Abstract
Background
Gestational diabetes mellitus (GDM) and insulin resistance (IR) increase the risk of adverse pregnancy outcomes. We aimed to examine the relationship of interstitial glucose assessed by continuous glucose monitoring (CGM) at early gestation, and the subsequent development of IR and GDM, and to determine 24-h interstitial glucose centile distributions in women with normal (non-IR and non-GDM) and suboptimal glycemic status (IR and/or GDM).
Methods
CGM measurements were taken for 3–10 days at 18–24 weeks’ gestation, followed by fasting serum insulin and oral glucose tolerance testing at 24–28 weeks’ gestation. IR and GDM were determined by the updated Homeostasis Model Assessment of IR score of ≥ 1.22 and 2013 World Health Organization criteria, respectively. Risks of IR and GDM were estimated using modified Poisson models, and hourly interstitial glucose centiles determined using Generalized Additive Models for Location, Scale and Shape.
Results
This prospective cohort study involved 167 pregnant women in Singapore, with a mean age of 31.7 years, body mass index of 22.9 kg/m2, and gestation of 20.3 weeks. 25% of women exhibited IR and 18% developed GDM. After confounders adjustment, women with suboptimal glycemic control, indicated by higher mean daily glucose (risk ratio 1.42; 95% confidence interval 1.16, 1.73), glucose management indicator (1.08; 1.03, 1.12), and J-index (1.04; 1.02, 1.06), as well as those with greater glycemic variability, indicated by higher standard deviation (1.69; 1.37, 2.09), coefficient of variation (1.03; 1.00, 1.06), and mean amplitude of glycemic excursions (1.4; 1.14, 1.35) derived from CGM in early gestation were associated with higher risks of developing IR in later gestation. These associations were similarly observed for the development of GDM. Centile curves showed that, compared to those with normal glycemic status, women with suboptimal glycemic status had higher glucose levels, with greater fluctuations throughout 24 h.
Conclusions
In pregnant women who subsequently developed IR and GDM, interstitial glucose levels assessed by CGM were elevated and varied greatly. This supports the potential use of CGM to screen for glycemic changes early in pregnancy.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13098-024-01508-4.
Keywords: Continuous glucose monitoring, Glycemic control/variability, Gestational diabetes mellitus, Insulin resistance
Background
To ensure sufficient glucose supply for the growing fetus, maternal insulin resistance (IR) increases during pregnancy, especially in the latter half of the second trimester due to placental hormone effects [1, 2]. High IR is associated with increased risk of adverse outcomes, including pre-eclampsia, cardiovascular issues in mothers [3], and large-for-gestational-age births and childhood obesity in offspring [3]. Moreover, elevated IR contributes to gestational diabetes mellitus (GDM) [4] development, which can lead to a range of both short- and long-term complications in mother–child [5]. The prevalence of GDM has been increasing and affects approximately 14% of pregnancies worldwide [6]. GDM is currently diagnosed based on an oral glucose tolerance test (OGTT) between 24 and 28 weeks’ gestation [7]. Studies have shown that glycemic dysregulation early in pregnancy heightens the risks of adverse maternal-child health outcomes [8], highlighting the need for early screening and intervention [9]. However, there are no widely recognized and accepted clinical tools available to detect early gestational glycemic changes prior to the onset of elevated IR or GDM development, which could enable timely intervention.
The use of continuous glucose monitoring (CGM) in women with GDM has been explored increasingly in recent years [10]. Compared to OGTT which only measures glucose tolerance at a single time point during pregnancy, CGM provides a dynamic overview of glucose fluctuations throughout 24 h. Hence, using CGM to identify glycemic control at earlier stages of pregnancy may facilitate timely interventions to prevent poor glycemic outcomes in later pregnancy. Indeed, studies have demonstrated that women with GDM exhibited poorer glycemic control and glycemic variability as assessed through CGM [10–15]. However, even though current guidelines have shed light on the glycemic targets for pregnant women with type one diabetes mellitus [16], there is a lack of evidence for CGM targets in women with normal pregnancies. Furthermore, these studies had either a cross-sectional or case–control design, and only assessed CGM-derived parameters after GDM diagnosis. Therefore, it remains uncertain if the poorer glycemic control and glycemic variability precedes GDM diagnosis and if these parameters can be used in early pregnancy to screen for subsequent GDM diagnosis. There has only been one previous study that assessed the use of CGM-derived parameters as a possible predictor of subsequent GDM diagnosis. This prospective observational study conducted in Singapore reported higher CGM-derived glycemic variability indices in the first and second trimesters in women who were subsequently diagnosed with GDM in the third trimester [17]. However, the study was constrained by a small sample size and limited GDM cases.
In this study with a larger dataset, we aimed to determine the potential use of CGM glycemic patterns as an early screening tool to identify pregnant women at-risk of developing abnormal glycemic states (IR and/or GDM). We hypothesized that interstitial glucose levels, assessed by CGM, will be elevated, and display significant variability over 24 h in pregnant women who will subsequently develop IR and GDM. To test this hypothesis, we sought (i) to examine the relationships of CGM-derived interstitial glucose measurements assessed at mean 20 weeks’ gestation with IR and GDM status ascertained at a mean of 25 weeks’ gestation, and (ii) to determine 24-h interstitial glucose centile distributions in women with normal (non-IR and non-GDM) and suboptimal (IR and/or GDM) glycemic status.
Methods
Study design
We used data from a prospective cohort study designed to examine nocturnal eating pattern and glucose metabolism among pregnant women (NCT03803345) [18]. The study was conducted at KK Women’s and Children’s Hospital (KKH), Singapore, and recruitment took place between March 2019 and October 2021. The study adhered to the principles outlined in the Declaration of Helsinki and the findings were reported following the Strengthening the Reporting of Observational Studies in Epidemiology guidelines [19].
Ethical approval
The Centralized Institutional Review Board of SingHealth approved the study (Reference 2018/2529). All participants provided written informed consent.
Participants
Women were eligible for the study if they were between 18- and 24-weeks’ gestation, aged 18 years and above, had Singapore citizenship or Singapore permanent residence status. We excluded women who were diagnosed with GDM at recruitment, had pre-existing Type 1 or 2 diabetes, were on routine night-shift work, used anticonvulsant medications or oral steroids in the past month, had known or suspected allergy to medical grade adhesives. Additionally, women who were diagnosed with chronic kidney disease, preeclampsia, or had multiple pregnancies were not included due to the lack of evidence supporting the accuracy of the CGM device (Freestyle Libre Pro, Abbott, Germany) during pregnancy.
Study procedures and data collection
The study procedures, detailed elsewhere [18], involved a baseline assessment between 18 and 24 weeks gestation. This included collection of data on sociodemographic characteristics (age, ethnicity, education), physical activity (assessed by the International Physical Activity Questionnaire-Short Form [20], allowing calculation of metabolic equivalent of task score [MET-min]), meal regularity (frequency of skipped and/or delayed meals per week) [21], history of GDM, and family diabetes history. Pre-pregnancy body mass index (BMI) was calculated using self-reported weight (kg) divided by height squared (m2) (measured by the SECA 213 stadiometer, Germany). Participants were fitted with a blind CGM sensor (Freestyle Libre Pro, Abbott, Germany) on the posterior upper arm, recording interstitial glucose levels every 15 min for up to 10 days. At 24 to 28 weeks’ gestation, participants underwent a 3-point (0, 1-, and 2-h) 75-g OGTT. Plasma glucose and fasting insulin were assessed using the Abbott Alinity c glucose enzymatic (Hexokinase) assay (Germany) and the Abbott Alinity insulin immunochemiluminometric assay (Germany), respectively. Blood samples were analyzed within one hour of collection at the KKH laboratory, following standardized clinical protocols.
Assessment of CGM parameters
We downloaded the interstitial glucose values from the LibreView software and used EasyGV (version 8) [22] to derive glycemic control and glycemic variability indices from at least 3 complete 24-h CGM readings. The glycemic control indices included mean daily glucose (mmol/L), glucose management indicator (GMI; mmol/mol) [23], J-index [24], percentage of time in range 3.5–7.8 mmol/L (TIR) [25], percentage of time above the target range 7.8 mmol/L (TAR) [25], and percentage of time below the target range 3.5 mmol/L (TBR) [25]. In this study, the term mean daily glucose is used to refer to mean interstitial glucose levels measured by CGM. The glycemic variability indices included standard deviation (SD; mmol/L), coefficient of variation (CV; %), and mean amplitude of glucose excursions (MAGE; mmol/L). MAGE quantifies blood glucose variability by calculating the average of significant upward or downward excursions that surpass a defined threshold [26–29]. This threshold is determined by the SD of blood glucose over a 24-h period.
Assessment of IR and GDM
Insulin resistance is determined using the HOMA calculator [30] where we categorized participants with IR if they had an updated Homeostasis Model Assessment for IR (HOMA2-IR) [30] score of at least 1.22, following cutoff points for prediabetes [31]. GDM diagnosis was based on the 2013 World Health Organization criteria [32]: fasting glucose ≥ 5.1 mmol/L, or 1-h glucose ≥ 10.0 mmol/L, or 2-h glucose ≥ 8.5 mmol/L. When OGTT results were unavailable, we retrieved GDM diagnosis from delivery records.
Statistical analysis
We performed statistical analyses using Stata version 16 (StataCorp LLC, College Station, TX) and R programming (R Foundation for Statistical Computing, Vienna, Austria). We compared baseline characteristics and CGM-derived glycemic values of participants based on their IR and GDM status using an independent t-test or Pearson’s Chi-squared test, as appropriate. In addition, we further compared the CGM values according to baseline characteristics. The CGM values were log(e)-transformed and presented in geometric mean, with 95% confidence intervals (CI).
We applied modified Poisson regression models with covariate adjustment to examine the associations of glycemic control and glycemic variability indices derived from CGM with the risk of IR and GDM. Results are presented as risk ratios (RRs) and 95% CI. Models were adjusted for age (continuous), ethnicity (Chinese vs. non-Chinese), years of education (continuous), parity (nulliparous vs. multiparous), history of GDM or family history of diabetes (no vs. yes), pre-pregnancy BMI (continuous), irregular meal (no < 3 times vs. yes ≥ 3 times skipped or delayed meals per week), and physical activity (< 600 vs. ≥ 600 MET-min/week) [33], These potential confounders were identified from previous literature [33–35] and based on the disjunctive cause criteria, [36] guided by a directed acyclic graph. We used Stata to implement the models.
We used generalized estimating equations (GEE) with an exchangeable covariance structure and an identity link function to examine the differences in predicted mean estimates of median interstitial glucose levels over 24 h based on glycemic status. GEE accounts for the non-independence of multiple interstitial glucose measurements. In the GEE model, we treated glycemic status as a covariate and adjusted for the same variables as in the modified Poisson models, plus an interaction term between glycemic status and time. Additionally, we performed a sensitivity analysis by including only participants with complete 10-day 24-h CGM readings. The interstitial glucose values were loge-transformed before analyses. Effect estimates are presented as geometric mean ratios (GMRs), with respective 95% CI. We used the geepack package in R [37–39] to implement the models.
To better understand the distribution of interstitial glucose levels by glycemic status, we fitted centile curves for the CGM readings against time in participants with normal (non-IR and non-GDM) and suboptimal (IR and/or GDM) glycemic status, using the generalized additive model for location, scale and shape (GAMLSS) [40] at the 2.5th, 5th, 25th, 50th, 75th, 95th and 97.5th percentile. Within the GAMLSS framework, we modelled predicted glucose levels as a non-linear function of time using the Box-Cox t (BCT) distribution [40] with four parameters: mu (μ), the median of the distribution; sigma (σ), approximately the coefficient of variation; nu (ν), which controls for skewness; and tau (τ), which controls for the kurtosis of the distribution. We used the gamlss package in R [40] to derive the curves.
Results
Participant characteristics
Of the 300 women enrolled, 172 women completed at least 3 days of CGM at 18–24 weeks gestation with an average of 828 CGM readings (200 to 1217 readings). After excluding five women without GDM status data and nine without IR status data, the final sample sizes were 167 for the GDM outcome analysis and 158 for the IR outcome analysis, respectively (Additional file 1). There were 112 women (67.1%) with at least 10 days of CGM data. 40 women out of 158 (25.3%) had IR, 30 out of 167 (18.0%) had GDM, and 62 out of 167 (37.1%) had suboptimal glycemic status (IR and/or GDM). Women with IR had fewer years of education (14.0 vs. 14.5 years) and a higher BMI before pregnancy (25.3 vs 22.0 kg/m2) than their non-IR counterparts. There were no significant differences in baseline characteristics between GDM and non-GDM women (Table 1). When comparing characteristics of included (n = 167) and excluded women (n = 133), included women were older (31.7 vs. 30.3 years) and had higher years of education (14.7 vs. 13.8 years) (Additional file 2).
Table 1.
Baseline characteristics of pregnant women by glycemic status
| Characteristics | Total (n = 167) | Non-IR (n = 118) | IR (n = 40) | p | Non-GDM (n = 137) | GDM (n = 30) | p |
|---|---|---|---|---|---|---|---|
| Gestation at enrolment, weeks | 20.3 ± 0.5 | 20.3 ± 0.4 | 20.4 ± 0.8 | 0.393 | 20.31 ± 0.5 | 20.5 ± 0.8 | 0.293 |
| Age, years | 31.7 ± 4.2 | 32.1 ± 4.3 | 30.8 ± 3.7 | 0.063 | 31.5 ± 4.1 | 32.8 ± 4.5 | 0.152 |
| Ethnicity, n (%) | 0.541 | 0.781 | |||||
| Chinese | 142 (85.0) | 102 (86.4) | 33 (82.5) | 116 (84.7) | 26 (86.7) | ||
| Non-Chinese | 25 (15.0) | 16 (13.6) | 7 (17.5) | 21 (15.3) | 4 (13.3) | ||
| Education, years | 14.7 ± 2.2 | 14.5 ± 2.3 | 14.0 ± 1.9 | 0.012 | 14.7 ± 2.1 | 14.6 ± 3.0 | 0.766 |
| Parity, n (%) | 0.870 | 0.455 | |||||
| Nulliparous | 107 (64.1) | 75 (63.6) | 26 (65.0) | 86 (62.8) | 21 (70.0) | ||
| Multiparous | 60 (35.9) | 43 (36.4) | 14 (35.0) | 51 (37.2) | 9 (30.0) | ||
| History of GDM or family history of diabetes, n (%) | 0.711 | 0.864 | |||||
| No or not applicable | 126 (75.4) | 88 (74.6) | 31 (77.5) | 103 (75.2) | 23 (76.7) | ||
| Yes | 41 (24.6) | 30 (25.4) | 9 (22.5) | 34 (24.8) | 7 (23.3) | ||
| Pre-pregnancy BMI, kg/m2 | 22.9 ± 3.9 | 22.0 ± 3.4 | 25.3 ± 4.2 | < 0.001 | 22.8 ± 4.0 | 23.5 ± 3.7 | 0.335 |
| Irregular meal, n (%) | 0.131 | 0.864 | |||||
| No | 130 (77.8) | 96 (81.4) | 28 (70.0) | 107 (78.1) | 23 (76.7) | ||
| Yes | 37 (22.2) | 22 (18.6) | 12 (30.0) | 30 (21.9) | 7 (23.3) | ||
| Physical activity, n (%) | 0.200 | 0.338 | |||||
| Active (≥ 600 MET-min/week) | 123 (73.7) | 82 (69.5) | 32 (80.0) | 103 (75.2) | 20 (66.7) | ||
| Inactive (< 600 MET-min/week) | 44 (26.3) | 36 (30.5) | 8 (20.0) | 34 (24.8) | 10 (33.3) |
Continuous data are presented in mean ± SD and categorical data are presented in frequency and percentages. Nine women without IR status data were excluded. IR insulin resistance based on HOMA2-IR at least 1.22. GDM, gestational diabetes mellitus based on 2013 WHO criteria, BMI body mass index, MET metabolic equivalent of task
p-value derived from independent t-test or Pearson’s Chi-squared test, as appropriate
CGM-derived glycemic measures, IR and GDM development
Glycemic control and glycemic variability indices derived from CGM were assessed at a mean of 20 weeks’ gestation, according to IR and GDM status ascertained at a mean of 25 weeks’ gestation (Table 2). Women with IR had poorer glycemic control, indicated by higher mean daily glucose levels, GMI, and J-index, as well as lower TBR (all p < 0.05). Women with GDM had poorer glycemic control, indicated by higher mean daily glucose, GMI, J-index, TAR, and lower TBR; these women also had a greater glycemic variability, indicated by higher SD, CV, and MAGE (all p < 0.05). After adjustment for socio-demographic and lifestyle factors, the majority of glycemic control and glycemic variability indices remained associated with the risks of IR and GDM. In particular, mean daily glucose and SD were associated with the highest risk of IR (1.42; 95% CI 1.16, 1.73, and 1.69; 95% CI 1.37, 2.09 respectively) and GDM (1.84; 95% CI 1.45, 2.33, and 2.37; 95% CI 1.66, 3.38 respectively) (Table 3). When comparing with baseline characteristics, the non-Chinese women have a lower TIR but higher TBR than Chinese women and women who skipped/delayed at least 3 meals per week have lower mean daily glucose, J-index, TIR and higher TBR than women with regular meals (all p < 0.05). (Additional file 3).
Table 2.
Comparison of CGM-derived glycemic control and variability indices in pregnant women based on glycemic status
| CGM Measures | Total (n = 167) | Non-IR (n = 118) | IR (n = 40) | p | Non-GDM (n = 137) | GDM (n = 30) | p |
|---|---|---|---|---|---|---|---|
| Glycemic control index | |||||||
| Mean daily glucose, mmol/L | 4.46 (4.37, 4.56) | 4.35 (4.26, 4.43) | 4.84 (4.57, 5.12) | 0.003 | 4.35 (4.27, 4.43) | 5.02 (4.69, 5.36) | 0.002 |
| GMI, mmol/mol | 33.79 (33.34, 34.24) | 33.21 (32.82, 33.61) | 35.60 (34.25, 36.99) | 0.003 | 33.23 (32.86, 33.61) | 36.44 (34.79, 38.16) | 0.002 |
| J-index | 9.86 (9.43, 10.32) | 9.29 (8.93, 9.67) | 11.68 (10.21, 13.36) | 0.017 | 9.26 (8.92, 9.61) | 13.17 (11.32, 15.31) | 0.006 |
| TIR, % | 77.82 (74.74, 81.02) | 76.86 (73.36, 80.51) | 81.91 (74.55, 89.99) | 0.073 | 77.63 (74.42, 80.97) | 78.68 (69.67, 88.86) | 0.587 |
| TAR, % | 0.93 (0.72, 1.21) | 0.74 (0.56, 0.97) | 1.52 (0.77, 3.01) | 0.092 | 0.64 (0.50, 0.82) | 2.70 (1.57, 4.65) | 0.033 |
| TBR, % | 10.83 (8.96, 13.09) | 12.34 (9.86, 15.44) | 6.50 (4.43, 9.53) | < 0.001 | 11.64 (9.50, 14.27) | 7.32 (4.37, 12.26) | 0.003 |
| Glycemic variability index | |||||||
| SD, mmol/L | 1.03 (1.00, 1.08) | 0.99 (0.96, 1.03) | 1.13 (1.01, 1.27) | 0.056 | 0.98 (0.95, 1.01) | 1.33 (1.18, 1.50) | 0.002 |
| CV, % | 23.19 (22.47, 23.92) | 22.88 (22.09, 23.69) | 23.44 (21.64, 25.39) | 0.457 | 22.52 (21.83, 23.24) | 26.46 (24.23, 28.90) | 0.009 |
| MAGE, mmol/L | 2.70 (2.60, 2.81) | 2.62 (2.51, 2.72) | 2.93 (2.62, 3.27) | 0.080 | 2.56 (2.47, 2.65) | 3.46 (3.07, 3.90) | 0.002 |
Data are presented as geometric mean (95% confidence interval). Nine women without IR status data were excluded. CGM, continuous glucose monitoring; IR, insulin resistance based on HOMA2-IR at least 1.22; GDM gestational diabetes mellitus based on 2013 WHO criteria, GMI, glucose management indicator, TIR percentage of time in range 3.5–7.8 mmol/L, TAR percentage of time above target range 7.8 mmol/L, TBR percentage of time below target range 3.5 mmol/L, SD standard deviation, CV coefficient of variation, MAGE mean amplitude of glycemic excursions
p-value derived from independent t-test from log-transformed CGM-derived glycemic indices
Table 3.
Associations between CGM-derived glycemic control and variability indices with risk of IR and GDM (n = 167)
| CGM Measures | Risk Ratio (95% CI) | |||
|---|---|---|---|---|
| IR | GDM | |||
| Model 1 (crude) | Model 2 (adjusted) | Model 1 (crude) | Model 2 (adjusted) | |
| Glycemic control index | ||||
| Mean daily glucose, mmol/L | 1.42 (1.21, 1.66) | 1.42 (1.16, 1.73) | 1.62 (1.33, 1.96) | 1.84 (1.45, 2.33) |
| GMI, mmol/mol | 1.08 (1.04, 1.11) | 1.08 (1.03, 1.12) | 1.11 (1.06, 1.15) | 1.14 (1.08, 1.20) |
| J-index | 1.04 (1.03, 1.06) | 1.04 (1.02, 1.06) | 1.06 (1.04, 1.08) | 1.07 (1.05, 1.09) |
| TIR, % | 1.01 (1.00, 1.03) | 1.01 (1.00, 1.03) | 1.01 (0.99, 1.03) | 1.00 (0.98, 1.03) |
| TAR, % | 1.02 (1.01, 1.03) | 1.02 (1.01, 1.03) | 1.03 (1.02, 1.04) | 1.04 (1.02, 1.06) |
| TBR, % | 0.98 (0.96, 0.99) | 0.98 (0.96, 0.99) | 0.97 (0.94, 1.00) | 0.97 (0.94, 0.99) |
| Glycemic variability index | ||||
| SD, mmol/L | 1.62 (1.32, 1.99) | 1.69 (1.37, 2.09) | 2.21 (1.63, 3.01) | 2.37 (1.66, 3.38) |
| CV, % | 1.02 (0.99, 1.05) | 1.03 (1.00, 1.06) | 1.06 (1.04, 1.09) | 1.07 (1.04, 1.10) |
| MAGE, mmol/L | 1.20 (1.11, 1.31) | 1.24 (1.14, 1.35) | 1.39 (1.21, 1.58) | 1.42 (1.23, 1.65) |
Data were analyzed using modified Poisson regression models to examine the associations between CGM indices with IR and GDM. Models 2 were adjusted for age, ethnicity, years of education, parity, history of GDM or family history of diabetes, pre-pregnancy body mass index, irregular meal, and physical activity. CI confidence interval, IR insulin resistance based on HOMA2-IR at least 1.22, GDM gestational diabetes mellitus based on 2013 WHO criteria, GMI glucose management indicator, TIR percentage of time in range 3.5–7.8 mmol/L, TAR percentage of time above target range 7.8 mmol/L, TBR percentage of time below target range 3.5 mmol/L, SD standard deviation, CV coefficient of variation, MAGE mean amplitude of glycemic excursions
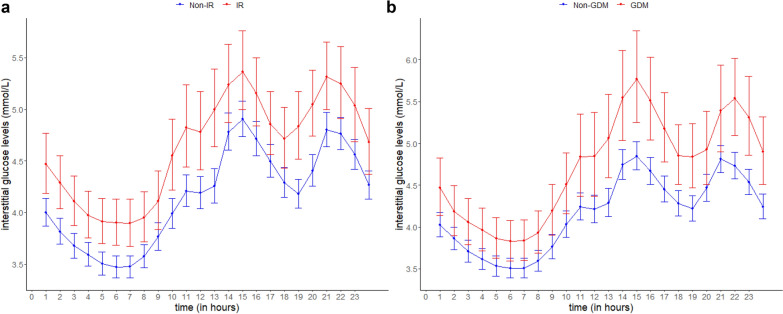
When interstitial glucose levels over a 24-h period were compared between women according to their glycemic status, those with IR showed 12% higher glucose levels (GMR 1.12; 95% CI 1.05, 1.19), equivalent to a difference of 0.45 mmol/L, than their counterparts without IR, after adjustment for potential confounders. Similarly, women with GDM showed 11% higher glucose levels over 24 h (1.11; 1.03, 1.20), equivalent to a difference of 0.46 mmol/L, compared to those without GDM. When the hourly adjusted means of the median interstitial glucose levels were plotted throughout 24 h, consistently higher glucose levels were observed in women who later developed IR or GDM compared to their normal counterparts (Fig. 1). When analysis was restricted to women with complete 10 day 24-h CGM readings (n = 112), women with IR and GDM respectively showed 12% (1.12; 1.03, 1.21) and 9% (1.09; 0.99, 1.20) higher glucose levels across 24 h than their normal counterparts (Additional file 4).
Fig. 1.
The predicted 24-h interstitial glucose levels for women by (a) IR (GMR 1.12; 95% CI 1.05, 1.19) and (b) GDM status (1.11; 1.03, 1.20) based on the GEE analysis. Red represents the women with IR or GDM and blue represents the women with non-IR or non-GDM. The circle markers and capped vertical lines represent the predicted mean daily glucose levels and the respective 95% CI based on the exponentiated log-transformed hourly median glucose values. Models were adjusted for age, ethnicity, years of education, parity, history of GDM or family history of diabetes, pre-pregnancy body mass index, irregular meal, physical activity, and an interaction term between glycemic status and time. CI confidence intervals, GDM gestational diabetes mellitus based on 2013 World Health Organization criteria, GEE generalized estimating equations, GMR geometrical mean ratio, IR insulin resistance based on HOMA2-IR of at least 1.22
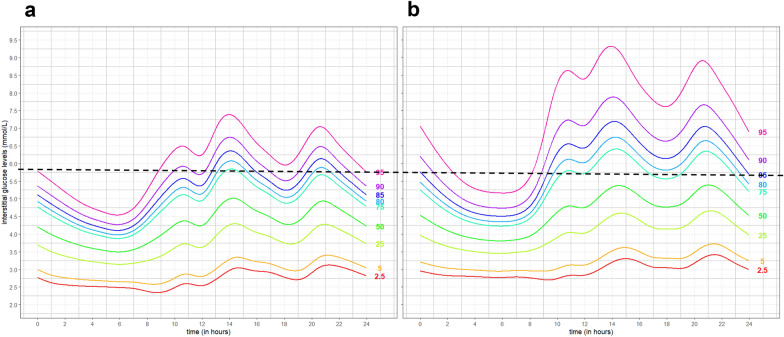
24-h interstitial glucose centile distributions in women with normal and suboptimal glycemic measures
Centile curves of the interstitial glucose readings for women with normal and suboptimal glycemic status were calculated using the BCT models (Fig. 2). These curves illustrate the higher glucose levels and greater fluctuations throughout 24 h in women with suboptimal glycemic status when compared to those with normal glycemic status. The centile curves demonstrated a distinct diurnal pattern of interstitial glucose levels for both groups of women. From midnight onwards, interstitial glucose levels gradually declined, reaching their lowest point at around 6 am. Starting from 7 am, there was a noticeable increase in glucose levels, peaking between 11 am and 2 pm. The glucose levels decreased until 7 pm, at which point they began to rise again, reaching a second peak at around 9 pm. In the subsequent hours, there was a tapering off as the levels decreased, aligned with the onset of the nocturnal phase. The 95th centile among pregnant women without IR and GDM corresponds to the 85th centile among those with IR and/or GDM. Thus, above this cutoff, pregnant women are three times more likely to have IR and/or GDM.
Fig. 2.
The centile curves of 24-h interstitial glucose levels from Box-Cox transformation among pregnant women with (a) non-IR and non-GDM, and (b) IR and/or GDM. A line across the 95th centile for pregnant women without IR and GDM corresponds to the 85th centile among those with IR and/or GDM, which suggests that those above the 95th centile are three times more likely to have IR and/or GDM. GDM, gestational diabetes mellitus based on 2013 World Health Organization criteria; IR, insulin resistance based on HOMA2-IR of at least 1.22
Discussion
Main findings
This study has shown that women who later experienced elevated IR or developed GDM exhibited higher pre-diagnosis interstitial glucose levels with greater variability throughout the 24-h period. The generated centile curves similarly showed elevated levels and variability in interstitial glucose among women with suboptimal glycemic status, setting them apart from those with normal status, even before IR or GDM diagnosis. These findings support the existing evidence advocating early CGM screening for subsequent glucose dysregulation and identify pregnant women at risk of developing elevated IR or GDM in later stages of pregnancy. Notably, most baseline characteristics, such as pre-pregnancy BMI, maternal age, parity, history of GDM or family history of diabetes, are not significantly associated with CGM parameters.
Comparison with other studies
We provide new evidence showing poorer glycemic control assessed by CGM in earlier gestation among women with elevated IR in later gestation. We also demonstrate poorer glycemic control in early gestation among women subsequently diagnosed with GDM. The only study which examined the prospective association between CGM-derived glycemic control and subsequent development of GDM showed a non-significant association [17]. However, in a previous observational study of pregnant women already diagnosed with GDM, mean daily glucose was reported to be higher compared to healthy pregnant women between 24 and 36 weeks of gestation using the flash glucose monitoring system [11]. Another study reported significant associations only in the second trimester, but not the third [12], while other studies reported null associations [25, 41, 42]. Our study was able to demonstrate the utility of using CGM to monitor glycemic control levels in pregnancy and address the gap in literature with regard to the prospective association of glycemic control with IR and GDM development. In the hyperglycemia and adverse pregnancy outcomes (HAPO) study, it was reported that there was a strong and continuous relationship between maternal blood glucose level and adverse maternal and neonatal outcomes [43]. Hence, monitoring glycemic control levels earlier in pregnancy using CGM may pave the way for earlier interventions and improve glycemic parameters, and thus maternal and neonatal outcomes. Notably, TBR was elevated in patients without IR or GDM at 12.3% and 11.6% respectively. Our findings were similar to a local observational study [17] with a smaller sample size of 39, which utilized early pregnancy CGM measurement in the first and second trimester to predict GDM diagnosis. In that study, the patients without GDM had a higher TBR of 30.2% compared to their GDM counterpart with a TBR of 26.9%. Currently, there is a lack of evidence on CGM targets including TBR for women with normal pregnancy [16]. However, other studies have suggested that more stringent targets [44] and closer monitoring of overnight glucose profiles may be required to improve outcomes in pregnant women with GDM [45].
At present, little is known about the association between glycemic variability and IR in pregnant women. This study provides new evidence, showing that women exhibiting greater glycemic variability are at an increased risk of developing IR. Our findings also reveal increased glycemic variability in women later diagnosed with GDM. This aligns with a prospective observational study conducted in Singapore in 2022, which identified higher first and second trimester glycemic variability indices (SD and MAGE) in women subsequently diagnosed with GDM, compared to those without a diagnosis [17]. However, it is noteworthy that in that study, the associations of glycemic variability indices with GDM development were inconsistent, perhaps as a result of the small sample size (n = 60). In contrast, our study consistently demonstrated universally elevated glycemic variability indices in women subsequently diagnosed with GDM. Other case–control studies also showed higher MAGE levels in pregnant women who were diagnosed with GDM than those who were not [10, 12]. Monitoring glycemic variability is especially important in pregnancy due to the established association of poorer glycemic variability with poorer pregnancy outcomes [46]. Poor glycemic variability as indicated by elevated MAGE has been associated with adverse maternal and neonatal outcomes, including large or small for gestational age, higher birth weight, and neonatal hypoglycemia [47]. As such, this study offers much-needed evidence supporting the use of CGM to monitor glycemic variability in early pregnancy and its association with subsequent development of IR and GDM.
The 24-h interstitial glucose centile curves showed higher mean daily glucose levels at all time points of the day and greater glucose fluctuations in women with suboptimal glycemic status (IR and/or GDM). The greatest glucose fluctuation was observed to be during the day, followed by a physiological dip in blood glucose level at night. This physiological dip is consistent with the effects of the circadian rhythm on glucose homeostasis as the metabolic demand of the body is lower during sleep. Similarly, a diurnal elevation can be attributed to factors such as increased food intake, physical activity, and other metabolic processes that are active during waking hours [48]. As such, this variation prompts the development of different blood glucose threshold levels for day and night to allow patients and their physicians to monitor glycemic control and glycemic variability. This may facilitate time-specific lifestyle interventions or medications when glycemic levels are poor. In addition, CGM measures were more clearly differentiated between GDM and non-GDM but not so clearly between IR and non-IR. It is plausible that the greater degree of overlap between non-IR and IR women is due to the compensatory hyperinsulinemia in IR women which helps to maintain lower interstitial glucose levels, compared to the decompensated status of those who developed GDM.
Additionally, the development of these centile curves provides a proof of concept for its potential utility as a reference curve to screen for abnormal glycemic regulations in early gestation. This paves the way for the comparison of an individual’s interstitial glucose levels to those of a reference population. Using the 95th centile for pregnant women without IR and GDM, levels above which corresponds to a three-fold increase in risk of IR and/or GDM. This provides a theoretical basis for future studies to validate the use of standardized centile curves for triaging and monitoring women with normal or suboptimal glycemic status. Early screening and intervention is essential to reduce the potential adverse health outcomes to both mother and child [9]. Hence, development of such standardized curves could potentially facilitate early pregnancy screening and interventions to reduce the adverse outcomes associated with suboptimal glycemic status.
Strengths and limitations
A major strength in our study lies in its prospective design, which enabled us to assess the associations between CGM-derived data at 18 to 24 weeks gestation and subsequently diagnosed IR and/or GDM at 24 to 28 weeks. To the best of our knowledge, this is the first study examining the association between CGM-derived data and subsequent development of IR in pregnant women. This is also the first study to have generated centile curves which could potentially differentiate between normal individuals and those at risk of developing IR and/or GDM later in pregnancy. While our study benefits from a comparatively larger sample size for women undergoing CGM than those reported in previous studies [12, 17], we acknowledge the necessity of expanding the sample size in future studies to enhance the generalizability and facilitate subgroup analyses. The association of fasting intervals, particularly from the perspective of time-restricted feeding, with CGM parameters was not explored in this study. Given the potential importance of such data, we recommend this as a promising direction for future research to gain deeper insights into how specific eating-fasting intervals influence glycemic trends and pregnancy outcomes. Furthermore, we were unable to consider maternal weight gain during pregnancy in the analysis due to incomplete data collected. While gestational weight gain has been reported as a risk factor for increased GV at late pregnancy in women with GDM, its effects in the early pregnancy remain less clear [25].
Conclusions
Taken together, higher mean daily glucose over 24 h as well as poorer glycemic control and glycemic variability in early gestation are associated with subsequent diagnosis of IR and GDM. The CGM-derived glycemic control and glycemic variability parameters along with the 24-h interstitial glucose centile curves demonstrate the potential utility of CGM as a tool in early pregnancy to screen for subsequent suboptimal glycemic status. This paves the way for early initiation of lifestyle interventions to improve glycemic regulation and reduce the risk of adverse maternal and neonatal outcomes.
Supplementary Information
Additional file 1. Participant recruitment
Additional file 2. Baseline characteristics of women included and excluded from the study
Additional file 3. Comparison of CGM-derived glycemic control and variability indices in pregnant women based on baseline characteristics
Additional file 4. The predicted 24-hour interstitial glucose levels for women with at least 10 days of CGM readings by (a) IR (GMR 1.12; 95% CI 1.03, 1.21) and (b) GDM status (1.09; 0.99, 1.20) based on the GEE analysis. Red represents the women with IR or GDM and blue represents the women with non-IR or non-GDM. The circle markers and capped vertical lines represent the predicted mean daily glucose levels and the respective 95% CI based on the exponentiated log-transformed hourly median glucose values. Models were adjusted for age, ethnicity, years of education, parity, history of GDM or family history of diabetes, pre-pregnancy body mass index, irregular meal, physical activity, and an interaction term between glycemic status and time. CI confidence intervals, GDM gestational diabetes mellitus based on 2013 World Health Organization criteria, GEE generalized estimating equations, GMR geometrical mean ratio, IR insulin resistance based on HOMA2-IR of at least 1.22.
Acknowledgements
We thank KKH for the institutional support received during this study. We also thank the pregnant women who participated in the study, and the clinical research coordinators, research officers, and healthcare providers who have been committed to this study.
Abbreviations
- GDM
Gestational diabetes mellitus
- IR
Insulin resistance
- CGM
Continuous glucose monitoring
- OGTT
Oral glucose tolerance test
- MET
Metabolic equivalent of task
- BMI
Body mass index
- GMI
Glucose management indicator
- TIR
Time in range
- TAR
Time above range
- TBR
Time below range
- SD
Standard deviation
- CV
Coefficient of variation
- MAGE
Mean amplitude of glucose excursions
- HOMA2-IR
Homeostasis Model Assessment for IR
- RR
Risk ratios
- CI
95% Confidence interval
- GEE
Generalized estimating equations
- GMR
Geometric mean ratios
- GAMLSS
Generalized additive model for location, scale and shape
- BCT
Box-Cox t
Author contributions
FY, NL, and SLL conceived and designed the study. CWK, JKYC and FY contributed to the study implementation. YBC advised on statistical analysis. RZT cleaned the data. RZT, LWC and SLL analyzed the data. CWK, HYT and JYQL wrote the first draft of the manuscript. All authors contributed to the interpretation of the results and critical revision of the manuscript for important intellectual content and approved the final version of the manuscript. The corresponding authors attest that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted. SLL obtained funding and is the study guarantor and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Funding
This study was supported by the Singapore Ministry of Health’s National Medical Research Council under its Open Fund-Young Individual Research Grant (NMRC/OFYIRG/0082/2018). CWK and JKYC are supported by the National Medical Research Council, Ministry of Health, Singapore (NMRC/MOH-000596-00 and NMRC/CSA-SI-008-2016, MOH-001266-01, MOH-001221-01 and MOH-000932-01, respectively). KMG is supported by the UK Medical Research Council (MC_UU_12011/4), the National Institute for Health Research (NIHR Senior Investigator (NF-SI-0515-10042) and NIHR Southampton Biomedical Research Centre (IS-BRC-1215-20004)), the European Union (Erasmus + Programme ImpENSA 598488-EPP-1-2018-1-DE-EPPKA2-CBHE-JP) and the British Heart Foundation (RG/15/17/3174, SP/F/21/150013). The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. For the purpose of Open Access, the author has applied a Creative Commons Attribution (CC BY) license to any Author Accepted Manuscript version arising from this submission.
Availability of data and materials
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Declarations
Ethics approval and consent to participate
The Centralized Institutional Review Board of SingHealth approved the study (Reference 2018/2529). All participants provided written informed consent.
Consent for publication
Consent for publication was obtained from all study participants.
Competing interests
KMG and FY received reimbursement for speaking at conferences sponsored by companies selling nutritional products. KMG is part of an academic consortium that received research funding from Abbott Nutrition, Nestle and Danone. All other authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Ngee Lek and See Ling Loy co-senior authors.
References
- 1.Sonagra AD, Biradar SM, Dattatreya K, Jayaprakash Murthy DS. Normal pregnancy- a state of insulin resistance. J Clin Diagn Res JCDR. 2014;8(11):CC01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marcinkevage JA, Narayan KMV. Gestational diabetes mellitus: taking it to heart. Prim Care Diabetes. 2011;5(2):81–8. [DOI] [PubMed] [Google Scholar]
- 3.Catalano PM, Ehrenberg HM. The short- and long-term implications of maternal obesity on the mother and her offspring. BJOG. 2006;113(10):1126–33. [DOI] [PubMed] [Google Scholar]
- 4.Catalano PM. Trying to understand gestational diabetes. Diabet Med. 2014;31(3):273–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ye W, Luo C, Huang J. Gestational diabetes mellitus and adverse pregnancy outcomes: systematic review and meta-analysis. BMJ. 2022;377: e067946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Juan J, Yang H. Prevalence, prevention, and lifestyle intervention of gestational diabetes mellitus in China. Int J Environ Res Public Health. 2020;17(24):9517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sirimarco MP, Guerra HM, Lisboa EG, Vernini JM, Cassetari BN, de Araujo Costa RA, et al. Diagnostic protocol for gestational diabetes mellitus (GDM) (IADPSG/ADA, 2011): influence on the occurrence of GDM and mild gestational hyperglycemia (MGH) and on the perinatal outcomes. Diabetol Metab Syndr. 2017;3(9):2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu B, Cai J, Xu Y, Long Y, Deng L, Lin S, et al. Early diagnosed gestational diabetes mellitus is associated with adverse pregnancy outcomes: a prospective cohort study. J Clin Endocrinol Metab. 2020;105(12):dgaa633. [DOI] [PubMed] [Google Scholar]
- 9.Clarke E, Cade TJ, Brennecke S. Early pregnancy screening for women at high-risk of GDM results in reduced neonatal morbidity and similar maternal outcomes to routine screening. J Pregnancy. 2020;2020: e9083264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Su JB, Wang XQ, Chen JF, Wu G, Jin Y, Xu F, et al. Glycemic variability in gestational diabetes mellitus and its association with β cell function. Endocrine. 2013;43(2):370–5. [DOI] [PubMed] [Google Scholar]
- 11.Nigam A, Sharma S, Varun N, Munjal Y, Prakash A. Comparative analysis of 2-week glycaemic profile of healthy versus mild gestational diabetic pregnant women using flash glucose monitoring system: an observational study. BJOG Int J Obstet Gynaecol. 2019;126(S4):27–33. [DOI] [PubMed] [Google Scholar]
- 12.Dalfrà MG, Chilelli NC, Di Cianni G, Mello G, Lencioni C, Biagioni S, et al. Glucose fluctuations during gestation: an additional tool for monitoring pregnancy complicated by diabetes. Int J Endocrinol. 2013;11(2013): e279021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dalfrà MG, Sartore G, Cianni GD, Mello G, Lencioni C, Ottanelli S, et al. Glucose variability in diabetic pregnancy. Diabetes Technol Ther. 2011;13(8):853–9. [DOI] [PubMed] [Google Scholar]
- 14.Durnwald C, Beck RW, Li Z, Norton E, Bergenstal RM, Johnson M, et al. Continuous glucose monitoring profiles in pregnancies with and without gestational diabetes mellitus. Diabetes Care. 2024;47(8):1333–41. [DOI] [PubMed] [Google Scholar]
- 15.Di Filippo D, Henry A, Bell C, Haynes S, Chang MHY, Darling J, et al. A new continuous glucose monitor for the diagnosis of gestational diabetes mellitus: a pilot study. BMC Pregnancy Childbirth. 2023;23(1):186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Battelino T, Danne T, Bergenstal RM, Amiel SA, Beck R, Biester T, et al. Clinical targets for continuous glucose monitoring data interpretation: recommendations from the international consensus on time in range. Diabetes Care. 2019;42(8):1593–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Quah PL, Tan LK, Lek N, Thain S, Tan KH. Glycemic variability in early pregnancy may predict a subsequent diagnosis of gestational diabetes. DMSO. 2022;28(15):4065–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Loy SL, Cheung YB, Chong M, Müller-Riemenschneider F, Lek N, Lee YS, et al. Maternal night-eating pattern and glucose tolerance during pregnancy: study protocol for a longitudinal study. BMJ Open. 2019;9(10): e030036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. Strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ. 2007;335(7624):806–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Craig CL, Marshall AL, Sjöström M, Bauman AE, Booth ML, Ainsworth BE, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35(8):1381–95. [DOI] [PubMed] [Google Scholar]
- 21.Loo RSX, Yap F, Ku CW, Cheung YB, Tan KH, Chan JKY, et al. Maternal meal irregularities during pregnancy and lifestyle correlates. Appetite. 2022;1(168):105747. [DOI] [PubMed] [Google Scholar]
- 22.Moscardó V, Giménez M, Oliver N, Hill NR. Updated software for automated assessment of glucose variability and quality of glycemic control in diabetes. Diabetes Technol Ther. 2020;22(10):701–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bergenstal RM, Beck RW, Close KL, Grunberger G, Sacks DB, Kowalski A, et al. Glucose management indicator (GMI): a new term for estimating A1C from continuous glucose monitoring. Diabetes Care. 2018;41(11):2275–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wójcicki JM. “J”-index. A new proposition of the assessment of current glucose control in diabetic patients. Horm Metab Res. 1995;27(1):41–2. [DOI] [PubMed] [Google Scholar]
- 25.Gáborová M, Doničová V, Bačová I, Pallayová M, Bona M, Peregrim I, et al. Glycaemic variability and risk factors of pregnant women with and without gestational diabetes mellitus measured by continuous glucose monitoring. Int J Environ Res Pub Health. 2021;18(7):3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Service FJ, Molnar GD, Rosevear JW, Ackerman E, Gatewood LC, Taylor WF. Mean amplitude of glycemic excursions, a measure of diabetic instability. Diabetes. 1970;19(9):644–55. [DOI] [PubMed] [Google Scholar]
- 27.Service FJ, Nelson RL. Characteristics of glycemic stability. Diabetes Care. 1980;3(1):58–62. [DOI] [PubMed] [Google Scholar]
- 28.Service FJ, O’Brien PC, Rizza RA. Measurements of glucose control. Diabetes Care. 1987;10(2):225–37. [DOI] [PubMed] [Google Scholar]
- 29.Hill NR, Oliver NS, Choudhary P, Levy JC, Hindmarsh P, Matthews DR. Normal reference range for mean tissue glucose and glycemic variability derived from continuous glucose monitoring for subjects without diabetes in different ethnic groups. Diabetes Technol Ther. 2011;13(9):921–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Levy JC, Matthews DR, Hermans MP. Correct homeostasis model assessment (HOMA) evaluation uses the computer program. Diabetes Care. 1998;21(12):2191–2. [DOI] [PubMed] [Google Scholar]
- 31.Elsafty A, Shamma S, Mahmoud M, Azzazy H. Specific cutoffs for HOMA1-IR, HOMA2-IR, HOMA1-%B, and HOMA2-%B in adult Egyptian patients. Am J Clin Pathol. 2018;21(150):S66–S66. [Google Scholar]
- 32.Chi C, Loy SL, Chan SY, Choong C, Cai S, Soh SE, et al. Impact of adopting the 2013 World Health Organization criteria for diagnosis of gestational diabetes in a multi-ethnic Asian cohort: a prospective study. BMC Pregnancy Childbirth. 2018;18(1):69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee KW, Ching SM, Ramachandran V, Yee A, Hoo FK, Chia YC, et al. Prevalence and risk factors of gestational diabetes mellitus in Asia: a systematic review and meta-analysis. BMC Pregnancy Childbirth. 2018;18(1):494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Berkowitz GS, Lapinski RH, Wein R, Lee D. Race/ethnicity and other risk factors for gestational diabetes. Am J Epidemiol. 1992;135(9):965–73. [DOI] [PubMed] [Google Scholar]
- 35.Zhang C, Ning Y. Effect of dietary and lifestyle factors on the risk of gestational diabetes: review of epidemiologic evidence. Am J Clin Nutr. 2011;94(6 Suppl):1975S-1979S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.VanderWeele TJ, Shpitser I. A new criterion for confounder selection. Biometrics. 2011;67(4):1406–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Højsgaard S, Halekoh U, Yan J. The R package geepack for generalized estimating equations. J Stat Softw. 2006;15:1–11. [Google Scholar]
- 38.Yan J, Fine J. Estimating equations for association structures. Stat Med. 2004;23(6):859–74. [DOI] [PubMed] [Google Scholar]
- 39.Yan J. Geepack: yet another package for generalized estimating equations. R-News. 2002;1(2):12–4. [Google Scholar]
- 40.Rigby RA, Stasinopoulos DM. Using the Box-Cox t distribution in GAMLSS to model skewness and kurtosis. Stat Model. 2006;6(3):209–29. [Google Scholar]
- 41.Cypryk K, Pertyńska-Marczewska M, Szymczak W, Wilcyński J, Lewiński A. Evaluation of metabolic control in women with gestational diabetes mellitus by the continuous glucose monitoring system: a pilot study. Endocr Pract. 2006;12(3):245–50. [DOI] [PubMed] [Google Scholar]
- 42.Mazze R, Yogev Y, Langer O. Measuring glucose exposure and variability using continuous glucose monitoring in normal and abnormal glucose metabolism in pregnancy. J Matern Fetal Neonatal Med. 2012;25(7):1171–5. [DOI] [PubMed] [Google Scholar]
- 43.HAPO Study Cooperative Research Group, Metzger BE, Lowe LP, Dyer AR, Trimble ER, Chaovarindr U, et al. Hyperglycemia and adverse pregnancy outcomes. N Engl J Med. 2008;358(19):1991–2002. [DOI] [PubMed] [Google Scholar]
- 44.Paramasivam SS, Chinna K, Singh AKK, Ratnasingam J, Ibrahim L, Lim LL, et al. Continuous glucose monitoring results in lower HbA1c in Malaysian women with insulin-treated gestational diabetes: a randomized controlled trial. Diabet Med. 2018;35(8):1118–29. [DOI] [PubMed] [Google Scholar]
- 45.Law GR, Alnaji A, Alrefaii L, Endersby D, Cartland SJ, Gilbey SG, et al. Suboptimal nocturnal glucose control is associated with large for gestational age in treated gestational diabetes mellitus. Diabetes Care. 2019;42(5):810–5. [DOI] [PubMed] [Google Scholar]
- 46.Yu W, Wu N, Li L, OuYang H, Qian M, Shen H. A review of research progress on glycemic variability and gestational diabetes. DMSO. 2020;13:2729–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yu F, Lv L, Liang Z, Wang Y, Wen J, Lin X, et al. Continuous glucose monitoring effects on maternal glycemic control and pregnancy outcomes in patients with gestational diabetes mellitus: a prospective cohort study. J Clin Endocrinol Metab. 2014;99(12):4674–82. [DOI] [PubMed] [Google Scholar]
- 48.Jarrett RJ, Baker IA, Keen H, Oakley NW. Diurnal variation in oral glucose tolerance: blood sugar and plasma insulin levels morning, afternoon, and evening. Br Med J. 1972;1(5794):199–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Participant recruitment
Additional file 2. Baseline characteristics of women included and excluded from the study
Additional file 3. Comparison of CGM-derived glycemic control and variability indices in pregnant women based on baseline characteristics
Additional file 4. The predicted 24-hour interstitial glucose levels for women with at least 10 days of CGM readings by (a) IR (GMR 1.12; 95% CI 1.03, 1.21) and (b) GDM status (1.09; 0.99, 1.20) based on the GEE analysis. Red represents the women with IR or GDM and blue represents the women with non-IR or non-GDM. The circle markers and capped vertical lines represent the predicted mean daily glucose levels and the respective 95% CI based on the exponentiated log-transformed hourly median glucose values. Models were adjusted for age, ethnicity, years of education, parity, history of GDM or family history of diabetes, pre-pregnancy body mass index, irregular meal, physical activity, and an interaction term between glycemic status and time. CI confidence intervals, GDM gestational diabetes mellitus based on 2013 World Health Organization criteria, GEE generalized estimating equations, GMR geometrical mean ratio, IR insulin resistance based on HOMA2-IR of at least 1.22.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.