Abstract
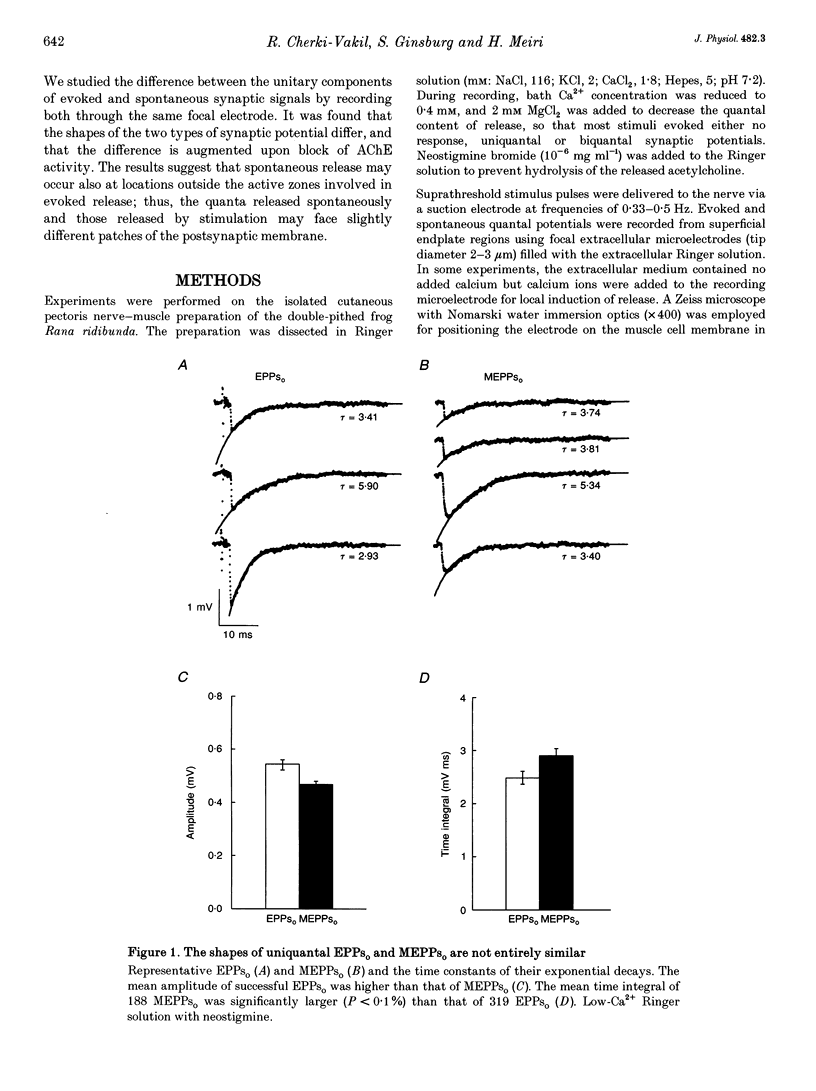
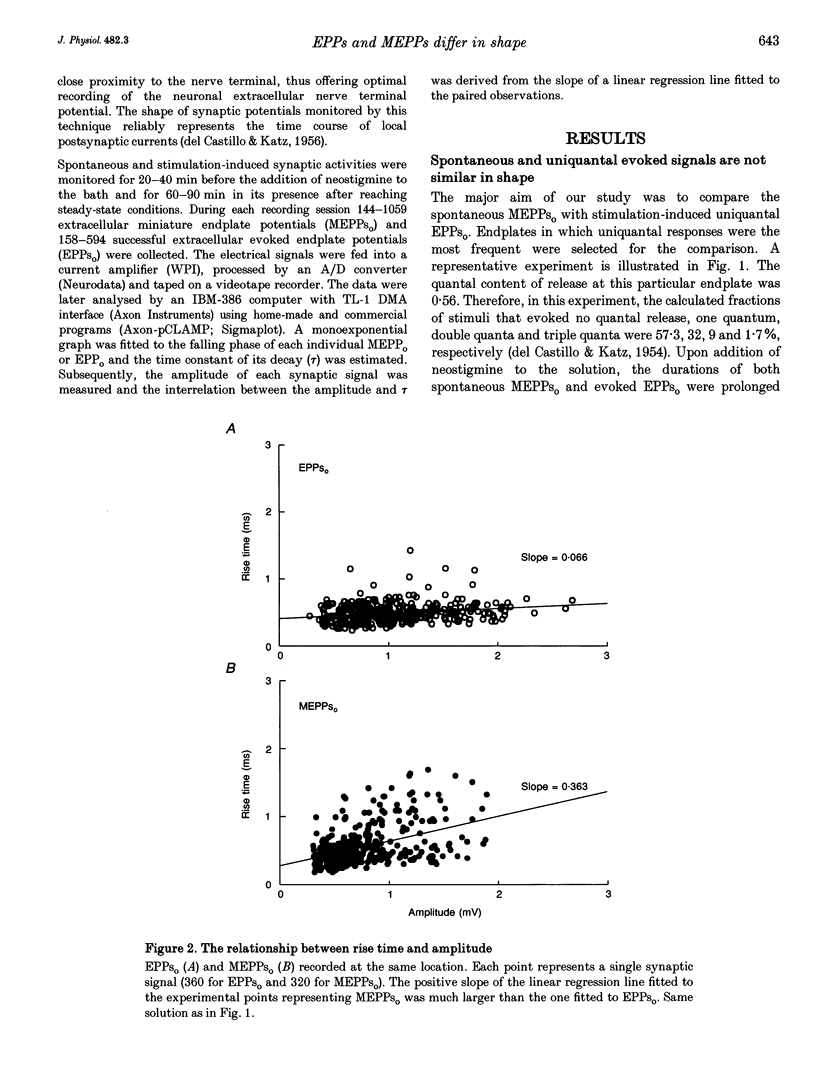
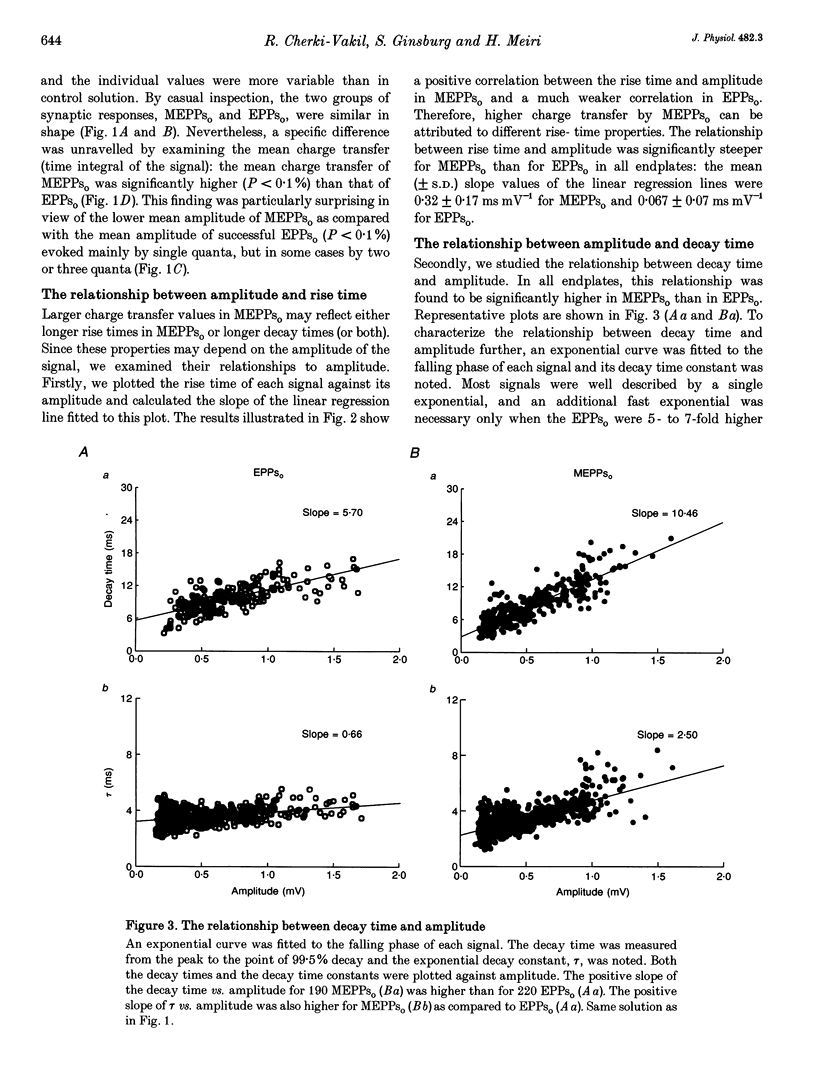
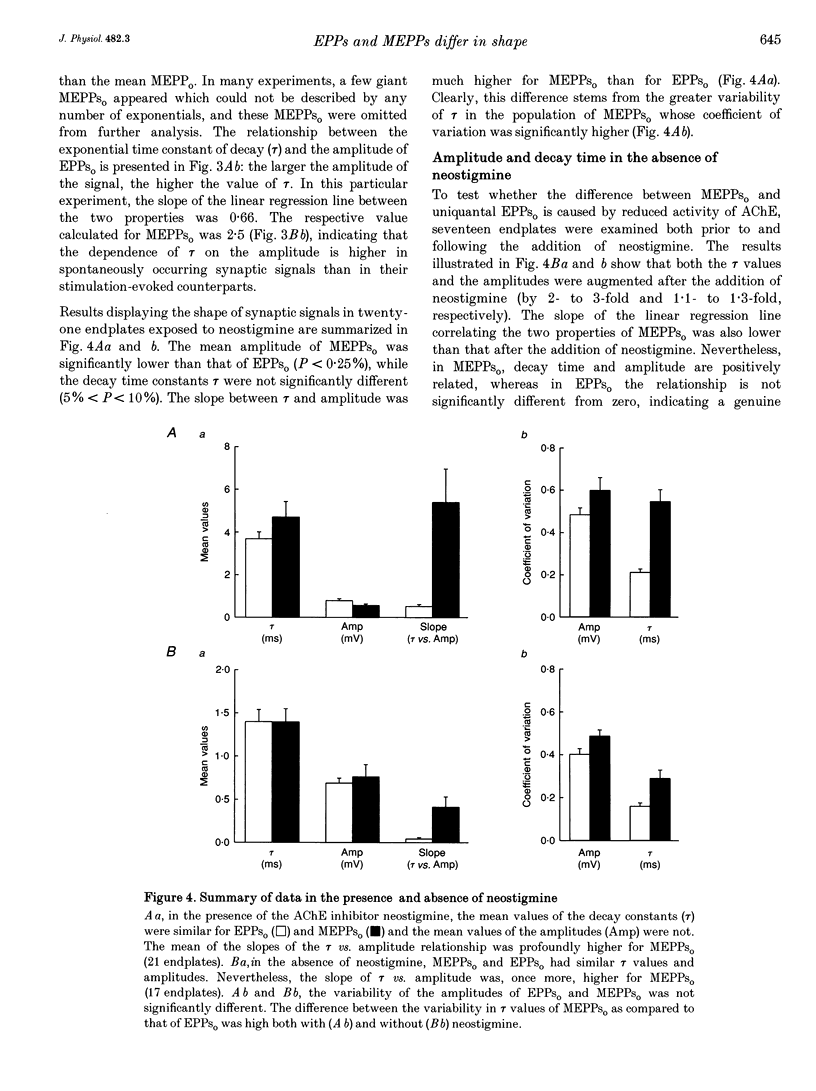
1. Spontaneous and stimulation-induced uniquantal synaptic activity at the frog cutaneous pectoris muscle, treated with neostigmine, was recorded by focal extracellular microelectrodes. A monoexponential curve was fitted to the decay of each synaptic response. 2. A highly significant positive relationship was found between the amplitude and the decay time constant of spontaneous extracellular miniature endplate potentials (MEPPs(o)), whereas the relationship displayed by evoked uniquantal extracellular endplate potentials (EPPs(o)) was only slightly greater than zero. 3. The difference did not stem from changes in the muscle membrane conductance or from inclusion of outstanding MEPPs(o) formed as a result of the block of acetylcholinesterase. 4. The dependence of the rise time on the amplitude was also stronger in MEPPs(o) than in EPPs(o). 5. In the absence of neostigmine, MEPPs(o) exhibited a positive correlation between decay time constant and amplitude, while EPPs(o) did not show such a correlation. 6. In view of previously published models of transmitter release, it is suggested that spontaneous secretion of quanta occurs both within and outside the active zones facing postsynaptic areas of variable receptor density.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams P. R. Acetylcholine receptor kinetics. J Membr Biol. 1981 Feb 28;58(3):161–174. doi: 10.1007/BF01870902. [DOI] [PubMed] [Google Scholar]
- Albuquerque E. X., Gage P. W. Differential effects of perhydrohistrionicotoxin on neurally and iontophoretically evoked endplate currents. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1596–1599. doi: 10.1073/pnas.75.3.1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceccarelli B., Fesce R., Grohovaz F., Haimann C. The effect of potassium on exocytosis of transmitter at the frog neuromuscular junction. J Physiol. 1988 Jul;401:163–183. doi: 10.1113/jphysiol.1988.sp017156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceccarelli B., Grohovaz F., Hurlbut W. P. Freeze-fracture studies of frog neuromuscular junctions during intense release of neurotransmitter. II. Effects of electrical stimulation and high potassium. J Cell Biol. 1979 Apr;81(1):178–192. doi: 10.1083/jcb.81.1.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colquhoun D., Sakmann B. Fast events in single-channel currents activated by acetylcholine and its analogues at the frog muscle end-plate. J Physiol. 1985 Dec;369:501–557. doi: 10.1113/jphysiol.1985.sp015912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEL CASTILLO J., KATZ B. Localization of active spots within the neuromuscular junction of the frog. J Physiol. 1956 Jun 28;132(3):630–649. doi: 10.1113/jphysiol.1956.sp005554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEL CASTILLO J., KATZ B. Quantal components of the end-plate potential. J Physiol. 1954 Jun 28;124(3):560–573. doi: 10.1113/jphysiol.1954.sp005129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FATT P., KATZ B. Spontaneous subthreshold activity at motor nerve endings. J Physiol. 1952 May;117(1):109–128. [PMC free article] [PubMed] [Google Scholar]
- Gage P. W. Generation of end-plate potentials. Physiol Rev. 1976 Jan;56(1):177–247. doi: 10.1152/physrev.1976.56.1.177. [DOI] [PubMed] [Google Scholar]
- Giniatullin R. A., Khazipov R. N., Vyskocil F. A correlation between quantal content and decay time of endplate currents in frog muscles with intact cholinesterase. J Physiol. 1993 Jul;466:95–103. [PMC free article] [PubMed] [Google Scholar]
- Glavinović M. I. Synaptic depression in frog neuromuscular junction. J Neurophysiol. 1987 Jul;58(1):230–246. doi: 10.1152/jn.1987.58.1.230. [DOI] [PubMed] [Google Scholar]
- Glavinović M. I. Variability of quantal events in control solution and after cholinesterase blockade in frog. Neuroscience. 1986 Feb;17(2):519–526. doi: 10.1016/0306-4522(86)90264-2. [DOI] [PubMed] [Google Scholar]
- Grinnell A. D., Pawson P. A. Dependence of spontaneous release at frog junctions on synaptic strength, external calcium and terminal length. J Physiol. 1989 Nov;418:397–410. doi: 10.1113/jphysiol.1989.sp017848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartzell H. C., Kuffler S. W., Yoshikami D. Post-synaptic potentiation: interaction between quanta of acetylcholine at the skeletal neuromuscular synapse. J Physiol. 1975 Oct;251(2):427–463. doi: 10.1113/jphysiol.1975.sp011102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuser J. E., Reese T. S., Dennis M. J., Jan Y., Jan L., Evans L. Synaptic vesicle exocytosis captured by quick freezing and correlated with quantal transmitter release. J Cell Biol. 1979 May;81(2):275–300. doi: 10.1083/jcb.81.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson M. B., Wong B. S., Morris C. E., Lecar H., Christian C. N. Successive openings of the same acetylcholine receptor channel are correlated in open time. Biophys J. 1983 Apr;42(1):109–114. doi: 10.1016/S0006-3495(83)84375-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B., Miledi R. The binding of acetylcholine to receptors and its removal from the synaptic cleft. J Physiol. 1973 Jun;231(3):549–574. doi: 10.1113/jphysiol.1973.sp010248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B., Miledi R. The effect of temperature on the synaptic delay at the neuromuscular junction. J Physiol. 1965 Dec;181(3):656–670. doi: 10.1113/jphysiol.1965.sp007790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y. I. Passively transferred Lambert-Eaton syndrome in mice receiving purified IgG. Muscle Nerve. 1986 Jul-Aug;9(6):523–530. doi: 10.1002/mus.880090608. [DOI] [PubMed] [Google Scholar]
- LILEY A. W. An investigation of spontaneous activity at the neuromuscular junction of the rat. J Physiol. 1956 Jun 28;132(3):650–666. doi: 10.1113/jphysiol.1956.sp005555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Land B. R., Harris W. V., Salpeter E. E., Salpeter M. M. Diffusion and binding constants for acetylcholine derived from the falling phase of miniature endplate currents. Proc Natl Acad Sci U S A. 1984 Mar;81(5):1594–1598. doi: 10.1073/pnas.81.5.1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Land B. R., Salpeter E. E., Salpeter M. M. Acetylcholine receptor site density affects the rising phase of miniature endplate currents. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3736–3740. doi: 10.1073/pnas.77.6.3736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Land B. R., Salpeter E. E., Salpeter M. M. Kinetic parameters for acetylcholine interaction in intact neuromuscular junction. Proc Natl Acad Sci U S A. 1981 Nov;78(11):7200–7204. doi: 10.1073/pnas.78.11.7200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linder T. M., Pennefather P., Quastel D. M. The time course of miniature endplate currents and its modification by receptor blockade and ethanol. J Gen Physiol. 1984 Mar;83(3):435–468. doi: 10.1085/jgp.83.3.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madsen B. W., Edeson R. O., Lam H. S., Milne R. K. Numerical simulation of miniature endplate currents. Neurosci Lett. 1984 Jul 13;48(1):67–74. doi: 10.1016/0304-3940(84)90290-8. [DOI] [PubMed] [Google Scholar]
- Madsen B. W., Edeson R. O., Milne R. K. Neurotransmission parameters estimated from miniature endplate current growth phase. Brain Res. 1987 Feb 3;402(2):387–392. doi: 10.1016/0006-8993(87)90052-7. [DOI] [PubMed] [Google Scholar]
- Magazanik L. G., Nikolsky E. E., Giniatullin R. A. End-plate currents evoked by paired stimuli in frog muscle fibres. Pflugers Arch. 1984 Jun;401(2):185–192. doi: 10.1007/BF00583880. [DOI] [PubMed] [Google Scholar]
- Magleby K. L., Stevens C. F. A quantitative description of end-plate currents. J Physiol. 1972 May;223(1):173–197. doi: 10.1113/jphysiol.1972.sp009840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magleby K. L., Stevens C. F. The effect of voltage on the time course of end-plate currents. J Physiol. 1972 May;223(1):151–171. doi: 10.1113/jphysiol.1972.sp009839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magleby K. L., Terrar D. A. Factors affecting the time course of decay of end-plate currents: a possible cooperative action of acetylcholine on receptors at the frog neuromuscular junction. J Physiol. 1975 Jan;244(2):467–495. doi: 10.1113/jphysiol.1975.sp010808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews-Bellinger J., Salpeter M. M. Distribution of acetylcholine receptors at frog neuromuscular junctions with a discussion of some physiological implications. J Physiol. 1978 Jun;279:197–213. doi: 10.1113/jphysiol.1978.sp012340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nystrom R. R., Ko C. P. Disruption of active zones in frog neuromuscular junctions following treatment with proteolytic enzymes. J Neurocytol. 1988 Feb;17(1):63–71. doi: 10.1007/BF01735378. [DOI] [PubMed] [Google Scholar]
- Pécot-Dechavassine M. Action of vinblastine on the spontaneous release of acetylcholine at the frog neuromuscular junction. J Physiol. 1976 Sep;261(1):31–48. doi: 10.1113/jphysiol.1976.sp011547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberry T. L. Quantitative simulation of endplate currents at neuromuscular junctions based on the reaction of acetylcholine with acetylcholine receptor and acetylcholinesterase. Biophys J. 1979 May;26(2):263–289. doi: 10.1016/S0006-3495(79)85249-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakmann B. Acetylcholine-induced ionic channels in rat skeletal muscle. Fed Proc. 1978 Oct;37(12):2654–2659. [PubMed] [Google Scholar]
- Wathey J. C., Nass M. M., Lester H. A. Numerical reconstruction of the quantal event at nicotinic synapses. Biophys J. 1979 Jul;27(1):145–164. doi: 10.1016/S0006-3495(79)85208-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wernig A., Stirner H. Quantum amplitude distributions point to functional unity of the synaptic 'active zone'. Nature. 1977 Oct 27;269(5631):820–822. doi: 10.1038/269820a0. [DOI] [PubMed] [Google Scholar]


