Abstract
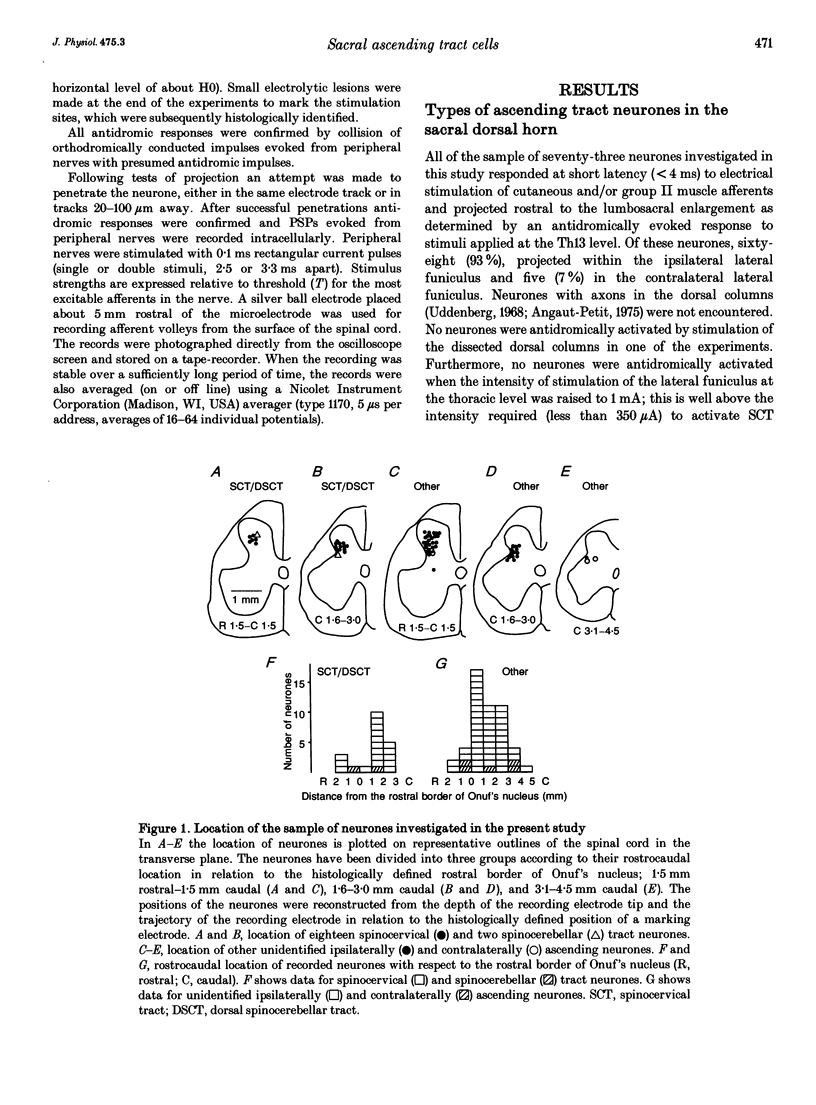
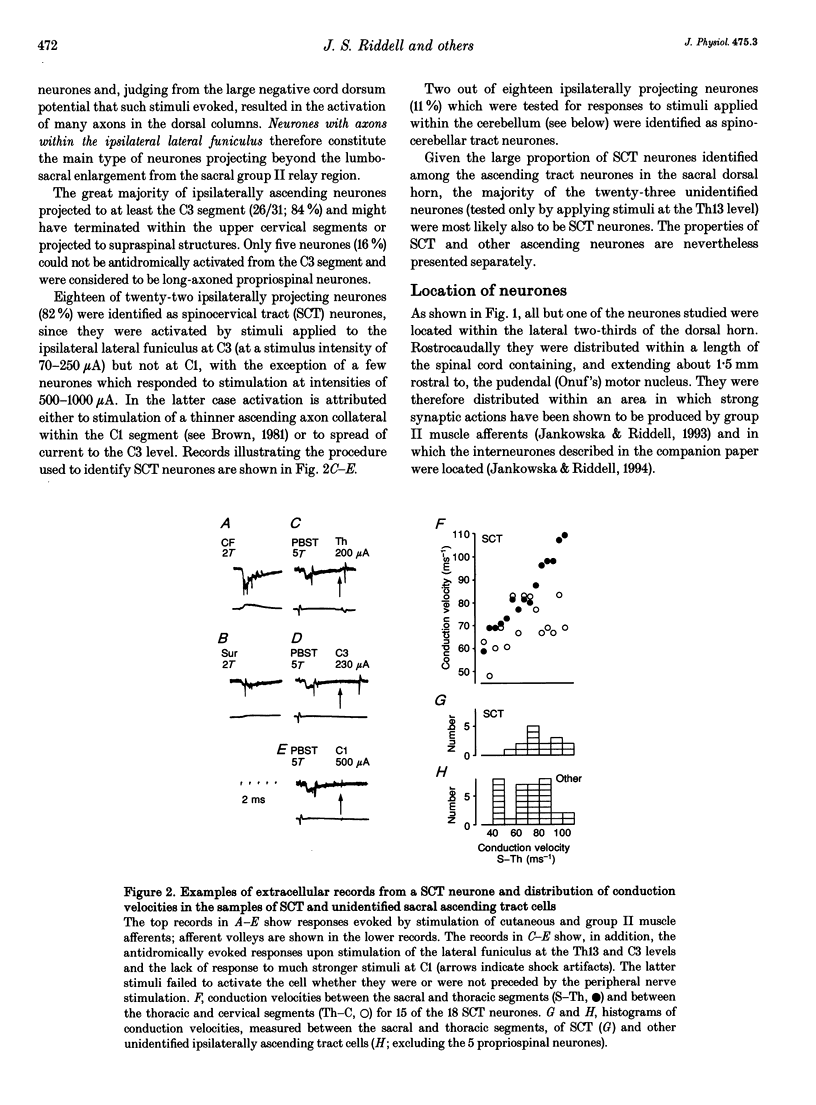
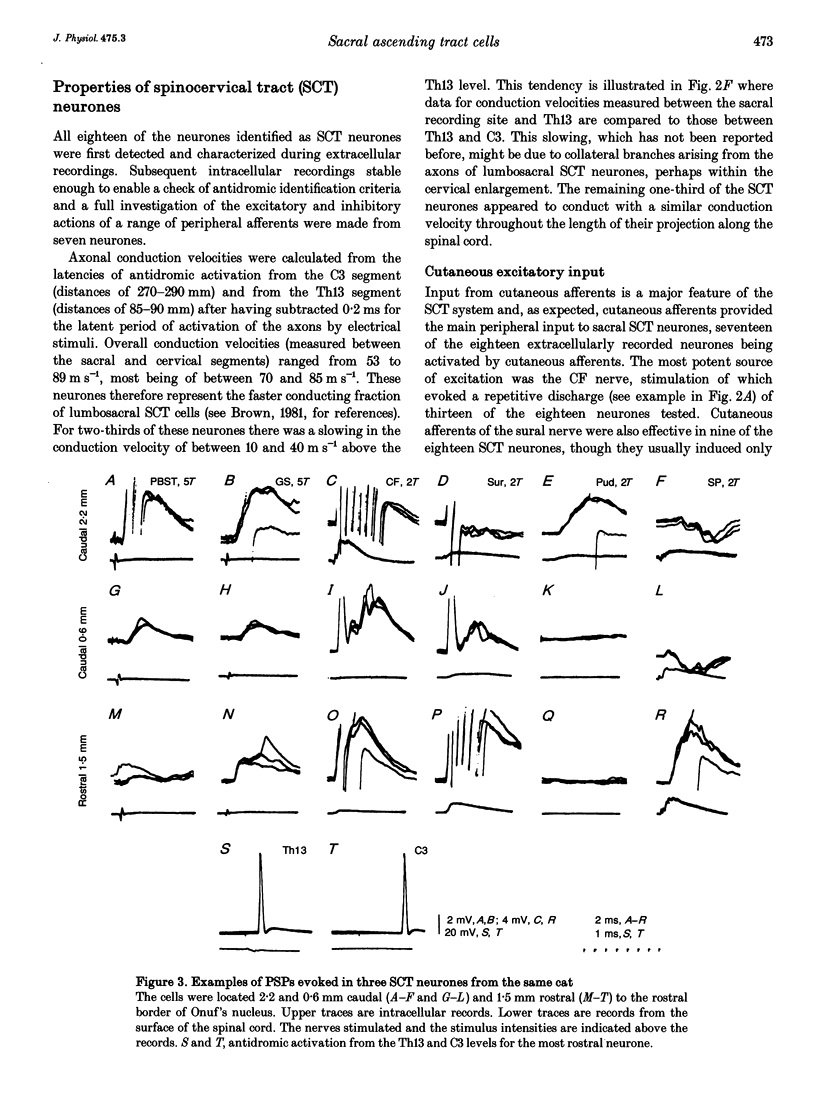
1. Ascending tract neurones located in the dorsal horn of sacral segments of the spinal cord have been investigated by extracellular and intracellular recording in the anaesthetized cat. The aim was to determine whether information from group II afferents that terminate within the sacral segments is conveyed to supraspinal structures and which types of neurones are involved. 2. A considerable proportion of ascending tract neurones found in the dorsal horn in the same segments as the pudendal (Onuf's) motor nucleus were excited by group II muscle afferents. The great majority (93%) of these neurones had axons ascending in ipsilateral funiculi. Spinocervical tract neurones constituted the largest proportion (82%) of such neurones, while very few spinocerebellar tract and propriospinal neurones and no postsynaptic dorsal column neurones were found among them. 3. In addition to activation by group II muscle afferents all of the neurones were strongly excited by cutaneous afferents. The most potent excitation was evoked by afferents of the posterior biceps-semitendinosus and gastrocnemius muscle nerves and by afferents of the cutaneous femoris, sural and pudendal nerves. The latencies of intracellularly recorded excitatory potentials were indicative of a high incidence of monosynaptic coupling between the afferents and ascending tract neurones. 4. The highly effective monosynaptic excitation of spinocervical tract neurones in the sacral segments by group II afferents is in contrast to the weak disynaptically mediated actions of group II afferents on such neurones in the L6-L7 segments but comparable to the actions of group II afferents on ascending tract neurones in the midlumbar segments. 5. Both the patterns of peripheral input and the latencies of synaptic actions in ascending tract neurones were similar to those in interneurones at the same locations (accompanying report). Similar information is therefore likely to be processed by both categories of neurones. 6. The role of sacral spinocervical tract neurones as a system for transmitting information from group II muscle afferents to supraspinal centres and the potential contribution of this system to the perception of limb position are discussed.
Full text
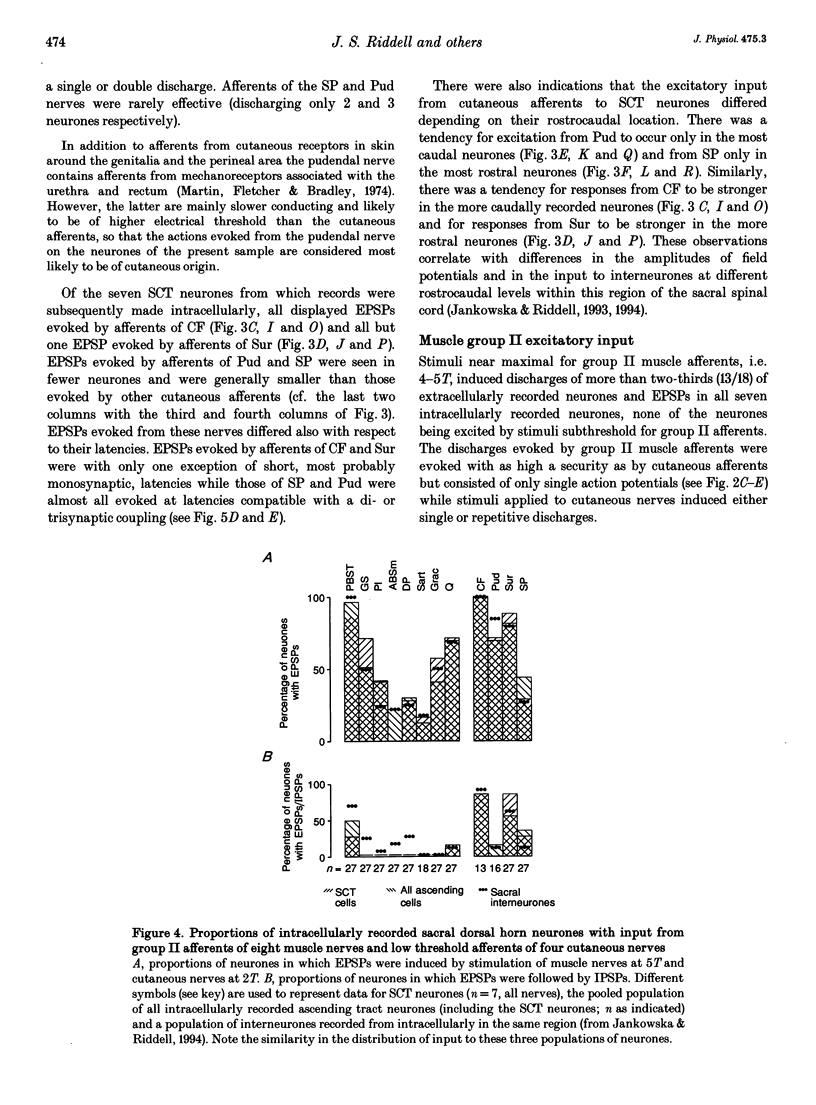
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angaut-Petit D. The dorsal column system: I. Existence of long ascending postsynaptic fibres in the cat's fasciculus gracilis. Exp Brain Res. 1975 May 22;22(5):457–470. doi: 10.1007/BF00237348. [DOI] [PubMed] [Google Scholar]
- Asif M., Edgley S. A. Projections of group II-activated midlumbar spinocerebellar tract neurones to the region of nucleus Z in the cat. J Physiol. 1992 Mar;448:565–578. doi: 10.1113/jphysiol.1992.sp019058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett G. J., Seltzer Z., Lu G. W., Nishikawa N., Dubner R. The cells of origin of the dorsal column postsynaptic projection in the lumbosacral enlargements of cats and monkeys. Somatosens Res. 1983;1(2):131–149. doi: 10.3109/07367228309144545. [DOI] [PubMed] [Google Scholar]
- Brown A. G., Franz D. N. Responses of spinocervical tract neurones to natural stimulation of identified cutaneous receptors. Exp Brain Res. 1969;7(3):231–249. doi: 10.1007/BF00239031. [DOI] [PubMed] [Google Scholar]
- Brown A. G., Fyffe R. E., Noble R., Rose P. K., Snow P. J. The density, distribution and topographical organization of spinocervical tract neurones in the cat. J Physiol. 1980 Mar;300:409–428. doi: 10.1113/jphysiol.1980.sp013169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. G., Koerber H. R., Noble R. Excitatory actions of single impulses in single hair follicle afferent fibres on spinocervical tract neurones in the cat. J Physiol. 1987 Jan;382:291–312. doi: 10.1113/jphysiol.1987.sp016368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. G. The spinocervical tract. Prog Neurobiol. 1981;17(1-2):59–96. doi: 10.1016/0301-0082(81)90004-6. [DOI] [PubMed] [Google Scholar]
- Burgess P. R., Wei J. Y., Clark F. J., Simon J. Signaling of kinesthetic information by peripheral sensory receptors. Annu Rev Neurosci. 1982;5:171–187. doi: 10.1146/annurev.ne.05.030182.001131. [DOI] [PubMed] [Google Scholar]
- Craig A. D., Jr Spinal and medullary input to the lateral cervical nucleus. J Comp Neurol. 1978 Oct 15;181(4):729–743. doi: 10.1002/cne.901810404. [DOI] [PubMed] [Google Scholar]
- Edgley S. A., Gallimore C. M. The morphology and projections of dorsal horn spinocerebellar tract neurones in the cat. J Physiol. 1988 Mar;397:99–111. doi: 10.1113/jphysiol.1988.sp016990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgley S. A., Jankowska E. An interneuronal relay for group I and II muscle afferents in the midlumbar segments of the cat spinal cord. J Physiol. 1987 Aug;389:647–674. doi: 10.1113/jphysiol.1987.sp016676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgley S. A., Jankowska E. Field potentials generated by group II muscle afferents in the middle lumbar segments of the cat spinal cord. J Physiol. 1987 Apr;385:393–413. doi: 10.1113/jphysiol.1987.sp016498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgley S. A., Jankowska E. Information processed by dorsal horn spinocerebellar tract neurones in the cat. J Physiol. 1988 Mar;397:81–97. doi: 10.1113/jphysiol.1988.sp016989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enevoldson T. P., Gordon G. Postsynaptic dorsal column neurons in the cat: a study with retrograde transport of horseradish peroxidase. Exp Brain Res. 1989;75(3):611–620. doi: 10.1007/BF00249912. [DOI] [PubMed] [Google Scholar]
- Enevoldson T. P., Gordon G. Spinocervical neurons and dorsal horn neurons projecting to the dorsal column nuclei through the dorsolateral fascicle: a retrograde HRP study in the cat. Exp Brain Res. 1989;75(3):621–630. doi: 10.1007/BF00249913. [DOI] [PubMed] [Google Scholar]
- Fern R., Harrison P. J., Riddell J. S. The dorsal column projection of muscle afferent fibres from the cat hindlimb. J Physiol. 1988 Jul;401:97–113. doi: 10.1113/jphysiol.1988.sp017153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu T. C., Santini M., Schomburg E. D. Characteristics and distribution of spinal focal synaptic potentials generated by group II muscle afferents. Acta Physiol Scand. 1974 Jul;91(3):298–313. doi: 10.1111/j.1748-1716.1974.tb05686.x. [DOI] [PubMed] [Google Scholar]
- Grant G., Wiksten B., Berkley K. J., Aldskogius H. The location of cerebellar-projecting neurons within the lumbosacral spinal cord in the cat. An anatomical study with HRP and retrograde chromatolysis. J Comp Neurol. 1982 Feb 1;204(4):336–348. doi: 10.1002/cne.902040405. [DOI] [PubMed] [Google Scholar]
- Grottel K., Huber J., Kowalski K. Functional properties of crossed spinocerebellar tract neurones with cell bodies in the S1 segment. Neurosci Res. 1991 Sep;11(4):286–291. doi: 10.1016/0168-0102(91)90011-m. [DOI] [PubMed] [Google Scholar]
- Gustafsson B., Jankowska E. Direct and indirect activation of nerve cells by electrical pulses applied extracellularly. J Physiol. 1976 Jun;258(1):33–61. doi: 10.1113/jphysiol.1976.sp011405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamann W. C., Hong S. K., Kniffki K. D., Schmidt R. F. Projections of primary afferent fibres from muscle to neurones of the spinocervical tract of the cat. J Physiol. 1978 Oct;283:369–378. doi: 10.1113/jphysiol.1978.sp012506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison P. J., Jankowska E. An intracellular study of descending and non-cutaneous afferent input to spinocervical tract neurones in the cat. J Physiol. 1984 Nov;356:245–261. doi: 10.1113/jphysiol.1984.sp015462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hongo T., Jankowska E., Lundberg A. Post-synaptic excitation and inhibition from primary afferents in neurones of the spinocervical tract. J Physiol. 1968 Dec;199(3):569–592. doi: 10.1113/jphysiol.1968.sp008669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska E., Rastad J., Zarzecki P. Segmental and supraspinal input to cells of origin of non-primary fibres in the feline dorsal columns. J Physiol. 1979 May;290(2):185–200. doi: 10.1113/jphysiol.1979.sp012767. [DOI] [PMC free article] [PubMed] [Google Scholar]
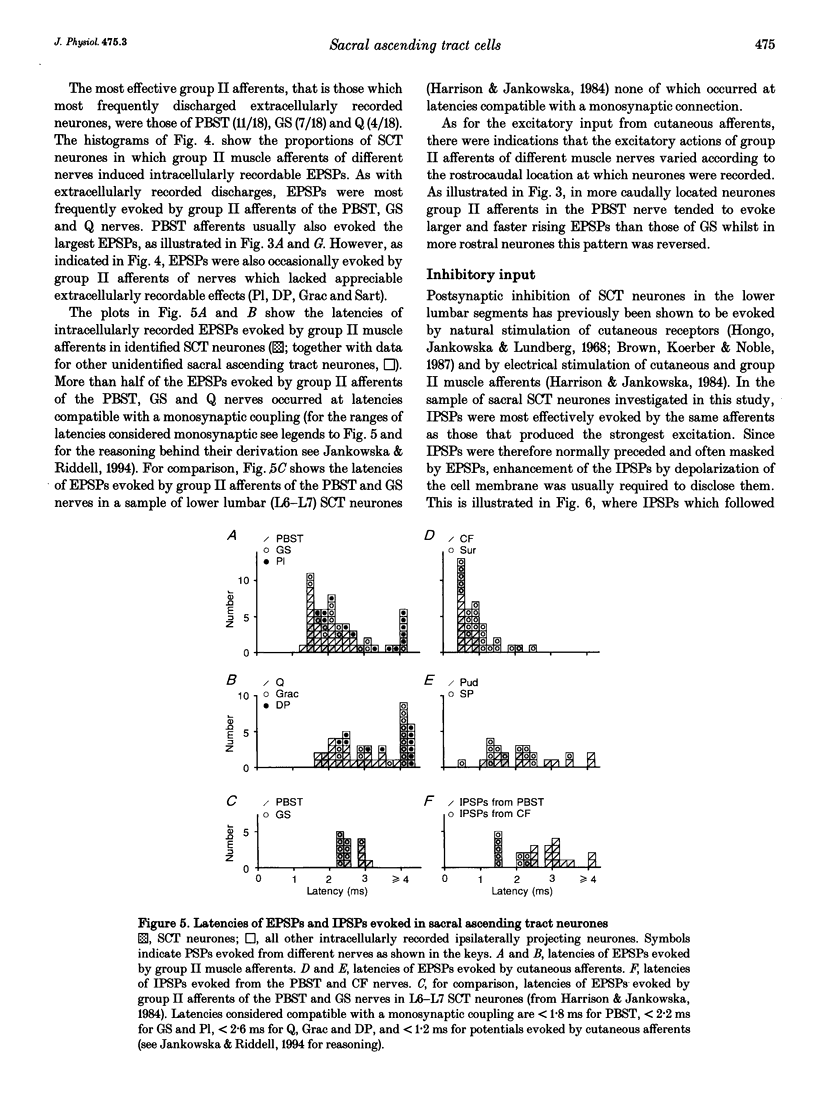
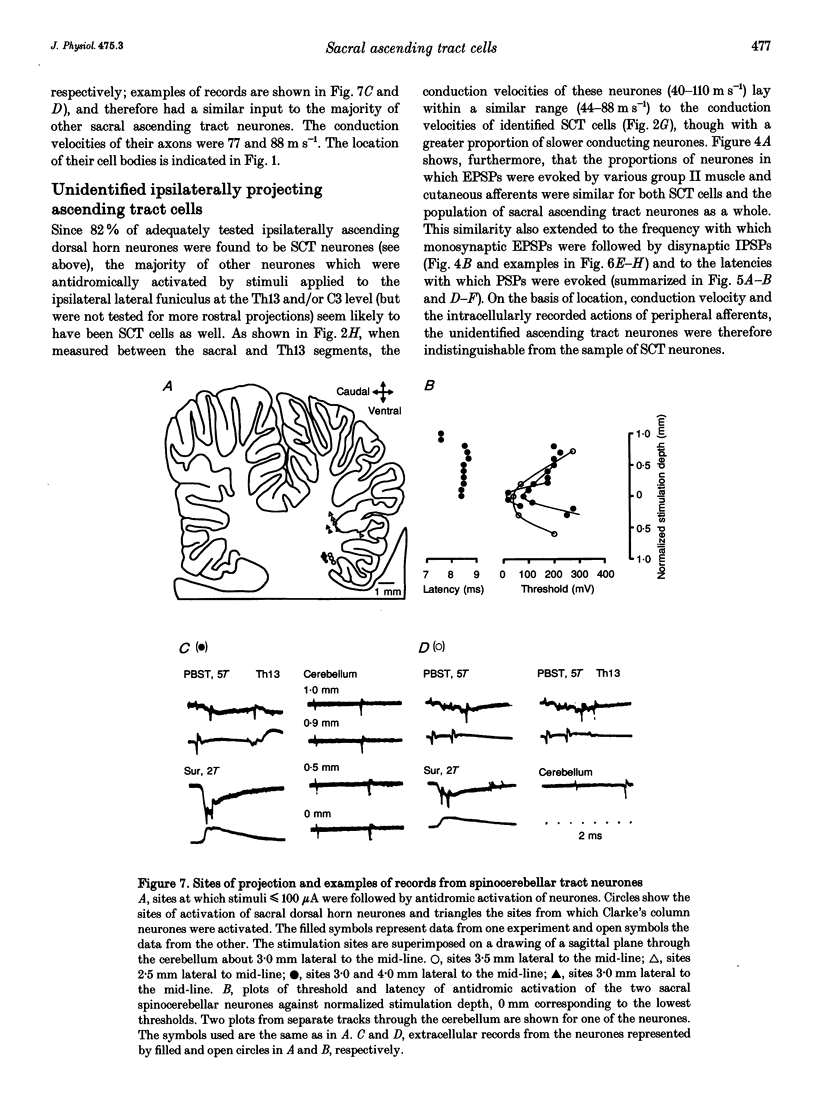
- Jankowska E., Riddell J. S. A relay for input from group II muscle afferents in sacral segments of the cat spinal cord. J Physiol. 1993 Jun;465:561–580. doi: 10.1113/jphysiol.1993.sp019693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska E., Riddell J. S. Interneurones in pathways from group II muscle afferents in sacral segments of the feline spinal cord. J Physiol. 1994 Mar 15;475(3):455–468. doi: 10.1113/jphysiol.1994.sp020085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska E., Roberts W. J. An electrophysiological demonstration of the axonal projections of single spinal interneurones in the cat. J Physiol. 1972 May;222(3):597–622. doi: 10.1113/jphysiol.1972.sp009817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson H., Silfvenius H. Axon-collateral activation by dorsal spinocerebellar tract fibres of group I relay cells of nucleus Z in the cat medulla oblongata. J Physiol. 1977 Feb;265(2):341–369. doi: 10.1113/jphysiol.1977.sp011720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LLOYD D. P. C., McINTYRE A. K. Dorsal column conduction of group I muscle afferent impulses and their relay through Clarke's column. J Neurophysiol. 1950 Jan;13(1):39–54. doi: 10.1152/jn.1950.13.1.39. [DOI] [PubMed] [Google Scholar]
- Landgren S., Silfvenius H. Nucleus Z, the medullary relay in the projection path to the cerebral cortex of group I muscle afferents from the cat's hind limb. J Physiol. 1971 Nov;218(3):551–571. doi: 10.1113/jphysiol.1971.sp009633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebman R. S., Levitt M. Position sense after combined spinal tractotomies and cerebellectomies in macaques. Exp Neurol. 1973 Jul;40(1):170–182. doi: 10.1016/0014-4886(73)90133-7. [DOI] [PubMed] [Google Scholar]
- Lundberg A. Function of the ventral spinocerebellar tract. A new hypothesis. Exp Brain Res. 1971;12(3):317–330. doi: 10.1007/BF00237923. [DOI] [PubMed] [Google Scholar]
- Martin W. D., Fletcher T. F., Bradley W. E. Innervation of feline perineal musculature. Anat Rec. 1974 Sep;180(1):15–29. doi: 10.1002/ar.1091800104. [DOI] [PubMed] [Google Scholar]
- Matsushita M., Hosoya Y., Ikeda M. Anatomical organization of the spinocerebellar system in the cat, as studied by retrograde transport of horseradish peroxidase. J Comp Neurol. 1979 Mar 1;184(1):81–106. doi: 10.1002/cne.901840106. [DOI] [PubMed] [Google Scholar]
- Matsushita M. Spinocerebellar projections from the lowest lumbar and sacral-caudal segments in the cat, as studied by anterograde transport of wheat germ agglutinin-horseradish peroxidase. J Comp Neurol. 1988 Aug 8;274(2):239–254. doi: 10.1002/cne.902740208. [DOI] [PubMed] [Google Scholar]
- Matthews P. B. Where does Sherrington's "muscular sense" originate? Muscles, joints, corollary discharges? Annu Rev Neurosci. 1982;5:189–218. doi: 10.1146/annurev.ne.05.030182.001201. [DOI] [PubMed] [Google Scholar]
- ROMANES G. J. The motor cell columns of the lumbo-sacral spinal cord of the cat. J Comp Neurol. 1951 Apr;94(2):313–363. doi: 10.1002/cne.900940209. [DOI] [PubMed] [Google Scholar]
- Rustioni A., Kaufman A. B. Identification of cells or origin of non-primary afferents to the dorsal column nuclei of the cat. Exp Brain Res. 1977 Jan 18;27(1):1–14. doi: 10.1007/BF00234821. [DOI] [PubMed] [Google Scholar]
- Uddenberg N. Functional organization of long, second-order afferents in the dorsal funiculus. Exp Brain Res. 1968;4(4):377–382. doi: 10.1007/BF00235702. [DOI] [PubMed] [Google Scholar]
- Xu Q., Grant G. Collateral projections of neurons from the lower part of the spinal cord to anterior and posterior cerebellar termination areas. A retrograde fluorescent double labeling study in the cat. Exp Brain Res. 1988;72(3):562–576. doi: 10.1007/BF00250601. [DOI] [PubMed] [Google Scholar]


