Abstract
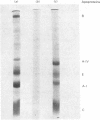
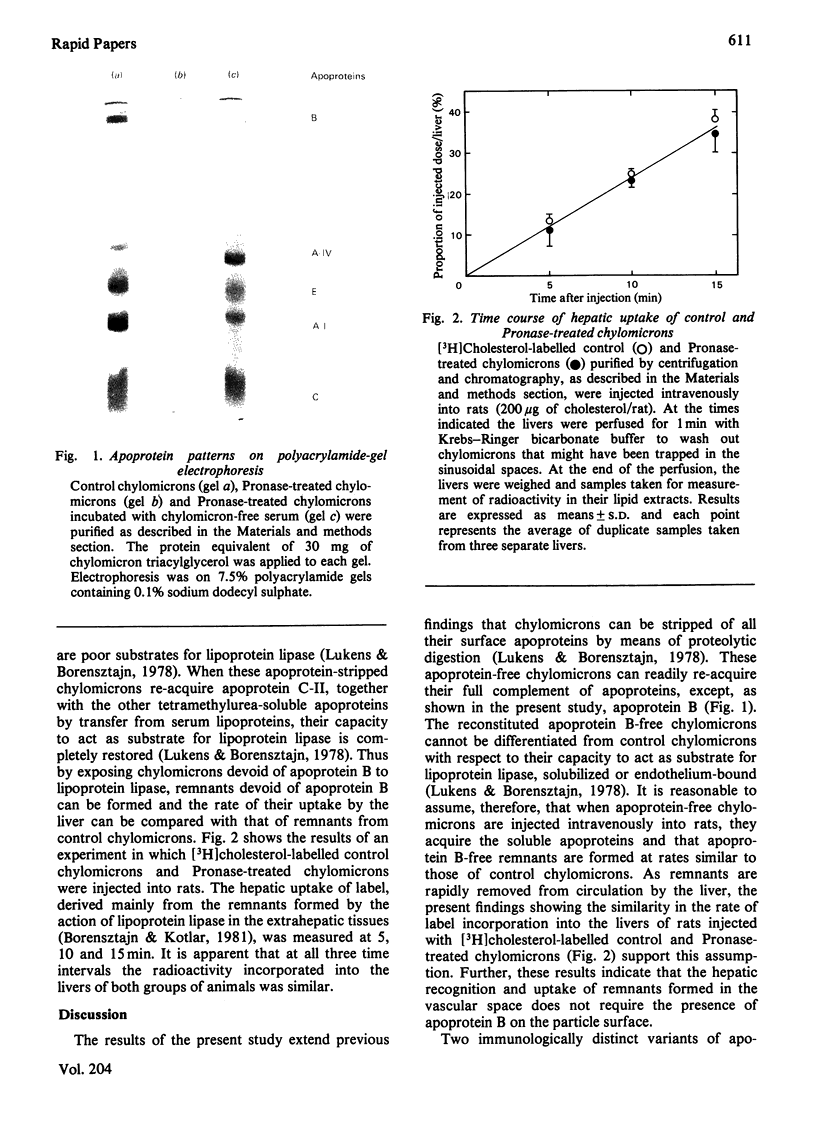
Rat lymph chylomicrons were treated with Pronase resulting in particles completely devoid of surface apoproteins. On re-incubation with serum, the Pronase-treated chylomicrons re-acquired, by transfer from other lipoproteins, all apoproteins except apoprotein B, which is water-insoluble and non-transferable. When two groups of rats were injected with [3H]cholesterol-labelled control or Pronase-treated chylomicrons, radioactivity was incorporated into the liver of both groups at similar rates. It is concluded that the remnants of the control and Pronase-treated chylomicrons formed in the vascular space were recognized and taken up by liver cells by a process that does not require apoprotein B.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Borensztajn J., Kotlar T. J. Hepatic uptake of phospholipid-depleted chylomicrons in vivo. Comparison with the uptake of chylomicron remnants. Biochem J. 1981 Dec 15;200(3):547–553. doi: 10.1042/bj2000547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borensztajn J., Kotlar T. J., McNeill B. J. Uptake of phospholipid-depleted chylomicrons by the perfused rat liver. Biochem J. 1980 Dec 15;192(3):845–851. doi: 10.1042/bj1920845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper A. D., Yu P. Y. Rates of removal and degradation of chylomicron remnants by isolated perfused rat liver. J Lipid Res. 1978 Jul;19(5):635–643. [PubMed] [Google Scholar]
- Fletcher M. J. A colorimetric method for estimating serum triglycerides. Clin Chim Acta. 1968 Nov;22(3):393–397. doi: 10.1016/0009-8981(68)90041-7. [DOI] [PubMed] [Google Scholar]
- Hui D. Y., Innerarity T. L., Mahley R. W. Lipoprotein binding to canine hepatic membranes. Metabolically distinct apo-E and apo-B,E receptors. J Biol Chem. 1981 Jun 10;256(11):5646–5655. [PubMed] [Google Scholar]
- Kovanen P. T., Brown M. S., Goldstein J. L. Increased binding of low density lipoprotein to liver membranes from rats treated with 17 alpha-ethinyl estradiol. J Biol Chem. 1979 Nov 25;254(22):11367–11373. [PubMed] [Google Scholar]
- Krishnaiah K. V., Walker L. F., Borensztajn J., Schonfeld G., Getz G. S. Apolipoprotein B variant derived from rat intestine. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3806–3810. doi: 10.1073/pnas.77.7.3806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lukens T. W., Borensztajn J. Action of liproprotein lipase on apoprotein-depleted chylomicrons. Biochem J. 1978 Oct 1;175(1):53–61. doi: 10.1042/bj1750053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mjos O. D., Faergeman O., Hamilton R. L., Havel R. J. Characterization of remnants produced during the metabolism of triglyceride-rich lipoproteins of blood plasma and intestinal lymph in the rat. J Clin Invest. 1975 Sep;56(3):603–615. doi: 10.1172/JCI108130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherrill B. C., Dietschy J. M. Characterization of the sinusoidal transport process responsible for uptake of chylomicrons by the liver. J Biol Chem. 1978 Mar 25;253(6):1859–1867. [PubMed] [Google Scholar]
- Sherrill B. C., Innerarity T. L., Mahley R. W. Rapid hepatic clearance of the canine lipoproteins containing only the E apoprotein by a high affinity receptor. Identity with the chylomicron remnant transport process. J Biol Chem. 1980 Mar 10;255(5):1804–1807. [PubMed] [Google Scholar]
- Smith L. C., Pownall H. J., Gotto A. M., Jr The plasma lipoproteins: structure and metabolism. Annu Rev Biochem. 1978;47:751–757. doi: 10.1146/annurev.bi.47.070178.003535. [DOI] [PubMed] [Google Scholar]
- Sparks C. E., Marsh J. B. Metabolic heterogeneity of apolipoprotein B in the rat. J Lipid Res. 1981 Mar;22(3):519–527. [PubMed] [Google Scholar]
- Tall A. R., Green P. H., Glickman R. M., Riley J. W. Metabolic fate of chylomicron phospholipids and apoproteins in the rat. J Clin Invest. 1979 Oct;64(4):977–989. doi: 10.1172/JCI109564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Windler E. E., Kovanen P. T., Chao Y. S., Brown M. S., Havel R. J., Goldstein J. L. The estradiol-stimulated lipoprotein receptor of rat liver. A binding site that membrane mediates the uptake of rat lipoproteins containing apoproteins B and E. J Biol Chem. 1980 Nov 10;255(21):10464–10471. [PubMed] [Google Scholar]
- Wu A. L., Windmueller H. G. Variant forms of plasma apolipoprotein B. Hepatic and intestinal biosynthesis and heterogeneous metabolism in the rat. J Biol Chem. 1981 Apr 25;256(8):3615–3618. [PubMed] [Google Scholar]