Abstract
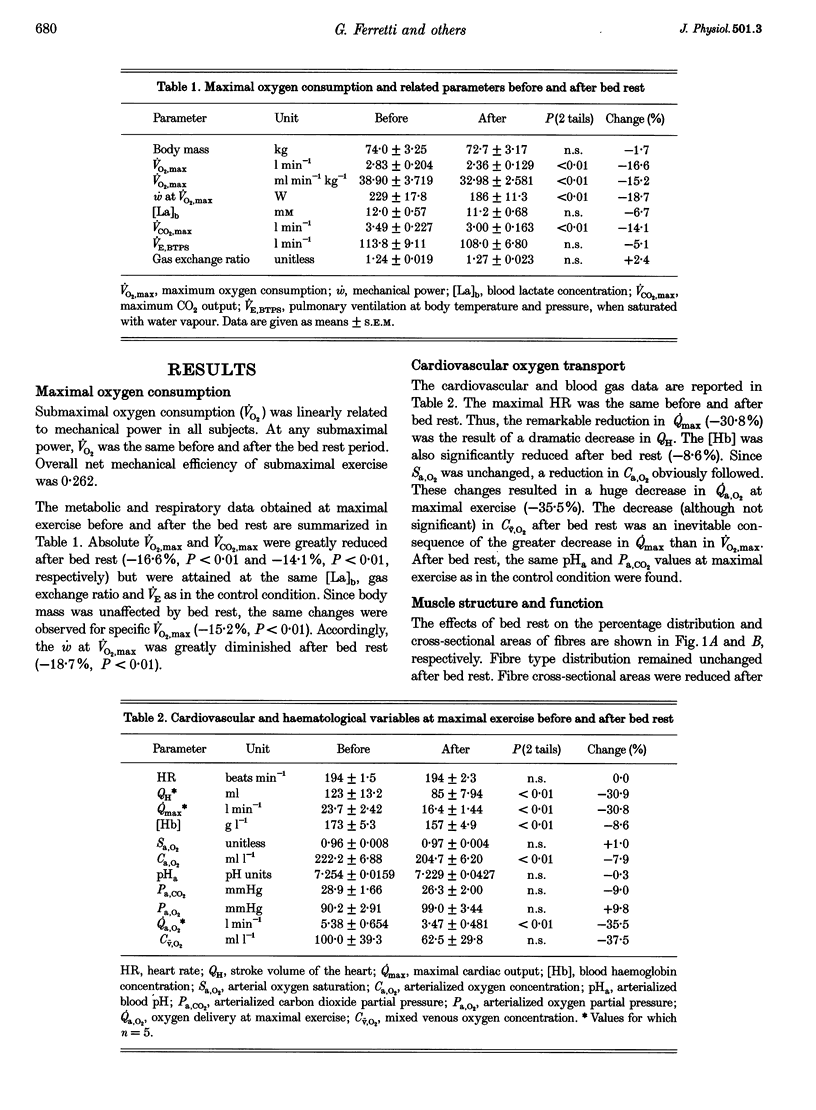
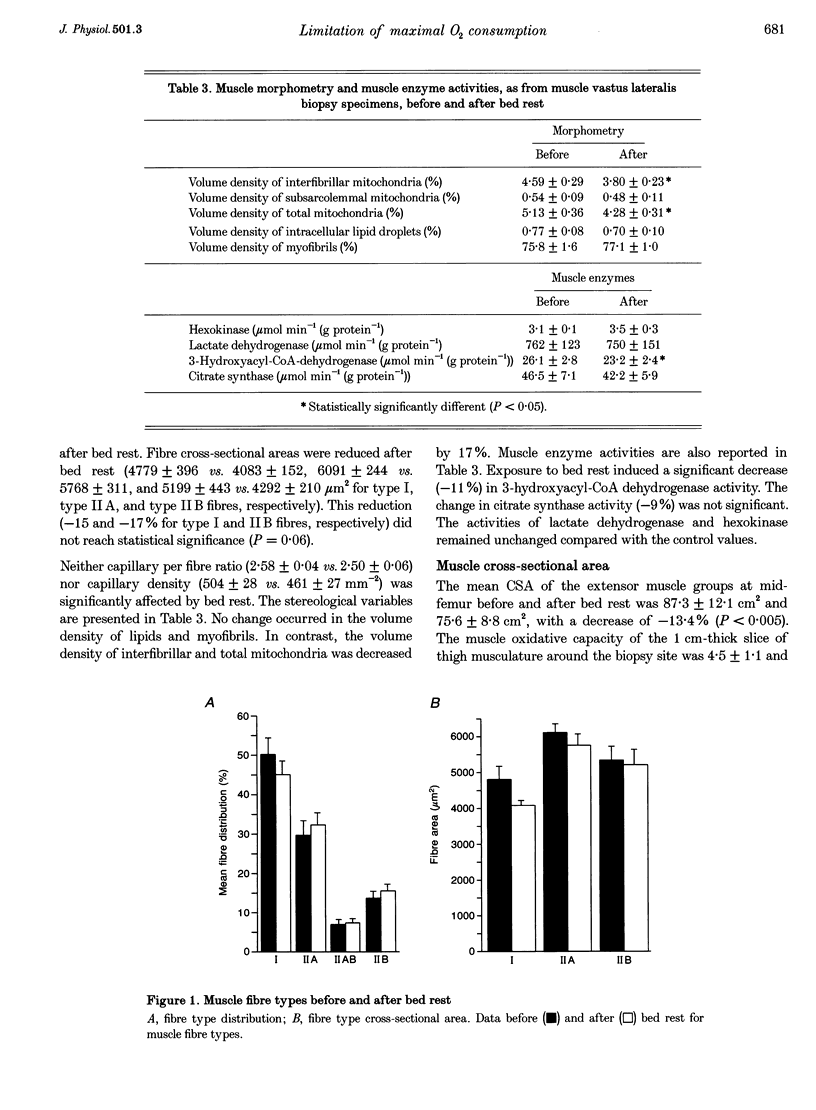
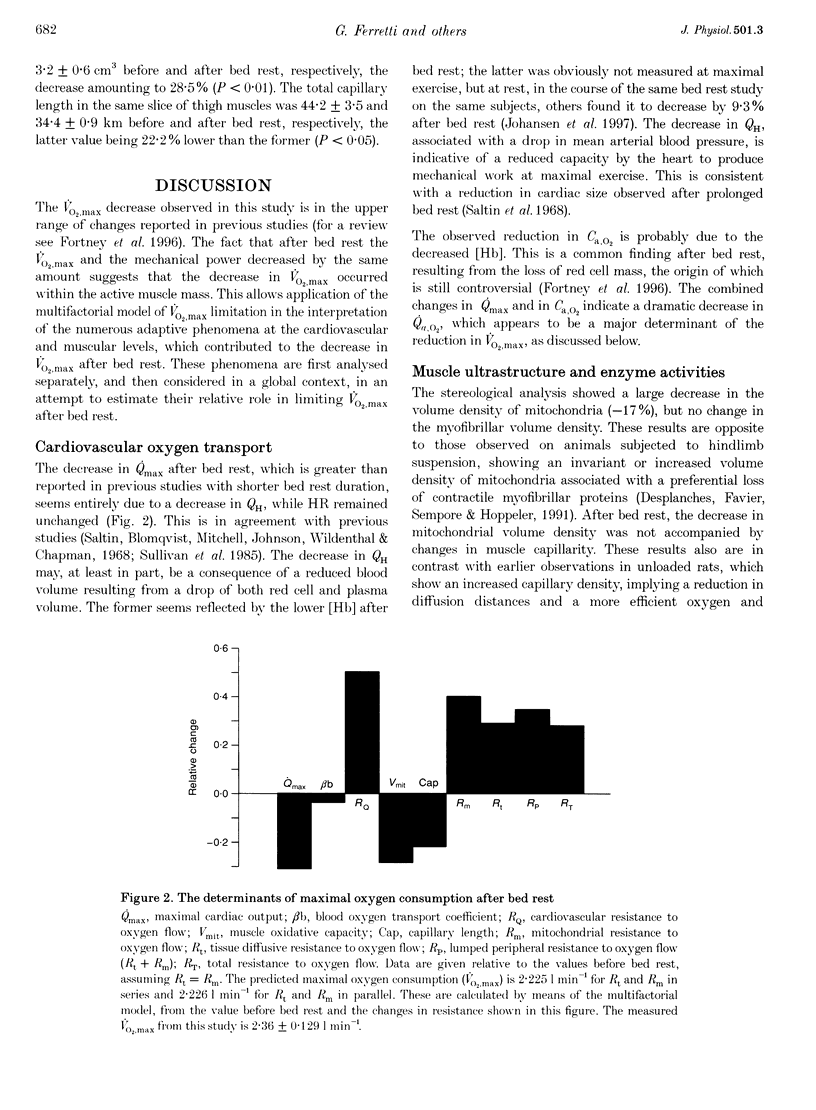
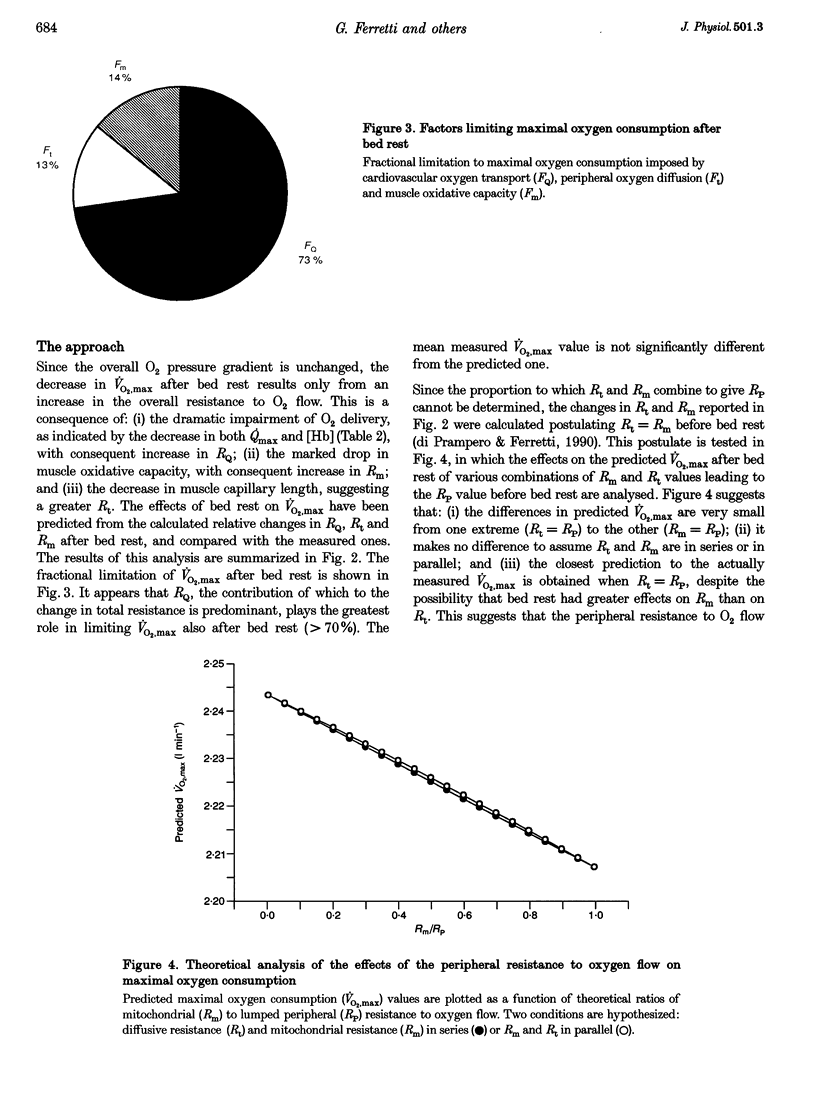
1. The effects of bed rest on the cardiovascular and muscular parameters which affect maximal O2 consumption (VO2,max) were studied. The fractional limitation of VO2,max imposed by these parameters after bed rest was analysed. 2. The VO2,max, by standard procedure, and the maximal cardiac output (Qmax), by the pulse contour method, were measured during graded cyclo-ergometric exercise on seven subjects before and after a 42-day head-down tilt bed rest. Blood haemoglobin concentration ([Hb]) and arterialized blood gas analysis were determined at the highest work load. 3. Muscle fibre types, oxidative enzyme activities, and capillary and mitochondrial densities were measured on biopsy samples from the vastus lateralis muscle before and at the end of bed rest. The measure of muscle cross-sectional area (CSA) by NMR imaging at the level of biopsy site allowed computation of muscle oxidative capacity and capillary length. 4. The VO2,max was reduced after bed rest (-16.6%). The concomitant decreases in Qmax (-30.8%), essentially due to a change in stroke volume, and in [Hb] led to a huge decrease in O2 delivery (-39.7%). 5. Fibre type distribution was unaffected by bed rest. The decrease in fibre area corresponded to the significant reduction in muscle CSA (-17%). The volume density of mitochondria was reduced after bed rest (-16.6%), as were the oxidative enzyme activities (-11%). The total mitochondrial volume was reduced by 28.5%. Capillary density was unchanged. Total capillary length was 22.2% lower after bed rest, due to muscle atrophy. 6. The interaction between these muscular and cardiovascular changes led to a smaller reduction in VO2,max than in cardiovascular O2 transport. Yet the latter appears to play the greatest role in limiting VO2,max after bed rest (> 70% of overall limitation), the remaining fraction being shared between peripheral O2 diffusion and utilization.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen P., Henriksson J. Capillary supply of the quadriceps femoris muscle of man: adaptive response to exercise. J Physiol. 1977 Sep;270(3):677–690. doi: 10.1113/jphysiol.1977.sp011975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonutto G., Girardis M., Tuniz D., di Prampero P. E. Noninvasive assessment of cardiac output from arterial pressure profiles during exercise. Eur J Appl Physiol Occup Physiol. 1995;72(1-2):18–24. doi: 10.1007/BF00964109. [DOI] [PubMed] [Google Scholar]
- Berg H. E., Dudley G. A., Hather B., Tesch P. A. Work capacity and metabolic and morphologic characteristics of the human quadriceps muscle in response to unloading. Clin Physiol. 1993 Jul;13(4):337–347. doi: 10.1111/j.1475-097x.1993.tb00334.x. [DOI] [PubMed] [Google Scholar]
- Berry P., Berry I., Manelfe C. Magnetic resonance imaging evaluation of lower limb muscles during bed rest--a microgravity simulation model. Aviat Space Environ Med. 1993 Mar;64(3 Pt 1):212–218. [PubMed] [Google Scholar]
- Blomqvist C. G., Saltin B. Cardiovascular adaptations to physical training. Annu Rev Physiol. 1983;45:169–189. doi: 10.1146/annurev.ph.45.030183.001125. [DOI] [PubMed] [Google Scholar]
- Brooke M. H., Kaiser K. K. Muscle fiber types: how many and what kind? Arch Neurol. 1970 Oct;23(4):369–379. doi: 10.1001/archneur.1970.00480280083010. [DOI] [PubMed] [Google Scholar]
- Desplanches D., Favier R., Sempore B., Hoppeler H. Whole body and muscle respiratory capacity with dobutamine and hindlimb suspension. J Appl Physiol (1985) 1991 Dec;71(6):2419–2424. doi: 10.1152/jappl.1991.71.6.2419. [DOI] [PubMed] [Google Scholar]
- Ekblom B. Factors determining maximal aerobic power. Acta Physiol Scand Suppl. 1986;556:15–19. [PubMed] [Google Scholar]
- Farhi L. E., Nesarajah M. S., Olszowka A. J., Metildi L. A., Ellis A. K. Cardiac output determination by simple one-step rebreathing technique. Respir Physiol. 1976 Oct;28(1):141–159. doi: 10.1016/0034-5687(76)90091-8. [DOI] [PubMed] [Google Scholar]
- Ferretti G., di Prampero P. E. Factors limiting maximal O2 consumption: effects of acute changes in ventilation. Respir Physiol. 1995 Feb;99(2):259–271. doi: 10.1016/0034-5687(94)00092-e. [DOI] [PubMed] [Google Scholar]
- Gollnick P. D., Armstrong R. B., Saubert C. W., 4th, Piehl K., Saltin B. Enzyme activity and fiber composition in skeletal muscle of untrained and trained men. J Appl Physiol. 1972 Sep;33(3):312–319. doi: 10.1152/jappl.1972.33.3.312. [DOI] [PubMed] [Google Scholar]
- Hather B. M., Adams G. R., Tesch P. A., Dudley G. A. Skeletal muscle responses to lower limb suspension in humans. J Appl Physiol (1985) 1992 Apr;72(4):1493–1498. doi: 10.1152/jappl.1992.72.4.1493. [DOI] [PubMed] [Google Scholar]
- Henriksson K. G. "Semi-open" muscle biopsy technique. A simple outpatient procedure. Acta Neurol Scand. 1979 Jun;59(6):317–323. [PubMed] [Google Scholar]
- Hikida R. S., Gollnick P. D., Dudley G. A., Convertino V. A., Buchanan P. Structural and metabolic characteristics of human skeletal muscle following 30 days of simulated microgravity. Aviat Space Environ Med. 1989 Jul;60(7):664–670. [PubMed] [Google Scholar]
- Hoppeler H., Mathieu O., Krauer R., Claassen H., Armstrong R. B., Weibel E. R. Design of the mammalian respiratory system. VI Distribution of mitochondria and capillaries in various muscles. Respir Physiol. 1981 Apr;44(1):87–111. doi: 10.1016/0034-5687(81)90078-5. [DOI] [PubMed] [Google Scholar]
- Howald H. Training-induced morphological and functional changes in skeletal muscle. Int J Sports Med. 1982 Feb;3(1):1–12. doi: 10.1055/s-2008-1026053. [DOI] [PubMed] [Google Scholar]
- Kaijser L. Limiting factors for aerobic muscle performance. The influence of varying oxygen pressure and temperature. Acta Physiol Scand Suppl. 1970;346:1–96. [PubMed] [Google Scholar]
- Kandarian S. C., Boushel R. C., Schulte L. M. Elevated interstitial fluid volume in rat soleus muscles by hindlimb unweighting. J Appl Physiol (1985) 1991 Sep;71(3):910–914. doi: 10.1152/jappl.1991.71.3.910. [DOI] [PubMed] [Google Scholar]
- Larsson L., Moss R. L. Maximum velocity of shortening in relation to myosin isoform composition in single fibres from human skeletal muscles. J Physiol. 1993 Dec;472:595–614. doi: 10.1113/jphysiol.1993.sp019964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lexell J., Taylor C. C. Variability in muscle fibre areas in whole human quadriceps muscle: how to reduce sampling errors in biopsy techniques. Clin Physiol. 1989 Aug;9(4):333–343. doi: 10.1111/j.1475-097x.1989.tb00987.x. [DOI] [PubMed] [Google Scholar]
- McDonald K. S., Fitts R. H. Effect of hindlimb unloading on rat soleus fiber force, stiffness, and calcium sensitivity. J Appl Physiol (1985) 1995 Nov;79(5):1796–1802. doi: 10.1152/jappl.1995.79.5.1796. [DOI] [PubMed] [Google Scholar]
- Ploutz-Snyder L. L., Tesch P. A., Crittenden D. J., Dudley G. A. Effect of unweighting on skeletal muscle use during exercise. J Appl Physiol (1985) 1995 Jul;79(1):168–175. doi: 10.1152/jappl.1995.79.1.168. [DOI] [PubMed] [Google Scholar]
- Saltin B. The interplay between peripheral and central factors in the adaptive response to exercise and training. Ann N Y Acad Sci. 1977;301:224–231. doi: 10.1111/j.1749-6632.1977.tb38201.x. [DOI] [PubMed] [Google Scholar]
- Sant'ana Pereira J. A., Wessels A., Nijtmans L., Moorman A. F., Sargeant A. J. New method for the accurate characterization of single human skeletal muscle fibres demonstrates a relation between mATPase and MyHC expression in pure and hybrid fibre types. J Muscle Res Cell Motil. 1995 Feb;16(1):21–34. doi: 10.1007/BF00125307. [DOI] [PubMed] [Google Scholar]
- Siggaard-Andersen O., Siggaard-Andersen M. The oxygen status algorithm: a computer program for calculating and displaying pH and blood gas data. Scand J Clin Lab Invest Suppl. 1990;203:29–45. doi: 10.3109/00365519009087489. [DOI] [PubMed] [Google Scholar]
- Sullivan M. J., Binkley P. F., Unverferth D. V., Ren J. H., Boudoulas H., Bashore T. M., Merola A. J., Leier C. V. Prevention of bedrest-induced physical deconditioning by daily dobutamine infusions. Implications for drug-induced physical conditioning. J Clin Invest. 1985 Oct;76(4):1632–1642. doi: 10.1172/JCI112148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi H., Wada M., Katsuta S. Expressions of myosin heavy chain IId isoform in rat soleus muscle during hindlimb suspension. Acta Physiol Scand. 1991 Sep;143(1):131–132. doi: 10.1111/j.1748-1716.1991.tb09209.x. [DOI] [PubMed] [Google Scholar]
- Thomason D. B., Booth F. W. Atrophy of the soleus muscle by hindlimb unweighting. J Appl Physiol (1985) 1990 Jan;68(1):1–12. doi: 10.1152/jappl.1990.68.1.1. [DOI] [PubMed] [Google Scholar]
- Turner D. L., Hoppeler H., Noti C., Gurtner H. P., Gerber H., Schena F., Kayser B., Ferretti G. Limitations to VO2max in humans after blood retransfusion. Respir Physiol. 1993 Jun;92(3):329–341. doi: 10.1016/0034-5687(93)90017-5. [DOI] [PubMed] [Google Scholar]
- Wagner P. D. Determinants of maximal oxygen transport and utilization. Annu Rev Physiol. 1996;58:21–50. doi: 10.1146/annurev.ph.58.030196.000321. [DOI] [PubMed] [Google Scholar]
- Weibel E. R., Taylor C. R., Hoppeler H. Variations in function and design: testing symmorphosis in the respiratory system. Respir Physiol. 1992 Mar;87(3):325–348. doi: 10.1016/0034-5687(92)90015-o. [DOI] [PubMed] [Google Scholar]
- di Prampero P. E., Ferretti G. Factors limiting maximal oxygen consumption in humans. Respir Physiol. 1990 May-Jun;80(2-3):113–127. doi: 10.1016/0034-5687(90)90075-a. [DOI] [PubMed] [Google Scholar]


