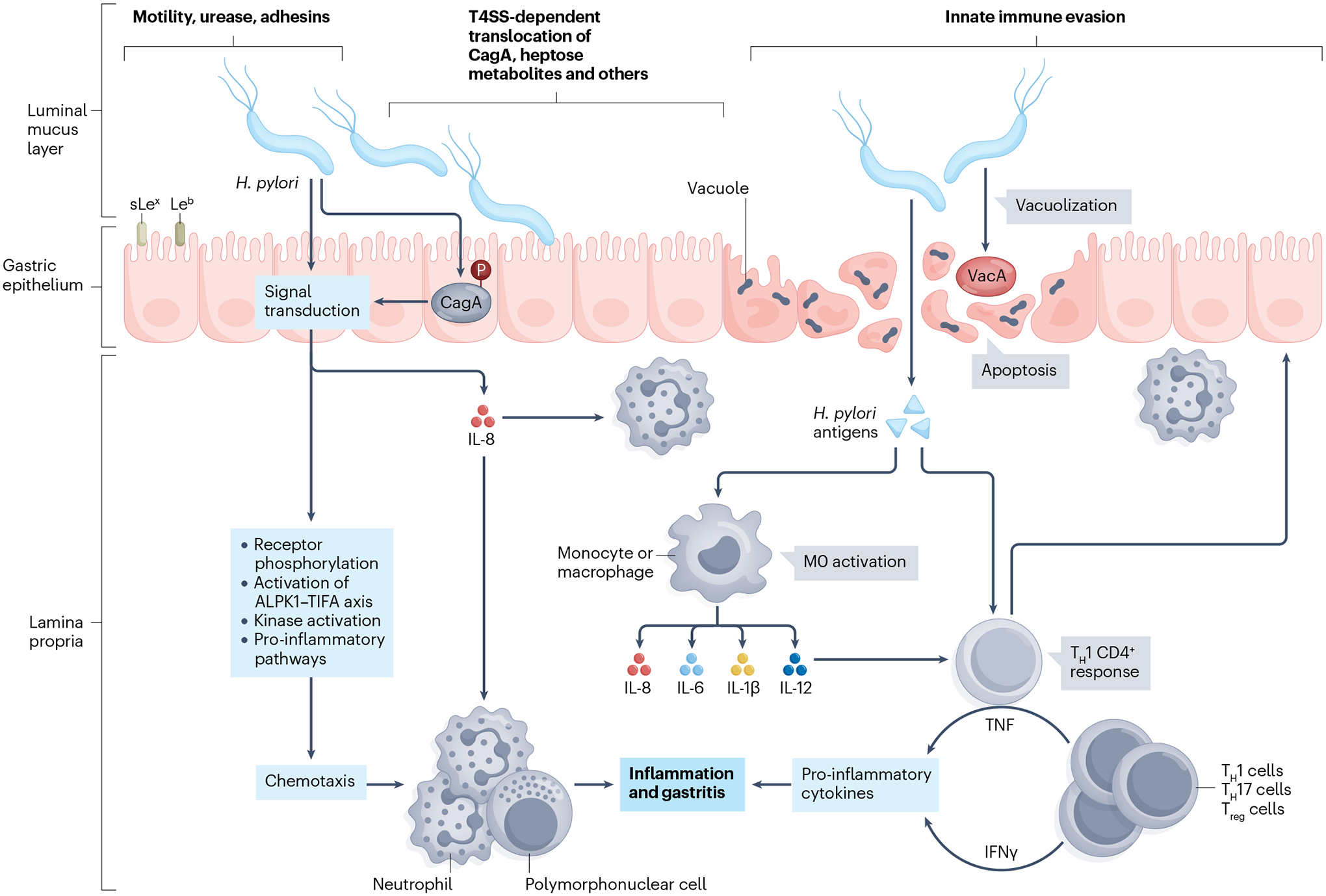
Key aspects of bacterial colonization involve flagellar motility, urease activity, mechanisms of adhesion and damage to the gastric epithelium via vacuolization. The Helicobacter pylori pathogenicity island exerts a key role in inflammation, composes a type IV secretory system (T4SS) and promotes the intracellular injection of cytotoxin-associated gene A (CagA) antigen. The host immune response is characterized by initial mucosal invasion with polymorphonuclear cells followed by activation of the innate and adaptive immune system with complex T helper 1 (TH1), TH17 and regulatory T (Treg) cell interactions. Leb, Lewis b blood group antigen; sLex, sialyl-Lewis x antigen.
Urease.
H. pylori produces abundant amounts of urease, aided by a unique system of accessory proteins that procure the required nickel, which is essential for urease holoenzyme activity, protects the bacterium from nickel toxicity and regulates urease activity by controlling urea influx into bacterial cells114,115. Urease is essential for colonization, most likely because the enzyme, by cleaving urea into ammonia and carbon dioxide, enables the bacteria to survive brief periods of exposure to very low pH values, which H. pylori may encounter in the gastric lumen during transmission116. Through urease activity, urea provides an always available nitrogen source for the organism.
Adhesion.
H. pylori can adhere to gastric epithelial cells by attaching surface molecules that are anchored on its outer membrane (adhesins) to host cell receptors. Adherence enables H. pylori to achieve high colonization despite epithelial cell shedding, mucus layer turnover and the physical force involved in gastric emptying, all of which act to reduce colonization117. The best-studied adhesins are encoded by members of the large hop superfamily of outer membrane protein-encoding genes. BabA mediates binding to Lewis b blood group antigens that are expressed on gastric epithelial cells118. The related SabA adhesin binds to host sialyl-Lewis x antigens, which are mainly expressed on epithelial cell surfaces under inflammatory conditions119. HopQ binds to multiple carcinoembryonic antigen-related cell adhesion molecules and seems to be important for Cag T4SS functionality120,121. AlpA and AlpB mediate binding to the extracellular matrix glycoprotein laminin122. Expression of adhesins varies widely between strains; the contribution of individual adhesins to bacteria–cell adherence and to pathogenesis continues to be studied.
cagPAI and its translocated effectors.
The ~37-kb cagPAI106 comprises ~26 genes that encode the elements of T4SS107. After cagPAI-carrying H. pylori attaches to a host cell, T4SS can translocate bacterial effector molecules into the host cell cytoplasm123, including the CagA protein, which is also encoded by the cagPAI. In addition, several other molecules can be translocated via T4SS, including heptose-containing lipopolysaccharide core precursors124,125, peptidoglycan fragments126 and bacterial DNA127. These molecules can interact with intracellular target molecules and profoundly affect intracellular signalling and cell function (Fig. 3).
After translocation, CagA undergoes tyrosine phosphorylation by cellular kinases128. The phosphorylated form can interact with multiple target molecules in the host cell, including SHP2 (ref. 129), PAR1 (refs. 130,131) and ASPP2 (ref. 132), contributing to increased cell motility, reduced cellular tight junctions, genome instability, nucleotide damage and activation of the Wnt signalling pathway that is relevant in local neoplasia formation133. Translocation of heptose-containing lipopolysaccharide core intermediates may be important in inducing pro-inflammatory responses by both epithelial and immune cells through the ALPK1–TIFA signalling pathway and may also induce mutagenic and oncogenic processes134–136. In addition, intracellular heptose signalling in macrophages may hamper antigen-presenting properties and subsequent T cell responses136.
Vacuolating cytotoxin.
Many H. pylori strains secrete vacuolating cytotoxin A (VacA), which is an oligomeric autotransporter protein toxin that can form anion-selective membrane channels137. The effects of VacA on cells include induction of large intracellular vacuoles derived from late endosomes, induction of apoptotic cell death (following mitochondrial membrane perturbation) or necrosis, induction of autophagy, and inhibition of T cell and B cell proliferation and effects on other immune cells138–140. Together, these effects downregulate immune responses to H. pylori infection and promote host tolerance to the organism. Expression of VacA is not essential for colonization, and its contribution to illness remains controversial.
Immune responses to H. pylori
Innate immune evasion.
The flagellins and lipopolysaccharides of H. pylori have evolved substantially differently from those of other Gram-negative bacteria and are largely not recognized by the human pattern recognition receptors TLR5 and TLR4, which signal danger to the host110,111. These and other structural variations may contribute to immune evasion by H. pylori and its success as a persistent colonizer.
Innate immune activation.
Contact between H. pylori and gastric epithelial and myeloid cells induces signalling through multiple innate pathways, leading to changes in cellular homeostasis and the release of cytokines and chemokines that trigger local and systemic inflammatory responses141–143. As canonical TLR4-dependent and TLR5-dependent signalling is evaded, most inflammatory signalling depends on the activity of an intact cagPAI144. The bacterial components transported into epithelial cells through T4SS engage multiple intracellular receptors. Many of the affected pathways converge on the activation of nuclear factor (NF)-κB, which leads to increased expression and release of IL-8 and other chemokines and cytokines145–147. IL-8 is a powerful attractant of neutrophils, which enter the gastric mucosa and are the defining element of the active component of chronic–active gastritis, the histological hallmark of H. pylori presence in the stomach148,149. Monocytes, macrophages and dendritic cells are also attracted to the H. pylori-colonized mucosa. Activation of phagocytic monocytes and macrophages seems to strongly depend on the delivery of heptose-containing lipopolysaccharide core intermediates via T4SS and the resulting signalling to the ALPK1–TIFA axis135. Dendritic cells can be reprogrammed by contact with the bacteria, for example, to produce IL-18, which drives the conversion of T cells to regulatory T (Treg) cells, suppressing immune activation150.
Adaptive immune response.
H. pylori invariably elicits a combined adaptive humoral and cellular immune response that is generally incapable of eradicating the bacteria. Colonization leads to formation of antibodies to many H. pylori antigens that have little effect on bacterial numbers151. In agreement with this apparent lack of a role of antibodies in protection against H. pylori, mice lacking antibody production can be successfully immunized against H. pylori152. H. pylori also induces the recruitment of T cells to the human gastric mucosa, including T helper 1 (TH1), TH17 and Treg cells. Experimental vaccination in mouse models suggests that both TH1 cells and TH17 cells can be important in mediating protection against H. pylori infection153. Furthermore, in mouse models, a protective effect of very early (neonatal) H. pylori infection against asthma was mediated by Treg cells accumulating in the lungs150,154,155, consistent with the hypothesis that H. pylori may downregulate systemic allergic responses through its recruitment of immunosuppressive Treg cells to the gastric mucosa and, potentially, other body sites such as the lung.
From chronic H. pylori colonization to illness
H. pylori colonization of the gastric mucosa induces a pro-inflammatory response of gastric epithelial cells, which recruits diverse immune cells to the submucosa156. The resulting condition is chronic–active gastritis, which is predominantly asymptomatic for decades of colonization in most patients. The severity of inflammation varies widely between individuals, depending on bacterial, host and environmental factors157 (Box 1).
The single most important determinant of the pro-inflammatory activity of an H. pylori strain is its possession of a functional cagPAI158. Expression of additional host-interaction factors, such as a portfolio of adhesins that fits the variable host receptor makeup and promotes strong binding to epithelial cells and, therefore, promotes crosstalk between the bacterium and the host cell, contributes to the response that a strain elicits in an individual host. Tolerogenic signalling contributes to the unusual accumulation and proliferation of gastric mucosa-associated lymphoid tissue. The decades-long inflammation in the gastric mucosa is thought to be an important driving force leading to gastric atrophy and, ultimately, gastric cancer as outlined by the Correa cascade159 (Fig. 4). The Correa cascade describes a multistage, multifactorial process starting with superficial gastritis, progressing to atrophic gastritis, intestinal metaplasia and dysplasia, and culminating in gastric adenocarcinoma. A key emerging concept is that chronic inflammation, gastric atrophy and consequent achlorhydria lead to an aberrant and dysbiotic gastric microbiome that drives the process towards gastric neoplasia160–162. Accumulating evidence suggests that, following H. pylori eradication, newly emerging components of the gastric microbiota might be involved in the oncogenic transformation of gastric epithelial cells160,163. In other individuals, peptic ulcer disease or the rare H. pylori-associated MALT lymphoma can develop4,5,14,164. The reasons why most individuals remain apparently asymptomatic throughout their lifetime, whereas others proceed to clinical sequelae of varying severity, remain to be fully elucidated. Clinically useful, early bacterial predictive markers that could inform the decision to prescribe eradication therapy have not been identified.
Fig. 4 |. Pathogenesis of gastric adenocarcinoma triggered by H. pylori.
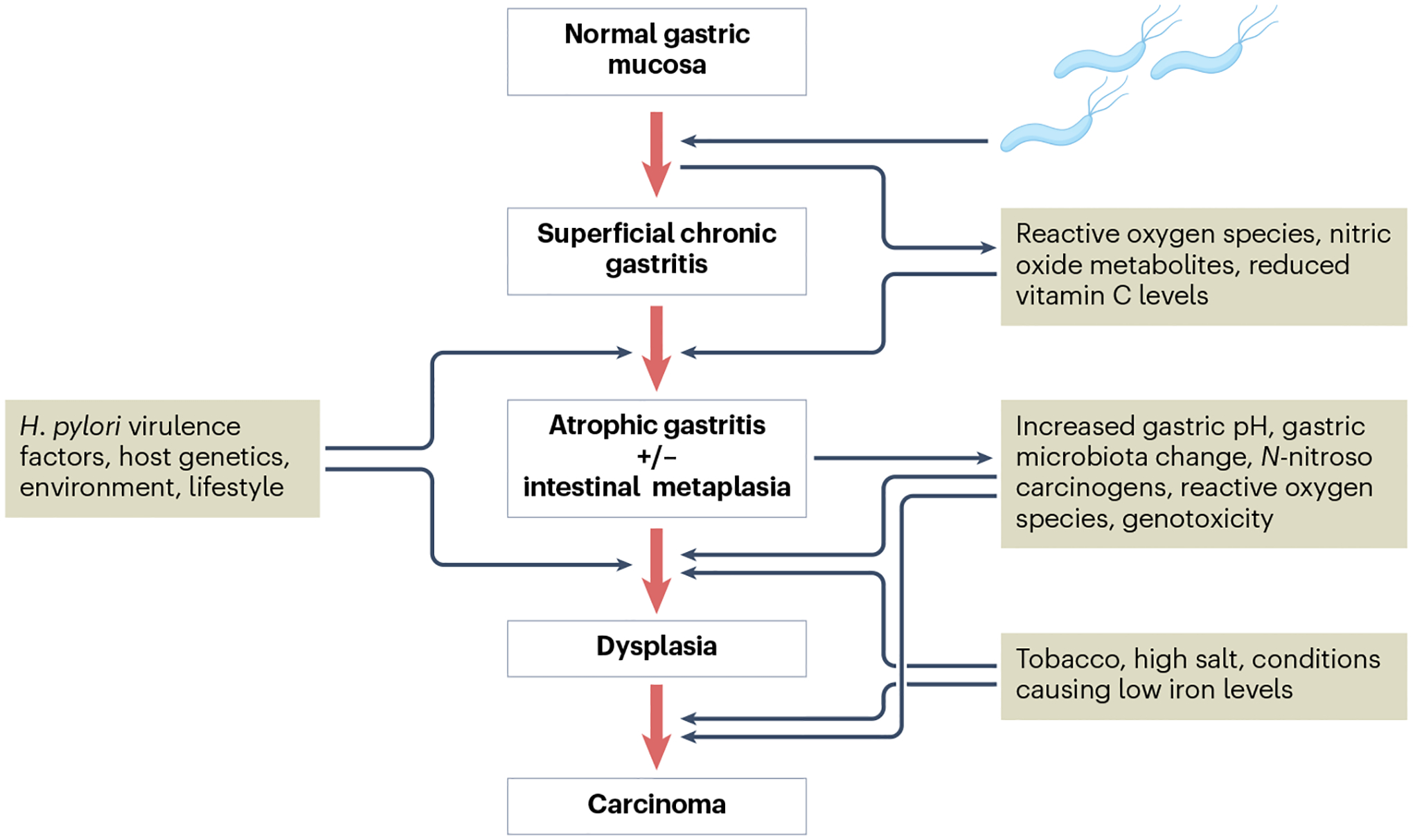
The Correa cascade describes the dynamic progress of gastric carcinogenesis along the stepwise evolution of chronic gastritis initiated by Helicobacter pylori infection. H. pylori causes chronic gastritis that is associated with the generation of reactive oxygen species and nitric oxide metabolites and a reduction in antioxidant vitamin C levels. The risk of gastric cancer is highest in individuals who have infection by more virulent H. pylori strains, have pro-inflammatory host genetic factors, poor diet (high salt, smoked foods), low iron levels, unhealthy lifestyle and/or smoking habit. In these individuals, sustained chronic inflammation leads to damage and loss of acid-producing parietal cells, which leads to hypochlorhydria and finally achlorhydria. The loss of acidity facilitates colonization by harmful pro-inflammatory gastric microbiota, which in turn may produce more genotoxic pro-inflammatory metabolites and carcinogens that act directly on malignant epithelial cell transformation in the stomach384–386.
It is now well established that the clinical outcome of H. pylori infection depends largely on the distribution and severity of H. pylori-induced gastritis165 (Table 1). Thus, peptic ulcers are more likely in individuals with an antral-predominant pattern of gastritis characterized by high acid secretion and relative sparing of gastric corpus with its high parietal cell mass. Parietal cells secrete gastric acid and patients with peptic ulcers have a higher parietal cell mass than healthy individuals without ulcers. By contrast, gastric cancer develops in the context of corpus-predominant gastritis, gastric atrophy and a profound loss of acid secretory capacity that precedes cancer by decades166. The chronically inflamed and achlorhydric environment is further exacerbated by an aberrant pro-inflammatory and genotoxic gastric microbiota that drives the neoplastic process even after loss of H. pylori infection160,161,167. Indeed, experimental work suggests that transplantation of the gastric microbiota from humans with intestinal metaplasia or gastric cancer into germ-free mice leads to the development of precancerous gastric changes168.
Diagnosis, screening and prevention
Diagnosis
Presentation.
In daily routine, acute infection with H. pylori remains mostly undiagnosed at any age. Naturally occurring acute infection in childhood is usually not captured and is supposed to frequently present with abdominal complaints with potentially diverse aetiologies169. In adults, the clinical presentation of acute infection can entail hypochlorhydria, epigastric pain and mild-to-moderate dyspeptic symptoms as described in case reports and from challenge studies in volunteers with H. pylori for vaccine development170–172. By contrast, most children with H. pylori infection remain asymptomatic and complications are infrequent173.
Once established, H. pylori infection is a persisting and not self-limiting condition in adults with the potential of severe complications in some individuals. PUD, gastric cancer and MALT lymphoma174, in decreasing order of incidence, are the most important complications in adults86.
Diagnostic tests.
An accurate diagnosis of H. pylori infection is required before commencing treatment42,175. Diagnostic methods for H. pylori detection include invasive and non-invasive test procedures70,176–181 (Fig. 5) (Tables 2 and 3).
Fig. 5 |. H. pylori diagnostic procedures.
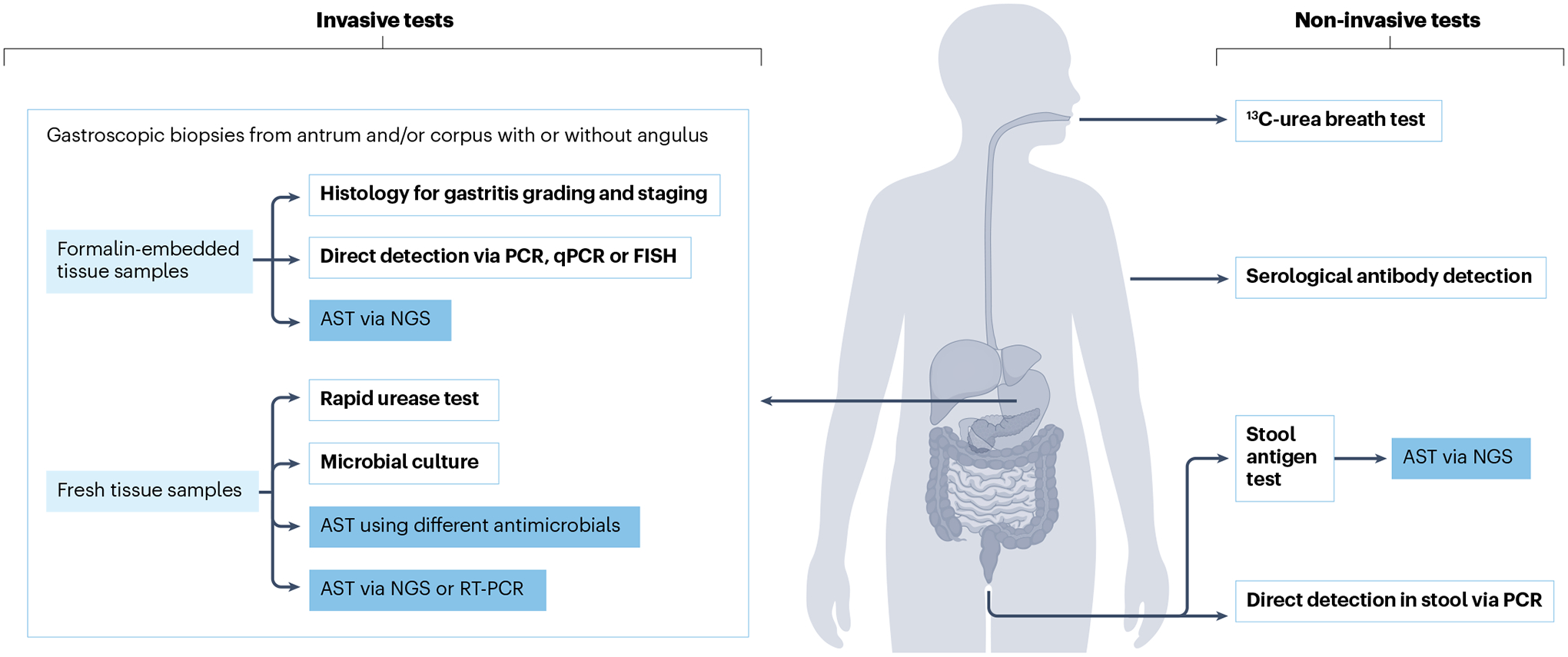
Diagnostic procedures are selected according to clinical scenarios. Non-invasive testing with the 13C-urea breath test and stool antigen test enables diagnosis of a current infection. Serological Helicobacter pylori antibody detection does not enable differentiation between current and previous H. pylori infection, necessitating confirmation by 13C-urea breath test or stool antigen test. All invasive tests are based on biopsy samples from gastroscopy. These enable histological assessment for gastritis grading and staging, direct H. pylori detection via PCR, microbial culture, rapid urease test, and molecular examinations. Antibiotic susceptibility testing (AST) can be performed from stool or biopsy samples using microbial culture, next-generation sequencing (NGS) or real-time PCR (RT-PCR) techniques. FISH, fluorescence in situ hybridization; qPCR, quantitative PCR.
Table 2 |.
Indications for H. pylori testing
| Indications for testing | Recommendationa | Refs. | |
|---|---|---|---|
| Strong | Weak | ||
| Active or history of peptic ulcer disease | x | 42,86 | |
| Low-grade gastric mucosa-associated lymphoid tissue lymphoma | x | 86,337,338 | |
| History of endoscopic resection of early gastric cancer | x | 86,219 | |
| Non-investigated dyspepsia in patients <50 years of age with no alarm symptoms | x | 42,86 | |
| Investigated non-ulcer dyspepsia (functional dyspepsia) | x | 42,86 | |
| First-degree relatives of patients with gastric cancer | x | 42,86,214 | |
| First-generation immigrant from an area with high prevalence of Helicobacter pylori infection | x | 42,86 | |
| Unexplained iron-deficiency anaemia when other causes have been excluded | x | 42,86 | |
| Immune thrombocytopenia in adults | x | 42,86 | |
| Long-term proton pump inhibitor use | x | 42,86 | |
| Long-term acetylsalicylic acid and long-term NSAIDs, in consideration of individual additional risks | x | 42,86,211 | |
Table 3 |.
Diagnostic methods for H. pylori detection
| Test | Sensitivity | Specificity | Clinical use | Comments | Refs. |
|---|---|---|---|---|---|
| Invasive methods | |||||
| Rapid urease test | 84–95% | 95–100% | Important for initial diagnosis; testing two biopsy samples improves sensitivity; provides rapid results | PPIs need to be stopped 14 days before testing; current or recent antibiotic therapy needs to be excluded | 86,179 |
| Microbial culture | 76–90% | 100% | Important for phenotypic susceptibility testing | Absolute specificity but costly; PPIs need to be stopped 14 days before testing; current or recent antibiotic therapy needs to be excluded | 86,179 |
| Histological assessment | 60–93% | >95% | Gold standard for diagnosis and assessment of mucosal changes | Based on updated Sydney system | 86,373 |
| Molecular testing (PCR methods and FISH) | 80–95% | 100% | Useful in initial diagnosis and follow-up; provides rapid results | High sensitivity and specificity; useful in gastrointestinal bleeding, virulence typing and detection of antibiotic resistance | 86,179,373–375 |
| Non-invasive methods | |||||
| UBT | 95–100% | 95–100% | Gold standard for non-invasive diagnosis; higher sensitivity and specificity than stool antigen test and serological assessment; for initial diagnosis and follow-up | PPIs need to be stopped 14 days before testing; current or recent antibiotic therapy needs to be excluded | 86,179,373 |
| Stool antigen test | >95% | >95% | Useful for initial diagnosis and follow-up; slightly lower sensitivity than UBT | Rapid, simple and inexpensive | 86,373 |
| Serological antibody detection | 74.4% | 59% | Useful for initial diagnosis in specific cases | Cheap, simple and rapid; highly variable results; ideal for epidemiological purposes; no need to stop PPI and useful in patients with gastrointestinal bleeding; cannot distinguish between active and previous infection | 86,179,376 |
FISH, fluorescence in situ hybridization; H. pylori, Helicobacter pylori; PPI, proton pump inhibitor; UBT, 13C-urea breath test.
Invasive tests require biopsy samples obtained during gastroduo-denoscopy and include the rapid urease test (RUT), histological assessment, bacterial culture and direct detection of H. pylori genetic material using PCR, quantitative PCR or fluorescence in situ hybridization. Non-invasive methods include the 13C-urea breath test (UBT), serological detection for anti-H. pylori antibodies, the stool antigen test (SAT) and direct detection of H. pylori genetic material in stool via PCR179.
RUT is a low-cost test with a specificity of 95–100%. False positive results are rare and can be explained by the presence of other urease-positive organisms such as Proteus mirabilis179. Current use of a proton pump inhibitor (PPI) may lead to false negative results in RUT as well as in all other diagnostic tests except for serological assessment175. Thus, PPI therapy should be interrupted 14 days before testing42.
Histological assessment on formalin-embedded samples is made according to the updated Sydney system, which provides information on H. pylori presence via direct visualization and on the extent of active and chronic inflammation and atrophy148,149,182. The histochemical method for assessment of H. pylori gastritis relies on haematoxylin and eosin and Giemsa stains for detection of H. pylori148. Gastritis severity is defined by the degree and extension of atrophy and/or intestinal metaplasia. Severe gastric atrophy is associated with an increased risk of gastric cancer and risk is best determined by changes according to the gastritis severity staging systems Operative Link on Gastritis Assessment (OLGA) and Operative Link on Gastritis/Intestinal Metaplasia Assessment (OLGIM)183,184.
Culture of H. pylori is 100% specific but has a relatively low sensitivity (<80%) strongly dependent on transport media and logistics and laboratory proficiency owing to the required laboratory expertise with special culture media. Limitations include costs and time constraints but microbial culture also enables phenotypical antimicrobial susceptibility testing (AST)180.
Molecular testing from formalin-embedded biopsy or RUT samples with PCR methods, of which quantitative PCR is most appropriate, and fluorescence in situ hybridization is highly accurate in the detection of H. pylori185 and can also be combined with molecular resistance testing186.
Serological assessment of serum IgG levels is used as a screening test in specific clinical scenarios but it cannot distinguish active and previous infections because of the prolonged persistence of H. pylori antibodies. A positive serological test should be confirmed with a test that indicates active infection187. Serological testing is the only method not influenced by current PPI intake. Next-generation blood tests to be used for screening in the consulting room became available in 2022 (ref. 187).
UBT uses stable isotope-labelled 13C-urea ingested with citric acid, which is then hydrolysed by bacterial urease and releases carbon dioxide and ammonia. UBT has high sensitivity and specificity (95–100%)181. SAT has a similar diagnostic accuracy as UBT. SAT is an immunological method based on monoclonal antibodies with which H. pylori antigens can be detected in stool samples180. UBT, SAT and histological assessment are the commonly used tests in clinical practice for diagnosis of H. pylori as they enable the detection of active infection. However, the availability of these tests depends on the status of regional health-care services, which can directly affect treatment decisions175,179.
The increase of antibiotic resistance to H. pylori worldwide188,189 demands AST in the individual patient to enable effective therapy choices following failed eradication treatment and to monitor antimicrobial resistance at the regional community level42. AST can be performed using phenotypic and genotypic approaches. Culture-based phenotypic testing requires fresh biopsy samples, but PCR-based genotypic testing can be done on fresh, formalin-embedded or RUT samples as well as on stool samples186,190–192. Clarithromycin resistance should be excluded before its empirical use in regions with known clarithromycin resistance rates of >15% or unknown resistance rates42. Molecular genotypic testing enables the detection of resistance against frequently used antibiotics. Clarithromycin resistance conferred by mutations in the gene encoding 23S rRNA are predominantly related to A2143G, A2142G and A2142C193. Levofloxacin resistance is conferred by point mutations in the gyrase gene gyrA194,195. The accuracy of the molecular detection methods for predicting antibiotic resistance varies between antibiotics, favouring clarithromycin and quinolone resistance detection194,196. Formalin-embedded biopsy samples enable genotypical resistance testing at a later time point after endoscopy197–200.
Infections with Helicobacter species other than H. pylori are rare and those most relevant refer to Helicobacter heilmannii, Helicobacter felis and Helicobacter suis201–203. Standard H. pylori diagnostic tests (UBT, SAT, serological assessment and immunohistochemistry) have low sensitivity for the detection of these species201. The clinical relevance of these often incidentally detected rare infections is low because of the low rates of complications.
Testing for eradication success after 4–6 weeks of antibiotic treatment is primarily — with some specific exceptions — performed with non-invasive diagnostic tests UBT and SAT (see Management section)42. PPI use has to be stopped 14 days before testing to exclude recrudescence of reduced bacterial density under acid suppressive therapy.
Indications for diagnostic testing.
Based on the rarity of complications in childhood, a diagnostic endoscopic examination and treatment is recommended only in those with suspected peptic ulcer disease169. In general, H. pylori detection in children is only recommended when complications arise169,204. In the group of patients with dyspepsia without alarm symptoms, such as anaemia, loss of weight or family history of gastric cancer, and age <45 years (45–55 years according to age-related gastric cancer incidence variation among world regions), non-invasive testing with UBT or SAT is the strategy of choice70,205,206. In patients aged >45 years or in the presence of alarm symptoms, endoscopy-based diagnosis is recommended to exclude mucosal changes207,208. H. pyloriassociated dyspepsia is an independent entity that resembles but is distinct from functional dyspepsia1,208. A test-and-treat strategy is the most cost-effective approach in patients with H. pylori infection and dyspepsia if H. pylori prevalence in the population is >5%. This strategy is superior to alternative therapies including PPIs70,209,210, and the therapeutic gain of H. pylori eradication for symptom relief compared with other therapeutic options is substantial. A randomized, double-blind, placebo-controlled trial for primary prevention of peptic ulcer bleeding in older patients who were prescribed aspirin in primary care lends support to an H. pylori test-and-treat strategy in patients starting aspirin treatment. Gastrointestinal bleeding episodes within a 2-year period were reduced by 65% in the H. pylori eradication group211.
Screening and prevention
H. pylori eradication as a strategy for preventing gastric cancer.
Gastric cancer incidence and mortality at the population level are reduced by H. pylori eradication but more epidemiological data are required. Meta-analyses of randomized controlled trials and observational studies have concluded that moderate evidence suggests that H. pylori eradication therapy reduces the incidence of gastric cancer in healthy individuals212,213, with an overall risk reduction of 46%212. In individuals with H. pylori infection and a family history of gastric cancer in first-degree relatives, H. pylori eradication treatment reduces the risk of gastric cancer, with an overall risk reduction of 55%214. A meta-analysis of randomized and observational cohorts that included five studies that considered baseline histological findings suggests that H. pylori eradication seems to be a primary preventive strategy in individuals with non-atrophic gastritis or multifocal atrophic gastritis without intestinal metaplasia, but not in those with intestinal metaplasia or dysplasia215. In another meta-analysis, H. pylori eradication was associated with improvement in the severity of atrophic gastritis with and without intestinal metaplasia compared with placebo216. Notably, eradicating H. pylori in patients treated for early-stage gastric cancer reduces rates of metachronous gastric cancer by ~50% (range 20–70%) according to two meta-analyses of randomized trials217,218. In a pivotal trial, the reduction in metachronous gastric cancer incidence following endoscopic removal of early gastric cancer in patients receiving H. pylori eradication compared to placebo219 suggests that eradication therapy may even work in the condition of severe atrophic gastritis220.
Diffuse and intestinal types of gastric cancer221 are two major histological entities that differ in epidemiology, pathogenesis and clinical course222. However, randomized and observational studies have been unable to separately calculate the risk effects for these histological types. Further data on the benefits or adverse effects of H. pylori eradication will come from ongoing trials in China223, UK (HPSS study)224, Korea (HELPER Study)224 and Latvia (GISTAR study)225.
Targeted test-and-treat strategies for H. pylori infection.
H. pylori test-and-treat strategies aim to decrease morbidity and mortality related to gastroduodenal disease (Box 2) according to the 2022 Maastricht VI/Florence guidelines86. This strategy is appropriate for individuals with non-investigated dyspepsia. Testing for H. pylori infection should also be performed in persons who use NSAIDs and have a history of peptic ulcer. In addition, evidence is accumulating that supports the eradication of H. pylori in individuals with non-ulcer dyspepsia226, idiopathic thrombocytopenic purpura227, and iron and vitamin B12 deficiency anaemia228,229. Consensus exists for eradicating H. pylori in all cases of MALT lymphoma, regardless of disease stage and prognostic factors230,231. Cure of H. pylori infection results in complete histological remission in most patients with localized MALT lymphoma232.
Box 2. Test-and-treat or endoscopy-based diagnosis in clinical management of H. pylori infection.
Test-and-treat
This strategy refers to non-invasive testing of patients with dyspeptic symptoms and without alarm symptoms, such as vomiting, weight loss or anaemia, at age 50 years (range 45–55 years because of increased individual risk of gastric cancer). The non-invasive 13C-urea breath test or stool antigen test are highly accurate in diagnosing current Helicobacter pylori infection376 and surrogate markers for the histological detection of H. pylori gastritis. Serious upper gastrointestinal lesions in patients with dyspepsia in this age group are very rare; thus, non-invasive testing as an initial management step is appropriate in areas of low or intermediate gastric cancer risk388,389. Test-and-treat is superior to other management options, including empirical proton pump inhibitor therapy in patients with dyspepsia, and is more cost-effective than empirical therapy and endoscopy-based management390,391.
Endoscopy-based diagnosis
This approach is required to exclude gastric preneoplastic conditions or malignant disease in patients with dyspeptic or other symptoms referred to the upper abdomen at age >50 years or at any age in the presence of alarm symptoms. A patient with symptoms related to ulcerogenic drug (NSAIDs) use should also be considered for endoscopy70,392. Endoscopy-based investigations are the most reassuring and should be considered in patients with anxiety393.
Test-and-treat for gastric cancer prevention
This strategy targets asymptomatic individuals at increased risk of gastric cancer owing to a first-degree relative with this malignancy. The non-invasive 13C-urea breath test or stool antigen test are appropriate for younger adults. Endoscopy-based investigations should be considered in individuals >45 years of age or earlier according to the age at which gastric cancer was diagnosed in the index patient394.
Population-based test-and-treat
This approach is recommended in regions with a high gastric cancer incidence. For this purpose, serological assessment combining the detection of anti-H. pylori antibodies with measurement of pepsinogen levels provides useful information on the aetiology and atrophy stage of chronic gastritis and helps direct further management of the disease17,395,396.
Serological assessment for gastric cancer screening.
A large body of research, particularly from East Asian populations at high risk, suggests that measurement of circulating pepsinogen levels is the most useful non-invasive test to define the status of the gastric mucosa (that is, whether it is atrophic)233,234. Experts from the Kyoto Global Consensus agreed that pepsinogen levels in conjunction with anti-H. pylori antibody levels are useful for identifying individuals at increased risk for gastric cancer1. Although there are still possibilities for optimization, the ABC (gastritis A, B, C and D) screening method based on this combined measurement is useful for the detection of an increased risk for both intestinal and diffuse types of gastric cancer235. The specific groups are defined as follows, where Hp indicates H. pylori infection and PG indicates pepsinogen: A [Hp−PG−], individuals without infection; B [Hp+PG−], without chronic atrophic gastritis (CAG); C [Hp+PG+], with CAG; and D [Hp−PG+], with severe CAG; the latter two groups carry the highest risk for gastric cancer236,237.
Population endoscopic screening for gastric cancer.
Around 75% of all new gastric cancer cases are diagnosed in East Asian populations238. Consequently, Japan and South Korea have established successful national screening programmes in individuals aged ≥40 years using either upper gastrointestinal series or upper endoscopy, depending on participant preference or comorbidities. Endoscopy has been the primary method for gastric cancer screening in Japan since 2017, and a study published in 2022 reported the benefits of this approach in the reduction of gastric cancer mortality239. In South Korea, the use of upper endoscopy has increased as this method is more accurate than upper gastrointestinal series for gastric cancer screening240.
Endoscopic surveillance of individuals at high risk.
Although H. pylori eradication can reverse multifocal gastric atrophy and, to some extent, intestinal metaplasia, some patients with these histological lesions might benefit from surveillance at regular intervals. According to the management of precancerous conditions and lesions in the stomach (MAPS II)241 European guidelines and the Maastricht VI/Florence consensus86, individuals with advanced stages of atrophic gastritis (severe atrophic changes with and without intestinal metaplasia in both antrum and corpus, OLGA/OLGIM stages III and IV) should be followed-up with a high-quality endoscopy every 3 years. Based on growing evidence, endoscopic surveillance should also be considered in individuals with intestinal metaplasia at a single location but with a family history of gastric cancer, in those with incomplete-type intestinal metaplasia and in those with persistent H. pylori gastritis. These recommendations are primarily intended for regions with low-to-moderate gastric cancer burden, where population-based screening is not practical or economically feasible but where subgroups at risk can be identified. Although the American Gastroenterological Association does not recommend routine use of endoscopic surveillance in patients with intestinal metaplasia242, a common denominator between American Gastroenterological Association and European MAPS II guidelines is that they are based on low-quality evidence, highlighting the need for well-designed, large and long-term trials.
Management
General aspects
H. pylori gastritis is an infectious disease and all adult individuals with the infection require therapy for cure if clinical symptoms and complications are present or for prevention if at risk for complications even if asymptomatic1,42,243. H. pylori test-and-treat strategies are selected according to diverse clinical scenarios17,42,206 (Box 2). In the paediatric population, H. pylori infection rarely leads to complications and requires specific management addressed in the joint ESPGHAN/NASPGHAN Guidelines that were updated in 2016 (ref. 169). All treatment discussions in this section relate to the disease in adults.
Treatment regimens for H. pylori eradication are based on the combination of a strong acid suppressant and antibiotics. First-line therapy is selected according to locoregional or individual H. pylori antibiotic resistance patterns244,245. Treatment failures induce resistance to several of the antibiotics used in first-line regimens and render further therapies more complex and costly42,206,246,247. Second-line therapy needs to consider the first-line regimen and antibiotic resistance status (Fig. 6). Confirmation of treatment success not earlier than 4 weeks after end of therapy is mandatory to guide further management and provides important information on the effectiveness of treatment regimens in defined regions42.
Fig. 6 |. Suggested H. pylori therapy algorithm.
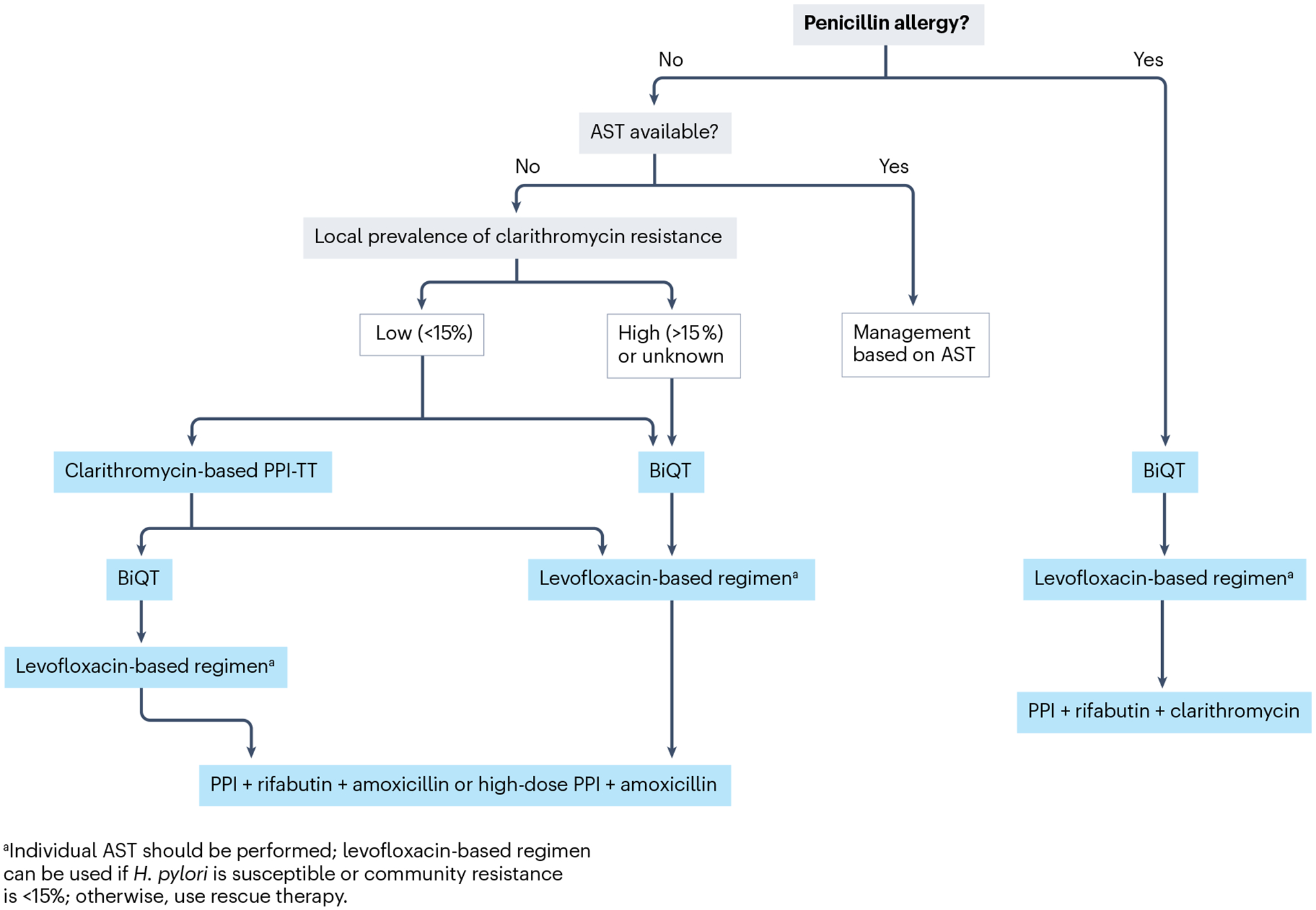
Helicobacter pylori therapy algorithm with the indication of regimens that consist of triple or quadruple combinations to be used in first-line and subsequently in case of failure. Proton pump inhibitors (PPIs) or, where available, potassium-competitive acid blockers are essential components for acid suppression to render antibiotics more effective. PPI can be substituted by potassium-competitive acid blockers where available. Antibiotics are selected according to individual antibiotic susceptibility testing (AST) or according to regional antibiotic susceptibility based on surveillance as well as according to local availability. Clarithromycin-based PPI triple therapy (PPI-TT) is a first-line therapy if local clarithromycin resistance prevalence is <15%. If clarithromycin resistance exceeds 15% or is unknown, the recommended first-line regimen is BiQT (PPI, bismuth, tetracycline and a nitroimidazole antibiotic). Levofloxacin-based regimens are recommended as second-line treatments if a first-line regimen with BiQT fails. Levoflaxin-based regimens include amoxicillin and PPI. If levofloxacin resistance in regional surveillance exceeds 15%, it is advisable to directly select third-line or fourth-line regimens as rescue therapy. The fourth-line regimen (rescue therapy) consists of PPI, rifabutin and amoxicillin (or clarithromycin in case of penicillin allergy).
PPI triple therapy
First-line setting.
The introduction of PPI-based triple therapies (PPI-TT) marked a turning point in the treatment of H. pylori infection owing to their superior efficacy compared with previous approaches. The three components of PPI-TT include a PPI, clarithromycin and amoxicillin or, alternatively, metronidazole as a substitute for either amoxicillin or clarithromycin. Seven-day PPI-TT obtained initial eradication rates of >90%248,249 and, between 1997 and 2005, became the most widely recommended first-line therapy globally42,206,247,250. Treatment duration has since been recommended to be extended to 14 days owing to a substantially higher efficacy compared to the 7-day duration42,244,247. Antibiotics used in first-line PPI-TT are clarithromycin, amoxicillin and metronidazole or, more restrictive, levofloxacin and, in selected cases, furazolidone. Treatment failures with PPI-TT occur with increasing frequency and are primarily related to antibiotic resistance, insufficient acid suppression and inadequate adherence to medications10,251–253. Acid suppression with PPI (omeprazole, esomeprazole, lansoprazole, pantoprazole or rabeprazole in double standard dose) is essential and aims to raise intragastric pH to 6 or higher, which optimizes the stability, bioavailability and efficacy of antibiotics254,255. A modestly higher acid-inhibiting effect is shown for second-generation PPIs (esomeprazole, rabeprazole)256. Increased intragastric pH (optimum pH >6) enables bacterial replication, which increases the susceptibility of H. pylori to antibiotics. This is particularly important for amoxicillin, which is highly acid sensitive254,255. Less effective acid suppressants, such as histamine 2 receptor antagonists, are no longer considered in H. pylori eradication regimens246,257. PPI efficacy is further increased by doubling the PPI standard dose and should always be considered if first-line therapy fails258–261.
Rapid metabolization of PPIs leads to reduced efficacy253,262,263. Rapid and ultrarapid metabolization of PPIs varies considerably among ethnic groups and occurs more frequently in white and African American populations, whereas slow metabolization is more frequent in Asian, including Japanese and Chinese, populations264–267. The efficacy of PPI metabolism depends on various genetic mutations related to CYP2C19 polymorphism and, to a minor extent, on CYP3A4 and gastric H+,K+-ATPase genotypes255,268. Apart from the use of PPI double standard dose in rapid metabolizers, better control of acidity has been reported by increasing PPI dosing frequency up to four times or by switching to a PPI less influenced by CYP2C19 genotypes268–271. No guideline is yet available recommending CYP2C19 genotyping to guide PPI prescription in clinical practice. The pharmacokinetic and pharmacodynamic properties of the second-generation PPIs esomeprazole and, in particular, rabeprazole are less influenced by variant CYP2C19 genotypes268,270,272,273.
To overcome inadequate adherence, careful patient instruction is appropriate on how to take medications and how to proceed in case of adverse events274,275. A history of penicillin allergy, availability of susceptibility testing, local prevalence of antibiotic resistance and history of prior eradication therapies should be considered when deciding on the initial therapy (Fig. 6).
Antibiotic resistance.
Antibiotic resistance is the most important factor in PPI-TT failure244. Clarithromycin resistance and metronidazole resistance are the most relevant resistances for PPI-TT failure42,247,276. Clarithromycin resistance has increased from 3% to 11% around the turn of the century and is now up to 15–30% worldwide188,189,248,277–279. In 2,852 treatment-naive patients from a European registry on H. pylori management (Hp-EuReg), resistance to clarithromycin, metronidazole and levofloxacin were 25%, 30% and 20%, respectively261,278. Resistances to tetracycline and amoxicillin were <1% in the same study. A WHO global priority list qualifies clarithromycin-resistant H. pylori infection as a high threat among community-acquired infections280, and international guidelines recommend abandoning clarithromycin-based regimens if regional resistance exceeds 15%244.
Among several modifications developed to overcome clarithromycin resistance, including sequential therapy (PPI-dual followed by PPI-TT) and hybrid therapy (PPI plus three antibiotics), only concomitant therapy (PPI plus three antibiotics simultaneously administered)244,281–283 was found to be superior to clarithromycin-based PPI-TT281,282. Concomitant therapy as an empirical first-line option should cautiously be considered in regions of clarithromycin resistance >15% and only used if individual AST or bismuth-based quadruple therapy (BiQT) are not locally available42,247,250. Levofloxacin as a component of PPI-TT is effective in first-line and second-line regimens in regions with low levofloxacin resistance284–286. However, levofloxacin resistance is now up to 20% in Europe and 18% in the Asia-Pacific region188,278,287. Although levofloxacin is not recommended as a first-line option, the high resistance restricts its use even in second-line regimens42,244,247. AST before using levofloxacin in empirical second-line regimens is advised189,244,278,287. Other quinolones, such as ciprofloxacin and moxifloxacin, which have reduced efficacy and/or less consistent results, are not an alternative to levofloxacin286,288. Sitafloxacin-based triple and dual regimens that have been successfully tested in Japan289 are not used as an alternative to levofloxacin in western countries42,244,247.
Metronidazole resistance is >25% in most areas of the world189,278 but has a minor effect on eradication efficacy when used in triple or quadruple regimens because of inconsistency between in vitro AST results and clinical efficacy and the synergism with co-administered drugs, in particular bismuth224,290,291. Resistance to amoxicillin and tetracycline is low (<2%) and these antibiotics remain a key component in standard PPI-TT and in BiQT, respectively, without the need for routine AST244,291. Rifabutin resistance is <1% and the H. pylori eradication rate of rifabutin-containing regimens is 73% according to a meta-analysis from 2020 (ref. 292). A rifabutin delayed-release preparation, combined with amoxicillin and omeprazole, obtained an eradication rate of 89%293 and FDA approval for use as a first-line therapy was granted in 2019 (ref. 294). Outside of the USA, rifabutin-containing regimens are recommended as rescue therapy only owing to the need of this drug for other critical infections and the risk of myelotoxicity in rare cases42,247. Furazolidone resistance is <5% and the drug is effective in triple and quadruple combinations; its use is limited to a few countries in Asia and South America195,295 and it may serve as rescue therapy in individual cases296.
For these antibiotic classes, mechanisms of resistance are related to drug-specific target gene mutations (macrolides and quinolones) or to detoxication (nitroimidazoles)296. H. pylori colonies may carry single-drug, multidrug or hetero resistance296,297. Isolates from antrum and corpus are reported to differ by up to 15% in AST, which may account for treatment failure if biopsy samples for AST are only taken from a single site in the stomach298,299.
Bismuth-based quadruple therapy
Bismuth has multiple beneficial properties in peptic ulcer healing that include a stimulating effect on prostaglandin synthesis, inactivation of pepsin, and bile acid binding but, most relevant in H. pylori eradication, is its bactericidal effect300,301. Bismuth subcitrate upregulates the expression of genes involved in H. pylori growth and metabolism and impedes proton entry, thereby preventing lowering of the bacterial cytoplasmic pH. These mechanisms are suggested to render antibiotics more effective302. Bismuth-based quadruple therapy (BiQT; PPI, bismuth, tetracycline and a nitroimidazole antibiotic), available either as individual components or as PPI plus a capsule containing all antibacterial components, has an eradication efficacy of 90%261,291,303,304.
BiQT is recommended as an empirical first-line therapy, does not require AST, is not affected by clarithromycin resistance and overcomes metronidazole resistance owing to synergism with bismuth, as documented by its consistently high therapeutic efficacy244,291,300. Bismuth added to clarithromycin-containing regimens increases eradication efficacy also in the presence of clarithromycin resistance but, in these combinations, offers no advantage over standard BiQT300,305,306. BiQT does not contain antibiotics that are essential for cure of other infections. BiQT is an effective rescue option with a success rate of >90% following previous treatment failures303,307,308.
Regimens with potassium-competitive acid blockers
Potassium-competitive acid blockers (P-CABs), a new class of acid inhibitors, have a more potent and durable effect on acid suppression than PPIs309,310. Vonoprazan-based triple therapy (V-TT) with clarithromycin and amoxicillin in first-line achieved an eradication rate of 92.6% versus 75.9% with PPI-TT, and 98% in second-line in Japan311, which was also confirmed in western countries312. In network meta-analyses, V-TT ranked best among all current first-line empirical therapies, which was also confirmed after the inclusion of a trial conducted in western countries313,314. Vonoprazan dual therapy, consisting of vonoprazan plus amoxicillin, provides an eradication rate of H. pylori similar to that of V-TT252. Increasing resistance and absence of new antibiotics set major expectations on P-CAB-based regimens, which are being investigated in several trials309 (Supplementary Table 1).
H. pylori eradication and rescue therapies
Management of refractory H. pylori needs to consider individual or local antibiotic resistance, facilities for AST, logistics, and drug availability244,246 (Fig. 6). Following PPI-TT failure, BiQT or a regimen with antibiotics selected following AST is recommended42,244,247. Empirical therapy, with careful consideration of previously taken medications, is a valid alternative to genotypic resistance-guided therapy of refractory H. pylori infection315. BiQT is currently the best empirical approach as it is not influenced by antibiotic resistance316. If BiQT fails in first-line, levofloxacin-based triple therapy is recommended. A meta-analysis including 25 trials with levofloxacin-based triple therapy in second-line treatment reports a cumulative eradication rate of 74.5% (95% CI 70.9–77.8)317. The PPI-amoxicillin, high-dose, dual therapy is another option, with a ≥81% eradication rate achieved as second-line treatment and with an efficacy comparable to other recommended therapies318,319.
P-CABs in triple therapies and in dual combination with amoxicillin already effectively used in Asian countries will become an important option once generally available and properly adapted to regional demands in first-line and second-line eradication regimens311,313. Rifabutin-based triple therapy is well documented as effective and should be kept as rescue therapy292. Surveillance programmes at the regional level, the introduction of antibiotic stewardship, regulations in the use of antimicrobials and increased public awareness are advised to control the increasing resistance of H. pylori244,320,321.
Adverse events
Overall, eradication regimens have a favourable safety profile, with usually mild and very few severe adverse events. Mostly mild-to-moderate adverse effects occur in 30–70% of patients and include taste disturbances, nausea, headache, diarrhoea and non-specific gastrointestinal symptoms with some variations according to type of eradication therapy282,291,322–324.
Diarrhoea varies in prevalence from >1% to 15% according to definitions applied, the population treated and type of therapy325. The non-recording of adverse effects as primary criteria in clinical trials accounts for the high variations. Darkening of the tongue and faeces is characteristic of bismuth salts326. Antibiotics affect gut microbiota and lead to mostly transient dysbiosis, bacterial resistance and overgrowth of opportunistic pathogens; however, rarely of Clostridioides difficile40,325,327.
Probiotics added to H. pylori therapies have a small and inconsistent effect on eradication rates but reduce adverse effects42, which has been shown in meta-analyses for individual probiotics as well as for mixtures322,328,329. In a new randomized controlled study, Saccharomyces boulardii combined with a mixture of probiotic bacteria modestly increased the eradication efficacy and reduced adverse effects330, whereas S. boulardii alone had no effect on eradication but remained effective in reducing adverse effects such as severe diarrhoea331. Defined probiotic mixtures have been shown to antagonize the harmful effects of antibiotics on the gut microbiota and their metabolic functions325.
Effects on peptic ulcer and MALT lymphoma
Successful H. pylori eradication achieves ulcer healing rates of >90% and continued acid inhibition with PPI is not required for uncomplicated duodenal ulcer42. Gastric ulcer requires prolonged acid inhibition for healing and endoscopic follow-up is needed to ensure complete ulcer healing and to exclude underlying gastric malignancy332. Management of bleeding peptic ulcers, both duodenal and gastric ulcers, requires immediate care by controlling and/or restoring cardiocirculatory and respiratory function and by performing emergency diagnostic endoscopic examination and endoscopic interventions according to standardized protocols333,334. PPI treatment is continued until complete healing is endoscopically documented44. H. pylori eradication should be initiated after the active bleeding phase is under control and oral nutrition can be resumed70,334. Patients with H. pylori infection exposed to ulcerogenic medications, in particular NSAIDs, are at an increased risk of complications56,335 and benefit from H. pylori testing and treatment42,70. Patients at high risk for rebleeding after H. pylori eradication, for example, those with continued NSAID use, require PPI maintenance therapy336.
H. pylori eradication is the standard-of-care initial therapy for MALT lymphomas in all stages and obtains 70–80% long-term remission in stage I disease337,338. Eradication therapy in patients negative for H. pylori after exclusion of the infection with routine diagnostics obtains cure in 30% of patients and should, therefore, always be considered as a first management step339.
Quality of life
Despite the vast number of H. pylori treatment studies, surprisingly few investigations have measured quality of life (QoL) outcomes. Several different questionnaires have been used to ascertain QoL metrics across the spectrum of diseases associated with H. pylori, and results have shown that eradication of H. pylori can either improve or worsen QoL, which may depend on the type of treatment used340. In a study from Japan, participants were included to survey improvement of GERD-related QoL measures following H. pylori treatment using a Japanese version of the QoL in reflux and dyspepsia score (QOLRAD-J) and Carlsson–Dent questionnaires341. GERD-related QoL scores improved following treatment and these were magnified among individuals with severe reflux symptoms. In another study, an 8-item Short-Form Health Survey and a modified Frequency Scale for GERD symptoms were used following H. pylori eradication342; QoL improved irrespective of treatment outcome. Finally, in a study from Thailand, patients with functional dyspepsia indicated that H. pylori infection, anxiety or depression were common, occurring in 23.3%, 23% and 7.3% of patients, respectively343. These findings suggest that eradication of H. pylori might not only improve functional dyspepsia but also potentially prevent the development of gastric cancer in some patients with functional dyspepsia by eliminating the chronic inflammatory process in the stomach.
In the UK, a randomized controlled study of QoL was conducted in 39,929 patients with dyspepsia following H. pylori therapy using a validated dyspepsia questionnaire and the psychological well-being index (PGWB) and reported no effect on QoL following therapy344. A further study in a smaller number of patients with functional dyspepsia similarly noted no improvement in QoL after eradication345. Other studies from Europe have reached different conclusions. In a study from Hungary, using the Functional Digestive Disorder Quality of Life system adapted from France to determine QoL in patients with functional dyspepsia, improvement of QoL was dependent on H. pylori therapy346. In a study from Croatia, the Gastrointestinal Symptom Rating Scale questionnaire was employed and improvement in QoL of patients with dyspepsia was found as early as 1 month into the year-long study347.
A group from Africa used the Short-Form Leeds Dyspepsia Questionnaire and the Short-Form Nepean Dyspepsia Index in health-care workers with dyspepsia and found reduced QoL in those with high dyspepsia prevalence348. In a study from Rwanda, dyspepsia was assessed within the general population using the Short-Form Nepean Dyspepsia Index questionnaire and noted improved QoL, which was dependent on H. pylori treatment349.
Finally, in a study examining potential detrimental consequences of eradication therapy, it was reported that patients with a duodenal ulcer in whom H. pylori eradication was successful were more likely to develop oesophagitis in the first year after treatment than those without H. pylori eradication350; however, in the subsequent 2 years, there was no difference between the groups. Collectively, these disparate results probably reflect differences in H. pylori treatment regimens, the QoL scoring systems used, varying genetic backgrounds of the host populations and differences in infecting H. pylori strains350.
Outlook
A vision for the future is to provide a healthy stomach free from H. pylori to all individuals. The expectation that H. pylori incidence will decrease to the point that the bacterium will disappear spontaneously within a foreseeable time frame is unlikely to occur351. A population-wide test-and-treat strategy should therefore remain a consideration. This strategy could confer a health benefit with the prevention of H. pylori-related complications in a considerable number of individuals. However, logistic limitations, substantial health costs and risks related to the massive use of antibiotics with the fear of aggravating antibiotic resistance would be disadvantages. Thus, the identification of individuals and population subsets with a higher-than-average risk of gastric cancer should be, for now, the primary target in prevention strategies. This is the case for first-degree family members of patients with gastric cancer and populations in world regions with high gastric cancer incidence. This approach is supported by favourable cost-effectiveness and advised by expert consensus reports and guidelines17,86. A new concept for comprehensive intrafamilial H. pylori management has been proposed for regions of high H. pylori prevalence352. It advises actively proceeding with test-and-treat strategies in family members living in the same household as the index patient diagnosed with H. pylori based on the rationale of predominant intrafamilial spreading of the infection, mainly in childhood.
Antibiotic resistance, with its dramatic increase, demands new antimicrobial drugs that can specifically target H. pylori and avoid cross-resistance effects and induction of antibiotic resistance in H. pylori and other bacteria (Box 3). Colloidal bismuth subcitrate remains a candidate, with a direct bactericidal effect by inducing cellular swelling, vacuolization, structural degradation and cell wall eruption of H. pylori353. For now, bismuth has the limitation of cure rates of <20% if used as monotherapy301.
Box 3. Potential therapeutic targets for non-antibiotic drugs against H. pylori infection.
Urease
Block the proton-gated urea channel, inhibit the activity of urease and block the production of urease.
Flagella
Inhibit motility, impair structure and production of flagella.
Adhesion factors
Reduce the adhesion of Helicobacter pylori to gastric mucosa.
Drug delivery into gastric mucus
Increase the delivery of antibiotics or new drugs into the firmly adherent mucus.
Urease is an essential factor for the survival of H. pylori in the acidic gastric environment, producing ammonia and carbonic acid to neutralize the acidic surroundings354. However, urease is a complex target composed of two subunits with 12 active sites354. The delineated structure of the proton-gated urea channel of urease355 and the development of a drug that can block the rapid influx of urea into the bacterium may offer a solution to inhibit the activity of urease.
Inhibition of motility by blocking the flagellar function of H. pylori might be worth considering as a therapeutic target. H. pylori flagella enable rapid movement through the viscous mucus, and disruption of a gene encoding cardiolipin synthase of H. pylori strain G27, shown in vitro, may abolish the biosynthesis of flagellum356. Furthermore, interference with the outer membrane proteins of H. pylori that confer adhesion to host glycans, mucins or gastric mucosa, such as BabA and SabA357, may impair H. pylori survival in the gastric mucosa. An anti-adhesion nanomedicine composed of H. pylori-mimicking outer membrane nanoparticles could compete with H. pylori and reduce its adhesion to gastric epithelial cells358.
Another approach is to increase the penetration of drugs into the gastric mucus layer, which includes the loosely adherent and firmly adherent mucus layers359. Conventional mucoadhesive particles usually attach to the loosely adherent layer only and are easily moved downstream to the lumen upon peristalsis359. Some potential mucuspenetrating polymeric nanoparticles can penetrate into the firmly adherent mucus layer359. Thus, delivery of selective antibiotics or other agents against H. pylori may be rendered more effective through the development of polymeric nanoparticles. H. pylori is a microaerobic organism and susceptible to increased oxygen levels, thus, the delivery of nanoparticles that include oxygen into the gastric mucus layer could render the bacterium vulnerable. In addition, multiple aspects of the interaction of H. pylori with the gastrointestinal microbiome are addressed by current research and will influence future research360–364. The exclusive property of H. pylori to colonize and infect the gastric mucosa affects the biodiversity of other gastric bacteria and their role in either enhancing or mitigating H. pylori-induced gastric inflammation; this needs to be further explored. H. pylori dominates the mucosa-associated community in the stomach but is less influential on the composition of bacterial communities in gastric juice365. In individuals with H. pylori infection, and even following eradication, the gastric microbial composition is dependent on the extent of gastric mucosal damage previously induced by H. pylori and gastric acidity366,367. In patients with atrophic gastritis, bacterial clusters comprising Peptostreptococcus, Streptococcus, Parvimonas, Prevotella, Rothia and Granulicatella with carcinogenetic potential become predominant, whereas the probiotic Faecalibacterium prausnitzii is depleted367. The development of targeted probiotics in the direction of antagonizing carcinogenic clusters in patients with atrophic gastritis following H. pylori eradication will serve an important purpose. It will be a further point of interest to understand the role of bacteria carried in the saliva and transiting the stomach and whether, in the absence of H. pylori, some of them become candidates for resilience and explain the entity of H. pylori-negative gastritis. The contributing role of bacteria in the development of severe gastritis into gastric cancer is shown in animal experiments and humans, and more insights are expected in this area161,368,369. Vaccine development should be pursued and lessons from previous failures will help in new approaches172.
Field and challenge studies should both be conducted after identification of effective vaccine candidates. A vaccine for preventive and therapeutic purposes might consider multiple epitopes to direct the immune response towards essential H. pylori functions such as epithelial cell adherence, proliferation and survival in the specific gastric niche230,370. Preliminary evidence shows that H. pylori reduces the efficacy of treatment with immune-checkpoint inhibitors in malignant diseases371,372. If these findings are confirmed in further investigations, a test-and-treat strategy for H. pylori could become a requirement before starting immune therapies in oncological diseases. Immune mechanisms involved in this phenomenon deserve further exploration.
Supplementary Material
Acknowledgements
The authors thank Y.-C. Chen and H.-T. Yu for help with figure design.
Footnotes
Competing interests
P.M. has consulted for Aboca, Bayer Healthcare, Cinclus, Imevax, Menarini Foundation and Phatom. P.M. has received honoraria for lectures from Allergosan, Biohit, Biocodex and Malesci. S.I.S. has received scientific support from Richen. C.S. has received speaker fees from Imevax, Falk Foundation and Lilly. S.S. is listed as an inventor on a patent application related to the use of bacterial motility inhibitors as potential treatment for Helicobacter pylori infection.
Supplementary information The online version contains supplementary material available at https://doi.org/10.1038/s41572-023-00431-8.
References
- 1.Sugano K et al. Kyoto global consensus report on Helicobacter pylori gastritis. Gut 64, 1353–1367 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Suerbaum S & Michetti P Helicobacter pylori infection. N. Engl. J. Med 347, 1175–1186 (2002). [DOI] [PubMed] [Google Scholar]
- 3.Peek RM Jr & Blaser MJ Helicobacter pylori and gastrointestinal tract adenocarcinomas. Nat. Rev. Cancer 2, 28–37 (2002). [DOI] [PubMed] [Google Scholar]
- 4.Amieva MR & El-Omar EM Host-bacterial interactions in Helicobacter pylori infection. Gastroenterology 134, 306–323 (2008). [DOI] [PubMed] [Google Scholar]
- 5.van Amsterdam K, van Vliet AH, Kusters JG & van der Ende A Of microbe and man: determinants of Helicobacter pylori-related diseases. FEMS Microbiol. Rev 30, 131–156 (2006). [DOI] [PubMed] [Google Scholar]
- 6.Kidd M & Modlin IM A century of Helicobacter pylori: paradigms lost-paradigms regained. Digestion 59, 1–15 (1998). [DOI] [PubMed] [Google Scholar]
- 7.Warren JR & Marshall B Unidentified curved bacilli on gastric epithelium in active chronic gastritis. Lancet 1, 1273–1275 (1983). [PubMed] [Google Scholar]; Discovery of H. pylori that starts worldwide research leading to the cure of peptic ulcer disease and identification of the main risk factor for gastric cancer.
- 8.Marshall BJ, Armstrong JA, McGechie DB & Glancy RJ Attempt to fulfil Koch’s postulates for pyloric Campylobacter. Med. J. Aust 142, 436–439 (1985). [DOI] [PubMed] [Google Scholar]
- 9.Morris A & Nicholson G Ingestion of Campylobacter pyloridis causes gastritis and raised fasting gastric pH. Am. J. Gastroenterol 82, 192–199 (1987). [PubMed] [Google Scholar]
- 10.Malfertheiner P, Link A & Selgrad M Helicobacter pylori: perspectives and time trends. Nat. Rev. Gastroenterol. Hepatol 11, 628–638 (2014). [DOI] [PubMed] [Google Scholar]
- 11.Goodwin CS & Armstrong JA Microbiological aspects of Helicobacter pylori (Campylobacter pylori). Eur. J. Clin. Microbiol. Infect. Dis 9, 1–13 (1990). [DOI] [PubMed] [Google Scholar]
- 12.Rauws EA & Tytgat GN Cure of duodenal ulcer associated with eradication of Helicobacter pylori. Lancet 335, 1233–1235 (1990). [DOI] [PubMed] [Google Scholar]; To our knowledge, first study to provide definitive proof that H. pylori eradication cures peptic ulcer disease.
- 13.Van der Hulst RW et al. Prevention of ulcer recurrence after eradication of Helicobacter pylori: a prospective long-term follow-up study. Gastroenterology 113, 1082–1086 (1997). [DOI] [PubMed] [Google Scholar]
- 14.Malfertheiner P, Chan FK & McColl KE Peptic ulcer disease. Lancet 374, 1449–1461 (2009). [DOI] [PubMed] [Google Scholar]
- 15.Pincock S Nobel Prize winners Robin Warren and Barry Marshall. Lancet 366, 1429 (2005). [DOI] [PubMed] [Google Scholar]
- 16.Hooi JKY et al. Global prevalence of Helicobacter pylori infection: systematic review and meta-analysis. Gastroenterology 153, 420–429 (2017). [DOI] [PubMed] [Google Scholar]
- 17.Liou JM et al. Screening and eradication of Helicobacter pylori for gastric cancer prevention: the Taipei global consensus. Gut 69, 2093–2112 (2020). [DOI] [PubMed] [Google Scholar]
- 18.Plummer M, Franceschi S, Vignat J, Forman D & de Martel C Global burden of gastric cancer attributable to Helicobacter pylori. Int. J. Cancer 136, 487–490 (2015). [DOI] [PubMed] [Google Scholar]
- 19.Malaty HM et al. Age at acquisition of Helicobacter pylori infection: a follow-up study from infancy to adulthood. Lancet 359, 931–935 (2002). [DOI] [PubMed] [Google Scholar]
- 20.Gao L, Weck MN, Nieters A & Brenner H Inverse association between a pro-inflammatory genetic profile and Helicobacter pylori seropositivity among patients with chronic atrophic gastritis: enhanced elimination of the infection during disease progression? Eur. J. Cancer 45, 2860–2866 (2009). [DOI] [PubMed] [Google Scholar]
- 21.Parsonnet J et al. Helicobacter pylori infection and the risk of gastric carcinoma. N. Engl. J. Med 325, 1127–1131 (1991). [DOI] [PubMed] [Google Scholar]
- 22.Eusebi LH, Zagari RM & Bazzoli F Epidemiology of Helicobacter pylori infection. Helicobacter 19, 1–5 (2014). [DOI] [PubMed] [Google Scholar]
- 23.Park JS, Jun JS, Seo JH, Youn HS & Rhee KH Changing prevalence of Helicobacter pylori infection in children and adolescents. Clin. Exp. Pediatr 64, 21–25 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yuan C et al. The global prevalence of and factors associated with Helicobacter pylori infection in children: a systematic review and meta-analysis. Lancet Child. Adolesc. Health 6, 185–194 (2022). [DOI] [PubMed] [Google Scholar]
- 25.Syam AF et al. Helicobacter pylori in the Indonesian Malay’s descendants might be imported from other ethnicities. Gut Pathog 13, 36 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liou JM et al. IL-1B-511 C–>T polymorphism is associated with increased host susceptibility to Helicobacter pylori infection in Chinese. Helicobacter 12, 142–149 (2007). [DOI] [PubMed] [Google Scholar]
- 27.Mayerle J et al. Identification of genetic loci associated with Helicobacter pylori serologic status. JAMA 309, 1912–1920 (2013). [DOI] [PubMed] [Google Scholar]
- 28.Lam SY et al. Toll-like receptor 1 locus re-examined in a genome-wide association study update on anti-Helicobacter pylori IgG titers. Gastroenterology 162, 1705–1715 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.El-Omar EM Genetic predisposition for Helicobacter pylori infection-the jury is still out! Gastroenterology 162, 1591–1593 (2022). [DOI] [PubMed] [Google Scholar]
- 30.Kayali S et al. Helicobacter pylori, transmission routes and recurrence of infection: state of the art. Acta Biomed 89, 72–76 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brown LM Helicobacter pylori: epidemiology and routes of transmission. Epidemiol. Rev 22, 283–297 (2000). [DOI] [PubMed] [Google Scholar]
- 32.Fox JG Non-human reservoirs of Helicobacter pylori. Aliment. Pharmacol. Ther 9, 93–103 (1995). [PubMed] [Google Scholar]
- 33.Parsonnet J, Shmuely H & Haggerty T Fecal and oral shedding of Helicobacter pylori from healthy infected adults. JAMA 282, 2240–2245 (1999). [DOI] [PubMed] [Google Scholar]
- 34.Weyermann M, Rothenbacher D & Brenner H Acquisition of Helicobacter pylori infection in early childhood: independent contributions of infected mothers, fathers, and siblings. Am. J. Gastroenterol 104, 182–189 (2009). [DOI] [PubMed] [Google Scholar]
- 35.Kivi M et al. Concordance of Helicobacter pylori strains within families. J. Clin. Microbiol 41, 5604–5608 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Georgopoulos SD et al. Helicobacter pylori infection in spouses of patients with duodenal ulcers and comparison of ribosomal RNA gene patterns. Gut 39, 634–638 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Luman W, Zhao Y, Ng HS & Ling KL Helicobacter pylori infection is unlikely to be transmitted between partners: evidence from genotypic study in partners of infected patients. Eur. J. Gastroenterol. Hepatol 14, 521–528 (2002). [DOI] [PubMed] [Google Scholar]
- 38.Schwarz S et al. Horizontal versus familial transmission of Helicobacter pylori. PLoS Pathog 4, e1000180 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Didelot X et al. Genomic evolution and transmission of Helicobacter pylori in two South African families. Proc. Natl Acad. Sci. USA 110, 13880–13885 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liou JM et al. Long-term changes of gut microbiota, antibiotic resistance, and metabolic parameters after Helicobacter pylori eradication: a multicentre, open-label, randomised trial. Lancet Infect. Dis 19, 1109–1120 (2019). [DOI] [PubMed] [Google Scholar]
- 41.Zhao JB et al. Whole family-based Helicobacter pylori eradication is a superior strategy to single-infected patient treatment approach: a systematic review and meta-analysis. Helicobacter 26, e12793 (2021). [DOI] [PubMed] [Google Scholar]
- 42.Malfertheiner P et al. Management of Helicobacter pylori infection — the Maastricht V/Florence consensus report. Gut 66, 6–30 (2017). [DOI] [PubMed] [Google Scholar]
- 43.Kuipers EJ, Thijs JC & Festen HP The prevalence of Helicobacter pylori in peptic ulcer disease. Aliment. Pharmacol. Ther 9, 59–69 (1995). [PubMed] [Google Scholar]
- 44.Lanas A & Chan FKL Peptic ulcer disease. Lancet 390, 613–624 (2017). [DOI] [PubMed] [Google Scholar]
- 45.Sipponen P et al. Cumulative 10-year risk of symptomatic duodenal and gastric ulcer in patients with or without chronic gastritis. A clinical follow-up study of 454 outpatients. Scand. J. Gastroenterol 25, 966–973 (1990). [DOI] [PubMed] [Google Scholar]
- 46.Schottker B, Adamu MA, Weck MN & Brenner H Helicobacter pylori infection is strongly associated with gastric and duodenal ulcers in a large prospective study. Clin. Gastroenterol. Hepatol 10, 487–493.e1 (2012). [DOI] [PubMed] [Google Scholar]
- 47.Xia B et al. Trends in the prevalence of peptic ulcer disease and Helicobacter pylori infection in family physician-referred uninvestigated dyspeptic patients in Hong Kong. Aliment. Pharmacol. Ther 22, 243–249 (2005). [DOI] [PubMed] [Google Scholar]
- 48.Perez-Aisa MA, Del Pino D, Siles M & Lanas A Clinical trends in ulcer diagnosis in a population with high prevalence of Helicobacter pylori infection. Aliment. Pharmacol. Ther 21, 65–72 (2005). [DOI] [PubMed] [Google Scholar]
- 49.Leow AH, Lim YY, Liew WC & Goh KL Time trends in upper gastrointestinal diseases and Helicobacter pylori infection in a multiracial Asian population — a 20-year experience over three time periods. Aliment. Pharmacol. Ther 43, 831–837 (2016). [DOI] [PubMed] [Google Scholar]
- 50.Azhari H et al. The global incidence of peptic ulcer disease is decreasing since the turn of the 21st century: a study of the Organisation for Economic Co-operation and Development (OECD). Am. J. Gastroenterol 117, 1419–1427 (2022). [DOI] [PubMed] [Google Scholar]
- 51.Yamamichi N et al. Inverse time trends of peptic ulcer and reflux esophagitis show significant association with reduced prevalence of Helicobacter pylori infection. Ann. Med 52, 506–514 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jiang JX et al. Downward trend in the prevalence of Helicobacter pylori infections and corresponding frequent upper gastrointestinal diseases profile changes in Southeastern China between 2003 and 2012. Springerplus 5, 1601 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xie X, Ren K, Zhou Z, Dang C & Zhang H The global, regional and national burden of peptic ulcer disease from 1990 to 2019: a population-based study. BMC Gastroenterol 22, 58 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Malfertheiner P & Schulz C Peptic ulcer: chapter closed? Dig. Dis 10.1159/000505367 (2020). [DOI] [PubMed] [Google Scholar]
- 55.Huang JQ, Sridhar S & Hunt RH Role of Helicobacter pylori infection and nonsteroidal anti-inflammatory drugs in peptic-ulcer disease: a meta-analysis. Lancet 359, 14–22 (2002). [DOI] [PubMed] [Google Scholar]
- 56.Venerito M et al. Contribution of Helicobacter pylori infection to the risk of peptic ulcer bleeding in patients on nonsteroidal anti-inflammatory drugs, antiplatelet agents, anticoagulants, corticosteroids and selective serotonin reuptake inhibitors. Aliment. Pharmacol. Ther 47, 1464–1471 (2018). [DOI] [PubMed] [Google Scholar]
- 57.Rosenstock S, Jorgensen T, Bonnevie O & Andersen L Risk factors for peptic ulcer disease: a population based prospective cohort study comprising 2416 Danish adults. Gut 52, 186–193 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zagari RM et al. Prevalence of upper gastrointestinal endoscopic findings in the community: a systematic review of studies in unselected samples of subjects. J. Gastroenterol. Hepatol 31, 1527–1538 (2016). [DOI] [PubMed] [Google Scholar]
- 59.Eslick G et al. Clinical and economic impact of “triple therapy” for Helicobacter pylori eradication on peptic ulcer disease in Australia. Helicobacter 25, e12751 (2020). [DOI] [PubMed] [Google Scholar]
- 60.Sung J, Kuipers E & El-Serag H Systematic review: the global incidence and prevalence of peptic ulcer disease. Aliment. Pharmacol. Ther 29, 938–946 (2009). [DOI] [PubMed] [Google Scholar]
- 61.Moss SF The clinical evidence linking Helicobacter pylori to gastric cancer. Cell Mol. Gastroenterol. Hepatol 3, 183–191 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.de Martel C, Georges D, Bray F, Ferlay J & Clifford GM Global burden of cancer attributable to infections in 2018: a worldwide incidence analysis. Lancet Glob. Health 8, e180–e190 (2020). [DOI] [PubMed] [Google Scholar]
- 63.Fann JC et al. Personalized risk assessment for dynamic transition of gastric neoplasms. J. Biomed. Sci 25, 84 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Arnold M et al. The burden of stomach cancer in indigenous populations: a systematic review and global assessment. Gut 63, 64–71 (2014). [DOI] [PubMed] [Google Scholar]
- 65.Kumar S, Metz DC, Ellenberg S, Kaplan DE & Goldberg DS Risk factors and incidence of gastric cancer after detection of Helicobacter pylori infection: a large cohort study. Gastroenterology 158, 527–536.e7 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gonzalez CA & Lopez-Carrillo L Helicobacter pylori, nutrition and smoking interactions: their impact in gastric carcinogenesis. Scand. J. Gastroenterol 45, 6–14 (2010). [DOI] [PubMed] [Google Scholar]
- 67.Venneman K et al. The epidemiology of Helicobacter pylori infection in Europe and the impact of lifestyle on its natural evolution toward stomach cancer after infection: a systematic review. Helicobacter 23, e12483 (2018). [DOI] [PubMed] [Google Scholar]
- 68.Wong F, Rayner-Hartley E & Byrne MF Extraintestinal manifestations of Helicobacter pylori: a concise review. World J. Gastroenterol 20, 11950–11961 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Takeuchi H & Okamoto A Helicobacter pylori infection and chronic immune thrombocytopenia. J. Clin. Med 11, 4822 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Malfertheiner P et al. Management of Helicobacter pylori infection — the Maastricht IV/Florence Consensus Report. Gut 61, 646–664 (2012). [DOI] [PubMed] [Google Scholar]
- 71.Gasbarrini A et al. Regression of autoimmune thrombocytopenia after eradication of Helicobacter pylori. Lancet 352, 878 (1998). [DOI] [PubMed] [Google Scholar]
- 72.Figura N et al. Extragastric manifestations of Helicobacter pylori infection. Helicobacter 15, 60–68 (2010). [DOI] [PubMed] [Google Scholar]
- 73.Franceschi F, Zuccala G, Roccarina D & Gasbarrini A Clinical effects of Helicobacter pylori outside the stomach. Nat. Rev. Gastroenterol. Hepatol 11, 234–242 (2014). [DOI] [PubMed] [Google Scholar]
- 74.Gravina AG et al. Extra-gastric manifestations of Helicobacter pylori infection. J. Clin. Med 9, 3887 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.McCune A et al. Reduced risk of atopic disorders in adults with Helicobacter pylori infection. Eur. J. Gastroenterol. Hepatol 15, 637–640 (2003). [DOI] [PubMed] [Google Scholar]
- 76.Chen Y & Blaser MJ Inverse associations of Helicobacter pylori with asthma and allergy. Arch. Intern. Med 167, 821–827 (2007). [DOI] [PubMed] [Google Scholar]
- 77.Blaser MJ, Chen Y & Reibman J Does Helicobacter pylori protect against asthma and allergy? Gut 57, 561–567 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Alvarez CS et al. Associations of Helicobacter pylori and hepatitis A seropositivity with asthma in the Hispanic Community Health Study/Study of Latinos (HCHS/SOL): addressing the hygiene hypothesis. Allergy Asthma Clin. Immunol 17, 120 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rokkas T, Pistiolas D, Sechopoulos P, Robotis I & Margantinis G Relationship between Helicobacter pylori infection and esophageal neoplasia: a meta-analysis. Clin. Gastroenterol. Hepatol 5, 1413–1417 (2007). [DOI] [PubMed] [Google Scholar]
- 80.Fischbach LA et al. The association between Barrett’s esophagus and Helicobacter pylori infection: a meta-analysis. Helicobacter 17, 163–175 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rubenstein JH et al. Association between Helicobacter pylori and Barrett’s esophagus, erosive esophagitis, and gastroesophageal reflux symptoms. Clin. Gastroenterol. Hepatol 12, 239–245 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Vicari JJ et al. The seroprevalence of cagA-positive Helicobacter pylori strains in the spectrum of gastroesophageal reflux disease. Gastroenterology 115, 50–57 (1998). [DOI] [PubMed] [Google Scholar]
- 83.Doorakkers E, Lagergren J, Santoni G, Engstrand L & Brusselaers N Helicobacter pylori eradication treatment and the risk of Barrett’s esophagus and esophageal adenocarcinoma. Helicobacter 25, e12688 (2020). [DOI] [PubMed] [Google Scholar]
- 84.Wang Z et al. Helicobacter pylori infection is associated with reduced risk of Barrett’s esophagus: an analysis of the Barrett’s and esophageal adenocarcinoma consortium. Am. J. Gastroenterol 113, 1148–1155 (2018). [DOI] [PubMed] [Google Scholar]
- 85.Zamani M, Alizadeh-Tabari S, Hasanpour AH, Eusebi LH & Ford AC Systematic review with meta-analysis: association of Helicobacter pylori infection with gastro-oesophageal reflux and its complications. Aliment. Pharmacol. Ther 54, 988–998 (2021). [DOI] [PubMed] [Google Scholar]
- 86.Malfertheiner P et al. Management of Helicobacter pylori infection: the Maastricht VI/Florence consensus report. Gut 10.1136/gutjnl-2022-327745 (2022). [DOI] [PubMed] [Google Scholar]
- 87.Tomb JF et al. The complete genome sequence of the gastric pathogen Helicobacter pylori. Nature 388, 539–547 (1997); erratum 389, 412 (1997). [DOI] [PubMed] [Google Scholar]
- 88.Alm RA et al. Genomic-sequence comparison of two unrelated isolates of the human gastric pathogen Helicobacter pylori. Nature 397, 176–180 (1999). [DOI] [PubMed] [Google Scholar]
- 89.Gressmann H et al. Gain and loss of multiple genes during the evolution of Helicobacter pylori. PLoS Genet 1, e43 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Krebes J et al. The complex methylome of the human gastric pathogen Helicobacter pylori. Nucleic Acids Res 42, 2415–2432 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ailloud F et al. Within-host evolution of Helicobacter pylori shaped by niche-specific adaptation, intragastric migrations and selective sweeps. Nat. Commun 10, 2273 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Jackson LK et al. Helicobacter pylori diversification during chronic infection within a single host generates sub-populations with distinct phenotypes. PLoS Pathog 16, e1008686 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Suerbaum S & Josenhans C Helicobacter pylori evolution and phenotypic diversification in a changing host. Nat. Rev. Microbiol 5, 441–452 (2007). [DOI] [PubMed] [Google Scholar]
- 94.Kang J & Blaser MJ Bacterial populations as perfect gases: genomic integrity and diversification tensions in Helicobacter pylori. Nat. Rev. Microbiol 4, 826–836 (2006). [DOI] [PubMed] [Google Scholar]
- 95.Garcia-Ortiz MV et al. Unexpected role for Helicobacter pylori DNA polymerase I as a source of genetic variability. PLoS Genet 7, e1002152 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hofreuter D, Odenbreit S & Haas R Natural transformation competence in Helicobacter pylori is mediated by the basic components of a type IV secretion system. Mol. Microbiol 41, 379–391 (2001). [DOI] [PubMed] [Google Scholar]
- 97.Stingl K, Muller S, Scheidgen-Kleyboldt G, Clausen M & Maier B Composite system mediates two-step DNA uptake into Helicobacter pylori. Proc. Natl Acad. Sci. USA 107, 1184–1189 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Suerbaum S et al. Free recombination within Helicobacter pylori. Proc. Natl Acad. Sci. USA 95, 12619–12624 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kennemann L et al. Helicobacter pylori genome evolution during human infection. Proc. Natl Acad. Sci. USA 108, 5033–5038 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bubendorfer S et al. Genome-wide analysis of chromosomal import patterns after natural transformation of Helicobacter pylori. Nat. Commun 7, 11995 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Falush D et al. Traces of human migrations in Helicobacter pylori populations. Science 299, 1582–1585 (2003). [DOI] [PubMed] [Google Scholar]
- 102.Linz B et al. An African origin for the intimate association between humans and Helicobacter pylori. Nature 445, 915–918 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Moodley Y et al. Age of the association between Helicobacter pylori and man. PLoS Pathog 8, e1002693 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ailloud F, Estibariz I & Suerbaum S Evolved to vary: genome and epigenome variation in the human pathogen Helicobacter pylori. FEMS Microbiol. Rev 45, fuaa042 (2021). [DOI] [PubMed] [Google Scholar]
- 105.Moodley Y et al. The peopling of the Pacific from a bacterial perspective. Science 323, 527–530 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Censini S et al. cag, a pathogenicity island of Helicobacter pylori, encodes type I-specific and disease-associated virulence factors. Proc. Natl Acad. Sci. USA 93, 14648–14653 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Olbermann P et al. A global overview of the genetic and functional diversity in the Helicobacter pylori cag pathogenicity island. PLoS Genet 6, e1001069 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Johnson KS & Otteman KM Colonization, localization, and inflammation: the roles of H. pylori chemotaxis in vivo. Curr. Opin. Microbiol 41, 51–57 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Josenhans C, Labigne A & Suerbaum S Comparative ultrastructural and functional studies of Helicobacter pylori and Helicobacter mustelae flagellin mutants: both flagellin subunits, FlaA and FlaB, are necessary for full motility in Helicobacter species. J. Bacteriol 177, 3010–3020 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lee SK et al. Helicobacter pylori flagellins have very low intrinsic activity to stimulate human gastric epithelial cells via TLR5. Microbes Infect 5, 1345–1356 (2003). [DOI] [PubMed] [Google Scholar]
- 111.Andersen-Nissen E et al. Evasion of Toll-like receptor 5 by flagellated bacteria. Proc. Natl Acad. Sci. USA 102, 9247–9252 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Schreiber S et al. The spatial orientation of Helicobacter pylori in the gastric mucus. Proc. Natl Acad. Sci. USA 101, 5024–5029 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Suerbaum S et al. Identification of antimotilins, novel inhibitors of Helicobacter pylori flagellar motility that inhibit stomach colonization in a mouse model. mBio 13, e0375521 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Mobley HL in Helicobacter pylori: Molecular and Cellular Biology (eds Achtman M & Suerbaum S) (Horizon Scientific Press, 2001). [Google Scholar]
- 115.Weeks DL, Eskandari S, Scott DR & Sachs G A H+-gated urea channel: the link between Helicobacter pylori urease and gastric colonization. Science 287, 482–485 (2000). [DOI] [PubMed] [Google Scholar]
- 116.Eaton KA, Brooks CL, Morgan DR & Krakowka S Essential role of urease in pathogenesis of gastritis induced by Helicobacter pylori in gnotobiotic piglets. Infect. Immun 59, 2470–2475 (1991). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kirschner DE & Blaser MJ The dynamics of Helicobacter pylori infection of the human stomach. J. Theor. Biol 176, 281–290 (1995). [DOI] [PubMed] [Google Scholar]
- 118.Borén T, Falk P, Roth KA, Larson G & Normark S Attachment of Helicobacter pylori to human gastric epithelium mediated by blood group antigens. Science 262, 1892–1895 (1993). [DOI] [PubMed] [Google Scholar]
- 119.Mahdavi J et al. Helicobacter pylori SabA adhesin in persistent infection and chronic inflammation. Science 297, 573–578 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Javaheri A et al. Helicobacter pylori adhesin HopQ engages in a virulence-enhancing interaction with human CEACAMs. Nat. Microbiol 2, 16189 (2016). [DOI] [PubMed] [Google Scholar]
- 121.Koniger V et al. Helicobacter pylori exploits human CEACAMs via HopQ for adherence and translocation of CagA. Nat. Microbiol 2, 16188 (2016). [DOI] [PubMed] [Google Scholar]
- 122.Senkovich OA et al. Helicobacter pylori AlpA and AlpB bind host laminin and influence gastric inflammation in gerbils. Infect. Immun 79, 3106–3116 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Odenbreit S et al. Translocation of Helicobacter pylori CagA into gastric epithelial cells by type IV secretion. Science 287, 1497–1500 (2000). [DOI] [PubMed] [Google Scholar]
- 124.Stein SC et al. Helicobacter pylori modulates host cell responses by CagT4SSdependent translocation of an intermediate metabolite of LPS inner core heptose biosynthesis. PLoS Pathog 13, e1006514 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Pfannkuch L et al. ADP heptose, a novel pathogen-associated molecular pattern identified in Helicobacter pylori. FASEB J 33, 9087–9099 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Viala J et al. Nod1 responds to peptidoglycan delivered by the Helicobacter pylori cag pathogenicity island. Nat. Immunol 5, 1166–1174 (2004). [DOI] [PubMed] [Google Scholar]
- 127.Varga MG et al. Pathogenic Helicobacter pylori strains translocate DNA and activate TLR9 via the cancer-associated cag type IV secretion system. Oncogene 35, 6262–6269 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Asahi M et al. Helicobacter pylori CagA protein can be tyrosine phosphorylated in gastric epithelial cells. J. Exp. Med 191, 593–602 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Higashi H et al. SHP-2 tyrosine phosphatase as an intracellular target of Helicobacter pylori CagA protein. Science 295, 683–686 (2002). [DOI] [PubMed] [Google Scholar]
- 130.Saadat I et al. Helicobacter pylori CagA targets PAR1/MARK kinase to disrupt epithelial cell polarity. Nature 447, 330–333 (2007). [DOI] [PubMed] [Google Scholar]
- 131.Nesic D et al. Helicobacter pylori CagA inhibits PAR1-MARK family kinases by mimicking host substrates. Nat. Struct. Mol. Biol 17, 130–132 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Buti L et al. Helicobacter pylori cytotoxin-associated gene A (CagA) subverts the apoptosis-stimulating protein of p53 (ASPP2) tumor suppressor pathway of the host. Proc. Natl Acad. Sci. USA 108, 9238–9243 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ding SZ, Goldberg JB & Hatakeyama M Helicobacter pylori infection, oncogenic pathways and epigenetic mechanisms in gastric carcinogenesis. Future Oncol 6, 851–862 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Bauer M et al. The ALPK1/TIFA/NF-κB axis links a bacterial carcinogen to R-loop-induced replication stress. Nat. Commun 11, 5117 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Faass L et al. Contribution of heptose metabolites and the cag pathogenicity island to the activation of monocytes/macrophages by Helicobacter pylori. Front. Immunol 12, 632154 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Coletta S et al. ADP-heptose enables Helicobacter pylori to exploit macrophages as a survival niche by suppressing antigen-presenting HLA-II expression. FEBS Lett 595, 2160–2168 (2021). [DOI] [PubMed] [Google Scholar]
- 137.Cover TL & Blaser MJ Purification and characterization of the vacuolating toxin from Helicobacter pylori. J. Biol. Chem 267, 10570–10575 (1992). [PubMed] [Google Scholar]
- 138.Cover TL & Blanke SR Helicobacter pylori VacA, a paradigm for toxin multifunctionality. Nat. Rev. Microbiol 3, 320–332 (2005). [DOI] [PubMed] [Google Scholar]
- 139.Foegeding NJ, Caston RR, McClain MS, Ohi MD & Cover TL An overview of Helicobacter pylori VacA toxin biology. Toxins 8, 173 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Altobelli A, Bauer M, Velez K, Cover TL & Muller A Helicobacter pylori VacA targets myeloid cells in the gastric lamina propria to promote peripherally induced regulatory T-cell differentiation and persistent infection. mBio 10, e00261–19 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Zhang X, Arnold IC & Muller A Mechanisms of persistence, innate immune activation and immunomodulation by the gastric pathogen Helicobacter pylori. Curr. Opin. Microbiol 54, 1–10 (2020). [DOI] [PubMed] [Google Scholar]
- 142.Gobert AP & Wilson KT Induction and regulation of the innate immune response in Helicobacter pylori infection. Cell Mol. Gastroenterol. Hepatol 13, 1347–1363 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Faass L, Hauke M, Stein SC & Josenhans C Innate immune activation and modulatory factors of Helicobacter pylori towards phagocytic and nonphagocytic cells. Curr. Opin. Immunol 82, 102301 (2023). [DOI] [PubMed] [Google Scholar]
- 144.de Bernard M & Josenhans C Pathogenesis of Helicobacter pylori infection. Helicobacter 19, 11–18 (2014). [DOI] [PubMed] [Google Scholar]
- 145.Crabtree JE et al. Interleukin-8 expression in Helicobacter pylori infected, normal, and neoplastic gastroduodenal mucosa. J. Clin. Pathol 47, 61–66 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Sharma SA, Tummuru MK, Blaser MJ & Kerr LD Activation of IL-8 gene expression by Helicobacter pylori is regulated by transcription factor nuclear factor-kappa B in gastric epithelial cells. J. Immunol 160, 2401–2407 (1998). [PubMed] [Google Scholar]
- 147.Maubach G, Vieth M, Boccellato F & Naumann M Helicobacter pylori-induced NF-κB: trailblazer for gastric pathophysiology. Trends Mol. Med 28, 210–222 (2022). [DOI] [PubMed] [Google Scholar]
- 148.Rugge M, Savarino E, Sbaraglia M, Bricca L & Malfertheiner P Gastritis: the clinico-pathological spectrum. Dig. Liver Dis 53, 1237–1246 (2021). [DOI] [PubMed] [Google Scholar]
- 149.Sipponen P, Kekki M & Siurala M The Sydney System: epidemiology and natural history of chronic gastritis. J. Gastroenterol. Hepatol 6, 244–251 (1991). [DOI] [PubMed] [Google Scholar]
- 150.Oertli M et al. DC-derived IL-18 drives Treg differentiation, murine Helicobacter pylori-specific immune tolerance, and asthma protection. J. Clin. Invest 122, 1082–1096 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Arshad U, Sarkar S, Alipour Talesh G & Sutton P A lack of role for antibodies in regulating Helicobacter pylori colonization and associated gastritis. Helicobacter 25, e12681 (2020). [DOI] [PubMed] [Google Scholar]
- 152.Ermak TH et al. Immunization of mice with urease vaccine affords protection against Helicobacter pylori infection in the absence of antibodies and is mediated by MHC class II-restricted responses. J. Exp. Med 188, 2277–2288 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.D’Elios MM & Czinn SJ Immunity, inflammation, and vaccines for Helicobacter pylori. Helicobacter 19, 19–26 (2014). [DOI] [PubMed] [Google Scholar]
- 154.Arnold IC et al. Helicobacter pylori infection prevents allergic asthma in mouse models through the induction of regulatory T cells. J. Clin. Invest 121, 3088–3093 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Kyburz A et al. Transmaternal Helicobacter pylori exposure reduces allergic airway inflammation in offspring through regulatory T cells. J. Allergy Clin. Immunol 143, 1496–1512.e11 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Cook KW et al. CCL20/CCR6-mediated migration of regulatory T cells to the Helicobacter pylori-infected human gastric mucosa. Gut 63, 1550–1559 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Robinson K et al. Helicobacter pylori-induced peptic ulcer disease is associated with inadequate regulatory T cell responses. Gut 57, 1375–1385 (2008). [DOI] [PubMed] [Google Scholar]
- 158.Backert S, Haas R, Gerhard M & Naumann M The Helicobacter pylori type IV secretion system encoded by the cag pathogenicity island: architecture, function, and signaling. Curr. Top. Microbiol. Immunol 413, 187–220 (2017). [DOI] [PubMed] [Google Scholar]
- 159.Correa P Human gastric carcinogenesis: a multistep and multifactorial process. First American Cancer Society award lecture on cancer epidemiology and prevention. Cancer Res 52, 6735 (1992). [PubMed] [Google Scholar]
- 160.Ferreira RM et al. Gastric microbial community profiling reveals a dysbiotic cancer-associated microbiota. Gut 67, 226–236 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Guo Y et al. Effect of Helicobacter pylori on gastrointestinal microbiota: a population-based study in Linqu, a high-risk area of gastric cancer. Gut 69, 1598–1607 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Kwon SK et al. Human gastric microbiota transplantation recapitulates premalignant lesions in germ-free mice. Gut 71, 1266–1276 (2022). [DOI] [PubMed] [Google Scholar]
- 163.Pereira-Marques J, Ferreira RM, Machado JC & Figueiredo C The influence of the gastric microbiota in gastric cancer development. Best Pract. Res. Clin. Gastroenterol 50–51, 101734 (2021). [DOI] [PubMed] [Google Scholar]
- 164.Bayerdorffer E et al. Regression of primary gastric lymphoma of mucosa-associated lymphoid tissue type after cure of Helicobacter pylori infection. MALT Lymphoma Study Group. Lancet 345, 1591–1594 (1995). [DOI] [PubMed] [Google Scholar]
- 165.Hunt RH et al. The stomach in health and disease. Gut 64, 1650–1668 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.El-Omar EM et al. Helicobacter pylori infection and chronic gastric acid hyposecretion. Gastroenterology 113, 15–24 (1997). [DOI] [PubMed] [Google Scholar]
- 167.Amieva M & Peek RM Jr. Pathobiology of Helicobacter pylori-induced gastric cancer. Gastroenterology 150, 64–78 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Kwon SK et al. Human gastric microbiota transplantation recapitulates premalignant lesions in germ-free mice. Gut 71, 1266–1276 (2021). [DOI] [PubMed] [Google Scholar]
- 169.Jones NL et al. Joint ESPGHAN/NASPGHAN guidelines for the management of Helicobacter pylori in children and adolescents (Update 2016). J. Pediatr. Gastroenterol. Nutr 64, 991–1003 (2017). [DOI] [PubMed] [Google Scholar]
- 170.Sobala GM et al. Acute Helicobacter pylori infection: clinical features, local and systemic immune response, gastric mucosal histology, and gastric juice ascorbic acid concentrations. Gut 32, 1415–1418 (1991). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Graham DY et al. Challenge model for Helicobacter pylori infection in human volunteers. Gut 53, 1235–1243 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Malfertheiner P et al. Efficacy, immunogenicity, and safety of a parenteral vaccine against Helicobacter pylori in healthy volunteers challenged with a Cag-positive strain: a randomised, placebo-controlled phase 1/2 study. Lancet Gastroenterol. Hepatol 3, 698–707 (2018). [DOI] [PubMed] [Google Scholar]
- 173.Spee LA, Madderom MB, Pijpers M, van Leeuwen Y & Berger MY Association between Helicobacter pylori and gastrointestinal symptoms in children. Pediatrics 125, e651–e669 (2010). [DOI] [PubMed] [Google Scholar]
- 174.Fischbach W, Goebeler-Kolve ME, Dragosics B, Greiner A & Stolte M Long term outcome of patients with gastric marginal zone B cell lymphoma of mucosa associated lymphoid tissue (MALT) following exclusive Helicobacter pylori eradication therapy: experience from a large prospective series. Gut 53, 34–37 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Malfertheiner P Diagnostic methods for H. pylori infection: choices, opportunities and pitfalls. United European Gastroenterol. J 3, 429–431 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Pilotto A & Franceschi M Helicobacter pylori infection in older people. World J. Gastroenterol 20, 6364–6373 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Uotani T & Graham DY Diagnosis of Helicobacter pylori using the rapid urease test. Ann. Transl Med 3, 9 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Smith SI et al. Helicobacter pylori infection in Africa: update of the current situation and challenges. Dig. Dis 40, 535–544 (2021). [DOI] [PubMed] [Google Scholar]
- 179.Bordin DS, Voynovan IN & Andreev DN Maev IV. Current Helicobacter pylori diagnostics. Diagnostics 11, 1458 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Miftahussurur M & Yamaoka Y Diagnostic methods of Helicobacter pylori infection for epidemiological studies: critical importance of indirect test validation. Biomed. Res. Int 2016, 4819423 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Talebi Bezmin Abadi A Diagnosis of Helicobacter pylori using invasive and noninvasive approaches. J. Pathog 2018, 9064952 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Dixon MF, Genta RM, Yardley JH & Correa P Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am. J. Surg. Pathol 20, 1161–1181 (1996). [DOI] [PubMed] [Google Scholar]
- 183.Rugge M et al. Gastritis staging in clinical practice: the OLGA staging system. Gut 56, 631–636 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Capelle LG et al. The staging of gastritis with the OLGA system by using intestinal metaplasia as an accurate alternative for atrophic gastritis. Gastrointest. Endosc 71, 1150–1158 (2010). [DOI] [PubMed] [Google Scholar]
- 185.Ajayi A, Jolaiya T & Smith SI Direct detection of Helicobacter pylori from biopsies of patients in Lagos, Nigeria using real-time PCR-a pilot study. BMC Res. Notes 14, 90 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Moss SF et al. Comparable results of Helicobacter pylori antibiotic resistance testing of stools vs gastric biopsies using next-generation sequencing. Gastroenterology 162, 2095–2097.e2 (2022). [DOI] [PubMed] [Google Scholar]
- 187.Schulz C, Kalali B, Link A, Gerhard M & Malfertheiner P New rapid Helicobacter pylori blood test based on dual detection of FliD and CagA antibodies for on-site testing. Clin. Gastroenterol. Hepatol 21, 229–231.e1 (2021). [DOI] [PubMed] [Google Scholar]
- 188.Megraud F et al. Helicobacter pylori resistance to antibiotics in Europe in 2018 and its relationship to antibiotic consumption in the community. Gut 70, 1815–1822 (2021). [DOI] [PubMed] [Google Scholar]
- 189.Savoldi A, Carrara E, Graham DY, Conti M & Tacconelli E Prevalence of antibiotic resistance in Helicobacter pylori: a systematic review and meta-analysis in World Health Organization Regions. Gastroenterology 155, 1372–1382.e17 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Hulten KG et al. Comparison of culture with antibiogram to next-generation sequencing using bacterial isolates and formalin-fixed, paraffin-embedded gastric biopsies. Gastroenterology 161, 1433–1442.e2 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Argueta AE, Alsamman MA, Moss SF & D’Agata EMC Impact of antimicrobial resistance rates on eradication of Helicobacter pylori in a US population. Gastroenterology 160, 2181–2183.e1 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.David YG & Steven FM Antimicrobial susceptibility testing for Helicobacter pylori is now widely available: when, how, why. Am. J. Gastroenterol 117, 524–528 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Hu Y, Zhang M, Lu B & Dai J Helicobacter pylori and antibiotic resistance, a continuing and intractable problem. Helicobacter 21, 349–363 (2016). [DOI] [PubMed] [Google Scholar]
- 194.Egli K et al. Comparison of the diagnostic performance of qPCR, sanger sequencing, and whole-genome sequencing in determining clarithromycin and levofloxacin resistance in Helicobacter pylori. Front. Cell Infect. Microbiol 10, 596371 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Zamani M, Rahbar A & Shokri-Shirvani J Resistance of Helicobacter pylori to furazolidone and levofloxacin: a viewpoint. World J. Gastroenterol 23, 6920–6922 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Wang YH et al. A systematic review and meta-analysis of genotypic methods for detecting antibiotic resistance in Helicobacter pylori. Helicobacter 23, e12467 (2018). [DOI] [PubMed] [Google Scholar]
- 197.Li Y et al. Detection of clarithromycin resistance in Helicobacter pylori following noncryogenic storage of rapid urease tests for 30 days. J. Dig. Dis 13, 54–59 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Chung WC et al. Dual-priming oligonucleotide-based multiplex PCR using tissue samples in rapid urease test in the detection of Helicobacter pylori infection. World J. Gastroenterol 20, 6547–6553 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Chung WC et al. Dual-priming oligonucleotide-based multiplex PCR using tissue samples from the rapid urease test kit for the detection of Helicobacter pylori in bleeding peptic ulcers. Dig. Liver Dis 48, 899–903 (2016). [DOI] [PubMed] [Google Scholar]
- 200.Chen T, Meng X, Zhang H, Tsang RW & Tsang TK Comparing multiplex PCR and rapid urease test in the detection of H. pylori in patients on proton pump inhibitors. Gastroenterol. Res. Pract 2012, 898276 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Goji S et al. Helicobacter suis-infected nodular gastritis and a review of diagnostic sensitivity for Helicobacter heilmannii-like organisms. Case Rep. Gastroenterol 9, 179–187 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Kobayashi M et al. Helicobacter heilmannii-like organisms in parietal cells: a diagnostic pitfall. Pathol. Int 66, 120–122 (2016). [DOI] [PubMed] [Google Scholar]
- 203.De Witte C, Schulz C, Smet A, Malfertheiner P & Haesebrouck F Other Helicobacters and gastric microbiota. Helicobacter 21 (Suppl. 1), 62–68 (2016). [DOI] [PubMed] [Google Scholar]
- 204.Seiichi K et al. The updated JSPGHAN guidelines for the management of Helicobacter pylori infection in childhood. Pediatr. Int 62, 1315–1331 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Moayyedi P et al. Guideline: management of dyspepsia. Am. J. Gastroenterol 112, 988–1013 (2017). [DOI] [PubMed] [Google Scholar]
- 206.Chey WD, Leontiadis GI, Howden CW & Moss SF ACG clinical guideline: treatment of Helicobacter pylori infection. Am. J. Gastroenterol 112, 212–239 (2017). [DOI] [PubMed] [Google Scholar]
- 207.Talley NJ How to manage the difficult-to-treat dyspeptic patient. Nat. Clin. Pract. Gastroenterol. Hepatol 4, 35–42 (2007). [DOI] [PubMed] [Google Scholar]
- 208.Koletzko L, Macke L, Schulz C & Malfertheiner P Helicobacter pylori eradication in dyspepsia: new evidence for symptomatic benefit. Best Pract. Res. Clin. Gastroenterol 40–41, 101637 (2019). [DOI] [PubMed] [Google Scholar]
- 209.Mahadeva S, Chia YC, Vinothini A, Mohazmi M & Goh KL Cost-effectiveness of and satisfaction with a Helicobacter pylori “test and treat” strategy compared with prompt endoscopy in young Asians with dyspepsia. Gut 57, 1214–1220 (2008). [DOI] [PubMed] [Google Scholar]
- 210.Malfertheiner P et al. Helicobacter pylori eradication is beneficial in the treatment of functional dyspepsia. Aliment. Pharmacol. Ther 18, 615–625 (2003). [DOI] [PubMed] [Google Scholar]
- 211.Hawkey C et al. Helicobacter pylori eradication for primary prevention of peptic ulcer bleeding in older patients prescribed aspirin in primary care (HEAT): a randomised, double-blind, placebo-controlled trial. Lancet 400, 1597–1606 (2022). [DOI] [PubMed] [Google Scholar]
- 212.Ford AC, Yuan Y, Forman D, Hunt R & Moayyedi P Helicobacter pylori eradication for the prevention of gastric neoplasia. Cochrane Database Syst. Rev 7, CD005583 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Ford AC, Yuan Y & Moayyedi P Helicobacter pylori eradication therapy to prevent gastric cancer: systematic review and meta-analysis. Gut 69, 2113–2121 (2020). [DOI] [PubMed] [Google Scholar]
- 214.Choi IJ et al. Family history of gastric cancer and Helicobacter pylori treatment. N. Engl. J. Med 382, 427–436 (2020). [DOI] [PubMed] [Google Scholar]
- 215.Rokkas T, Rokka A & Portincasa P A systematic review and meta-analysis of the role of Helicobacter pylori eradication in preventing gastric cancer. Ann. Gastroenterol 30, 414–423 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Khan MY et al. Effectiveness of Helicobacter pylori eradication in preventing metachronous gastric cancer and preneoplastic lesions. A systematic review and meta-analysis. Eur. J. Gastroenterol. Hepatol 32, 686–694 (2020). [DOI] [PubMed] [Google Scholar]
- 217.Zhao B et al. Does Helicobacter pylori eradication reduce the incidence of metachronous gastric cancer after curative endoscopic resection of early gastric cancer: a systematic review and meta-analysis. J. Clin. Gastroenterol 54, 235–241 (2020). [DOI] [PubMed] [Google Scholar]
- 218.Fan F, Wang Z, Li B & Zhang H Effects of eradicating Helicobacter pylori on metachronous gastric cancer prevention: a systematic review and meta-analysis. J. Eval. Clin. Pract 26, 308–315 (2020). [DOI] [PubMed] [Google Scholar]
- 219.Choi IJ et al. Helicobacter pylori therapy for the prevention of metachronous gastric cancer. N. Engl. J. Med 378, 1085–1095 (2018). [DOI] [PubMed] [Google Scholar]
- 220.Malfertheiner P Helicobacter pylori treatment for gastric cancer prevention. N. Engl. J. Med 378, 1154–1156 (2018). [DOI] [PubMed] [Google Scholar]
- 221.Lauren P The two histological main types of gastric carcinoma: diffuse and so-called intestinal-type carcinoma. An attempt at a histo-clinical classification. Acta Pathol. Microbiol. Scand 64, 31–49 (1965). [DOI] [PubMed] [Google Scholar]
- 222.Ma J, Shen H, Kapesa L & Zeng S Lauren classification and individualized chemotherapy in gastric cancer. Oncol. Lett 11, 2959–2964 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Pan KF et al. A large randomised controlled intervention trial to prevent gastric cancer by eradication of Helicobacter pylori in Linqu County, China: baseline results and factors affecting the eradication. Gut 65, 9–18 (2016). [DOI] [PubMed] [Google Scholar]
- 224.Herrero R, Park JY & Forman D The fight against gastric cancer — the IARC Working Group report. Best Pract. Res. Clin. Gastroenterol 28, 1107–1114 (2014). [DOI] [PubMed] [Google Scholar]
- 225.Leja M et al. Multicentric randomised study of Helicobacter pylori eradication and pepsinogen testing for prevention of gastric cancer mortality: the GISTAR study. BMJ Open 7, e016999 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Ford AC, Tsipotis E, Yuan Y, Leontiadis GI & Moayyedi P Efficacy of Helicobacter pylori eradication therapy for functional dyspepsia: updated systematic review and meta-analysis. Gut 10.1136/gutjnl-2021-326583 (2022). [DOI] [PubMed] [Google Scholar]
- 227.Kim BJ, Kim HS, Jang HJ & Kim JH Helicobacter pylori eradication in idiopathic thrombocytopenic purpura: a meta-analysis of randomized trials. Gastroenterol. Res. Pract 2018, 6090878 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Hudak L, Jaraisy A, Haj S & Muhsen K An updated systematic review and meta-analysis on the association between Helicobacter pylori infection and iron deficiency anemia. Helicobacter 22, 12330 (2017). [DOI] [PubMed] [Google Scholar]
- 229.Malfertheiner P, Selgrad M & Bornschein J Helicobacter pylori: clinical management. Curr. Opin. Gastroenterol 28, 608–614 (2012). [DOI] [PubMed] [Google Scholar]
- 230.Ferreri AJ, Govi S & Ponzoni M The role of Helicobacter pylori eradication in the treatment of diffuse large B-cell and marginal zone lymphomas of the stomach. Curr. Opin. Oncol 25, 470–479 (2013). [DOI] [PubMed] [Google Scholar]
- 231.Salar A Gastric MALT lymphoma and Helicobacter pylori. Med. Clin 152, 65–71 (2019). [DOI] [PubMed] [Google Scholar]
- 232.Wundisch T et al. Long-term follow-up of gastric MALT lymphoma after Helicobacter pylori eradication. J. Clin. Oncol 23, 8018–8024 (2005). [DOI] [PubMed] [Google Scholar]
- 233.Miki K Gastric cancer screening using the serum pepsinogen test method. Gastric Cancer 9, 245–253 (2006). [DOI] [PubMed] [Google Scholar]
- 234.Sui Z et al. Risk for gastric cancer in patients with gastric atrophy: a systematic review and meta-analysis. Transl Cancer Res 9, 1618–1624 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Yoshida T et al. Cancer development based on chronic active gastritis and resulting gastric atrophy as assessed by serum levels of pepsinogen and Helicobacter pylori antibody titer. Int. J. Cancer 134, 1445–1457 (2014). [DOI] [PubMed] [Google Scholar]
- 236.Miki K Gastric cancer screening by combined assay for serum anti-Helicobacter pylori IgG antibody and serum pepsinogen levels — “ABC method”. Proc. Jpn Acad. Ser. B Phys. Biol. Sci 87, 405–414 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Miki K, Fujishiro M, Kodashima S & Yahagi N Long-term results of gastric cancer screening using the serum pepsinogen test method among an asymptomatic middle-aged Japanese population. Dig. Endosc 21, 78–81 (2009). [DOI] [PubMed] [Google Scholar]
- 238.Sung H et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin 71, 209–249 (2021). [DOI] [PubMed] [Google Scholar]
- 239.Mabe K et al. Endoscopic screening for gastric cancer in Japan: current status and future perspectives. Dig. Endosc 34, 412–419 (2022). [DOI] [PubMed] [Google Scholar]
- 240.Ryu JE et al. Trends in the performance of the Korean National Cancer Screening Program for Gastric Cancer from 2007 to 2016. Cancer Res. Treat 54, 842–849 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Pimentel-Nunes P et al. Management of epithelial precancerous conditions and lesions in the stomach (MAPS II): European Society of Gastrointestinal Endoscopy (ESGE), European Helicobacter and Microbiota Study Group (EHMSG), European Society of Pathology (ESP), and Sociedade Portuguesa de Endoscopia Digestiva (SPED) guideline update 2019. Endoscopy 51, 365–388 (2019). [DOI] [PubMed] [Google Scholar]
- 242.Gupta S et al. AGA clinical practice guidelines on management of gastric intestinal metaplasia. Gastroenterology 158, 693–702 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Lee YC et al. Association between Helicobacter pylori eradication and gastric cancer incidence: a systematic review and meta-analysis. Gastroenterology 150, 1113–1124.e5 (2016). [DOI] [PubMed] [Google Scholar]
- 244.Fallone CA, Moss SF & Malfertheiner P Reconciliation of recent Helicobacter pylori treatment guidelines in a time of increasing resistance to antibiotics. Gastroenterology 157, 44–53 (2019). [DOI] [PubMed] [Google Scholar]
- 245.El-Serag HB et al. Houston consensus conference on testing for Helicobacter pylori infection in the United States. Clin. Gastroenterol. Hepatol 16, 992–1002.e6 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Shah SC, Iyer PG & Moss SF AGA clinical practice update on the management of refractory Helicobacter pylori infection: expert review. Gastroenterology 160, 1831–1841 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Fallone CA et al. The Toronto consensus for the treatment of Helicobacter pylori infection in adults. Gastroenterology 151, 51–69.e14 (2016). [DOI] [PubMed] [Google Scholar]
- 248.Lind T et al. The MACH2 study: role of omeprazole in eradication of Helicobacter pylori with 1-week triple therapies. Gastroenterology 116, 248–253 (1999). [DOI] [PubMed] [Google Scholar]
- 249.Bazzoli F et al. Evaluation of short-term low-dose triple therapy for the eradication of Helicobacter pylori by factorial design in a randomized, double-blind, controlled study. Aliment. Pharmacol. Ther 12, 439–445 (1998). [DOI] [PubMed] [Google Scholar]
- 250.Mahachai V et al. Helicobacter pylori management in ASEAN: the Bangkok consensus report. J. Gastroenterol. Hepatol 33, 37–56 (2018). [DOI] [PubMed] [Google Scholar]
- 251.Boyanova L, Hadzhiyski P, Gergova R & Markovska R Evolution of Helicobacter pylori resistance to antibiotics: a topic of increasing concern. Antibiotics 12, 332 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Furuta T et al. Dual therapy with vonoprazan and amoxicillin is as effective as triple therapy with vonoprazan, amoxicillin and clarithromycin for eradication of Helicobacter pylori. Digestion 101, 743–751 (2020). [DOI] [PubMed] [Google Scholar]
- 253.Lima JJ et al. Clinical pharmacogenetics implementation consortium (CPIC) guideline for CYP2C19 and proton pump inhibitor dosing. Clin. Pharmacol. Ther 109, 1417–1423 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Erah PO, Goddard AF, Barrett DA, Shaw PN & Spiller RC The stability of amoxycillin, clarithromycin and metronidazole in gastric juice: relevance to the treatment of Helicobacter pylori infection. J. Antimicrob. Chemother 39, 5–12 (1997). [DOI] [PubMed] [Google Scholar]
- 255.Furuta T & Graham DY Pharmacologic aspects of eradication therapy for Helicobacter pylori infection. Gastroenterol. Clin. North Am 39, 465–480 (2010). [DOI] [PubMed] [Google Scholar]
- 256.McNicholl AG, Linares PM, Nyssen OP, Calvet X & Gisbert JP Meta-analysis: esomeprazole or rabeprazole vs. first-generation pump inhibitors in the treatment of Helicobacter pylori infection. Aliment. Pharmacol. Ther 36, 414–425 (2012). [DOI] [PubMed] [Google Scholar]
- 257.Gisbert JP Potent gastric acid inhibition in Helicobacter pylori eradication. Drugs 65, 83–96 (2005). [DOI] [PubMed] [Google Scholar]
- 258.Villoria A, Garcia P, Calvet X, Gisbert JP & Vergara M Meta-analysis: high-dose proton pump inhibitors vs. standard dose in triple therapy for Helicobacter pylori eradication. Aliment. Pharmacol. Ther 28, 868–877 (2008). [DOI] [PubMed] [Google Scholar]
- 259.Miehlke S et al. An increasing dose of omeprazole combined with amoxycillin cures Helicobacter pylori infection more effectively. Aliment. Pharmacol. Ther 11, 323–329 (1997). [DOI] [PubMed] [Google Scholar]
- 260.Gao W, Zhang X, Yin Y, Yu S & Wang L Different dose of new generation proton pump inhibitors for the treatment of Helicobacter pylori infection: a meta-analysis. Int. J. Immunopathol. Pharmacol 35, 20587384211030397 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Nyssen OP et al. European Registry on Helicobacter pylori management (Hp-EuReg): patterns and trends in first-line empirical eradication prescription and outcomes of 5 years and 21 533 patients. Gut 70, 40–54 (2021). [DOI] [PubMed] [Google Scholar]
- 262.Scordo MG, Caputi AP, D’Arrigo C, Fava G & Spina E Allele and genotype frequencies of CYP2C9, CYP2C19 and CYP2D6 in an Italian population. Pharmacol. Res 50, 195–200 (2004). [DOI] [PubMed] [Google Scholar]
- 263.El Rouby N, Lima JJ & Johnson JA Proton pump inhibitors: from CYP2C19 pharmacogenetics to precision medicine. Expert Opin. Drug Metab. Toxicol 14, 447–460 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Strom CM et al. Testing for variants in CYP2C19: population frequencies and testing experience in a clinical laboratory. Genet. Med 14, 95–100 (2012). [DOI] [PubMed] [Google Scholar]
- 265.Sugimoto K, Uno T, Yamazaki H & Tateishi T Limited frequency of the CYP2C19*17 allele and its minor role in a Japanese population. Br. J. Clin. Pharmacol 65, 437–439 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Yusuf I et al. Ethnic and geographical distributions of CYP2C19 alleles in the populations of Southeast Asia. Adv. Exp. Med. Biol 531, 37–46 (2003). [DOI] [PubMed] [Google Scholar]
- 267.Xie HG Genetic variations of S-mephenytoin 4’-hydroxylase (CYP2C19) in the Chinese population. Life Sci 66, PL175–PL181 (2000). [DOI] [PubMed] [Google Scholar]
- 268.Morino Y et al. Influence of cytochrome P450 2C19 genotype on Helicobacter pylori proton pump inhibitor-amoxicillin-clarithromycin eradication therapy: a meta-analysis. Front. Pharmacol 12, 759249 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Sugimoto M & Furuta T Efficacy of tailored Helicobacter pylori eradication therapy based on antibiotic susceptibility and CYP2C19 genotype. World J. Gastroenterol 20, 6400–6411 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Zhang HJ et al. Effects of genetic polymorphisms on the pharmacokinetics and pharmacodynamics of proton pump inhibitors. Pharmacol. Res 152, 104606 (2020). [DOI] [PubMed] [Google Scholar]
- 271.Sugimoto M et al. Rabeprazole 10 mg q.d.s. decreases 24-h intragastric acidity significantly more than rabeprazole 20 mg b.d. or 40 mg o.m., overcoming CYP2C19 genotype. Aliment. Pharmacol. Ther 36, 627–634 (2012). [DOI] [PubMed] [Google Scholar]
- 272.Saitoh T et al. Effects of rabeprazole, lansoprazole and omeprazole on intragastric pH in CYP2C19 extensive metabolizers. Aliment. Pharmacol. Ther 16, 1811–1817 (2002). [DOI] [PubMed] [Google Scholar]
- 273.Sahara S et al. Twice-daily dosing of esomeprazole effectively inhibits acid secretion in CYP2C19 rapid metabolisers compared with twice-daily omeprazole, rabeprazole or lansoprazole. Aliment. Pharmacol. Ther 38, 1129–1137 (2013). [DOI] [PubMed] [Google Scholar]
- 274.Graham DY et al. Factors influencing the eradication of Helicobacter pylori with triple therapy. Gastroenterology 102, 493–496 (1992). [DOI] [PubMed] [Google Scholar]
- 275.Zhou BG et al. Effect of enhanced patient instructions on Helicobacter pylori eradication: A systematic review and meta-analysis of randomized controlled trials. Helicobacter 27, e12869 (2022). [DOI] [PubMed] [Google Scholar]
- 276.Graham DY & Fischbach L Helicobacter pylori treatment in the era of increasing antibiotic resistance. Gut 59, 1143–1153 (2010). [DOI] [PubMed] [Google Scholar]
- 277.Meyer JM et al. Risk factors for Helicobacter pylori resistance in the United States: the surveillance of H. pylori antimicrobial resistance partnership (SHARP) study, 1993–1999. Ann. Intern. Med 136, 13–24 (2002). [DOI] [PubMed] [Google Scholar]
- 278.Bujanda L et al. Antibiotic resistance prevalence and trends in patients infected with Helicobacter pylori in the period 2013–2020: results of the European Registry on H. pylori management (Hp-EuReg). Antibiotics 10, 1058 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Camargo MC et al. The problem of Helicobacter pylori resistance to antibiotics: a systematic review in Latin America. Am. J. Gastroenterol 109, 485–495 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Tacconelli E et al. Discovery, research, and development of new antibiotics: the WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect. Dis 18, 318–327 (2018). [DOI] [PubMed] [Google Scholar]
- 281.Molina-Infante J et al. Optimised empiric triple and concomitant therapy for Helicobacter pylori eradication in clinical practice: the OPTRICON study. Aliment. Pharmacol. Ther 41, 581–589 (2015). [DOI] [PubMed] [Google Scholar]
- 282.Liou JM et al. Concomitant, bismuth quadruple, and 14-day triple therapy in the first-line treatment of Helicobacter pylori: a multicentre, open-label, randomised trial. Lancet 388, 2355–2365 (2016). [DOI] [PubMed] [Google Scholar]
- 283.Crowe SE Helicobacter pylori infection. N. Engl. J. Med 380, 1158–1165 (2019). [DOI] [PubMed] [Google Scholar]
- 284.Romano M et al. Empirical levofloxacin-containing versus clarithromycin-containing sequential therapy for Helicobacter pylori eradication: a randomised trial. Gut 59, 1465–1470 (2010). [DOI] [PubMed] [Google Scholar]
- 285.Federico A et al. Efficacy of 5-day levofloxacin-containing concomitant therapy in eradication of Helicobacter pylori infection. Gastroenterology 143, 55–61.e1 (2012). [DOI] [PubMed] [Google Scholar]
- 286.Gisbert JP Optimization strategies aimed to increase the efficacy of Helicobacter pylori eradication therapies with quinolones. Molecules 25, 5084 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Kuo YT et al. Primary antibiotic resistance in Helicobacter pylori in the Asia-Pacific region: a systematic review and meta-analysis. Lancet Gastroenterol. Hepatol 2, 707–715 (2017). [DOI] [PubMed] [Google Scholar]
- 288.An Y et al. Fourth-generation quinolones in the treatment of Helicobacter pylori infection: a meta-analysis. World J. Gastroenterol 24, 3302–3312 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Sugimoto M et al. High Helicobacter pylori cure rate with sitafloxacin-based triple therapy. Aliment. Pharmacol. Ther 42, 477–483 (2015). [DOI] [PubMed] [Google Scholar]
- 290.Megraud F Antibiotic resistance is the key element in treatment of Helicobacter pylori infection. Gastroenterology 155, 1300–1302 (2018). [DOI] [PubMed] [Google Scholar]
- 291.Malfertheiner P et al. Helicobacter pylori eradication with a capsule containing bismuth subcitrate potassium, metronidazole, and tetracycline given with omeprazole versus clarithromycin-based triple therapy: a randomised, open-label, non-inferiority, phase 3 trial. Lancet 377, 905–913 (2011). [DOI] [PubMed] [Google Scholar]
- 292.Gisbert JP Rifabutin for the treatment of Helicobacter pylori infection: a review. Pathogens 10, 15 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Graham DY et al. Rifabutin-based triple therapy (RHB-105) for Helicobacter pylori eradication: a double-blind, randomized, controlled trial. Ann. Intern. Med 172, 795–802 (2020). [DOI] [PubMed] [Google Scholar]
- 294.FDA. TALICIA (Omeprazole Magnesium, Amoxicillin and Rifabutin) Delayed-release Capsules [Package Insert] Raleigh, NC: RedHill Biopharma Inc. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/213004lbl.pdf (2019). [Google Scholar]
- 295.Ji CR et al. Safety of furazolidone-containing regimen in Helicobacter pylori infection: a systematic review and meta-analysis. BMJ Open 10, e037375 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Treiber G, Ammon S, Malfertheiner P & Klotz U Impact of furazolidone-based quadruple therapy for eradication of Helicobacter pylori after previous treatment failures. Helicobacter 7, 225–231 (2002). [DOI] [PubMed] [Google Scholar]
- 297.Tshibangu-Kabamba E & Yamaoka Y Helicobacter pylori infection and antibiotic resistance - from biology to clinical implications. Nat. Rev. Gastroenterol. Hepatol 18, 613–629 (2021). [DOI] [PubMed] [Google Scholar]
- 298.Wang YH et al. Characteristics of Helicobacter pylori heteroresistance in gastric biopsies and its clinical Relevance. Front. Cell Infect. Microbiol 11, 819506 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Selgrad M et al. Different antibiotic susceptibility between antrum and corpus of the stomach, a possible reason for treatment failure of Helicobacter pylori infection. World J. Gastroenterol 20, 16245–16251 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Malfertheiner P Infection: bismuth improves PPI-based triple therapy for H. pylori eradication. Nat. Rev. Gastroenterol. Hepatol 7, 538–539 (2010). [DOI] [PubMed] [Google Scholar]
- 301.Dore MP, Lu H & Graham DY Role of bismuth in improving Helicobacter pylori eradication with triple therapy. Gut 65, 870–878 (2016). [DOI] [PubMed] [Google Scholar]
- 302.Marcus EA, Sachs G & Scott DR Colloidal bismuth subcitrate impedes proton entry into Helicobacter pylori and increases the efficacy of growth-dependent antibiotics. Aliment. Pharmacol. Ther 42, 922–933 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Nyssen OP et al. European registry on Helicobacter pylori management: single-capsule bismuth quadruple therapy is effective in real-world clinical practice. United European Gastroenterol. J 9, 38–46 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Zagari RM et al. The “three-in-one” formulation of bismuth quadruple therapy for Helicobacter pylori eradication with or without probiotics supplementation: efficacy and safety in daily clinical practice. Helicobacter 23, e12502 (2018). [DOI] [PubMed] [Google Scholar]
- 305.Zhang W et al. Bismuth, lansoprazole, amoxicillin and metronidazole or clarithromycin as first-line Helicobacter pylori therapy. Gut 64, 1715–1720 (2015). [DOI] [PubMed] [Google Scholar]
- 306.Bang CS et al. Amoxicillin or tetracycline in bismuth-containing quadruple therapy as first-line treatment for Helicobacter pylori infection. Gut Microbes 11, 1314–1323 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Delchier JC, Malfertheiner P & Thieroff-Ekerdt R Use of a combination formulation of bismuth, metronidazole and tetracycline with omeprazole as a rescue therapy for eradication of Helicobacter pylori. Aliment. Pharmacol. Ther 40, 171–177 (2014). [DOI] [PubMed] [Google Scholar]
- 308.Chen Q et al. Rescue therapy for Helicobacter pylori eradication: a randomized non-inferiority trial of amoxicillin or tetracycline in bismuth quadruple therapy. Am. J. Gastroenterol 111, 1736–1742 (2016). [DOI] [PubMed] [Google Scholar]
- 309.Hunt RH & Scarpignato C Potent acid suppression with PPIs and P-CABs: what’s new? Curr. Treat. Options Gastroenterol 16, 570–590 (2018). [DOI] [PubMed] [Google Scholar]
- 310.Abdel-Aziz Y, Metz DC & Howden CW Review article: potassium-competitive acid blockers for the treatment of acid-related disorders. Aliment. Pharmacol. Ther 53, 794–809 (2021). [DOI] [PubMed] [Google Scholar]
- 311.Murakami K et al. Vonoprazan, a novel potassium-competitive acid blocker, as a component of first-line and second-line triple therapy for Helicobacter pylori eradication: a phase III, randomised, double-blind study. Gut 65, 1439–1446 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Chey WD et al. S1382 Vonoprazan dual and triple therapy for Helicobacter pylori eradication. J. Am. Coll. Gastroenterol 116, S634 (2021). [Google Scholar]
- 313.Rokkas T et al. Comparative effectiveness of multiple different first-line treatment regimens for Helicobacter pylori infection: a network meta-analysis. Gastroenterology 161, 495–507.e4 (2021). [DOI] [PubMed] [Google Scholar]
- 314.Malfertheiner P et al. Potassium-competitive acid blocker and proton pump inhibitor–based regimens for first-line Helicobacter pylori eradication: a network meta-analysis. Gastro. Hep. Adv 1, 824–834 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Liou JM et al. Efficacies of genotypic resistance-guided vs empirical therapy for refractory Helicobacter pylori infection. Gastroenterology 155, 1109–1119 (2018). [DOI] [PubMed] [Google Scholar]
- 316.Yu L et al. Susceptibility-guided therapy for Helicobacter pylori infection treatment failures. Ther. Adv. Gastroenterol 12, 1756284819874922 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Chen PY et al. Systematic review with meta-analysis: the efficacy of levofloxacin triple therapy as the first- or second-line treatments of Helicobacter pylori infection. Aliment. Pharmacol. Ther 44, 427–437 (2016). [DOI] [PubMed] [Google Scholar]
- 318.Gao CP et al. PPI-amoxicillin dual therapy for Helicobacter pylori infection: an update based on a systematic review and meta-analysis. Helicobacter 25, e12692 (2020). [DOI] [PubMed] [Google Scholar]
- 319.Gao W et al. Eradication rate and safety of a “simplified rescue therapy”: 14-day vonoprazan and amoxicillin dual regimen as rescue therapy on treatment of Helicobacter pylori infection previously failed in eradication: a real-world, retrospective clinical study in China. Helicobacter 27, e12918 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Shiotani A, Roy P, Lu H & Graham DY Helicobacter pylori diagnosis and therapy in the era of antimicrobial stewardship. Ther. Adv. Gastroenterol 14, 17562848211064080 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Graham DY & Liou JM Primer for development of guidelines for Helicobacter pylori therapy using antimicrobial stewardship. Clin. Gastroenterol. Hepatol 20, 973–983.e1 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.McFarland LV, Huang Y, Wang L & Malfertheiner P Systematic review and meta-analysis: Multi-strain probiotics as adjunct therapy for Helicobacter pylori eradication and prevention of adverse events. United European Gastroenterol. J 4, 546–561 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Fernandez-Salazar L et al. Effectiveness and safety of high-dose dual therapy: results of the European Registry on the management of Helicobacter pylori infection (Hp-EuReg). J. Clin. Med 11, 3544 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Liou JM et al. Second-line levofloxacin-based quadruple therapy versus bismuth-based quadruple therapy for Helicobacter pylori eradication and long-term changes to the gut microbiota and antibiotic resistome: a multicentre, open-label, randomised controlled trial. Lancet Gastroenterol. Hepatol 8, 228–241 (2023). [DOI] [PubMed] [Google Scholar]
- 325.Guillemard E et al. A randomised, controlled trial: effect of a multi-strain fermented milk on the gut microbiota recovery after Helicobacter pylori therapy. Nutrients 13, 3171 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Ford AC et al. Adverse events with bismuth salts for Helicobacter pylori eradication: systematic review and meta-analysis. World J. Gastroenterol 14, 7361–7370 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Kumar S, Metz DC, Kaplan DE & Goldberg DS Treatment of Helicobacter pylori is not associated with future clostridium difficile infection. Am. J. Gastroenterol 115, 716–722 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Lu M et al. Efficacy of probiotic supplementation therapy for Helicobacter pylori eradication: a meta-analysis of randomized controlled trials. PLoS ONE 11, e0163743 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Szajewska H, Horvath A & Kolodziej M Systematic review with meta-analysis: Saccharomyces boulardii supplementation and eradication of Helicobacter pylori infection. Aliment. Pharmacol. Ther 41, 1237–1245 (2015). [DOI] [PubMed] [Google Scholar]
- 330.Viazis N et al. A four-probiotics regimen combined with a standard Helicobacter pylorieradication treatment reduces side effects and increases eradication rates. Nutrients 14, 632 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Zhao Y et al. Saccharomyces boulardii combined with quadruple therapy for Helicobacter pylori eradication decreased the duration and severity of diarrhea: a multi-center prospective randomized controlled trial. Front. Med 8, 776955 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Malfertheiner P et al. Helicobacter pylori eradication and gastric ulcer healing — comparison of three pantoprazole-based triple therapies. Aliment. Pharmacol. Ther 17, 1125–1135 (2003). [DOI] [PubMed] [Google Scholar]
- 333.Gralnek IM et al. Endoscopic diagnosis and management of nonvariceal upper gastrointestinal hemorrhage (NVUGIH): European Society of Gastrointestinal Endoscopy (ESGE) Guideline — Update 2021. Endoscopy 53, 300–332 (2021). [DOI] [PubMed] [Google Scholar]
- 334.Barkun AN et al. Management of nonvariceal upper gastrointestinal bleeding: guideline recommendations from the International Consensus Group. Ann. Intern. Med 171, 805–822 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Sostres C et al. Peptic ulcer bleeding risk. The role of Helicobacter pylori infection in NSAID/low-dose aspirin users. Am. J. Gastroenterol 110, 684–689 (2015). [DOI] [PubMed] [Google Scholar]
- 336.Malfertheiner P et al. Current concepts in the management of Helicobacter pylori infection: the Maastricht III Consensus Report. Gut 56, 772–781 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Ruskone-Fourmestraux A et al. EGILS consensus report. Gastric extranodal marginal zone B-cell lymphoma of MALT. Gut 60, 747–758 (2011). [DOI] [PubMed] [Google Scholar]
- 338.Raderer M, Kiesewetter B & Ferreri AJ Clinicopathologic characteristics and treatment of marginal zone lymphoma of mucosa-associated lymphoid tissue (MALT lymphoma). CA Cancer J. Clin 66, 153–171 (2016). [DOI] [PubMed] [Google Scholar]
- 339.Jung K, Kim DH, Seo HI, Gong EJ & Bang CS Efficacy of eradication therapy in Helicobacter pylori-negative gastric mucosa-associated lymphoid tissue lymphoma: a meta-analysis. Helicobacter 26, e12774 (2021). [DOI] [PubMed] [Google Scholar]
- 340.Laine L & Dhir V Helicobacter pylori eradication does not worsen quality of life related to reflux symptoms: a prospective trial. Aliment. Pharmacol. Ther 16, 1143–1148 (2002). [DOI] [PubMed] [Google Scholar]
- 341.Hirata K et al. Improvement of reflux symptom related quality of life after Helicobacter pylori eradication therapy. J. Clin. Biochem. Nutr 52, 172–178 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Taguchi H et al. Helicobacter pylori eradication improves the quality of life regardless of the treatment outcome: a multicenter prospective cohort study. Medicine 96, e9507 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Piriyapong K, Tangaroonsanti A, Mahachai V & Vilaichone RK Helicobacter pylori infection impacts on functional dyspepsia in Thailand. Asian Pac. J. Cancer Prev 15, 10887–10891 (2014). [DOI] [PubMed] [Google Scholar]
- 344.Moayyedi P et al. Effect of population screening and treatment for Helicobacter pylori on dyspepsia and quality of life in the community: a randomised controlled trial. Leeds HELP Study Group. Lancet 355, 1665–1669 (2000). [DOI] [PubMed] [Google Scholar]
- 345.Bektas M, Soykan I, Altan M, Alkan M & Ozden A The effect of Helicobacter pylori eradication on dyspeptic symptoms, acid reflux and quality of life in patients with functional dyspepsia. Eur. J. Intern. Med 20, 419–423 (2009). [DOI] [PubMed] [Google Scholar]
- 346.Buzas GM Quality of life in patients with functional dyspepsia: short- and long-term effect of Helicobacter pylori eradication with pantoprazole, amoxicillin, and clarithromycin or cisapride therapy: a prospective, parallel-group study. Curr. Ther. Res. Clin. Exp 67, 305–320 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Mestrovic A, Bozic J, Vukojevic K & Tonkic A Impact of different Helicobacter pylori eradication therapies on gastrointestinal symptoms. Medicina 57, 803 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Bitwayiki R et al. Dyspepsia prevalence and impact on quality of life among Rwandan healthcare workers: a cross-sectional survey. S. Afr. Med. J 105, 1064–1069 (2015). [DOI] [PubMed] [Google Scholar]
- 349.Kabakambira JD et al. Efficacy of Helicobacter pylori eradication regimens in Rwanda: a randomized controlled trial. BMC Gastroenterol 18, 134 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Labenz J et al. Curing Helicobacter pylori infection in patients with duodenal ulcer may provoke reflux esophagitis. Gastroenterology 112, 1442–1447 (1997). [DOI] [PubMed] [Google Scholar]
- 351.Zamani M et al. Systematic review with meta-analysis: the worldwide prevalence of Helicobacter pylori infection. Aliment. Pharmacol. Ther 47, 868–876 (2018). [DOI] [PubMed] [Google Scholar]
- 352.Ding SZ et al. Chinese consensus report on family-based Helicobacter pylori infection control and management (2021 edition). Gut 71, 238–253 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Chiang TH et al. Bismuth salts with versus without acid suppression for Helicobacter pylori infection: a transmission electron microscope study. Helicobacter 26, e12801 (2021). [DOI] [PubMed] [Google Scholar]
- 354.Kafarski P & Talma M Recent advances in design of new urease inhibitors: a review. J. Adv. Res 13, 101–112 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Strugatsky D et al. Structure of the proton-gated urea channel from the gastric pathogen Helicobacter pylori. Nature 493, 255–258 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Chu JK et al. Loss of a cardiolipin synthase in Helicobacter pylori G27 blocks flagellum assembly. J. Bacteriol 201, e00372–19 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Doohan D, Rezkitha YAA, Waskito LA, Yamaoka Y & Miftahussurur M Helicobacter pylori BabA-SabA key roles in the adherence phase: the synergic mechanism for successful colonization and disease development. Toxins 13, 485 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Zhang Y et al. Inhibition of pathogen adhesion by bacterial outer membrane-coated nanoparticles. Angew. Chem. Int. Ed. Engl 58, 11404–11408 (2019). [DOI] [PubMed] [Google Scholar]
- 359.Ensign LM, Cone R & Hanes J Oral drug delivery with polymeric nanoparticles: the gastrointestinal mucus barriers. Adv. Drug Deliv. Rev 64, 557–570 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Yang C et al. Effects of non-viable Lactobacillus reuteri combining with 14-day standard triple therapy on Helicobacter pylori eradication: a randomized double-blind placebo-controlled trial. Helicobacter 26, e12856 (2021). [DOI] [PubMed] [Google Scholar]
- 361.Liang B et al. Current and future perspectives for Helicobacter pylori treatment and management: from antibiotics to probiotics. Front. Cell Infect. Microbiol 12, 1042070 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Yang YJ & Sheu BS Metabolic interaction of Helicobacter pylori infection and gut microbiota. Microorganisms 4, 15 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Yang I, Nell S & Suerbaum S Survival in hostile territory: the microbiota of the stomach. FEMS Microbiol. Rev 37, 736–761 (2013). [DOI] [PubMed] [Google Scholar]
- 364.Chen CC et al. The interplay between Helicobacter pylori and gastrointestinal microbiota. Gut Microbes 13, 1–22 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Schulz C et al. The active bacterial assemblages of the upper GI tract in individuals with and without Helicobacter infection. Gut 67, 216–225 (2018). [DOI] [PubMed] [Google Scholar]
- 366.Sun QH et al. Microbiome changes in the gastric mucosa and gastric juice in different histological stages of Helicobacter pylori-negative gastric cancers. World J. Gastroenterol 28, 365–380 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Sung JJY et al. Gastric microbes associated with gastric inflammation, atrophy and intestinal metaplasia 1 year after Helicobacter pylori eradication. Gut 69, 1572–1580 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Guo Y, Cao XS, Zhou MG & Yu B Gastric microbiota in gastric cancer: different roles of Helicobacter pylori and other microbes. Front. Cell Infect. Microbiol 12, 1105811 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.Coker OO et al. Mucosal microbiome dysbiosis in gastric carcinogenesis. Gut 67, 1024–1032 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370.Vaillant L, Oster P, McMillan B, Orozco Fernandez E & Velin D GM-CSF is key in the efficacy of vaccine-induced reduction of Helicobacter pylori infection. Helicobacter 27, e12875 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.Oster P et al. Helicobacter pylori infection has a detrimental impact on the efficacy of cancer immunotherapies. Gut 71, 457–466 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Oster P, Vaillant L, McMillan B & Velin D The efficacy of cancer immunotherapies is compromised by Helicobacter pylori infection. Front. Immunol 13, 899161 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Abadi AT & Kusters JG Management of Helicobacter pylori infections. BMC Gastroenterol 16, 94 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Celiberto F et al. The state of the art of molecular fecal investigations for Helicobacter pylori (H. pylori) antibiotic resistances. Int. J. Mol. Sci 24, 4361 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Ranjbar R, Ebrahimi A & Sahebkar A Helicobacter pylori infection: conventional and molecular strategies for bacterial diagnosis and antibiotic resistance testing. Curr. Pharm. Biotechnol 10.2174/1389201023666220920094342 (2022). [DOI] [PubMed] [Google Scholar]
- 376.Best LM et al. Non-invasive diagnostic tests for Helicobacter pylori infection. Cochrane Database Syst. Rev 3, CD012080 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.No authors listed. Schistosomes, liver flukes and Helicobacter pylori. IARC Monogr. Eval. Carcinog. Risks Hum 61, 1–241 (1994). [PMC free article] [PubMed] [Google Scholar]
- 378.Wotherspoon AC et al. Regression of primary low-grade B-cell gastric lymphoma of mucosa-associated lymphoid tissue type after eradication of Helicobacter pylori. Lancet 342, 575–577 (1993). [DOI] [PubMed] [Google Scholar]
- 379.Rugge M et al. OLGA staging for gastritis: a tutorial. Dig. Liver Dis 40, 650–658 (2008). [DOI] [PubMed] [Google Scholar]
- 380.Chiang TH et al. Mass eradication of Helicobacter pylori to reduce gastric cancer incidence and mortality: a long-term cohort study on Matsu Islands. Gut 70, 243–250 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381.Du MQ & Isaccson PG Gastric MALT lymphoma: from aetiology to treatment. Lancet Oncol 3, 97–104 (2002). [DOI] [PubMed] [Google Scholar]
- 382.Glupczynski Y, Megraud F, Lopez-Brea M & Andersen LP European multicentre survey of in vitro antimicrobial resistance in Helicobacter pylori. Eur. J. Clin. Microbiol. Infect. Dis 20, 820–823 (2001). [DOI] [PubMed] [Google Scholar]
- 383.Li WQ et al. Effects of Helicobacter pylori treatment and vitamin and garlic supplementation on gastric cancer incidence and mortality: follow-up of a randomized intervention trial. BMJ 366, l5016 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384.Wang Z et al. Changes of the gastric mucosal microbiome associated with histological stages of gastric carcinogenesis. Front. Microbiol 11, 997 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Song P, Wu L & Guan W Dietary nitrates, nitrites, and nitrosamines intake and the risk of gastric cancer: a meta-analysis. Nutrients 7, 9872–9895 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386.Lucker S et al. A Nitrospira metagenome illuminates the physiology and evolution of globally important nitrite-oxidizing bacteria. Proc. Natl Acad. Sci. USA 107, 13479–13484 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387.Rudnicka K, Backert S & Chmiela M Genetic polymorphisms in inflammatory and other regulators in gastric cancer: risks and clinical consequences. Curr. Top. Microbiol. Immunol 421, 53–76 (2019). [DOI] [PubMed] [Google Scholar]
- 388.Ford AC, Marwaha A, Lim A & Moayyedi P What is the prevalence of clinically significant endoscopic findings in subjects with dyspepsia? Systematic review and meta-analysis. Clin. Gastroenterol. Hepatol 8, 830–837 (2010). [DOI] [PubMed] [Google Scholar]
- 389.Gisbert JP & Calvet X Helicobacter pylori “test-and-treat” strategy for management of dyspepsia: a comprehensive review. Clin. Transl Gastroenterol 4, e32 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 390.Beresniak A et al. Helicobacter pylori “test-and-treat” strategy with urea breath test: a cost-effective strategy for the management of dyspepsia and the prevention of ulcer and gastric cancer in Spain — results of the Hp-Breath initiative. Helicobacter 25, e12693 (2020). [DOI] [PubMed] [Google Scholar]
- 391.Pritchard DM et al. Cost-effectiveness modelling of use of urea breath test for the management of Helicobacter pylori-related dyspepsia and peptic ulcer in the UK. BMJ Open Gastroenterol 8, e000685 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392.Kawasaki K et al. Low-dose aspirin and non-steroidal anti-inflammatory drugs increase the risk of bleeding in patients with gastroduodenal ulcer. Dig. Dis. Sci 60, 1010–1015 (2015). [DOI] [PubMed] [Google Scholar]
- 393.Eusebi LH, Black CJ, Howden CW & Ford AC Effectiveness of management strategies for uninvestigated dyspepsia: systematic review and network meta-analysis. BMJ 367, l6483 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 394.Wu R et al. Prevalence of gastric cancer precursors in gastroscopy-screened adults by family history of gastric cancer and of cancers other than gastric. BMC Cancer 20, 1110 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 395.De RV et al. Pepsinogens to distinguish patients with gastric intestinal metaplasia and Helicobacter pylori infection among populations at risk for gastric cancer. Clin. Transl Gastroenterol 7, e183 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396.Zagari RM et al. Systematic review with meta-analysis: diagnostic performance of the combination of pepsinogen, gastrin-17 and anti-Helicobacter pylori antibodies serum assays for the diagnosis of atrophic gastritis. Aliment. Pharmacol. Ther 46, 657–667 (2017). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


