Abstract
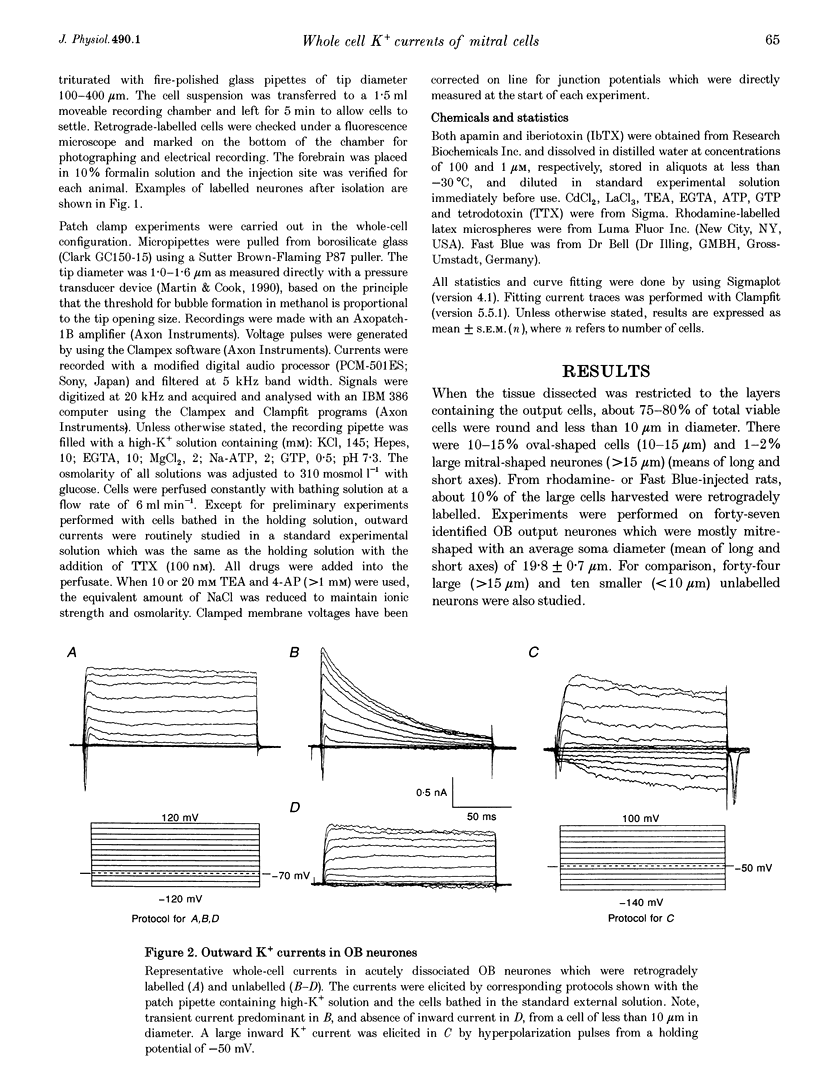
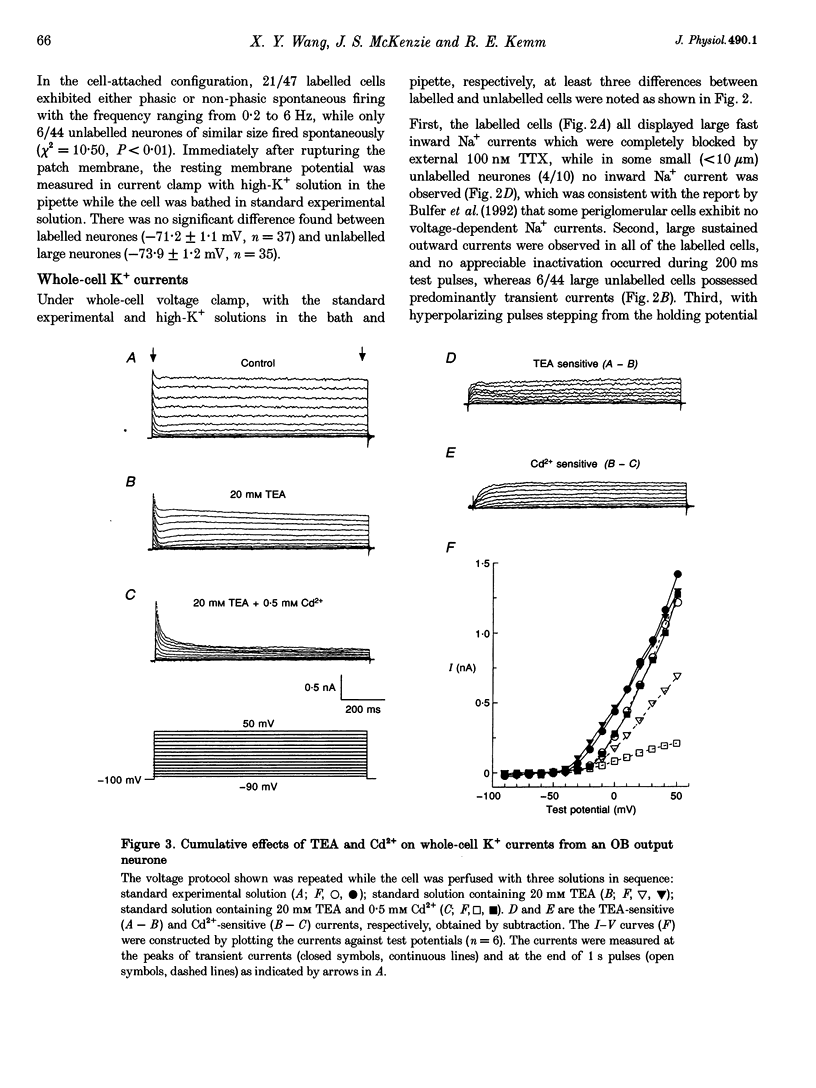
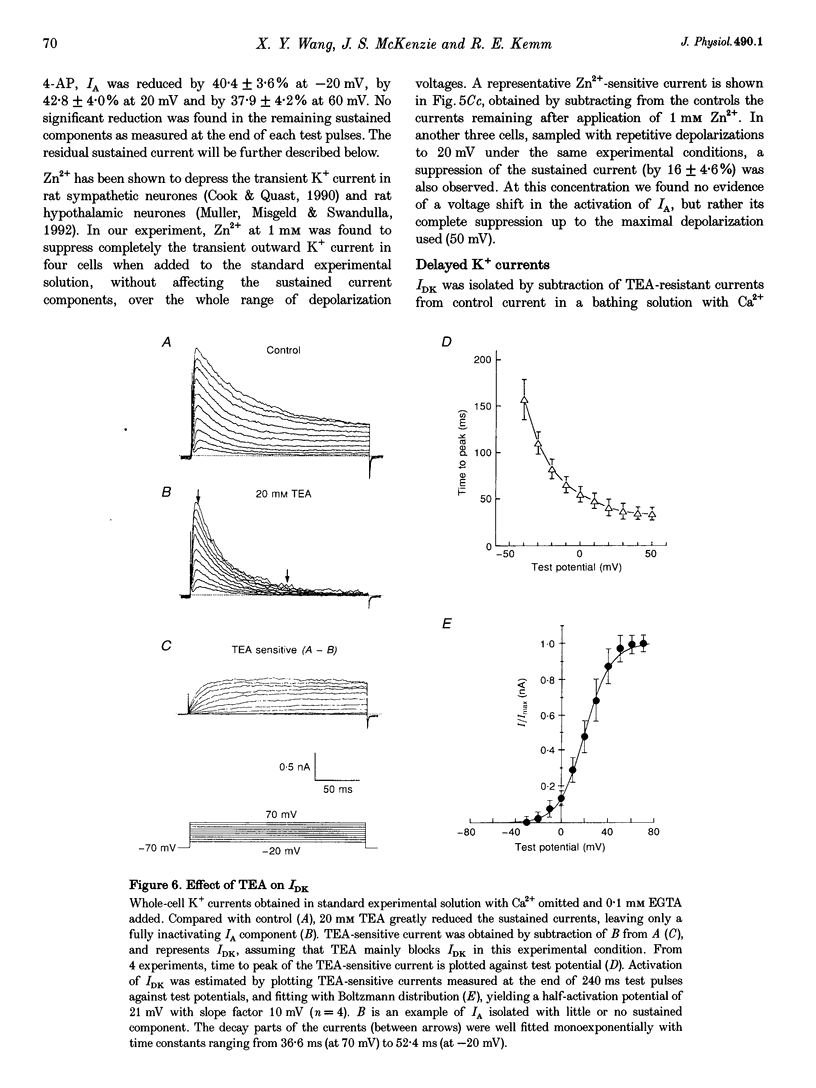
1. Voltage-gated whole-cell K+ currents have been investigated in olfactory bulb (OB) output (mitral/tufted) neurones from neonatal rats, which were retrogradely labelled by rhodamine or Fast Blue and identified after enzymatic dissociation. Forty-five per cent of labelled neurones exhibited either phasic or non-phasic spontaneous firing in cell-attached configuration. 2. Four outward K+ currents have been identified in all such identified OB output neurones. They are the transient (IA), the delayed rectifier (IDK), and two Ca(2+)-dependent (IK(Ca)) currents. No inward rectifier was detected. 3. The IA was activated at around -45 mV and reached its peak within 3-10 ms. The decay phase could be described by single exponential distribution with the time constant of 45.2 +/- 3.8 ms at depolarizations 10-60 mV from a holding potential of -70 mV. Its activation and steady-state inactivation processes could be fitted with Boltzmann equations yielding half-maximal activation potentials of 7.6 +/- 0.4 and -47.4 +/- 0.2 mV, respectively. It was sensitive to block by 4-AP (1 mM) and by Zn2+ (1 mM). 4. The IDK was activated at potentials more positive than -30 mV, with half-maximal activation at 21 mV. It was sustained during 1 s test pulses without apparent decay. It was blocked by TEA at a concentration of 20 mM. About 8% of the sustained current, in 11/24 cells tested, was found to resist block by a combination of all pharmacological agents tested. 5. Apamin at 100 nM blocked a TEA-insensitive component which accounted for about 23% of the maximal sustained currents. Iberiotoxin (IbTX), which has been found to block maxi K+ currents more selectively than does charybdotoxin, reversibly blocked Ca(2+)-activated K+ current, with a half-maximal dose of about 100 nM in 8/13 OB output neurones tested. This accounted for 20% of the maximal sustained K+ current. The effect of IbTX was not observed in the presence of 20 mM external TEA. 6. Direct evidence is provided in this study regarding kinetic and pharmacological properties of four types of outward K+ channels in OB output neurones.
Full text
PDF











Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akaike N., Kostyuk P. G., Osipchuk Y. V. Dihydropyridine-sensitive low-threshold calcium channels in isolated rat hypothalamic neurones. J Physiol. 1989 May;412:181–195. doi: 10.1113/jphysiol.1989.sp017610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alzheimer C. A novel voltage-dependent cation current in rat neocortical neurones. J Physiol. 1994 Sep 1;479(Pt 2):199–205. doi: 10.1113/jphysiol.1994.sp020288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardoni R., Belluzzi O. Modifications of A-current kinetics in mammalian central neurones induced by extracellular zinc. J Physiol. 1994 Sep 15;479(Pt 3):389–400. doi: 10.1113/jphysiol.1994.sp020304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belluzzi O., Sacchi O., Wanke E. A fast transient outward current in the rat sympathetic neurone studied under voltage-clamp conditions. J Physiol. 1985 Jan;358:91–108. doi: 10.1113/jphysiol.1985.sp015542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhalla U. S., Bower J. M. Exploring parameter space in detailed single neuron models: simulations of the mitral and granule cells of the olfactory bulb. J Neurophysiol. 1993 Jun;69(6):1948–1965. doi: 10.1152/jn.1993.69.6.1948. [DOI] [PubMed] [Google Scholar]
- Bufler J., Zufall F., Franke C., Hatt H. Patch-clamp recordings of spiking and nonspiking interneurons from rabbit olfactory bulb slices: membrane properties and ionic currents. J Comp Physiol A. 1992 Feb;170(2):145–152. doi: 10.1007/BF00196896. [DOI] [PubMed] [Google Scholar]
- Butler A., Tsunoda S., McCobb D. P., Wei A., Salkoff L. mSlo, a complex mouse gene encoding "maxi" calcium-activated potassium channels. Science. 1993 Jul 9;261(5118):221–224. doi: 10.1126/science.7687074. [DOI] [PubMed] [Google Scholar]
- Candia S., Garcia M. L., Latorre R. Mode of action of iberiotoxin, a potent blocker of the large conductance Ca(2+)-activated K+ channel. Biophys J. 1992 Aug;63(2):583–590. doi: 10.1016/S0006-3495(92)81630-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crest M., Gola M. Large conductance Ca(2+)-activated K+ channels are involved in both spike shaping and firing regulation in Helix neurones. J Physiol. 1993 Jun;465:265–287. doi: 10.1113/jphysiol.1993.sp019676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cull-Candy S. G., Marshall C. G., Ogden D. Voltage-activated membrane currents in rat cerebellar granule neurones. J Physiol. 1989 Jul;414:179–199. doi: 10.1113/jphysiol.1989.sp017683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan T. M., Dagan D., Levitan I. B. Properties and modulation of a calcium-activated potassium channel in rat olfactory bulb neurons. J Neurophysiol. 1993 May;69(5):1433–1442. doi: 10.1152/jn.1993.69.5.1433. [DOI] [PubMed] [Google Scholar]
- Foehring R. C., Surmeier D. J. Voltage-gated potassium currents in acutely dissociated rat cortical neurons. J Neurophysiol. 1993 Jul;70(1):51–63. doi: 10.1152/jn.1993.70.1.51. [DOI] [PubMed] [Google Scholar]
- Galvan M., Sedlmeir C. Outward currents in voltage-clamped rat sympathetic neurones. J Physiol. 1984 Nov;356:115–133. doi: 10.1113/jphysiol.1984.sp015456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvez A., Gimenez-Gallego G., Reuben J. P., Roy-Contancin L., Feigenbaum P., Kaczorowski G. J., Garcia M. L. Purification and characterization of a unique, potent, peptidyl probe for the high conductance calcium-activated potassium channel from venom of the scorpion Buthus tamulus. J Biol Chem. 1990 Jul 5;265(19):11083–11090. [PubMed] [Google Scholar]
- Hamilton K. A., Kauer J. S. Intracellular potentials of salamander mitral/tufted neurons in response to odor stimulation. Brain Res. 1985 Jul 8;338(1):181–185. doi: 10.1016/0006-8993(85)90265-3. [DOI] [PubMed] [Google Scholar]
- Jahr C. E., Nicoll R. A. An intracellular analysis of dendrodendritic inhibition in the turtle in vitro olfactory bulb. J Physiol. 1982 May;326:213–234. doi: 10.1113/jphysiol.1982.sp014187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz L. C., Burkhalter A., Dreyer W. J. Fluorescent latex microspheres as a retrograde neuronal marker for in vivo and in vitro studies of visual cortex. Nature. 1984 Aug 9;310(5977):498–500. doi: 10.1038/310498a0. [DOI] [PubMed] [Google Scholar]
- Kunze W. A., Shafton A. D., Kemm R. E., McKenzie J. S. Olfactory bulb output neurons excited from a basal forebrain magnocellular nucleus. Brain Res. 1992 Jun 26;583(1-2):327–331. doi: 10.1016/s0006-8993(10)80044-7. [DOI] [PubMed] [Google Scholar]
- Lang D. G., Ritchie A. K. Large and small conductance calcium-activated potassium channels in the GH3 anterior pituitary cell line. Pflugers Arch. 1987 Dec;410(6):614–622. doi: 10.1007/BF00581321. [DOI] [PubMed] [Google Scholar]
- Martin D. K., Cook D. I. A direct-reading device for measurement of patch-clamp micropipette tip diameters. Pflugers Arch. 1990 Nov;417(3):255–258. doi: 10.1007/BF00370989. [DOI] [PubMed] [Google Scholar]
- Mori K. Membrane and synaptic properties of identified neurons in the olfactory bulb. Prog Neurobiol. 1987;29(3):275–320. doi: 10.1016/0301-0082(87)90024-4. [DOI] [PubMed] [Google Scholar]
- Müller T. H., Misgeld U., Swandulla D. Ionic currents in cultured rat hypothalamic neurones. J Physiol. 1992 May;450:341–362. doi: 10.1113/jphysiol.1992.sp019130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Numann R. E., Wadman W. J., Wong R. K. Outward currents of single hippocampal cells obtained from the adult guinea-pig. J Physiol. 1987 Dec;393:331–353. doi: 10.1113/jphysiol.1987.sp016826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogata N., Tatebayashi H. Differential inhibition of a transient K+ current by chlorpromazine and 4-aminopyridine in neurones of the rat dorsal root ganglia. Br J Pharmacol. 1993 Aug;109(4):1239–1246. doi: 10.1111/j.1476-5381.1993.tb13755.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyama Y., Harata N., Akaike N. Delayed potassium current and non-specific outward current in pyramidal neurones acutely dissociated from rat hippocampus. Brain Res. 1991 Dec 24;568(1-2):350–354. doi: 10.1016/0006-8993(91)91425-z. [DOI] [PubMed] [Google Scholar]
- Robitaille R., Garcia M. L., Kaczorowski G. J., Charlton M. P. Functional colocalization of calcium and calcium-gated potassium channels in control of transmitter release. Neuron. 1993 Oct;11(4):645–655. doi: 10.1016/0896-6273(93)90076-4. [DOI] [PubMed] [Google Scholar]
- Rudy B. Diversity and ubiquity of K channels. Neuroscience. 1988 Jun;25(3):729–749. doi: 10.1016/0306-4522(88)90033-4. [DOI] [PubMed] [Google Scholar]
- Segal M., Barker J. L. Rat hippocampal neurons in culture: potassium conductances. J Neurophysiol. 1984 Jun;51(6):1409–1433. doi: 10.1152/jn.1984.51.6.1409. [DOI] [PubMed] [Google Scholar]
- Silva N. L., Pechura C. M., Barker J. L. Postnatal rat nigrostriatal dopaminergic neurons exhibit five types of potassium conductances. J Neurophysiol. 1990 Jul;64(1):262–272. doi: 10.1152/jn.1990.64.1.262. [DOI] [PubMed] [Google Scholar]
- Surmeier D. J., Bargas J., Kitai S. T. Two types of A-current differing in voltage-dependence are expressed by neurons of the rat neostriatum. Neurosci Lett. 1989 Sep 11;103(3):331–337. doi: 10.1016/0304-3940(89)90122-5. [DOI] [PubMed] [Google Scholar]
- Talukder G., Harrison N. L. On the mechanism of modulation of transient outward current in cultured rat hippocampal neurons by di- and trivalent cations. J Neurophysiol. 1995 Jan;73(1):73–79. doi: 10.1152/jn.1995.73.1.73. [DOI] [PubMed] [Google Scholar]
- Tauc M., Congar P., Poncet V., Merot J., Vita C., Poujeol P. Toxin pharmacology of the large-conductance Ca(2+)-activated K+ channel in the apical membrane of rabbit proximal convoluted tubule in primary culture. Pflugers Arch. 1993 Oct;425(1-2):126–133. doi: 10.1007/BF00374512. [DOI] [PubMed] [Google Scholar]
- Wellis D. P., Scott J. W., Harrison T. A. Discrimination among odorants by single neurons of the rat olfactory bulb. J Neurophysiol. 1989 Jun;61(6):1161–1177. doi: 10.1152/jn.1989.61.6.1161. [DOI] [PubMed] [Google Scholar]
- Wisgirda M. E., Dryer S. E. Divalent cations selectively alter the voltage dependence of inactivation of A-currents in chick autonomic neurons. Pflugers Arch. 1993 Jun;423(5-6):418–426. doi: 10.1007/BF00374936. [DOI] [PubMed] [Google Scholar]
- Yu G. Z., Kaba H., Saito H., Seto K. Heterogeneous characteristics of mitral cells in the rat olfactory bulb. Brain Res Bull. 1993;31(6):701–706. doi: 10.1016/0361-9230(93)90144-z. [DOI] [PubMed] [Google Scholar]



