Abstract
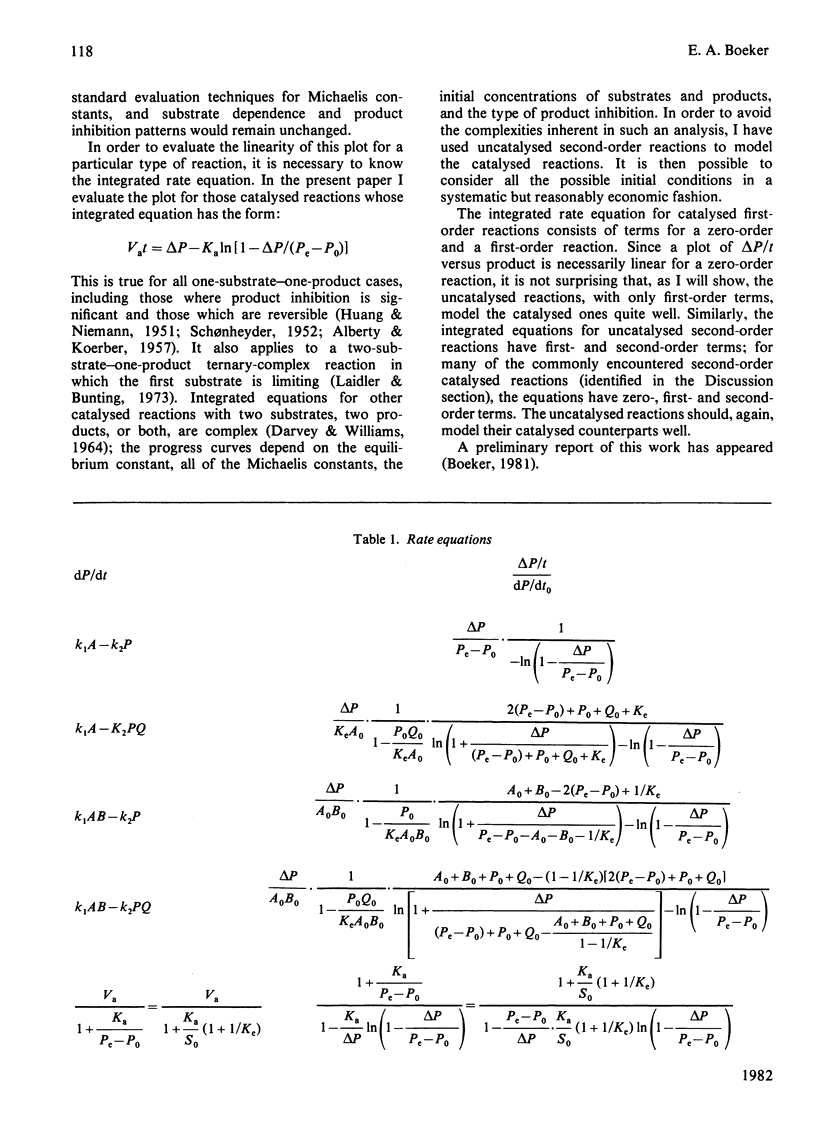
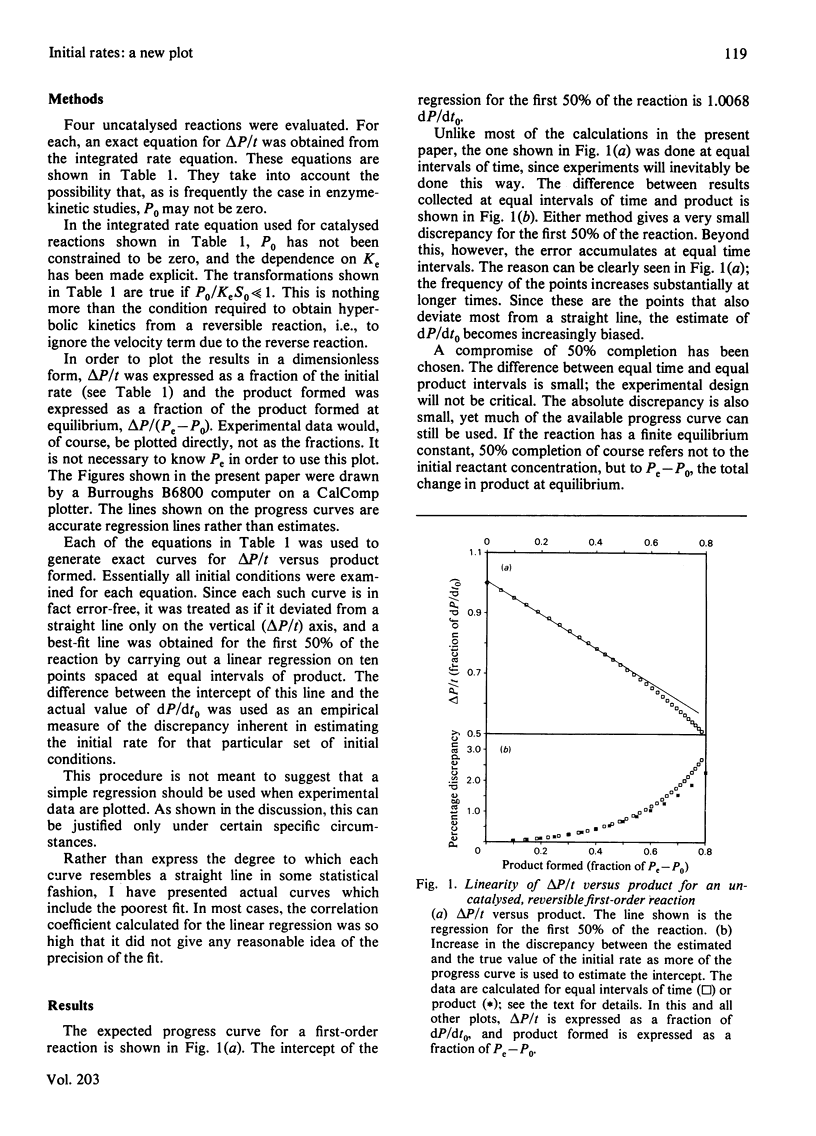
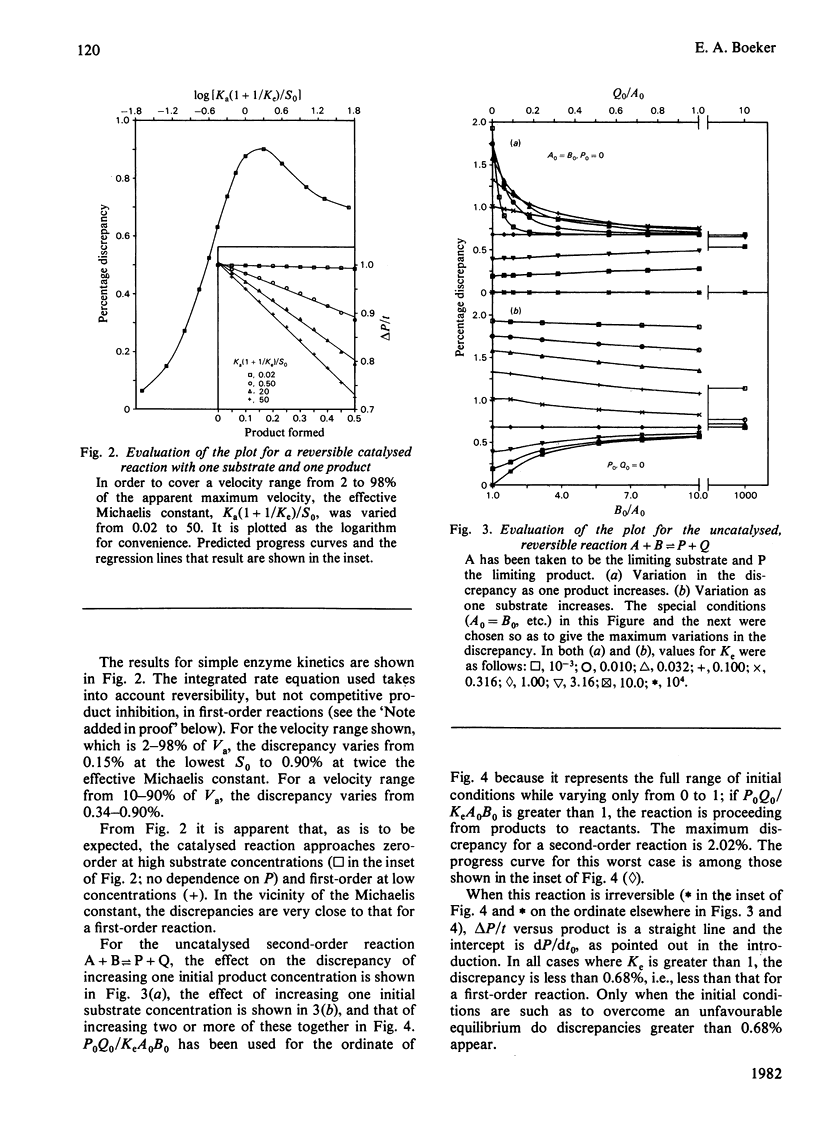
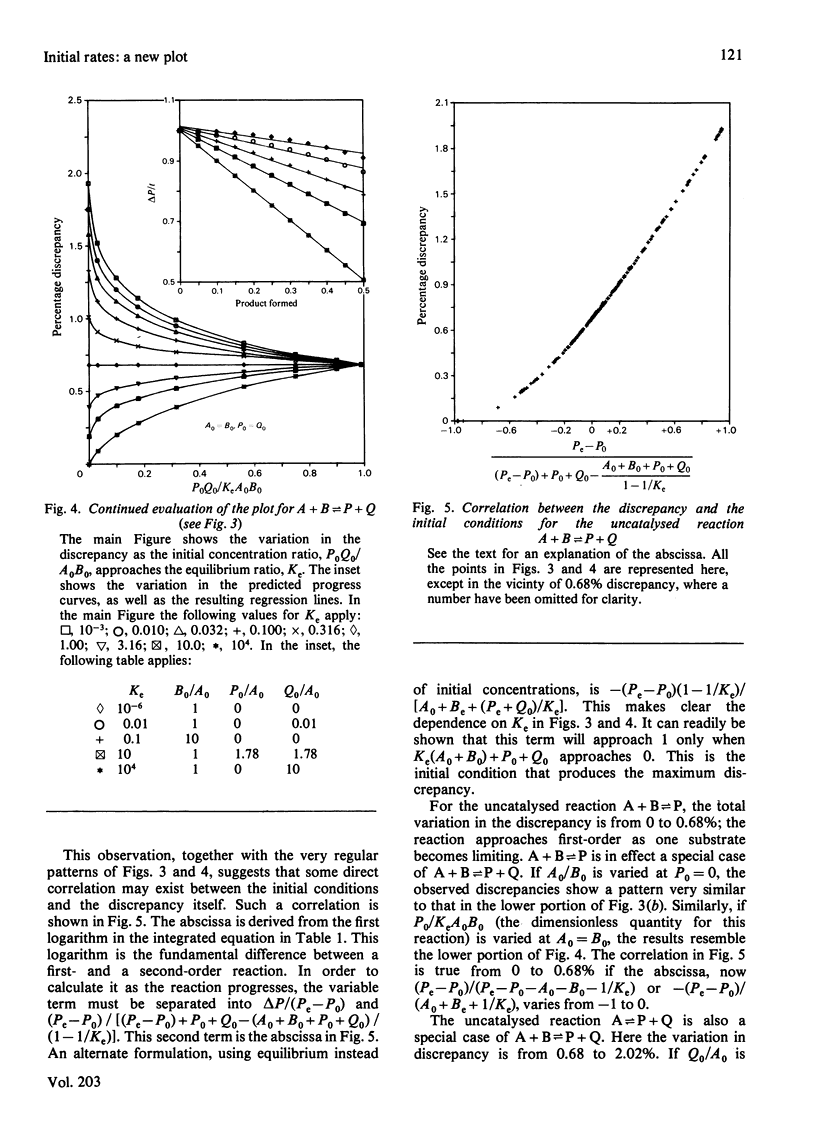
Excellent estimations of initial rates can be obtained from plots of delta P/t versus product formed (where P is the instantaneous concentration of the product). delta P/t is the chord from P0,t0 to P,t on an ordinary P-versus-t plot. When the chord is plotted as a function of product, the intercept at P0 of the resulting curve is necessarily dP/dt0. This curve approximates to a straight line extremely closely in all cases tested thus far. If delta P/t versus product is calculated from the integrated rate equation for a first-order reaction, and if a straight line is fitted through points representing the first 50% of the reaction, the discrepancy between the true initial rate and dP/dt0 estimated from the plot is 0.68%. For the most common form of the integrated rate equation for catalysed reactions the discrepancy varies between 0 and 0.90%. Because of the complexities of the integrated rate equations, catalysed second-order reactions have not been evaluated directly; uncatalysed reactions have been done instead. For a reaction with one reactant and two products, the discrepancy varies from 0.68 to 2.02%. For two reactants and one product, it varies from 0 to 0.68%; for two and two, 0 to 2.02%. The larger discrepancies occur only when unfavourable equilibrium constants are being overcome by the initial conditions.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cornish-Bowden A. The use of the direct linear plot for determining initial velocities. Biochem J. 1975 Aug;149(2):305–312. doi: 10.1042/bj1490305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DARVEY I. G., WILLIAMS J. F. INTEGRATED STEADY-STATE RATE EQUATIONS FOR ENZYME-CATALYZED REACTIONS. Biochim Biophys Acta. 1964 Apr 6;85:1–10. doi: 10.1016/0926-6569(64)90161-0. [DOI] [PubMed] [Google Scholar]
- GOLDENBERG H. Rectification of nonlinear enzyme activity curves. I. Preliminary. Arch Biochem Biophys. 1954 Sep;52(1):288–291. doi: 10.1016/0003-9861(54)90116-2. [DOI] [PubMed] [Google Scholar]
- Lee H. J., Wilson I. B. Enzymic parameters: measurement of V and Km. Biochim Biophys Acta. 1971 Sep 22;242(3):519–522. doi: 10.1016/0005-2744(71)90144-6. [DOI] [PubMed] [Google Scholar]
- SCHØNHEYDER F. Kinetics of 'acid' phosphatase action. Biochem J. 1952 Jan;50(3):378–384. doi: 10.1042/bj0500378. [DOI] [PMC free article] [PubMed] [Google Scholar]


