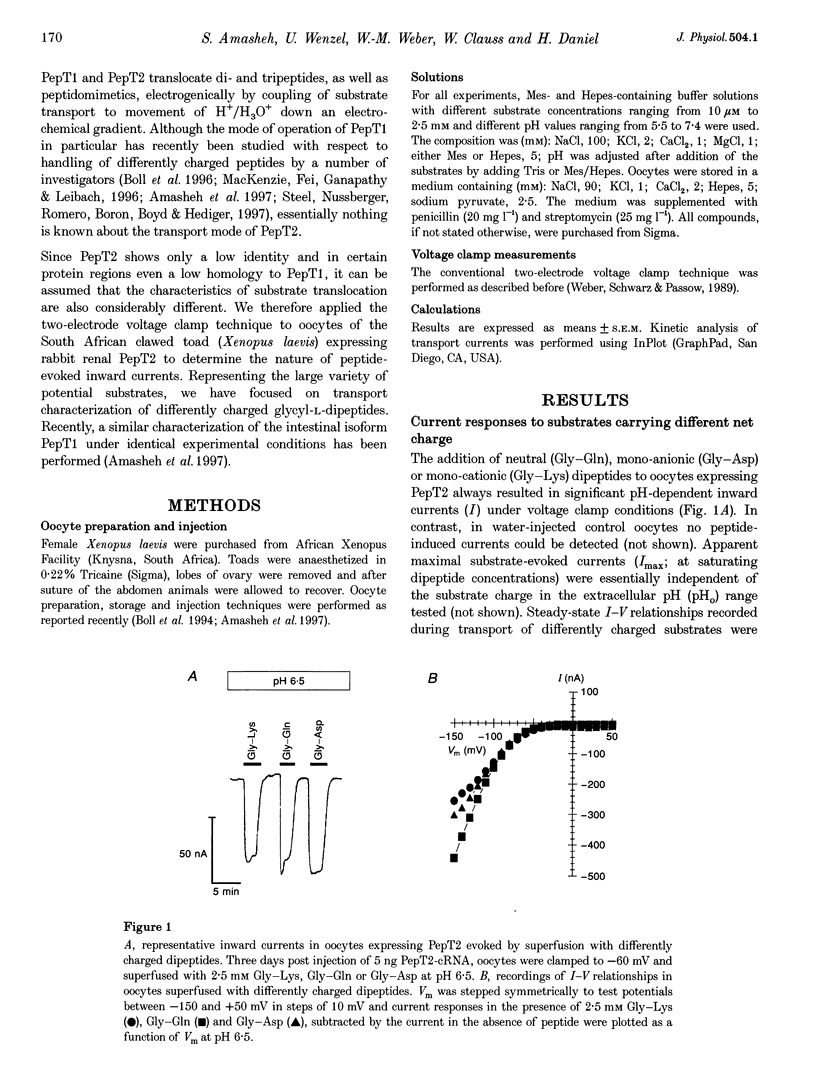
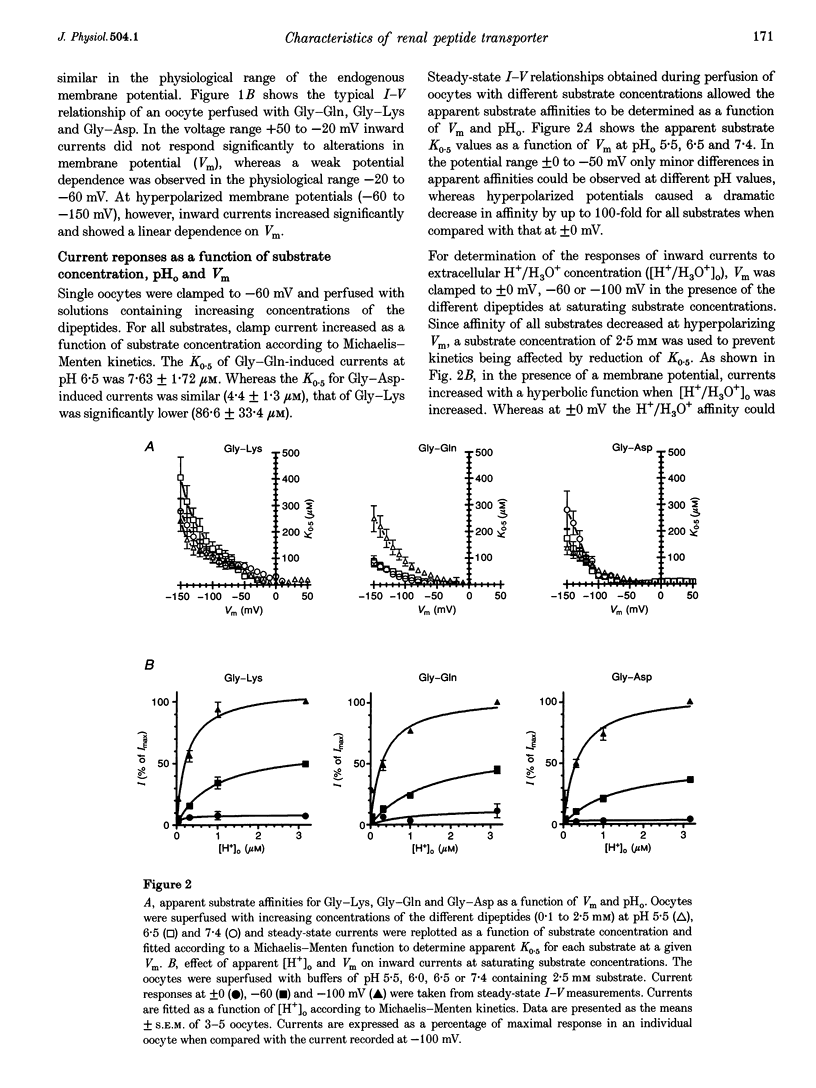
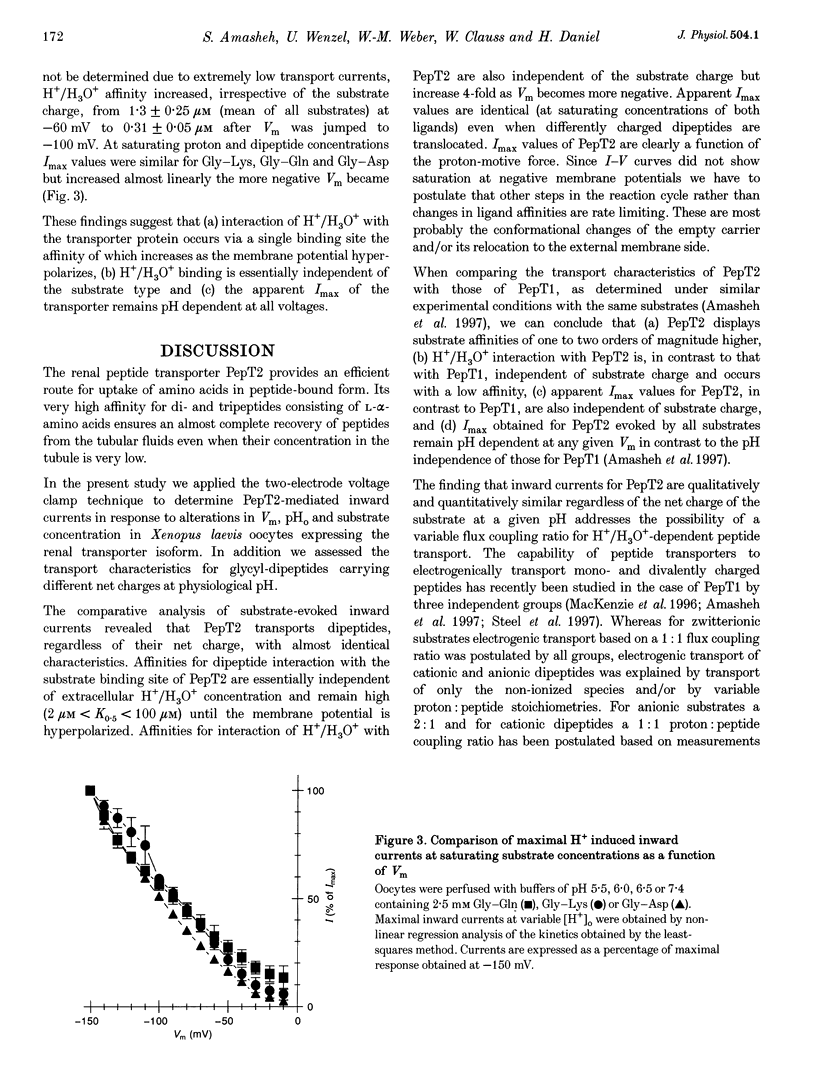
Abstract
1. To gain information on the mode of operation of the renal proton-coupled peptide transporter PepT2, voltage clamp studies were performed in Xenopus laevis oocytes expressing the rabbit renal PepT2. 2. Using differently charged glycyl-dipeptides we show that PepT2 translocates these dipeptides by an electrogenic pH-dependent process that is essentially independent of the substrate net charge. The apparent substrate affinities are in the micromolar range (2-50 microM) between pH 5.5 and 7.4 and membrane potentials of +/- 0 to -50 mV. 3. Maximal substrate-evoked inward currents (Imax) are affected by membrane voltage (Vm) and extracellular pH (pHo). Potential-dependent interactions of H+/H3O+ with PepT2 seem to be mediated by a single low affinity binding site and PepT2 remains pH dependent at all voltages. 4. The effects of voltage on apparent Imax and substrate affinity display an inverse relationship. As Vm is altered from -50 to -150 mV substrate affinities decrease 10- to 50-fold whereas apparent Imax increases almost 10-fold. 5. Even at saturating H+/H3O+ and dipeptide concentrations the I-V curves did not show saturation at negative membrane potentials, suggesting that other steps in the reaction cycle and not the ligand affinity changes are rate limiting. These are possibly the conformational changes of the empty and/or loaded transporters. 6. These findings demonstrate that not only substrate affinities but also other kinetic characteristics of PepT2 differ markedly from those of the intestinal peptide transporter isoform PepT1.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amasheh S., Wenzel U., Boll M., Dorn D., Weber W., Clauss W., Daniel H. Transport of charged dipeptides by the intestinal H+/peptide symporter PepT1 expressed in Xenopus laevis oocytes. J Membr Biol. 1997 Feb 1;155(3):247–256. doi: 10.1007/s002329900177. [DOI] [PubMed] [Google Scholar]
- Boll M., Herget M., Wagener M., Weber W. M., Markovich D., Biber J., Clauss W., Murer H., Daniel H. Expression cloning and functional characterization of the kidney cortex high-affinity proton-coupled peptide transporter. Proc Natl Acad Sci U S A. 1996 Jan 9;93(1):284–289. doi: 10.1073/pnas.93.1.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boll M., Markovich D., Weber W. M., Korte H., Daniel H., Murer H. Expression cloning of a cDNA from rabbit small intestine related to proton-coupled transport of peptides, beta-lactam antibiotics and ACE-inhibitors. Pflugers Arch. 1994 Nov;429(1):146–149. doi: 10.1007/BF02584043. [DOI] [PubMed] [Google Scholar]
- Boorer K. J., Frommer W. B., Bush D. R., Kreman M., Loo D. D., Wright E. M. Kinetics and specificity of a H+/amino acid transporter from Arabidopsis thaliana. J Biol Chem. 1996 Jan 26;271(4):2213–2220. doi: 10.1074/jbc.271.4.2213. [DOI] [PubMed] [Google Scholar]
- Daniel H. Function and molecular structure of brush border membrane peptide/H+ symporters. J Membr Biol. 1996 Dec;154(3):197–203. doi: 10.1007/s002329900144. [DOI] [PubMed] [Google Scholar]
- Daniel H., Morse E. L., Adibi S. A. The high and low affinity transport systems for dipeptides in kidney brush border membrane respond differently to alterations in pH gradient and membrane potential. J Biol Chem. 1991 Oct 25;266(30):19917–19924. [PubMed] [Google Scholar]
- Fei Y. J., Kanai Y., Nussberger S., Ganapathy V., Leibach F. H., Romero M. F., Singh S. K., Boron W. F., Hediger M. A. Expression cloning of a mammalian proton-coupled oligopeptide transporter. Nature. 1994 Apr 7;368(6471):563–566. doi: 10.1038/368563a0. [DOI] [PubMed] [Google Scholar]
- Liang R., Fei Y. J., Prasad P. D., Ramamoorthy S., Han H., Yang-Feng T. L., Hediger M. A., Ganapathy V., Leibach F. H. Human intestinal H+/peptide cotransporter. Cloning, functional expression, and chromosomal localization. J Biol Chem. 1995 Mar 24;270(12):6456–6463. doi: 10.1074/jbc.270.12.6456. [DOI] [PubMed] [Google Scholar]
- Liu W., Liang R., Ramamoorthy S., Fei Y. J., Ganapathy M. E., Hediger M. A., Ganapathy V., Leibach F. H. Molecular cloning of PEPT 2, a new member of the H+/peptide cotransporter family, from human kidney. Biochim Biophys Acta. 1995 May 4;1235(2):461–466. doi: 10.1016/0005-2736(95)80036-f. [DOI] [PubMed] [Google Scholar]
- Mackenzie B., Fei Y. J., Ganapathy V., Leibach F. H. The human intestinal H+/oligopeptide cotransporter hPEPT1 transports differently-charged dipeptides with identical electrogenic properties. Biochim Biophys Acta. 1996 Oct 23;1284(2):125–128. doi: 10.1016/s0005-2736(96)00170-8. [DOI] [PubMed] [Google Scholar]
- Miyamoto Y., Ganapathy V., Leibach F. H. Identification of histidyl and thiol groups at the active site of rabbit renal dipeptide transporter. J Biol Chem. 1986 Dec 5;261(34):16133–16140. [PubMed] [Google Scholar]
- Saito H., Okuda M., Terada T., Sasaki S., Inui K. Cloning and characterization of a rat H+/peptide cotransporter mediating absorption of beta-lactam antibiotics in the intestine and kidney. J Pharmacol Exp Ther. 1995 Dec;275(3):1631–1637. [PubMed] [Google Scholar]
- Shan S. O., Herschlag D. The change in hydrogen bond strength accompanying charge rearrangement: implications for enzymatic catalysis. Proc Natl Acad Sci U S A. 1996 Dec 10;93(25):14474–14479. doi: 10.1073/pnas.93.25.14474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silbernagl S., Ganapathy V., Leibach F. H. H+ gradient-driven dipeptide reabsorption in proximal tubule of rat kidney. Studies in vivo and in vitro. Am J Physiol. 1987 Sep;253(3 Pt 2):F448–F457. doi: 10.1152/ajprenal.1987.253.3.F448. [DOI] [PubMed] [Google Scholar]
- Steel A., Nussberger S., Romero M. F., Boron W. F., Boyd C. A., Hediger M. A. Stoichiometry and pH dependence of the rabbit proton-dependent oligopeptide transporter PepT1. J Physiol. 1997 Feb 1;498(Pt 3):563–569. doi: 10.1113/jphysiol.1997.sp021883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner H. Y., Naider F., Becker J. M. The PTR family: a new group of peptide transporters. Mol Microbiol. 1995 Jun;16(5):825–834. doi: 10.1111/j.1365-2958.1995.tb02310.x. [DOI] [PubMed] [Google Scholar]
- Weber W. M., Schwarz W., Passow H. Endogenous D-glucose transport in oocytes of Xenopus laevis. J Membr Biol. 1989 Oct;111(1):93–102. doi: 10.1007/BF01869212. [DOI] [PubMed] [Google Scholar]


