Abstract
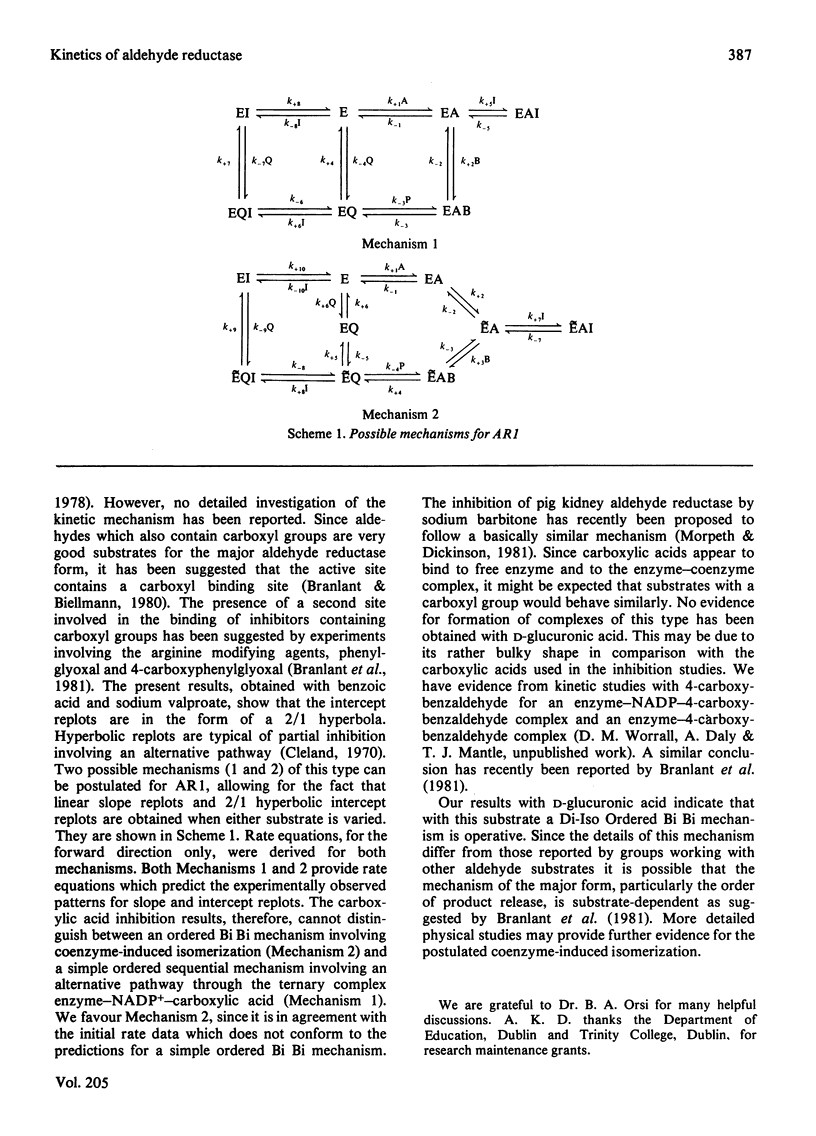
The steady-state kinetics of the major form of ox kidney aldehyde reductase with d-glucuronic acid have been determined at pH7. Initial rate and product inhibition studies performed in both directions are consistent with a Di-Iso Ordered Bi Bi mechanism. The mechanism of inhibition by sodium valproate and benzoic acid is shown to involve flux through an alternative pathway.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams M. J., Buehner M., Chandrasekhar K., Ford G. C., Hackert M. L., Liljas A., Rossmann M. G., Smiley I. E., Allison W. S., Everse J. Structure-function relationships in lactate dehydrogenase. Proc Natl Acad Sci U S A. 1973 Jul;70(7):1968–1972. doi: 10.1073/pnas.70.7.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachur N. R. Cytoplasmic aldo-keto reductases: a class of drug metabolizing enzymes. Science. 1976 Aug 13;193(4253):595–597. doi: 10.1126/science.959821. [DOI] [PubMed] [Google Scholar]
- Branlant G., Biellmann J. F. Purification and some properties of aldehyde reductases from pig liver. Eur J Biochem. 1980 Apr;105(3):611–621. doi: 10.1111/j.1432-1033.1980.tb04539.x. [DOI] [PubMed] [Google Scholar]
- Branlant G., Tritsch D., Biellmann J. F. Evidence for the presence of anion-recognition sites in pig-liver aldehyde reductase. Modification by phenyl glyoxal and p-carboxyphenyl glyoxal of an arginyl residue located close to the substrate-binding site. Eur J Biochem. 1981 Jun 1;116(3):505–512. doi: 10.1111/j.1432-1033.1981.tb05365.x. [DOI] [PubMed] [Google Scholar]
- Bronaugh R. L., Erwin V. G. Further characterization of a reduced nicotinamide-adenine dinucleotide phosphate-dependent aldehyde reductase from bovine brain. Inhibition by phenothiazine derivatives. Biochem Pharmacol. 1972 May 15;21(10):1457–1464. doi: 10.1016/0006-2952(72)90370-x. [DOI] [PubMed] [Google Scholar]
- CLELAND W. W. The kinetics of enzyme-catalyzed reactions with two or more substrates or products. I. Nomenclature and rate equations. Biochim Biophys Acta. 1963 Jan 8;67:104–137. doi: 10.1016/0006-3002(63)91800-6. [DOI] [PubMed] [Google Scholar]
- Chatterjee I. B. Evolution and the biosynthesis of ascorbic acid. Science. 1973 Dec 21;182(4118):1271–1272. doi: 10.1126/science.182.4118.1271. [DOI] [PubMed] [Google Scholar]
- Cleland W. W. The statistical analysis of enzyme kinetic data. Adv Enzymol Relat Areas Mol Biol. 1967;29:1–32. doi: 10.1002/9780470122747.ch1. [DOI] [PubMed] [Google Scholar]
- DALZIEL K. KINETIC STUDIES OF LIVER ALCOHOL DEHYDROGENASE AND PH EFFECTS WITH COENZYME PREPARATIONS OF HIGH PURITY. J Biol Chem. 1963 Aug;238:2850–2858. [PubMed] [Google Scholar]
- Davidson W. S., Flynn T. G. Kinetics and mechanism of action of aldehyde reductase from pig kidney. Biochem J. 1979 Feb 1;177(2):595–601. doi: 10.1042/bj1770595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRIEDEN C. Glutamic dehydrogenase. III. The order of substrate addition in the enzymatic reaction. J Biol Chem. 1959 Nov;234:2891–2896. [PubMed] [Google Scholar]
- Flynn T. G., Shires J., Walton D. J. Properties of the nicotinamide adenine dinucleotide phosphate-dependent aldehyde reductase from pig kidney. Amino acid composition, reactivity of cysteinyl residues, and stereochemistry of D-glyceraldehyde reduction. J Biol Chem. 1975 Apr 25;250(8):2933–2940. [PubMed] [Google Scholar]
- HAY G. W., LEWIS B. A., SMITH F. THIN-FILM CHROMATOGRAPHY IN THE STUDY OF CARBOHYDRATES. J Chromatogr. 1963 Aug;11:479–486. doi: 10.1016/s0021-9673(01)80949-3. [DOI] [PubMed] [Google Scholar]
- Mannervik B., Górna-Hall B., Bártfai T. The steady-state kinetics of glyoxalase I from porcine erythrocytes. Evidence for a random-pathway mechanism involving one- and two-substrate branches. Eur J Biochem. 1973 Aug 17;37(2):270–281. doi: 10.1111/j.1432-1033.1973.tb02985.x. [DOI] [PubMed] [Google Scholar]
- Matthews D. A., Alden R. A., Bolin J. T., Filman D. J., Freer S. T., Hamlin R., Hol W. G., Kisliuk R. L., Pastore E. J., Plante L. T. Dihydrofolate reductase from Lactobacillus casei. X-ray structure of the enzyme methotrexate.NADPH complex. J Biol Chem. 1978 Oct 10;253(19):6946–6954. [PubMed] [Google Scholar]
- Morpeth F. F., Dickinson F. M. Kinetic studies of the mechanism of pig kidney aldehyde reductase. Biochem J. 1981 Feb 1;193(2):485–492. doi: 10.1042/bj1930485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morpeth F. F., Dickinson F. M. Some properties of pig kidney-cortex aldehyde reductase. Biochem J. 1980 Nov 1;191(2):619–626. doi: 10.1042/bj1910619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ris M. M., Deitrich R. A., Von Wartburg J. P. Inhibition of aldehyde reductase isoenzymes in human and rat brain. Biochem Pharmacol. 1975 Oct 15;24(20):1865–1869. doi: 10.1016/0006-2952(75)90405-0. [DOI] [PubMed] [Google Scholar]
- Rivett A. J., Tipton K. F. Kinetic studies of the reduction of succinic semialdehyde by rat-brain aldehyde reductase. Eur J Biochem. 1981 Sep 1;118(3):635–639. doi: 10.1111/j.1432-1033.1981.tb05566.x. [DOI] [PubMed] [Google Scholar]
- Toews C. J. The kinetics and reaction mechanism of the nicotinamide-adenine dinucleotide phosphate-specific glycerol dehydrogenase of rat skeletal muscle. Biochem J. 1967 Dec;105(3):1067–1073. doi: 10.1042/bj1051067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner A. J., Hick P. E. Inhibition of aldehyde reductase by acidic metabolites of the biogenic amines. Biochem Pharmacol. 1975 Sep 15;24(18):1731–1733. doi: 10.1016/0006-2952(75)90016-7. [DOI] [PubMed] [Google Scholar]
- WILKINSON G. N. Statistical estimations in enzyme kinetics. Biochem J. 1961 Aug;80:324–332. doi: 10.1042/bj0800324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WINER A. D., SCHWERT G. W. Lactic dehydrogenase. IV. The influence of pH on the kinetics of the reaction. J Biol Chem. 1958 Apr;231(2):1065–1083. [PubMed] [Google Scholar]
- Wermuth B., Münch J. D., von Wartburg J. P. Purification and properties of NADPH-dependent aldehyde reductase from human liver. J Biol Chem. 1977 Jun 10;252(11):3821–3828. [PubMed] [Google Scholar]
- Whittle S. R., Turner A. J. Effects of the anticonvulsant sodium valproate on gamma-aminobutyrate and aldehyde metabolism in ox brain. J Neurochem. 1978 Dec;31(6):1453–1459. doi: 10.1111/j.1471-4159.1978.tb06572.x. [DOI] [PubMed] [Google Scholar]


