Abstract
Contemporary societies exhibit delayed reproductive age and increased life expectancy. While the male reproductive system demonstrates relatively delayed aging compared to that of females, increasing age substantially impacts its function. A characteristic manifestation is age-induced testosterone decline. Testosterone, a crucial male sex hormone, plays pivotal roles in spermatogenesis and sexual function, and contributes significantly to metabolism, psychology, and cardiovascular health. Aging exerts profound effects on the hypothalamic-pituitary–gonadal axis and Leydig cells, precipitating testosterone reduction, which adversely affects male health. Exogenous testosterone supplementation can partially ameliorate age-related testosterone deficiency; however, its long-term safety remains contentious. Preserving endogenous testosterone production capacity during the aging process warrants further investigation as a potential intervention strategy.
Keywords: Aging, Testosterone, Leydig cell, Sertoli cell, Mitochondrial dysfunction
Introduction
Aging exerts a profound impact on testicular function, and substantial clinical evidence indicates that aging is accompanied by a decline in serum testosterone levels [1–3].Studies have demonstrated that serum testosterone levels in men begin to decline gradually from age 35 [4]. Another research indicates that in men aged 40–70 years, total serum testosterone decreases at a rate of 0.4% annually, while free testosterone shows a more pronounced decline of 1.3% per year [2]. Beyond its crucial roles in male sexual function and reproduction, testosterone influences mood, cognition, metabolism, immune function, bone mineral density maintenance, and the cardiovascular system [5–8]. Low testosterone levels can severely affect the health of aging males, increasing the risk of diabetes [9, 10], dementia [11], cardiovascular disease [12], and mortality [13]. In addition, low testosterone levels can adversely affect male fertility [14]. Given the vital role testosterone plays in the body, McBride et al. proposed that its decline drives the onset of overall male senescence [15]. With population aging, the prevalence of age-related conditions such as late-onset hypogonadism will further increase. Given testosterone's importance for male reproductive health and overall well-being, this review investigates the primary mechanisms underlying age-induced testosterone decline, intervention strategies, and future research priorities.
Normal testosterone biosynthetic pathway
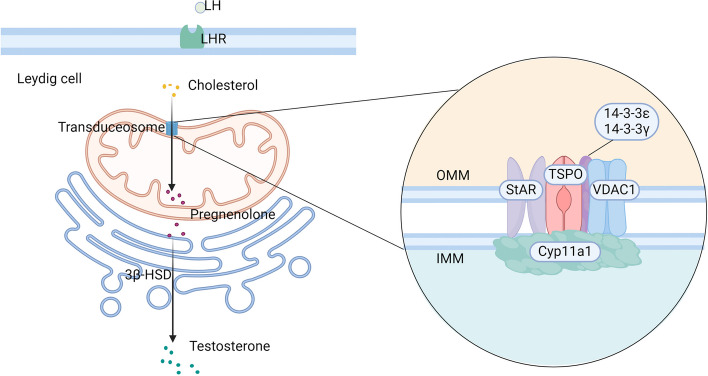
Leydig cells (LCs) are the primary source of testosterone synthesis in males. The hypothalamus secretes gonadotropin-releasing hormone (GnRH), which acts on the pituitary gland to stimulate the release of luteinizing hormone (LH). LH binds to LH receptors on LCs, triggering the production of cyclic adenosine monophosphate (cAMP) and initiating steroidogenesis (Fig. 1). The rate-limiting step in steroidogenesis is the transport of cholesterol to the inner mitochondrial membrane [16, 17]. This transport occurs through a multiprotein complex called the transduceosome, formed by protein–protein interactions between cytosolic and outer mitochondrial membrane proteins [18]. The transduceosome comprises the steroidogenic acute regulatory protein (StAR), translocator protein (TSPO), voltage-dependent anion channel 1 (VDAC1) [19–21], and potentially the 14–3-3γ and 14–3-3ε adaptor proteins, which act as negative regulators[22, 23] (Fig. 2). StAR acts on mitochondria, triggering cholesterol translocation across the membrane. TSPO, an outer mitochondrial membrane protein with high cholesterol affinity, plays a crucial role in steroidogenesis. The 14–3-3ε protein hinders effective TSPO-VDAC1 interaction, thereby impacting the rate of cholesterol entry into mitochondria [22, 23]. Once in the mitochondrial inner membrane, cholesterol is metabolized to pregnenolone by the enzyme cytochrome P450 side-chain cleavage enzyme (P450scc or Cyp11a1) and subsequently converted to testosterone by 3β-hydroxysteroid dehydrogenase enzymes (3β-HSD) in the mitochondria and smooth endoplasmic reticulum.
Fig. 1.
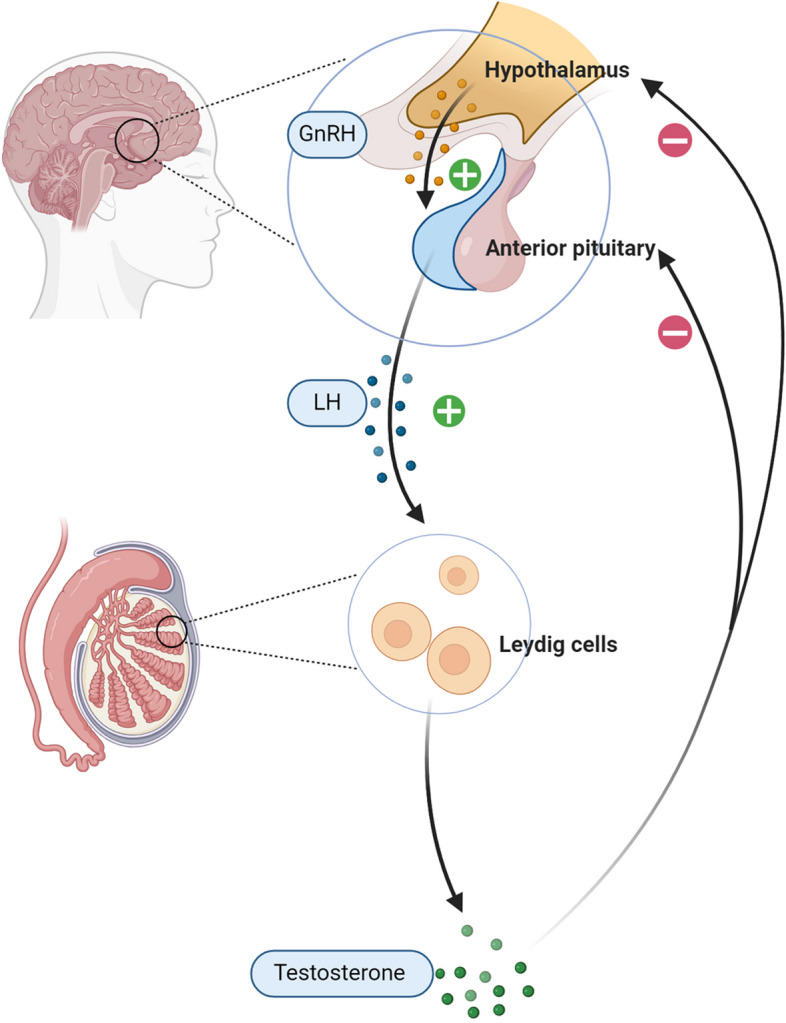
Hypothalamic-Pituitary–Testicular Axis. The hypothalamus secretes gonadotropin-releasing hormone (GnRH), which stimulates the pituitary gland to release luteinizing hormone (LH). LH binds to receptors on Leydig cells (LCs), leading to the production of cyclic adenosine monophosphate (cAMP) and the initiation of steroidogenesis. cAMP, cyclic adenosine monophosphate; GnRH, gonadotropin-releasing hormone; LCs, Leydig cells; LH, luteinizing hormone; Created in BioRender.com
Fig. 2.
A Brief Schematic Diagram of Testosterone Synthesis in Leydig Cells. LH binds to receptors on LCs, triggering steroidogenesis. Cholesterol is transported from the OMM to the IMM via the transduceosome, a complex that includes StAR, TSPO, and VDAC1. StAR facilitates cholesterol translocation across the mitochondrial membrane. TSPO, with a high affinity for cholesterol, plays a central role in this process. The 14–3-3γ and 14–3-3ε adaptor proteins, part of the transduceosome, act as negative regulators. Specifically, 14–3-3ε impairs the interaction between TSPO and VDAC1, slowing cholesterol entry into mitochondria. Once inside the IMM, cholesterol is converted by Cyp11a1 into pregnenolone, which is then metabolized into testosterone by 3β-HSD. LCs, Leydig cells; LH, luteinizing hormone; OMM, outer mitochondrial membrane; IMM, inner mitochondrial membrane; StAR, steroidogenic acute regulatory protein; TSPO, translocator protein; VDAC1, voltage-dependent anion channel 1; Created in BioRender.com
Impact of aging on LCs and testosterone synthesis
Aging can impair testosterone synthesis through its effects on the hypothalamic-pituitary–gonadal axis and direct actions on LCs [2, 24]. The impacts of aging on LCs can be broadly categorized into intrinsic and extrinsic factors. Intrinsic factors include mitochondrial dysfunction, impaired autophagy, and redox imbalance. Extrinsic factors comprise the senescence-associated secretory phenotype (SASP), which includes a myriad of inflammatory cytokines, chemokines, extracellular matrix remodeling, and growth factors released by senescent cells. The SASP can disrupt tissue homeostasis and remodel the tissue microenvironment, exerting deleterious systemic effects.
Changes in the hypothalamic-pituitary–testicular axis
In males over 35 years old, aging leads to alterations in the hypothalamic-pituitary–testicular axis, primarily manifesting as decreased GnRH secretion and reduced LC responsiveness to LH stimulation [25]. Early biomathematical models predicted a 33–50% decline in GnRH secretion in males from ages 20 to 80 years [26, 27]. A clinical study in 2020 partially corroborated these predictions. The study evaluated 40 healthy men aged 19–73 years using selective GnRH receptor antagonists, steroidogenesis inhibitors, LH secretion suppressants, and recombinant human LH to delineate the roles of the hypothalamus, pituitary gland, and testes in age-related testosterone decline. The study found that increasing age led to decreased GnRH outflow, while pituitary responsiveness to GnRH remained normal. Reduced GnRH outflow was the primary cause of decreased LH secretion in older individuals, accompanied by attenuated responsiveness of LC to LH [28]. Decreases in GnRH neuronal number and/or function underlie reduced GnRH outflow, but clinical studies elucidating age-related changes in human GnRH neurons are lacking due to their sparse distribution and low quantity[29]. However, animal studies have demonstrated a decline in hypothalamic GnRH neuronal numbers in aging male rats [30].
Changes in testicular microenvironment
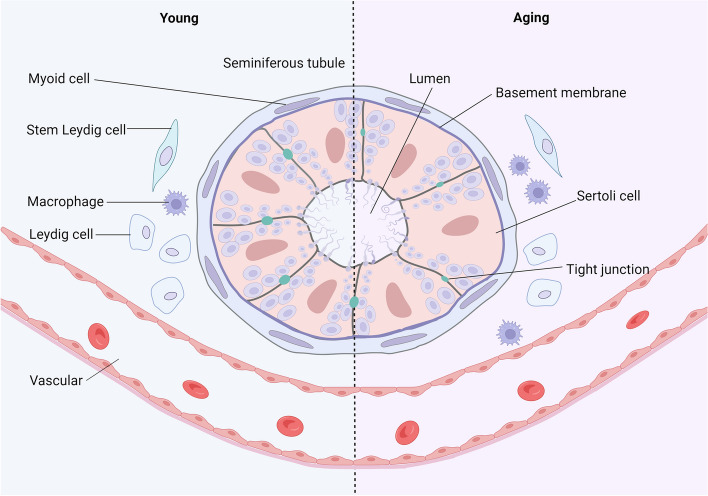
The testicular microenvironment is primarily composed of peritubular myoid cells, macrophages, LCs, Sertoli cells, vasculature, and their secreted cytokines [31] (Fig. 3). A stable testicular microenvironment is a prerequisite for the normal survival and function of LC. Dysregulation of this microenvironment during aging plays a crucial role in LC dysfunction [32]. Researchers obtained LCs from organ donors of different ages and stimulated them with human chorionic gonadotropin (hCG) in vitro. Surprisingly, the testosterone production capacity of the cultured LCs was unaffected by aging, suggesting that age-related changes in the microenvironment of LC may significantly impact testosterone biosynthesis [33].
Fig. 3.
Testicular Microenvironment and Age-related Changes. The testicular microenvironment consists of peritubular myoid cells, macrophages, Leydig cells, Sertoli cells, vasculature, and their secreted cytokines. Aging significantly impacts the testicular microenvironment. It is characterized by an increased population of macrophages, particularly those exhibiting a pro-inflammatory phenotype. This deterioration of the testicular microenvironment adversely affects other cell types. Sertoli cells show a decrease in both quantity and metabolic capabilities, alongside degeneration of tight junctions. Additionally, Leydig cells experience a decline in both number and function. Created in BioRender.com
Macrophages
Testicular macrophages constitute the largest immune cell population in the mammalian testis [34] and play critical roles during testicular development and aging [35]. Under homeostatic conditions, resident macrophages closely associate with LCs and modulate steroidogenesis. Additionally, macrophages mediate both acute and chronic inflammation [36]. Upon tissue injury, macrophages become activated, initiating chemotaxis, phagocytosis, and the production of reactive oxygen species (ROS) and pro-inflammatory factors, thereby suppressing testosterone production [37]. Chronic inflammation is a hallmark of aging [38], and inflammatory factors are elevated in aged testicular tissue [39]. A 2022 study employed single-cell RNA sequencing on testicular cells from organ donors, revealing the upregulation of inflammation-induced genes as a common feature of aged testicular cells [40]. Animal studies have also demonstrated increased macrophage number with a pro-inflammatory phenotype in aged mouse testes. Inflammation-associated cytokine genes, such as TNF-α, IL-1β, IL-6, and IL-8, are significantly upregulated in these macrophages with age, potentially disrupting the testicular microenvironment and impairing LC function [41]. Single-cell transcriptomic atlases of aged mouse testes have further corroborated an increase in pro-inflammatory macrophage subsets [42]. Moreover, macrophages modulate the proliferation and differentiation of Sertoli cells, an effect that is enhanced with aging, and age-related macrophage changes can suppress Sertoli cell proliferation [41].
Sertoli cells
Sertoli cells play crucial regulatory roles in testicular differentiation and development [43, 44], and the size of the Sertoli cell population determines the numbers of germ cells and LCs [45]. Findings from human testicular biopsy demonstrate a positive correlation between Sertoli cell and LC numbers across all ages [33]. Corroborating these observations, complete ablation of Sertoli cells in juvenile mice severely impairs the differentiation and development of the adult LC population [46]. In adult mice, complete depletion of Sertoli cells results in a 75% reduction in the number of LCs [43]. Sertoli cells are the most age-sensitive cell type in the testis, and recent studies suggest that age-induced downregulation of Wilms' tumor 1 (WT1) may be a potential underlying mechanism [47]. WT1 is a transcriptional regulator with multifaceted roles in development, tissue homeostasis, and disease pathogenesis [48]. Age-related changes in Sertoli cell number and function impact overall testicular physiology and exacerbate the effects of aging on other cell types [49–51]. Multiple studies have demonstrated a marked age-associated decline in human Sertoli cell numbers [40, 49, 50, 52, 53]. A recent single-cell RNA sequencing study on testes from patients with late-onset hypogonadism (LOH) identified Sertoli cells as key metabolic coordinators in the testicular microenvironment, with aged Sertoli cells exhibiting reduced cholesterol efflux capability leading to cholesterol accumulation [54]. Additionally, aging induces alterations in Sertoli cell junctions; aged Sertoli cells display degeneration of tight junctions, compromising the integrity of the blood-testis barrier and further disrupting the testicular microenvironment [55–57], a process driven in part by WT1 downregulation [47]. Consequently, age-related changes in Sertoli cells create a cascade of effects that ultimately compromise LC function and testicular testosterone production.
Changes in LC number
Owing to the scarcity of fresh, disease-free adult testicular tissue, evidence regarding the effect of aging on LC number in humans primarily comes from testicular biopsies of organ donors. Several studies have reported an age-associated decline in the number of LC. Neaves et al. performed testicular biopsies on 30 men aged 20–76 who died from trauma or heart disease and found a 44% reduction in the total number of LCs in older males [58]. Mularoni et al. biopsied testicular tissue from 24 organ donors and observed no significant change in LC number between ages 19–45, but a significant decrease thereafter [33]. However, contradictory findings were reported by Gougeon et al., who biopsied testes from 26 men aged 16–80 and found no significant change in LC number with aging [49]. While these studies provide evidence of age-related changes in LC number through testicular biopsies, their main limitations include relatively small sample sizes and insufficient information on potential confounding variables.
Given these limitations in human studies, animal models have been extensively utilized to further investigate age-related changes in LC number and function. The brown Norwegian rat (Rattus norvegicus) exhibits an age-related decline in testosterone levels, similar to that of human males, making it a widely used animal model for studying aging in men [59, 60]. Related studies suggest that the age-related decline in testosterone levels is primarily driven by reduced cellular function rather than a decrease in the number of LCs [61, 62]. However, contrary findings have been reported in non-human primates (NHPs), which share genetic and physiological similarities with humans and represent an ideal model for studying testicular aging in higher primates [63]. A recent 2023 study in male cynomolgus macaques demonstrated a significant age-related reduction in the number of LCs [47]. Additionally, stem Leydig cells (SLCs), the upstream progenitors of LCs, are also affected by aging, exhibiting decreased proliferative and differentiation capabilities during the aging process [41, 64], which may contribute to the reduced number of LCs observed in aged males.
Changes in LC function
LC aging
Aging exerts significant impacts on the morphology and function of LC. Morphologically, aged LCs appear normal but exhibit signs of dedifferentiation and degeneration, including poorly developed endoplasmic reticulum and mitochondria, increased lipofuscin granules, abnormal cytoplasmic lipid droplets, and multiple nuclei [56, 65]. These structural alterations are accompanied by notable functional changes. Functionally, the steroidogenic capacity of aged LCs declines [40]. Aging adversely affects multiple steps in the testosterone biosynthetic pathway, with aged human LCs showing reduced responsiveness to LH stimulation [28]. Aged Brown Norway rats also exhibit decreased cAMP production upon LH stimulation [66], downregulation of cholesterol transporters StAR and TSPO [67, 68], and reduced expression of steroidogenic enzymes [69], potentially due to increased oxidative stress [24, 70, 71]. However, some studies report no age-related changes in human steroidogenic enzyme mRNA levels [33].
Insulin-like factor 3 (INSL3), exclusively secreted by mature LCs, serves as a reliable indicator of LCs' number and function [72]. Studies have demonstrated a progressive age-related decline in serum insulin-like factor 3 (INSL3) levels in adult men [73, 74], providing further evidence of diminished LC function with aging. Cellular senescence is a highly stable state of cell cycle arrest [75], characterized by a decline in cellular function. The accumulation of senescent cells drives age-related tissue dysfunction [76, 77]. A recent single-cell analysis of aged human testes also revealed reduced LCs' function and an increased proportion of senescent LCs in aged males [78]. Concurrently, senescent cells exhibit a SASP [38], which can potentiate and propagate senescence through both autocrine and paracrine mechanisms, exacerbating damage [79].
Mitochondrial dysfunction
Mitochondria serve as the powerhouse of the cell and play essential roles in several key cellular processes, such as apoptosis [80], ROS production [81], and inflammation [82]. Mitochondria are also crucial for steroid hormone biosynthesis, as the initial steps of steroidogenesis take place within these organelles. Cholesterol transport to the inner mitochondrial membrane is the rate-limiting and decisive step in steroidogenesis [16, 17]. With aging, multiple mechanisms contribute to mitochondrial dysfunction, including the accumulation of mitochondrial DNA (mtDNA) mutations, instability of respiratory chain complexes, imbalance in mitochondrial quality control, dysregulation of nutrient-sensing, and calcium overload [38, 83, 84]. Reduced mitochondrial respiratory capacity and mitochondrial membrane potential (MMP) are key features of aging-induced mitochondrial dysfunction [83]. These changes severely impact steroidogenesis [85, 86], while also compromising energy production, increasing ROS generation, and potentially inducing mitochondrial membrane permeability, leading to inflammation and cell death [38]. Indeed, mitochondrial dysfunction is a hallmark of aging and drives the functional decline of tissues and organs [38, 87].
At the ultrastructural level, aged rat LCs exhibit an increased number of dysfunctional mitochondria, with loss of mitochondrial cristae and swollen appearance [88]. Aged LCs demonstrate increased mitochondrial mass and mitochondrial DNA (mtDNA) copy number, reduced mitochondrial autophagy (mitophagy), and abnormal mitochondrial dynamics [89]. The primary cause of increased mitochondrial mass is the accumulation of dysfunctional mitochondria due to impaired mitophagy [90, 91]. Mitochondrial dynamics are crucial for maintaining mitochondrial number, shape, and distribution [83], and play an essential role in steroidogenesis [92–94]. In summary, age-induced mitochondrial dysfunction significantly impairs testosterone biosynthesis.
Endoplasmic reticulum stress
The endoplasmic reticulum (ER) regulates various cellular pathways, including protein synthesis and quality control, calcium storage and release, and lipid biosynthesis [95, 96]. It is also a crucial organelle for steroidogenesis [97]. ER stress occurs when misfolded and unfolded proteins accumulate, disrupting protein homeostasis [98]. Various conditions can trigger ER stress, such as redox alterations, energy depletion, and calcium homeostasis disturbances [99]. ER stress activates the unfolded protein response (UPR), which triggers pathways to restore homeostasis; however, prolonged stress can induce apoptosis [100]. During aging, the protective UPR response declines while pro-apoptotic signaling increases [101, 102]. Aging may enhance the accumulation of misfolded proteins, and ER stress is a hallmark of aging [103]. The UPR is implicated in protein folding, mitochondrial dysfunction, oxidative stress, and autophagy [104, 105]. ER stress regulates the maintenance of male testicular cell homeostasis and apoptosis [106], potentially contributing to LC depletion. Heat-induced ER stress suppresses 3β-HSD expression and testosterone production in mouse LCs, which can be restored by ER stress inhibitors [107]. Aged mouse testes and senescent TM3 LCs exhibit increased ER stress, reduced testosterone secretion, and enhanced steroidogenesis upon ER stress inhibition [108].
Autophagy dysfunction
Autophagy is a major degradative pathway in eukaryotic cells that recycles cytoplasmic components and eliminates damaged or redundant organelles and misfolded proteins [109]. It is a core mechanism for maintaining cellular and organismal homeostasis [110]. Autophagy can be classified into microautophagy, macroautophagy, and chaperone-mediated autophagy, with macroautophagy (hereafter referred to as autophagy) being the most prevalent form [111]. Autophagy is highly active in LCs [112], starting in SLCs and gradually increasing during differentiation, peaking in adult LCs, and declining in aged LCs [88, 113–115]. Additionally, autophagy plays an important role in regulating steroidogenesis [116]. Impaired autophagy is observed in the testicular tissues of azoospermic patients with low testosterone levels, suggesting a link between autophagy and steroidogenesis [114]. A 2023 study demonstrated that autophagy in human testes activates autophagosome formation to degrade lipid droplets (LDs), releasing free cholesterol as a substrate for testosterone synthesis [117].
Knocking out autophagy-related (Atg) genes in Drosophila to inhibit autophagy resulted in significant cholesterol accumulation in LDs and decreased steroid production [118]. Scavenger receptor class B type I (SR-BI) is a key receptor for cholesterol uptake in LC [119]. Further research revealed that impaired autophagy affects cholesterol uptake in LCs by downregulating SR-BI, as autophagy degrades the SR-BI negative regulator Na + /H + exchange regulatory factor 2 (NHERF2) [118]. Autophagy impairment is a hallmark of aging, with autophagic activity declining with aging [38, 111]. Animal studies have also shown reduced autophagy levels in aged rat LCs [88]. Therefore, age-related autophagy impairment may contribute to the decline in testosterone production.
Oxidative stress
Redox processes are ubiquitous in fundamental life activities, from bioenergetics to metabolism and life functions; redox homeostasis is at the core of life [120]. An imbalance between ROS and antioxidants can lead to oxidative stress, where excessive oxidants can damage biomolecules and even cause cell death. Oxidative stress is a hallmark of aging and a major contributor to age-related diseases [121]. Numerous studies have shown that oxidative damage accumulates in tissues with age [122]. Several mechanisms contribute to elevated oxidative stress levels during aging, with mitochondrial dysfunction and depletion of endogenous antioxidants as key drivers [123]. LCs exhibit age-related changes, with decreased levels of antioxidants such as superoxide dismutase (SOD), glutathione peroxidase (GPx), and glutathione (GSH), along with an increase in ROS. Aged Brown Norway rat LCs demonstrate significantly higher ROS levels than their younger counterparts [124–126].
Elevated ROS levels and activation of the oxidative damage-associated p38 MAPK signaling pathway were also observed in the LCs of premature aging mouse models, with partial restoration of steroidogenic capacity upon treatment with p38 MAPK inhibitors [39]. Excessive ROS can damage LCs and even induce cell death, adversely impacting testosterone production. Ferroptosis, a recently discovered form of programmed cell death, is driven by lipid peroxidation resulting from excessive ROS, reflecting a state of dysregulated cellular metabolism and redox imbalance [127, 128]. Age-induced ROS overload may contribute to LC death through ferroptosis induction. Moreover, ROS can damage key steroidogenic enzymes, such as the P450 enzymes [129]. Furthermore, oxidative stress is closely interlinked with mitochondrial dysfunction, inflammation, and cellular senescence, exerting reciprocal influences. Given these interconnections, oxidative stress likely plays a crucial role in the age-related decline of testosterone production[130].
Treatment strategies
Various therapies have been developed to address testosterone decline. These include drugs targeting the gonadal axis, stem cell therapies, physical interventions, and testosterone replacement formulations—the latter being the most common clinical approach (summarized in Table 1).
Table 1.
Summary of Treatment Strategies for Improving Testosterone Levels
| Intervention Strategy | Main Mechanism | Current Evidence | Clinical Application | Sources |
|---|---|---|---|---|
| Lifestyle Modifications (e.g., Exercise) | Enhances overall health and reduces inflammation, indirectly improving testosterone levels | Studies suggest moderate exercise can improve testosterone, especially in older men | Supplementary strategy for managing testosterone decline, suitable for health management | [131] [132] |
| LIPUS | Provides non-invasive physical stimulation to enhance testosterone secretion | Early studies show it can improve testosterone synthesis in aging Leydig cells, but more research is needed | Potential non-pharmacological strategy, pending further clinical evidence | [133] |
| Stem Cell Transplantation | Transplantation of SLCs restores Leydig cell function and increases testosterone synthesis | Animal studies show effective testosterone increase; clinical studies still limited | Promising for reversing age- or damage-related testosterone decline in the future | [134] [135] [136] |
| TRT | Provides exogenous testosterone to compensate for age- or disease-related testosterone deficiency | Multiple RCTs confirm effectiveness, but concerns about misuse and long-term safety exist | Commonly used for age-related testosterone decline; safety needs monitoring |
[139] |
| SERMs | Blocks estrogen’s negative feedback on the HPG axis, stimulating testosterone production | Systematic reviews show it raises testosterone but increases thrombosis risk and reduces bone density with long-term use | Potential TRT alternative, requires more long-term safety and efficacy studies | [140] [141] |
| Melatonin | Protects Leydig cells through antioxidant and anti-inflammatory effects, delaying aging processes | Animal studies show protective effects, but convincing evidence of testosterone elevation is lacking | May help improve Leydig cell function; more clinical studies needed for validation |
[144] |
| TSPO Ligands | Activates TSPO protein, promoting cholesterol transport to mitochondria, enhancing testosterone synthesis | Animal studies show increased testosterone in aged rats, but TSPO is expressed in multiple tissues, posing a challenge | Promising for endogenous testosterone enhancement, but tissue-specific activation is a challenge | [67] [145] |
| VDAC1 Peptide | Binds to 14–3-3ε, reducing its interaction with VDAC1, increasing cholesterol transport to mitochondria, enhancing testosterone synthesis | Animal studies show subcutaneous and oral administration safely increases testosterone levels in male rats | Promising strategy for testosterone increase; more research needed for clinical application | [146] [147] |
HPG Hypothalamic-Pituitary–Gonadal, LIPUS Low-Intensity Pulsed Ultrasound, RCT Randomized Controlled Trial, StAR Steroidogenic Acute Regulatory protein, SLCs Stem Leydig Cells, TSPO Translocator Protein, VDAC1 Voltage-Dependent Anion Channel 1
Non-pharmacological interventions
Lifestyle modifications
Accumulating evidence over the past decades has established skeletal muscle as an endocrine organ that produces and releases cytokines and other peptides, particularly during muscle contraction, thereby exerting systemic anti-inflammatory effects [148, 149]. Regular physical activity significantly reduces the risk of age-related diseases and mortality [150]. A meta-analysis involving 3,439,874 participants followed for a mean of 12.3 years found that 150 min of moderate-intensity aerobic exercise per week provides substantial health benefits for adults [151]. Exercise has also been shown to improve testosterone levels, especially in older individuals [131]. Short-term moderate exercise was found to transiently elevate serum testosterone levels by 39% and free testosterone index by 23% in seven elderly men (70 ± 4 years). These levels returned to baseline 4 h after exercise [132]. Another study demonstrated a significant increase in total testosterone levels with exercise in 202 patients (mean age: 51.8 years, average BMI: 28.5 kg/m2) followed for approximately 15 weeks [131]. While higher-level clinical studies are still needed, current evidence suggests that exercise is a relatively safe and effective approach for improving testosterone levels and maintaining overall health.
Physical therapy
Low-intensity pulsed ultrasound (LIPUS) is a form of ultrasound that delivers pulsed low-intensity energy, with minimal thermal and acoustic effects, providing non-invasive physical stimulation [152]. LIPUS is currently used to treat male erectile dysfunction and has proven effective in improving mild to moderate ED [153]. Recent studies have investigated the effects of LIPUS on aged human LCs isolated from testicular tissues, which demonstrated improved aging phenotypes, increased expression of key steroidogenic pathways, and enhanced testosterone secretion upon LIPUS stimulation [133]. LIPUS represents a potential therapeutic approach for improving testosterone production, although additional studies are required to confirm its safety and efficacy. Current cell experiments suggest that LIPUS is a potential therapy for mitigating testosterone decline. However, reliable animal studies and clinical trials are still needed to confirm its safety and efficacy.
Stem cell transplantation
SLCs can proliferate and differentiate into LCs [154, 155], making SLC transplantation a potential method for ameliorating the effects of aging on LCs. In animal studies, when SLCs were transplanted into testes with impaired or aged LCs, they could engraft in the interstitial compartment, differentiate into LCs, and increase testosterone production in mice [134]. Testosterone secretion, regulated by the hypothalamic-pituitary gonadal (HPG) axis, was partially restored when murine SLCs were transplanted into rat testes with LCs ablated by ethylene dimethane sulfonate (EDS), as the SLCs differentiated into LCs [135]. A study evaluated the effects of SLC transplantation in a non-human primate model. SLCs were isolated from aged cynomolgus monkey testes and autologously transplanted into the testicular interstitium, where the SLCs differentiated into LCs in vivo and partially restored normal testosterone secretion rhythm [156]. Although SLC transplantation has made significant progress in mouse and non-human primate models, showing promise for restoring endogenous testosterone levels, further detailed studies are needed to pave the way for clinical trials and applications.
Pharmacological therapy
Testosterone preparations
Testosterone replacement therapy (TRT) is widely used for exogenous testosterone supplementation, with various formulations available, including oral, injectable, transdermal, and transmucosal preparations [136, 157]. Several randomized controlled trial (RCT) studies have demonstrated that TRT is an effective treatment for testosterone decline [137]. The TTrials, an RCT focused on testosterone therapy in elderly men with low testosterone, included 788 men aged 65 and above (average age 72) with testosterone deficiency. The 12-month intervention showed that TRT effectively improved testosterone levels, raising median serum testosterone levels to the normal range [138]. However, the clinical misuse of testosterone preparations remains a concern, with testosterone prescriptions increasing 11-fold between 2001 and 2011 [158]. Furthermore, TRT raises concerns regarding the suppression of endogenous testosterone production and unclear long-term risks, such as prostate cancer, erythrocytosis, and cardiovascular diseases [139, 159–162]. Current research data indicate that three years of testosterone therapy does not increase the risk of cardiovascular disease [159, 163–165]. Nonetheless, the incidence of atrial fibrillation, acute kidney injury, and pulmonary embolism is slightly higher in the testosterone treatment group [159]. Further studies with longer observation periods are required to mitigate concerns regarding its long-term usage risks. Moreover, TRT interferes with the feedback mechanism of endogenous testosterone on the HPG axis. Exogenous testosterone suppresses the production of GnRH, LH, and follicle-stimulating hormone (FSH), significantly inhibiting spermatogenesis [140]. Consequently, TRT is contraindicated in patients seeking fertility [141].
Antiestrogens
Antiestrogen agents are classified into selective estrogen receptor modulators (SERMs) and aromatase inhibitors (AIs). SERMs prevent estrogen's negative feedback on the HPG axis, while AIs inhibit the aromatization of testosterone to estrogens. In an RCT, researchers evaluated the effect of clomiphene citrate (a SERM medication) on obesity-related testosterone decline. After 12 weeks, participants receiving clomiphene citrate showed increased serum testosterone levels [166]. A systematic review of related studies indicated that both SERMs and AIs raise serum testosterone, with SERMs performing better and showing potential as a TRT alternative [167]. However, SERMs may increase the risk of venous thrombosis [168], and long-term use could reduce bone density, raising the risk of fractures. Although SERMs show promise as an alternative to TRT, there are currently no clinical studies targeting testosterone decline in middle-aged and older men. Additionally, the long-term risks and benefits remain unclear, requiring further high-quality, long-duration RCTs for confirmation.
Antioxidants and anti-inflammatory agents
Melatonin exhibits significant antioxidant properties and can protect mitochondrial function [169–171]. Animal studies involving the overexpression of sheep melatonin genes, elevating endogenous melatonin levels, have demonstrated an increase in testosterone levels. Further research indicated that melatonin targets the mitochondrial apoptotic pathway, inhibiting LC apoptosis and upregulating the expression of genes related to testosterone synthesis [142]. Melatonin treatment reduced the levels of oxidative stress in testicular tissue [143]. Additionally, melatonin exhibits anti-inflammatory effects, with exogenous melatonin reducing the levels of inflammatory markers in humans [144]. Studies have shown that long-term timed melatonin administration does not alter the secretion patterns of testosterone in healthy males [172]. However, convincing evidence regarding the elevation of testosterone levels is still lacking. Moreover, certain medicinal plants and extracts possess antioxidant activities that may protect LCs [173]. Although such drugs have theoretically and in animal models shown the potential to increase testosterone levels, high-quality clinical studies remain lacking.
Other strategies
TSPO is a protein located on the outer mitochondrial membrane and plays a crucial role in transporting cholesterol from the outer to the inner mitochondrial membrane [174]. TSPO ligands enhance cholesterol uptake and subsequent transport to the inner mitochondrial membrane. Studies in aged rats have demonstrated that TSPO ligands significantly increase testosterone production by LCs [67], concurrent with elevated LH levels, suggesting a dual mechanism: direct LC stimulation and enhanced LH secretion [145]. Therefore, TSPO ligands hold promise as a potential therapy for enhancing endogenous testosterone production. However, since TSPO is expressed in multiple tissues [175], the specific activation of TSPO in LCs remains a challenge. 14–3-3 proteins bind to VDAC1, reducing cholesterol input and limiting testosterone synthesis [176]. Researchers have designed a VDAC1 peptide that binds to 14–3-3ε, blocking the interaction between 14–3-3ε and VDAC1, thereby limiting testosterone synthesis [146]. Subcutaneous injection and oral administration of the VDAC1 peptide elevated testosterone levels in male rats, demonstrating safety and efficacy [147]. These drugs are still in the animal testing phase, and more research is needed to support their clinical application .
Conclusion and future directions
In conclusion, aging affects testosterone synthesis through various pathways, including alterations in the HPG axis, testicular microenvironment, LC number and function, consequently impacting male reproductive function and quality of life. Understanding the mechanisms underlying age-related testosterone decline is fundamental for developing relevant diagnostic and therapeutic strategies, necessitating further research to elucidate its exact mechanisms. While existing treatment strategies can improve testosterone levels to some extent, safely and effectively enhancing endogenous testosterone levels remains a focus of future research. Stem cell transplantation and biologics targeting specific steps in testosterone production hold promise as therapeutic approaches, but further animal and clinical studies are needed to support their clinical application.
Authors’ contributions
Authors' contribution Haoyang Cheng: Conceptualization, Writing – original draft, Writing – review & editing. Xiaoyan Zhang: Writing – review & editing. Yongheng Li: Writing – review & editing. Dezhong Cao: Writing – review & editing. Chenglong Luo: Writing – review & editing. Qi Zhang: Writing – review & editing. Sizheng Zhang: Writing – review & editing. Yongzheng Jiao: Conceptualization, Funding acquisition, Project administration, Writing – review & editing.
Funding
Funding was received for the research, authorship, and/or publication of this article. This work was supported by the National Natural Science Foundation of China (No. 82074446, 82474523) and the Scientific and Technological Innovation Project of the China Academy of Chinese Medical Sciences (No. CI2021A02202).
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
Not Applicable.
Consent for publication
Not Applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Haoyang Cheng and Xiaoyan Zhang contributed equally to this work.
References
- 1.Fabbri E, An Y, Gonzalez-Freire M, Zoli M, Maggio M, Studenski SA, et al. Bioavailable Testosterone Linearly Declines Over A Wide Age Spectrum in Men and Women From The Baltimore Longitudinal Study of Aging. J Gerontol A Biol Sci Med Sci. 2016;71(9):1202–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu FC, Tajar A, Pye SR, Silman AJ, Finn JD, O’Neill TW, et al. Hypothalamic-pituitary-testicular axis disruptions in older men are differentially linked to age and modifiable risk factors: the European Male Aging Study. J Clin Endocrinol Metab. 2008;93(7):2737–45. [DOI] [PubMed] [Google Scholar]
- 3.Li H, Gu Y, Shang X, Zhou Y, Zhang H, Zuo L, et al. Decreased testosterone secretion index and free testosterone level with multiple symptoms for late-onset hypogonadism identification: a nationwide multicenter study with 5980 aging males in China. Aging (Albany NY). 2020;12(24):26012–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Handelsman DJ, Yeap B, Flicker L, Martin S, Wittert GA, Ly LP. Age-specific population centiles for androgen status in men. Eur J Endocrinol. 2015;173(6):809–17. [DOI] [PubMed] [Google Scholar]
- 5.Zitzmann M. Testosterone and the brain. Aging Male. 2006;9(4):195–9. [DOI] [PubMed] [Google Scholar]
- 6.Shigehara K, Izumi K, Kadono Y, Mizokami A. Testosterone and Bone Health in Men: A Narrative Review. J Clin Med. 2021;10(3):520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaur H, Werstuck GH. The Effect of Testosterone on Cardiovascular Disease and Cardiovascular Risk Factors in Men: A Review of Clinical and Preclinical Data. CJC Open. 2021;3(10):1238–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mohamad NV, Wong SK, Wan Hasan WN, Jolly JJ, Nur-Farhana MF, Ima-Nirwana S, et al. The relationship between circulating testosterone and inflammatory cytokines in men. Aging Male. 2019;22(2):129–40. [DOI] [PubMed] [Google Scholar]
- 9.Ding EL, Song Y, Malik VS, Liu S. Sex differences of endogenous sex hormones and risk of type 2 diabetes: a systematic review and meta-analysis. JAMA. 2006;295(11):1288–99. [DOI] [PubMed] [Google Scholar]
- 10.Wittert G, Bracken K, Robledo KP, Grossmann M, Yeap BB, Handelsman DJ, et al. Testosterone treatment to prevent or revert type 2 diabetes in men enrolled in a lifestyle programme (T4DM): a randomised, double-blind, placebo-controlled, 2-year, phase 3b trial. Lancet Diabetes Endocrinol. 2021;9(1):32–45. [DOI] [PubMed] [Google Scholar]
- 11.Marriott RJ, Murray K, Flicker L, Hankey GJ, Matsumoto AM, Dwivedi G, et al. Lower serum testosterone concentrations are associated with a higher incidence of dementia in men: The UK Biobank prospective cohort study. Alzheimers Dement. 2022;18(10):1907–18. [DOI] [PubMed] [Google Scholar]
- 12.Chen Z, Zhang E, Gan L, Jiang G, Duan Q, Huang M, et al. Analysis of the association between testosterone and cardiovascular disease potential risk factor apolipoprotein B in adult males without cancer: national health and nutrition examination survey 2011–2016. Front Endocrinol (Lausanne). 2024;15:1304344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yeap BB, Marriott RJ, Antonio L, Chan YX, Raj S, Dwivedi G, et al. Serum Testosterone is Inversely and Sex Hormone-binding Globulin is Directly Associated with All-cause Mortality in Men. J Clin Endocrinol Metab. 2021;106(2):e625–37. [DOI] [PubMed] [Google Scholar]
- 14.Ohlander SJ, Lindgren MC, Lipshultz LI. Testosterone and Male Infertility. Urol Clin North Am. 2016;43(2):195–202. [DOI] [PubMed] [Google Scholar]
- 15.McBride JA, Carson CC 3rd, Coward RM. Testosterone deficiency in the aging male. Ther Adv Urol. 2016;8(1):47–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jefcoate C. High-flux mitochondrial cholesterol trafficking, a specialized function of the adrenal cortex. J Clin Invest. 2002;110(7):881–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Simpson ER, Waterman MR. Regulation by ACTH of steroid hormone biosynthesis in the adrenal cortex. Can J Biochem Cell Biol. 1983;61(7):692–707. [DOI] [PubMed] [Google Scholar]
- 18.Rone MB, Midzak AS, Issop L, Rammouz G, Jagannathan S, Fan J, et al. Identification of a dynamic mitochondrial protein complex driving cholesterol import, trafficking, and metabolism to steroid hormones. Mol Endocrinol. 2012;26(11):1868–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fan J, Papadopoulos V. Evolutionary origin of the mitochondrial cholesterol transport machinery reveals a universal mechanism of steroid hormone biosynthesis in animals. PLoS ONE. 2013;8(10): e76701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miller WL. Mechanism of StAR’s regulation of mitochondrial cholesterol import. Mol Cell Endocrinol. 2007;265–266:46–50. [DOI] [PubMed] [Google Scholar]
- 21.Miller WL. Steroidogenic acute regulatory protein (StAR), a novel mitochondrial cholesterol transporter. Biochimica et Biophysica Acta (BBA)-Molecular and Cell Biology of Lipids. 2007;1771(6):663–76. [DOI] [PubMed] [Google Scholar]
- 22.Aghazadeh Y, Rone MB, Blonder J, Ye X, Veenstra TD, Hales DB, et al. Hormone-induced 14-3-3γ adaptor protein regulates steroidogenic acute regulatory protein activity and steroid biosynthesis in MA-10 Leydig cells. J Biol Chem. 2012;287(19):15380–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aghazadeh Y, Martinez-Arguelles DB, Fan J, Culty M, Papadopoulos V. Induction of androgen formation in the male by a TAT-VDAC1 fusion peptide blocking 14-3-3ɛ protein adaptor and mitochondrial VDAC1 interactions. Mol Ther. 2014;22(10):1779–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang Y, Chen F, Ye L, Zirkin B, Chen H. Steroidogenesis in Leydig cells: effects of aging and environmental factors. Reproduction. 2017;154(4):R111–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Anawalt BD, Matsumoto AM. Aging and androgens: Physiology and clinical implications. Rev Endocr Metab Disord. 2022;23(6):1123–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Keenan DM, Veldhuis JD. Age-dependent regression analysis of male gonadal axis. Am J Physiol Regul Integr Comp Physiol. 2009;297(5):R1215–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Keenan DM, Takahashi PY, Liu PY, Roebuck PD, Nehra AX, Iranmanesh A, et al. An ensemble model of the male gonadal axis: illustrative application in aging men. Endocrinology. 2006;147(6):2817–28. [DOI] [PubMed] [Google Scholar]
- 28.Roelfsema F, Liu PY, Takahashi PY, Yang RJ, Veldhuis JD. Dynamic Interactions Between LH and Testosterone in Healthy Community-Dwelling Men: Impact of Age and Body Composition. J Clin Endocrinol Metab. 2020;105(3):e628–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Campbell RE, Coolen LM, Hoffman GE, Hrabovszky E. Highlights of neuroanatomical discoveries of the mammalian gonadotropin-releasing hormone system. J Neuroendocrinol. 2022;34(5): e13115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gruenewald DA, Naai MA, Marck BT, Matsumoto AM. Age-related decrease in hypothalamic gonadotropin-releasing hormone (GnRH) gene expression, but not pituitary responsiveness to GnRH, in the male Brown Norway rat. J Androl. 2000;21(1):72–84. [PubMed] [Google Scholar]
- 31.Hai Y, Hou J, Liu Y, Liu Y, Yang H, Li Z, et al. The roles and regulation of Sertoli cells in fate determinations of spermatogonial stem cells and spermatogenesis. Semin Cell Dev Biol. 2014;29:66–75. [DOI] [PubMed] [Google Scholar]
- 32.Curley M, Milne L, Smith S, Jørgensen A, Frederiksen H, Hadoke P, et al. A young testicular microenvironment protects Leydig cells against age-related dysfunction in a mouse model of premature aging. Faseb j. 2019;33(1):978–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mularoni V, Esposito V, Di Persio S, Vicini E, Spadetta G, Berloco P, et al. Age-related changes in human Leydig cell status. Hum Reprod. 2020;35(12):2663–76. [DOI] [PubMed] [Google Scholar]
- 34.Meinhardt A, Wang M, Schulz C, Bhushan S. Microenvironmental signals govern the cellular identity of testicular macrophages. J Leukoc Biol. 2018;104(4):757–66. [DOI] [PubMed] [Google Scholar]
- 35.Chi A, Yang B, Dai H, Li X, Mo J, Gao Y, et al. Stem Leydig cells support macrophage immunological homeostasis through mitochondrial transfer in mice. Nat Commun. 2024;15(1):2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee JW, Chun W, Lee HJ, Min JH, Kim SM, Seo JY, et al. The Role of Macrophages in the Development of Acute and Chronic Inflammatory Lung Diseases. Cells. 2021;10(4):897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hales DB. Testicular macrophage modulation of Leydig cell steroidogenesis. J Reprod Immunol. 2002;57(1–2):3–18. [DOI] [PubMed] [Google Scholar]
- 38.López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. Hallmarks of aging: An expanding universe. Cell. 2023;186(2):243–78. [DOI] [PubMed] [Google Scholar]
- 39.Zhao Y, Liu X, Qu Y, Wang L, Geng D, Chen W, et al. The roles of p38 MAPK → COX2 and NF-κB → COX2 signal pathways in age-related testosterone reduction. Sci Rep. 2019;9(1):10556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nie X, Munyoki SK, Sukhwani M, Schmid N, Missel A, Emery BR, et al. Single-cell analysis of human testis aging and correlation with elevated body mass index. Dev Cell. 2022;57(9):1160-76.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shao J, Wang J, Wen X, Xie J, Huang F, Guan X, et al. Effects of aging and macrophages on mice stem Leydig cell proliferation and differentiation in vitro. Front Endocrinol (Lausanne). 2023;14:1139281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang W, Xia S, Xiao W, Song Y, Tang L, Cao M, et al. A single-cell transcriptomic landscape of mouse testicular aging. J Adv Res. 2023;53:219–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rebourcet D, O’Shaughnessy PJ, Monteiro A, Milne L, Cruickshanks L, Jeffrey N, et al. Sertoli cells maintain Leydig cell number and peritubular myoid cell activity in the adult mouse testis. PLoS ONE. 2014;9(8):e105687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.O’Donnell L, Smith LB, Rebourcet D. Sertoli cells as key drivers of testis function. Semin Cell Dev Biol. 2022;121:2–9. [DOI] [PubMed] [Google Scholar]
- 45.Rebourcet D, Darbey A, Monteiro A, Soffientini U, Tsai YT, Handel I, et al. Sertoli Cell Number Defines and Predicts Germ and Leydig Cell Population Sizes in the Adult Mouse Testis. Endocrinology. 2017;158(9):2955–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rebourcet D, O’Shaughnessy PJ, Pitetti JL, Monteiro A, O’Hara L, Milne L, et al. Sertoli cells control peritubular myoid cell fate and support adult Leydig cell development in the prepubertal testis. Development. 2014;141(10):2139–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Huang D, Zuo Y, Zhang C, Sun G, Jing Y, Lei J, et al. A single-nucleus transcriptomic atlas of primate testicular aging reveals exhaustion of the spermatogonial stem cell reservoir and loss of Sertoli cell homeostasis. Protein Cell. 2023;14(12):888–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hastie ND. Wilms’ tumour 1 (WT1) in development, homeostasis and disease. Development. 2017;144(16):2862–72. [DOI] [PubMed] [Google Scholar]
- 49.Petersen PM, Seierøe K, Pakkenberg B. The total number of Leydig and Sertoli cells in the testes of men across various age groups - a stereological study. J Anat. 2015;226(2):175–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Johnson L, Zane RS, Petty CS, Neaves WB. Quantification of the human Sertoli cell population: its distribution, relation to germ cell numbers, and age-related decline. Biol Reprod. 1984;31(4):785–95. [DOI] [PubMed] [Google Scholar]
- 51.Paniagua R, Martín A, Nistal M, Amat P. Testicular involution in elderly men: comparison of histologic quantitative studies with hormone patterns. Fertil Steril. 1987;47(4):671–9. [DOI] [PubMed] [Google Scholar]
- 52.Xia Y, Zhu WJ, Hao SF, Liang WB, Li J. Stereological analysis of age-related changes of testicular peritubular cells in men. Arch Gerontol Geriatr. 2012;55(1):116–9. [DOI] [PubMed] [Google Scholar]
- 53.Dakouane M, Bicchieray L, Bergere M, Albert M, Vialard F, Selva J. A histomorphometric and cytogenetic study of testis from men 29–102 years old. Fertil Steril. 2005;83(4):923–8. [DOI] [PubMed] [Google Scholar]
- 54.Deng Z, Zhao L, Li S, Chen X, Ling X, Zheng J, et al. Targeting dysregulated phago-/auto-lysosomes in Sertoli cells to ameliorate late-onset hypogonadism. Nat Aging. 2024;4(5):647–63. [DOI] [PubMed] [Google Scholar]
- 55.Jiang H, Zhu WJ, Li J, Chen QJ, Liang WB, Gu YQ. Quantitative histological analysis and ultrastructure of the aging human testis. Int Urol Nephrol. 2014;46(5):879–85. [DOI] [PubMed] [Google Scholar]
- 56.Paniagua R, Nistal M, Sáez FJ, Fraile B. Ultrastructure of the aging human testis. J Electron Microsc Tech. 1991;19(2):241–60. [DOI] [PubMed] [Google Scholar]
- 57.Levy S, Serre V, Hermo L, Robaire B. The effects of aging on the seminiferous epithelium and the blood-testis barrier of the Brown Norway rat. J Androl. 1999;20(3):356–65. [PubMed] [Google Scholar]
- 58.Neaves WB, Johnson L, Porter JC, Parker CR Jr, Petty CS. Leydig cell numbers, daily sperm production, and serum gonadotropin levels in aging men. J Clin Endocrinol Metab. 1984;59(4):756–63. [DOI] [PubMed] [Google Scholar]
- 59.Wang C, Leung A, Sinha-Hikim AP. Reproductive aging in the male brown-Norway rat: a model for the human. Endocrinology. 1993;133(6):2773–81. [DOI] [PubMed] [Google Scholar]
- 60.Zirkin BR, Santulli R, Strandberg JD, Wright WW, Ewing LL. Testicular steroidogenesis in the aging brown Norway rat. J Androl. 1993;14(2):118–23. [PubMed] [Google Scholar]
- 61.Chen H, Hardy MP, Huhtaniemi I, Zirkin BR. Age-related decreased Leydig cell testosterone production in the brown Norway rat. J Androl. 1994;15(6):551–7. [PubMed] [Google Scholar]
- 62.Chen H, Huhtaniemi I, Zirkin BR. Depletion and repopulation of Leydig cells in the testes of aging brown Norway rats. Endocrinology. 1996;137(8):3447–52. [DOI] [PubMed] [Google Scholar]
- 63.Fayomi AP, Orwig KE. Spermatogonial stem cells and spermatogenesis in mice, monkeys and men. Stem Cell Res. 2018;29:207–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yao S, Wei X, Deng W, Wang B, Cai J, Huang Y, et al. Nestin-dependent mitochondria-ER contacts define stem Leydig cell differentiation to attenuate male reproductive ageing. Nat Commun. 2022;13(1):4020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Paniagua R, Amat P, Nistal M, Martin A. Ultrastructure of Leydig cells in human ageing testes. J Anat. 1986;146:173–83. [PMC free article] [PubMed] [Google Scholar]
- 66.Chen H, Hardy MP, Zirkin BR. Age-related decreases in Leydig cell testosterone production are not restored by exposure to LH in vitro. Endocrinology. 2002;143(5):1637–42. [DOI] [PubMed] [Google Scholar]
- 67.Chung JY, Chen H, Midzak A, Burnett AL, Papadopoulos V, Zirkin BR. Drug ligand-induced activation of translocator protein (TSPO) stimulates steroid production by aged brown Norway rat Leydig cells. Endocrinology. 2013;154(6):2156–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Luo L, Chen H, Zirkin BR. Leydig cell aging: steroidogenic acute regulatory protein (StAR) and cholesterol side-chain cleavage enzyme. J Androl. 2001;22(1):149–56. [PubMed] [Google Scholar]
- 69.Luo L, Chen H, Zirkin BR. Temporal relationships among testosterone production, steroidogenic acute regulatory protein (StAR), and P450 side-chain cleavage enzyme (P450scc) during Leydig cell aging. J Androl. 2005;26(1):25–31. [PubMed] [Google Scholar]
- 70.Beattie MC, Adekola L, Papadopoulos V, Chen H, Zirkin BR. Leydig cell aging and hypogonadism. Exp Gerontol. 2015;68:87–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ajayi AF, Onaolapo MC, Omole AI, Adeyemi WJ, Oluwole DT. Mechanism associated with changes in male reproductive functions during ageing process. Exp Gerontol. 2023;179: 112232. [DOI] [PubMed] [Google Scholar]
- 72.Ivell R, Wade JD, Anand-Ivell R. INSL3 as a biomarker of Leydig cell functionality. Biol Reprod. 2013;88(6):147. [DOI] [PubMed] [Google Scholar]
- 73.Anand-Ivell R, Wohlgemuth J, Haren MT, Hope PJ, Hatzinikolas G, Wittert G, et al. Peripheral INSL3 concentrations decline with age in a large population of Australian men. Int J Androl. 2006;29(6):618–26. [DOI] [PubMed] [Google Scholar]
- 74.Anand-Ivell R, Heng K, Severn K, Antonio L, Bartfai G, Casanueva FF, et al. Association of age, hormonal, and lifestyle factors with the Leydig cell biomarker INSL3 in aging men from the European Male Aging Study cohort. Andrology. 2022;10(7):1328–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chaib S, Tchkonia T, Kirkland JL. Cellular senescence and senolytics: the path to the clinic. Nat Med. 2022;28(8):1556–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ogrodnik M, Evans SA, Fielder E, Victorelli S, Kruger P, Salmonowicz H, et al. Whole-body senescent cell clearance alleviates age-related brain inflammation and cognitive impairment in mice. Aging Cell. 2021;20(2):e13296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Anderson R, Lagnado A, Maggiorani D, Walaszczyk A, Dookun E, Chapman J, et al. Length-independent telomere damage drives post-mitotic cardiomyocyte senescence. Embo j. 2019;38(5):e100492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.He J, Li J, Li Y, Xu Z, Ma M, Chen H, et al. Single-cell transcriptomics identifies senescence-associated secretory phenotype (SASP) features of testicular aging in human. Aging (Albany NY). 2024;16(4):3350–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kumari R, Jat P. Mechanisms of Cellular Senescence: Cell Cycle Arrest and Senescence Associated Secretory Phenotype. Front Cell Dev Biol. 2021;9: 645593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gahl RF, Dwivedi P, Tjandra N. Bcl-2 proteins bid and bax form a network to permeabilize the mitochondria at the onset of apoptosis. Cell Death Dis. 2016;7(10): e2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Shadel GS, Horvath TL. Mitochondrial ROS signaling in organismal homeostasis. Cell. 2015;163(3):560–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Marchi S, Guilbaud E, Tait SWG, Yamazaki T, Galluzzi L. Mitochondrial control of inflammation. Nat Rev Immunol. 2023;23(3):159–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Miwa S, Kashyap S, Chini E, von Zglinicki T. Mitochondrial dysfunction in cell senescence and aging. J Clin Invest. 2022;132(13):e158447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mikhed Y, Daiber A, Steven S. Mitochondrial Oxidative Stress, Mitochondrial DNA Damage and Their Role in Age-Related Vascular Dysfunction. Int J Mol Sci. 2015;16(7):15918–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Midzak AS, Chen H, Aon MA, Papadopoulos V, Zirkin BR. ATP synthesis, mitochondrial function, and steroid biosynthesis in rodent primary and tumor Leydig cells. Biol Reprod. 2011;84(5):976–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Allen JA, Shankara T, Janus P, Buck S, Diemer T, Hales KH, et al. Energized, polarized, and actively respiring mitochondria are required for acute Leydig cell steroidogenesis. Endocrinology. 2006;147(8):3924–35. [DOI] [PubMed] [Google Scholar]
- 87.Akbari M, Kirkwood TBL, Bohr VA. Mitochondria in the signaling pathways that control longevity and health span. Ageing Res Rev. 2019;54: 100940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Li WR, Chen L, Chang ZJ, Xin H, Liu T, Zhang YQ, et al. Autophagic deficiency is related to steroidogenic decline in aged rat Leydig cells. Asian J Androl. 2011;13(6):881–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sokanovic SJ, Baburski AZ, Kojic Z, Medar MLJ, Andric SA, Kostic TS. Aging-Related Increase of cGMP Disrupts Mitochondrial Homeostasis in Leydig Cells. J Gerontol A Biol Sci Med Sci. 2021;76(2):177–86. [DOI] [PubMed] [Google Scholar]
- 90.Dalle Pezze P, Nelson G, Otten EG, Korolchuk VI, Kirkwood TB, von Zglinicki T, et al. Dynamic modelling of pathways to cellular senescence reveals strategies for targeted interventions. PLoS Comput Biol. 2014;10(8): e1003728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Fielder E, Wan T, Alimohammadiha G, Ishaq A, Low E, Weigand BM, et al. Short senolytic or senostatic interventions rescue progression of radiation-induced frailty and premature ageing in mice. Elife. 2022;11:e75492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Garza S, Sottas C, Gukasyan HJ, Papadopoulos V. In vitro and in vivo studies on the effect of a mitochondrial fusion promoter on Leydig cell integrity and function. Front Toxicol. 2024;6:1357857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Duarte A, Poderoso C, Cooke M, Soria G, Cornejo Maciel F, Gottifredi V, et al. Mitochondrial fusion is essential for steroid biosynthesis. PLoS ONE. 2012;7(9): e45829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Garza S, Chen L, Galano M, Cheung G, Sottas C, Li L, et al. Mitochondrial dynamics, Leydig cell function, and age-related testosterone deficiency. Faseb j. 2022;36(12): e22637. [DOI] [PubMed] [Google Scholar]
- 95.Celik C, Lee SYT, Yap WS, Thibault G. Endoplasmic reticulum stress and lipids in health and diseases. Prog Lipid Res. 2023;89: 101198. [DOI] [PubMed] [Google Scholar]
- 96.Naidoo N. The endoplasmic reticulum stress response and aging. Rev Neurosci. 2009;20(1):23–38. [DOI] [PubMed] [Google Scholar]
- 97.Yu C, Jiang F, Zhang M, Luo D, Shao S, Zhao J, et al. HC diet inhibited testosterone synthesis by activating endoplasmic reticulum stress in testicular Leydig cells. J Cell Mol Med. 2019;23(5):3140–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chen X, Shi C, He M, Xiong S, Xia X. Endoplasmic reticulum stress: molecular mechanism and therapeutic targets. Signal Transduct Target Ther. 2023;8(1):352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kaufman RJ. Orchestrating the unfolded protein response in health and disease. J Clin Invest. 2002;110(10):1389–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bhattarai KR, Riaz TA, Kim HR, Chae HJ. The aftermath of the interplay between the endoplasmic reticulum stress response and redox signaling. Exp Mol Med. 2021;53(2):151–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Naidoo N, Ferber M, Master M, Zhu Y, Pack AI. Aging impairs the unfolded protein response to sleep deprivation and leads to proapoptotic signaling. J Neurosci. 2008;28(26):6539–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Paz Gavilán M, Vela J, Castaño A, Ramos B, del Río JC, Vitorica J, et al. Cellular environment facilitates protein accumulation in aged rat hippocampus. Neurobiol Aging. 2006;27(7):973–82. [DOI] [PubMed] [Google Scholar]
- 103.Martínez G, Duran-Aniotz C, Cabral-Miranda F, Vivar JP, Hetz C. Endoplasmic reticulum proteostasis impairment in aging. Aging Cell. 2017;16(4):615–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wang M, Kaufman RJ. Protein misfolding in the endoplasmic reticulum as a conduit to human disease. Nature. 2016;529(7586):326–35. [DOI] [PubMed] [Google Scholar]
- 105.Dombroski BA, Nayak RR, Ewens KG, Ankener W, Cheung VG, Spielman RS. Gene expression and genetic variation in response to endoplasmic reticulum stress in human cells. Am J Hum Genet. 2010;86(5):719–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Karna KK, Shin YS, Choi BR, Kim HK, Park JK. The Role of Endoplasmic Reticulum Stress Response in Male Reproductive Physiology and Pathology: A Review. World J Mens Health. 2020;38(4):484–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kim JH, Park SJ, Kim TS, Kim JM, Lee DS. Testosterone production by a Leydig tumor cell line is suppressed by hyperthermia-induced endoplasmic reticulum stress in mice. Life Sci. 2016;146:184–91. [DOI] [PubMed] [Google Scholar]
- 108.Gao L, Gao D, Zhang J, Li C, Wu M, Xiao Y, et al. Age-related endoplasmic reticulum stress represses testosterone synthesis via attenuation of the circadian clock in Leydig cells. Theriogenology. 2022;189:137–49. [DOI] [PubMed] [Google Scholar]
- 109.Feng Y, He D, Yao Z, Klionsky DJ. The machinery of macroautophagy. Cell Res. 2014;24(1):24–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Klionsky DJ, Petroni G, Amaravadi RK, Baehrecke EH, Ballabio A, Boya P, et al. Autophagy in major human diseases. Embo j. 2021;40(19): e108863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Aman Y, Schmauck-Medina T, Hansen M, Morimoto RI, Simon AK, Bjedov I, et al. Autophagy in healthy aging and disease. Nat Aging. 2021;1(8):634–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Yi J, Tang XM. Functional implication of autophagy in steroid-secreting cells of the rat. Anat Rec. 1995;242(2):137–46. [DOI] [PubMed] [Google Scholar]
- 113.Chen Y, Wang J, Xu D, Xiang Z, Ding J, Yang X, et al. m(6)A mRNA methylation regulates testosterone synthesis through modulating autophagy in Leydig cells. Autophagy. 2021;17(2):457–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Gao F, Li G, Liu C, Gao H, Wang H, Liu W, et al. Autophagy regulates testosterone synthesis by facilitating cholesterol uptake in Leydig cells. J Cell Biol. 2018;217(6):2103–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Midzak AS, Chen H, Papadopoulos V, Zirkin BR. Leydig cell aging and the mechanisms of reduced testosterone synthesis. Mol Cell Endocrinol. 2009;299(1):23–31. [DOI] [PubMed] [Google Scholar]
- 116.Yan Q, Zhang Y, Wang Q, Yuan L. Autophagy: A Double-Edged Sword in Male Reproduction. Int J Mol Sci. 2022;23(23):15273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Esmaeilian Y, Hela F, Bildik G, İltumur E, Yusufoglu S, Yildiz CS, et al. Autophagy regulates sex steroid hormone synthesis through lysosomal degradation of lipid droplets in human ovary and testis. Cell Death Dis. 2023;14(5):342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Texada MJ, Malita A, Rewitz K. Autophagy regulates steroid production by mediating cholesterol trafficking in endocrine cells. Autophagy. 2019;15(8):1478–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.DeAngelis AM, Roy-O’Reilly M, Rodriguez A. Genetic alterations affecting cholesterol metabolism and human fertility. Biol Reprod. 2014;91(5):117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Sies H, Berndt C, Jones DP. Oxidative Stress. Annu Rev Biochem. 2017;86:715–48. [DOI] [PubMed] [Google Scholar]
- 121.Luo J, Mills K, le Cessie S, Noordam R, van Heemst D. Ageing, age-related diseases and oxidative stress: What to do next? Ageing Res Rev. 2020;57: 100982. [DOI] [PubMed] [Google Scholar]
- 122.Campisi J, Kapahi P, Lithgow GJ, Melov S, Newman JC, Verdin E. From discoveries in ageing research to therapeutics for healthy ageing. Nature. 2019;571(7764):183–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Vatner SF, Zhang J, Oydanich M, Berkman T, Naftalovich R, Vatner DE. Healthful aging mediated by inhibition of oxidative stress. Ageing Res Rev. 2020;64: 101194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Chen H, Cangello D, Benson S, Folmer J, Zhu H, Trush MA, et al. Age-related increase in mitochondrial superoxide generation in the testosterone-producing cells of Brown Norway rat testes: relationship to reduced steroidogenic function? Exp Gerontol. 2001;36(8):1361–73. [DOI] [PubMed] [Google Scholar]
- 125.Chen H, Pechenino AS, Liu J, Beattie MC, Brown TR, Zirkin BR. Effect of glutathione depletion on Leydig cell steroidogenesis in young and old brown Norway rats. Endocrinology. 2008;149(5):2612–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Luo L, Chen H, Trush MA, Show MD, Anway MD, Zirkin BR. Aging and the brown Norway rat leydig cell antioxidant defense system. J Androl. 2006;27(2):240–7. [DOI] [PubMed] [Google Scholar]
- 127.Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, Gleason CE, et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149(5):1060–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Jiang X, Stockwell BR, Conrad M. Ferroptosis: mechanisms, biology and role in disease. Nat Rev Mol Cell Biol. 2021;22(4):266–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Hanukoglu I. Antioxidant protective mechanisms against reactive oxygen species (ROS) generated by mitochondrial P450 systems in steroidogenic cells. Drug Metab Rev. 2006;38(1–2):171–96. [DOI] [PubMed] [Google Scholar]
- 130.Chen H, Jin S, Guo J, Kombairaju P, Biswal S, Zirkin BR. Knockout of the transcription factor Nrf2: Effects on testosterone production by aging mouse Leydig cells. Mol Cell Endocrinol. 2015;409:113–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Corona G, Rastrelli G, Morelli A, Sarchielli E, Cipriani S, Vignozzi L, et al. Treatment of Functional Hypogonadism Besides Pharmacological Substitution. World J Mens Health. 2020;38(3):256–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zmuda JM, Thompson PD, Winters SJ. Exercise increases serum testosterone and sex hormone-binding globulin levels in older men. Metabolism. 1996;45(8):935–9. [DOI] [PubMed] [Google Scholar]
- 133.Han S, Luo J, Xu S, Zhao L, Yao C, Xu J, et al. Low-Intensity Pulsed Ultrasound Alleviates Human Testicular Leydig Cell Senescence In Vitro. Int J Mol Sci. 2022;24(1):418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Jiang MH, Cai B, Tuo Y, Wang J, Zang ZJ, Tu X, et al. Characterization of Nestin-positive stem Leydig cells as a potential source for the treatment of testicular Leydig cell dysfunction. Cell Res. 2014;24(12):1466–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Zang ZJ, Wang J, Chen Z, Zhang Y, Gao Y, Su Z, et al. Transplantation of CD51(+) Stem Leydig Cells: A New Strategy for the Treatment of Testosterone Deficiency. Stem Cells. 2017;35(5):1222–32. [DOI] [PubMed] [Google Scholar]
- 136.Salonia A, Bettocchi C, Boeri L, Capogrosso P, Carvalho J, Cilesiz NC, et al. European Association of Urology Guidelines on Sexual and Reproductive Health-2021 Update: Male Sexual Dysfunction. Eur Urol. 2021;80(3):333–57. [DOI] [PubMed] [Google Scholar]
- 137.Corona G, Torres LO, Maggi M. Testosterone Therapy: What We Have Learned From Trials. J Sex Med. 2020;17(3):447–60. [DOI] [PubMed] [Google Scholar]
- 138.Snyder PJ, Bhasin S, Cunningham GR, Matsumoto AM, Stephens-Shields AJ, Cauley JA, et al. Lessons From the Testosterone Trials. Endocr Rev. 2018;39(3):369–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Rambhatla A, Mills JN, Rajfer J. The Role of Estrogen Modulators in Male Hypogonadism and Infertility. Rev Urol. 2016;18(2):66–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Samplaski MK, Loai Y, Wong K, Lo KC, Grober ED, Jarvi KA. Testosterone use in the male infertility population: prescribing patterns and effects on semen and hormonal parameters. Fertil Steril. 2014;101(1):64–9. [DOI] [PubMed] [Google Scholar]
- 141.Corona G, Goulis DG, Huhtaniemi I, Zitzmann M, Toppari J, Forti G, et al. European Academy of Andrology (EAA) guidelines on investigation, treatment and monitoring of functional hypogonadism in males: Endorsing organization: European Society of Endocrinology. Andrology. 2020;8(5):970–87. [DOI] [PubMed] [Google Scholar]
- 142.Yang M, Guan S, Tao J, Zhu K, Lv D, Wang J, et al. Melatonin promotes male reproductive performance and increases testosterone synthesis in mammalian Leydig cells†. Biol Reprod. 2021;104(6):1322–36. [DOI] [PubMed] [Google Scholar]
- 143.Dehdari Ebrahimi N, Shojaei-Zarghani S, Taherifard E, Dastghaib S, Parsa S, Mohammadi N, et al. Protective effects of melatonin against physical injuries to testicular tissue: A systematic review and meta-analysis of animal models. Front Endocrinol (Lausanne). 2023;14:1123999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Cho JH, Bhutani S, Kim CH, Irwin MR. Anti-inflammatory effects of melatonin: A systematic review and meta-analysis of clinical trials. Brain Behav Immun. 2021;93:245–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Chen F, Lu H, Chen P, Zhao X, Guan X, Liang Q, et al. Acute effects of the translocator protein drug ligand FGIN-1-27 on serum testosterone and luteinizing hormone levels in male Sprague-Dawley rats†. Biol Reprod. 2019;100(3):824–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Aghazadeh Y, Martinez-Arguelles DB, Fan J, Culty M, Papadopoulos V. Induction of androgen formation in the male by a TAT-VDAC1 fusion peptide blocking 14-3-3ɛ protein adaptor and mitochondrial VDAC1 interactions. Mol Ther. 2014;22(10):1779–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Martinez-Arguelles DB, Nedow JW, Gukasyan HJ, Papadopoulos V. Oral administration of VDAC1-derived small molecule peptides increases circulating testosterone levels in male rats. Front Endocrinol (Lausanne). 2022;13:1003017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Pedersen BK, Febbraio MA. Muscles, exercise and obesity: skeletal muscle as a secretory organ. Nat Rev Endocrinol. 2012;8(8):457–65. [DOI] [PubMed] [Google Scholar]
- 149.Fiuza-Luces C, Santos-Lozano A, Joyner M, Carrera-Bastos P, Picazo O, Zugaza JL, et al. Exercise benefits in cardiovascular disease: beyond attenuation of traditional risk factors. Nat Rev Cardiol. 2018;15(12):731–43. [DOI] [PubMed] [Google Scholar]
- 150.Furman D, Campisi J, Verdin E, Carrera-Bastos P, Targ S, Franceschi C, et al. Chronic inflammation in the etiology of disease across the life span. Nat Med. 2019;25(12):1822–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Wahid A, Manek N, Nichols M, Kelly P, Foster C, Webster P, et al. Quantifying the Association Between Physical Activity and Cardiovascular Disease and Diabetes: A Systematic Review and Meta-Analysis. J Am Heart Assoc. 2016;5(9):e002495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Jiang X, Savchenko O, Li Y, Qi S, Yang T, Zhang W, et al. A Review of Low-Intensity Pulsed Ultrasound for Therapeutic Applications. IEEE Trans Biomed Eng. 2019;66(10):2704–18. [DOI] [PubMed] [Google Scholar]
- 153.Cui W, Li H, Guan R, Li M, Yang B, Xu Z, et al. Efficacy and safety of novel low-intensity pulsed ultrasound (LIPUS) in treating mild to moderate erectile dysfunction: a multicenter, randomized, double-blind, sham-controlled clinical study. Transl Androl Urol. 2019;8(4):307–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Davidoff MS, Middendorff R, Enikolopov G, Riethmacher D, Holstein AF, Müller D. Progenitor cells of the testosterone-producing Leydig cells revealed. J Cell Biol. 2004;167(5):935–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Stanley E, Lin CY, Jin S, Liu J, Sottas CM, Ge R, et al. Identification, proliferation, and differentiation of adult Leydig stem cells. Endocrinology. 2012;153(10):5002–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Xia K, Chen H, Wang J, Feng X, Gao Y, Wang Y, et al. Restorative functions of Autologous Stem Leydig Cell transplantation in a Testosterone-deficient non-human primate model. Theranostics. 2020;10(19):8705–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Barbonetti A, D’Andrea S, Francavilla S. Testosterone replacement therapy. Andrology. 2020;8(6):1551–66. [DOI] [PubMed] [Google Scholar]
- 158.Handelsman DJ. Global trends in testosterone prescribing, 2000–2011: expanding the spectrum of prescription drug misuse. Med J Aust. 2013;199(8):548–51. [DOI] [PubMed] [Google Scholar]
- 159.Lincoff AM, Bhasin S, Flevaris P, Mitchell LM, Basaria S, Boden WE, et al. Cardiovascular Safety of Testosterone-Replacement Therapy. N Engl J Med. 2023;389(2):107–17. [DOI] [PubMed] [Google Scholar]
- 160.Bhasin S, Travison TG, Pencina KM, O’Leary M, Cunningham GR, Lincoff AM, et al. Prostate Safety Events During Testosterone Replacement Therapy in Men With Hypogonadism: A Randomized Clinical Trial. JAMA Netw Open. 2023;6(12):e2348692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Haddad RM, Kennedy CC, Caples SM, Tracz MJ, Boloña ER, Sideras K, et al. Testosterone and cardiovascular risk in men: a systematic review and meta-analysis of randomized placebo-controlled trials. Mayo Clin Proc. 2007;82(1):29–39. [DOI] [PubMed] [Google Scholar]
- 162.Corona GG, Rastrelli G, Maseroli E, Sforza A, Maggi M. Testosterone Replacement Therapy and Cardiovascular Risk: A Review. World J Mens Health. 2015;33(3):130–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Alexander GC, Iyer G, Lucas E, Lin D, Singh S. Cardiovascular Risks of Exogenous Testosterone Use Among Men: A Systematic Review and Meta-Analysis. Am J Med. 2017;130(3):293–305. [DOI] [PubMed] [Google Scholar]
- 164.Corona G, Maseroli E, Rastrelli G, Isidori AM, Sforza A, Mannucci E, et al. Cardiovascular risk associated with testosterone-boosting medications: a systematic review and meta-analysis. Expert Opin Drug Saf. 2014;13(10):1327–51. [DOI] [PubMed] [Google Scholar]
- 165.Hudson J, Cruickshank M, Quinton R, Aucott L, Aceves-Martins M, Gillies K, et al. Adverse cardiovascular events and mortality in men during testosterone treatment: an individual patient and aggregate data meta-analysis. Lancet Healthy Longev. 2022;3(6):e381–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Soares AH, Horie NC, Chiang LAP, Caramelli B, Matheus MG, Campos AH, et al. Effects of clomiphene citrate on male obesity-associated hypogonadism: a randomized, double-blind, placebo-controlled study. Int J Obes (Lond). 2018;42(5):953–63. [DOI] [PubMed] [Google Scholar]
- 167.Awouters M, Vanderschueren D, Antonio L. Aromatase inhibitors and selective estrogen receptor modulators: Unconventional therapies for functional hypogonadism? Andrology. 2020;8(6):1590–7. [DOI] [PubMed] [Google Scholar]
- 168.Rastrelli G, Vignozzi L, Corona G, Maggi M. Pharmacotherapy of male hypogonadism. Curr Opin Pharmacol. 2023;68: 102323. [DOI] [PubMed] [Google Scholar]
- 169.Cipolla-Neto J, Amaral FGD. Melatonin as a Hormone: New Physiological and Clinical Insights. Endocr Rev. 2018;39(6):990–1028. [DOI] [PubMed] [Google Scholar]
- 170.Martín M, Macías M, Escames G, Reiter RJ, Agapito MT, Ortiz GG, et al. Melatonin-induced increased activity of the respiratory chain complexes I and IV can prevent mitochondrial damage induced by ruthenium red in vivo. J Pineal Res. 2000;28(4):242–8. [DOI] [PubMed] [Google Scholar]
- 171.Jou MJ, Jou SB, Chen HM, Lin CH, Peng TI. Critical role of mitochondrial reactive oxygen species formation in visible laser irradiation-induced apoptosis in rat brain astrocytes (RBA-1). J Biomed Sci. 2002;9(6 Pt 1):507–16. [DOI] [PubMed] [Google Scholar]
- 172.Luboshitzky R, Levi M, Shen-Orr Z, Blumenfeld Z, Herer P, Lavie P. Long-term melatonin administration does not alter pituitary-gonadal hormone secretion in normal men. Hum Reprod. 2000;15(1):60–5. [DOI] [PubMed] [Google Scholar]
- 173.Martin LJ, Touaibia M. Improvement of Testicular Steroidogenesis Using Flavonoids and Isoflavonoids for Prevention of Late-Onset Male Hypogonadism. Antioxidants (Basel). 2020;9(3):237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.therapy V, Aghazadeh Y, Fan J, Campioli E, Zirkin B, Midzak A. Translocator protein-mediated pharmacology of cholesterol transport and steroidogenesis. Mol Cell Endocrinol. 2015;408:90–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Rupprecht R, Rammes G, Eser D, Baghai TC, Schüle C, Nothdurfter C, et al. Translocator protein (18 kD) as target for anxiolytics without benzodiazepine-like side effects. Science. 2009;325(5939):490–3. [DOI] [PubMed] [Google Scholar]
- 176.Aghazadeh Y, Ye X, Blonder J, Papadopoulos V. Protein modifications regulate the role of 14-3-3γ adaptor protein in cAMP-induced steroidogenesis in MA-10 Leydig cells. J Biol Chem. 2014;289(38):26542–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.