Abstract
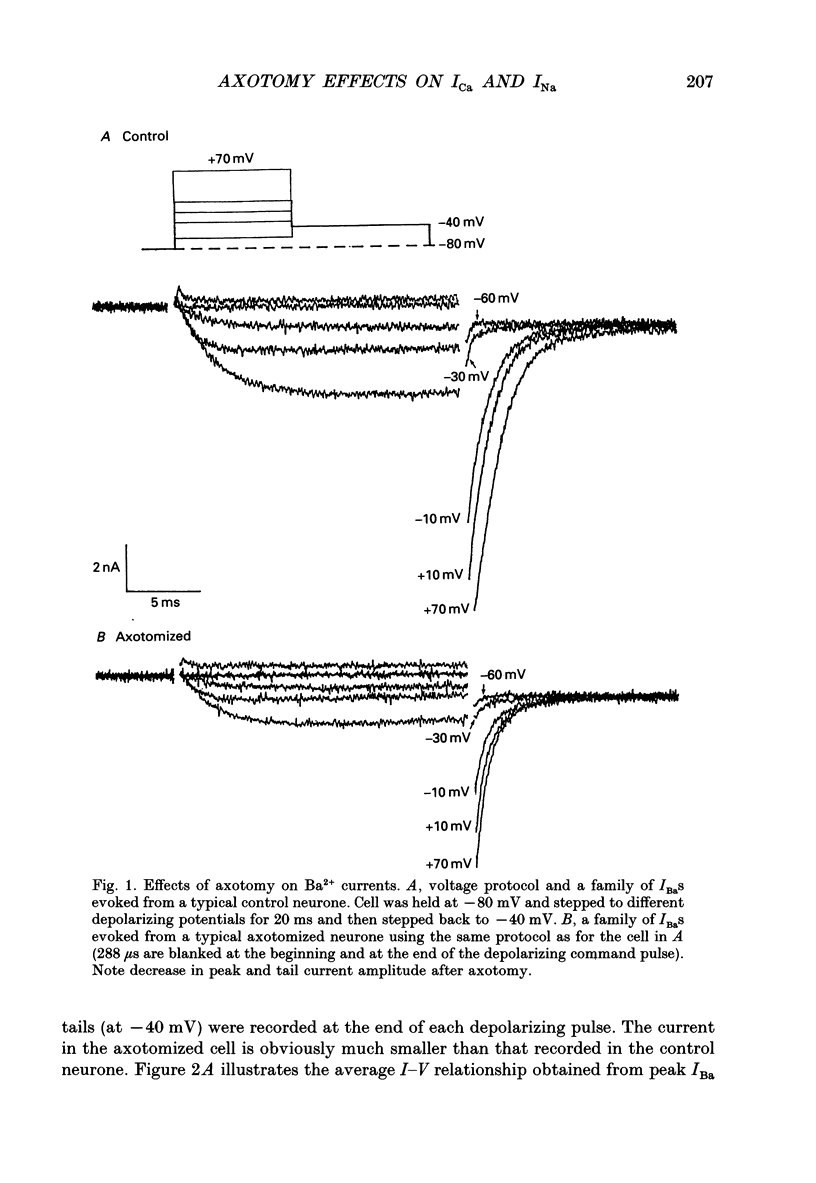
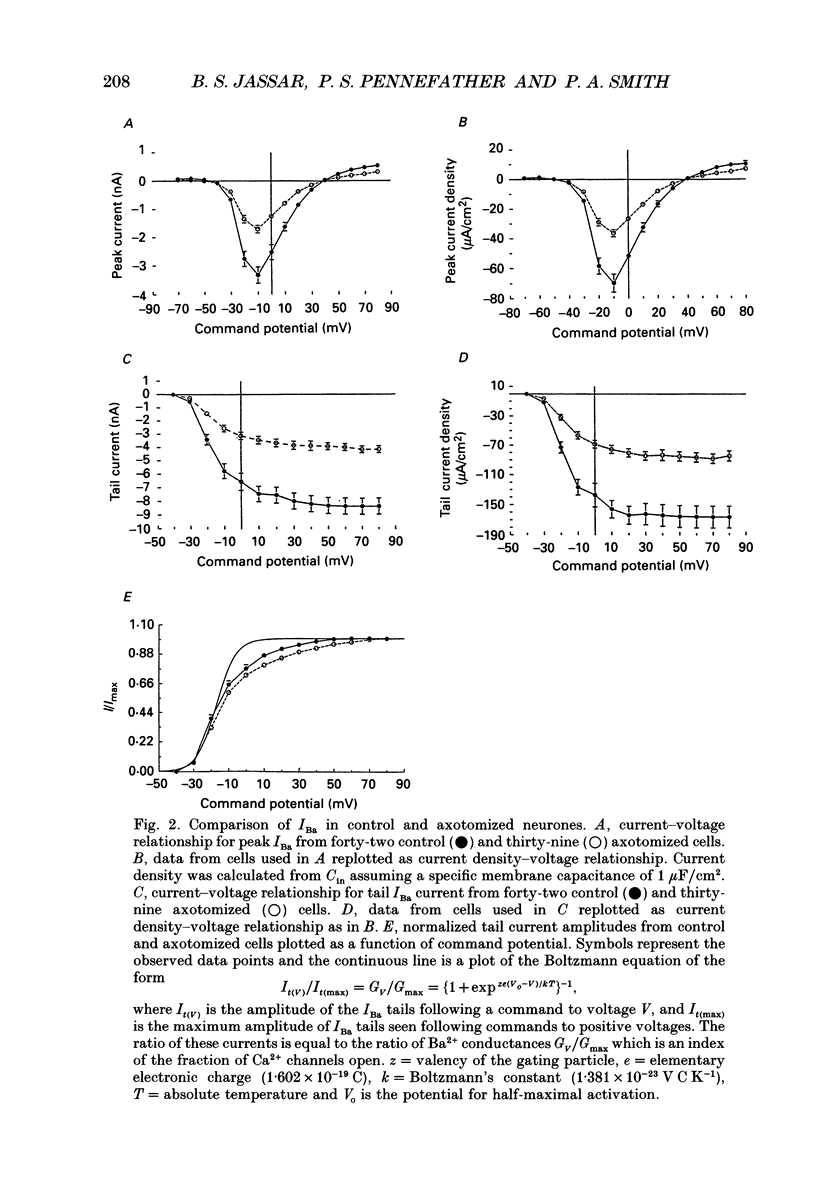
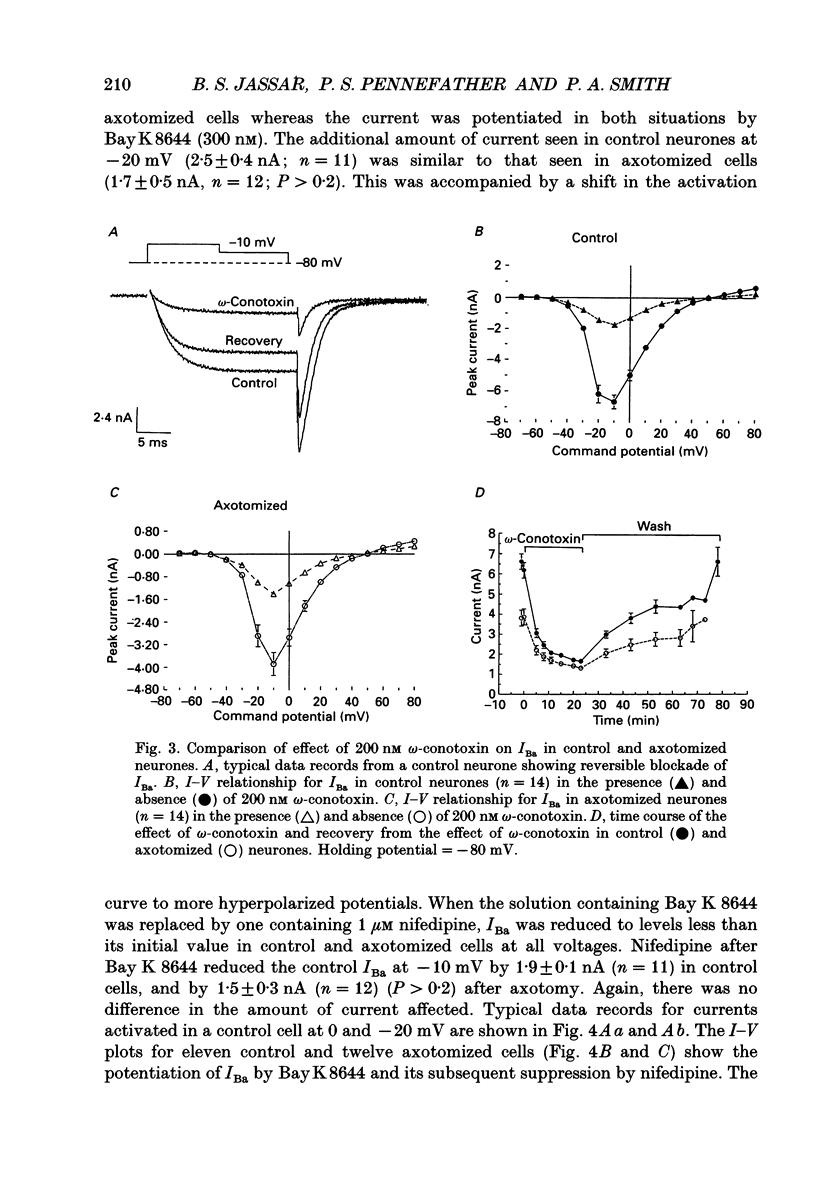
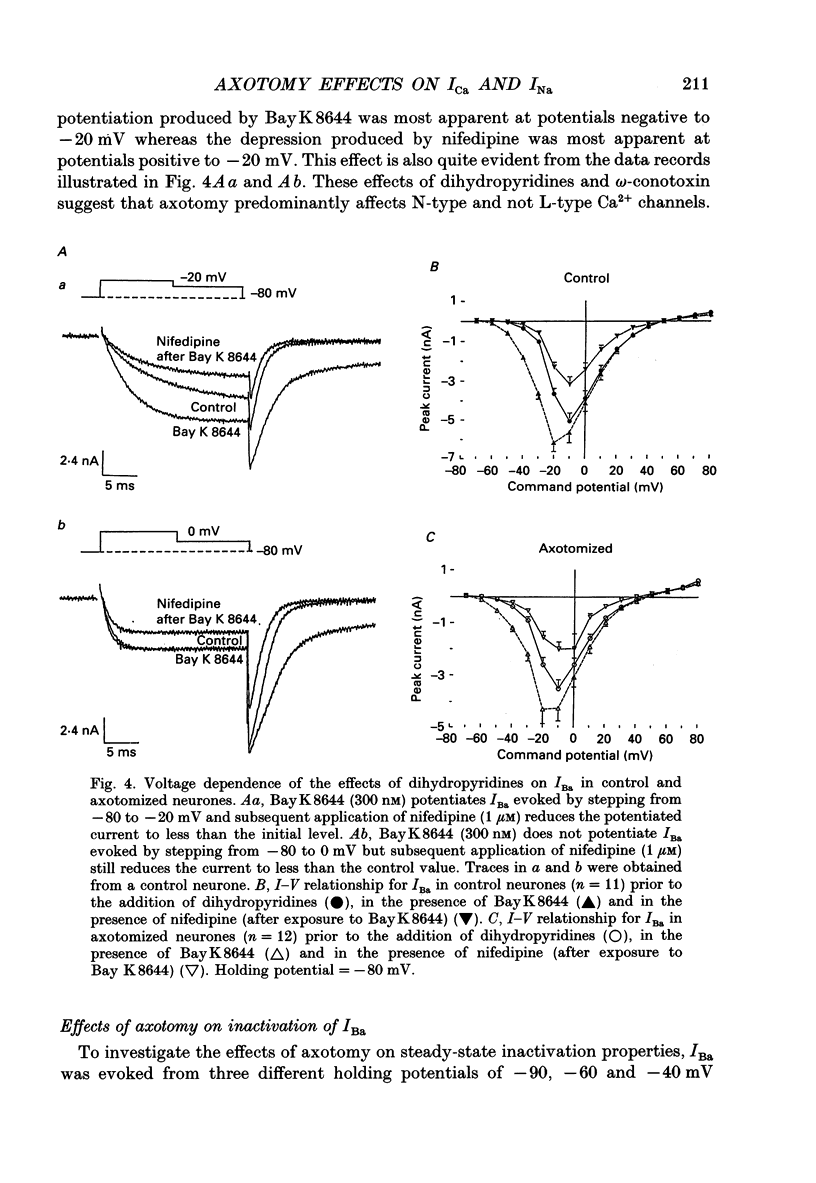
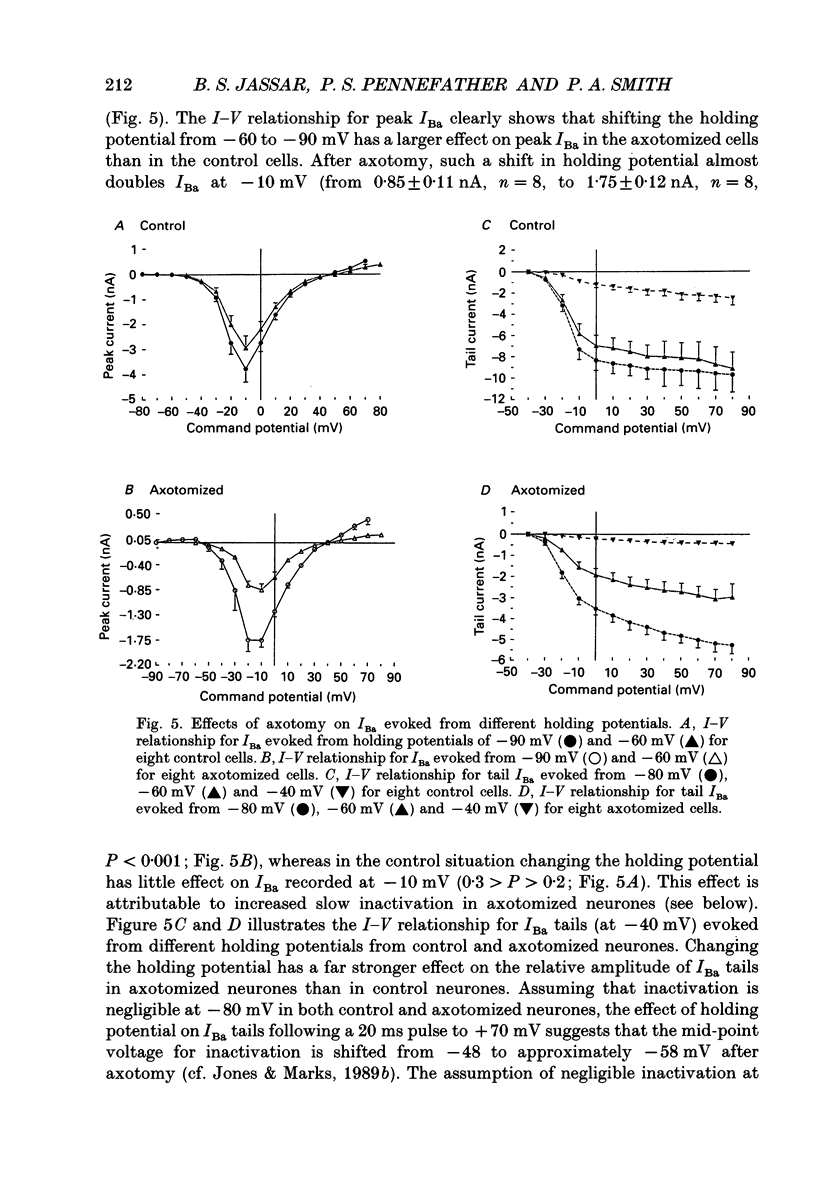
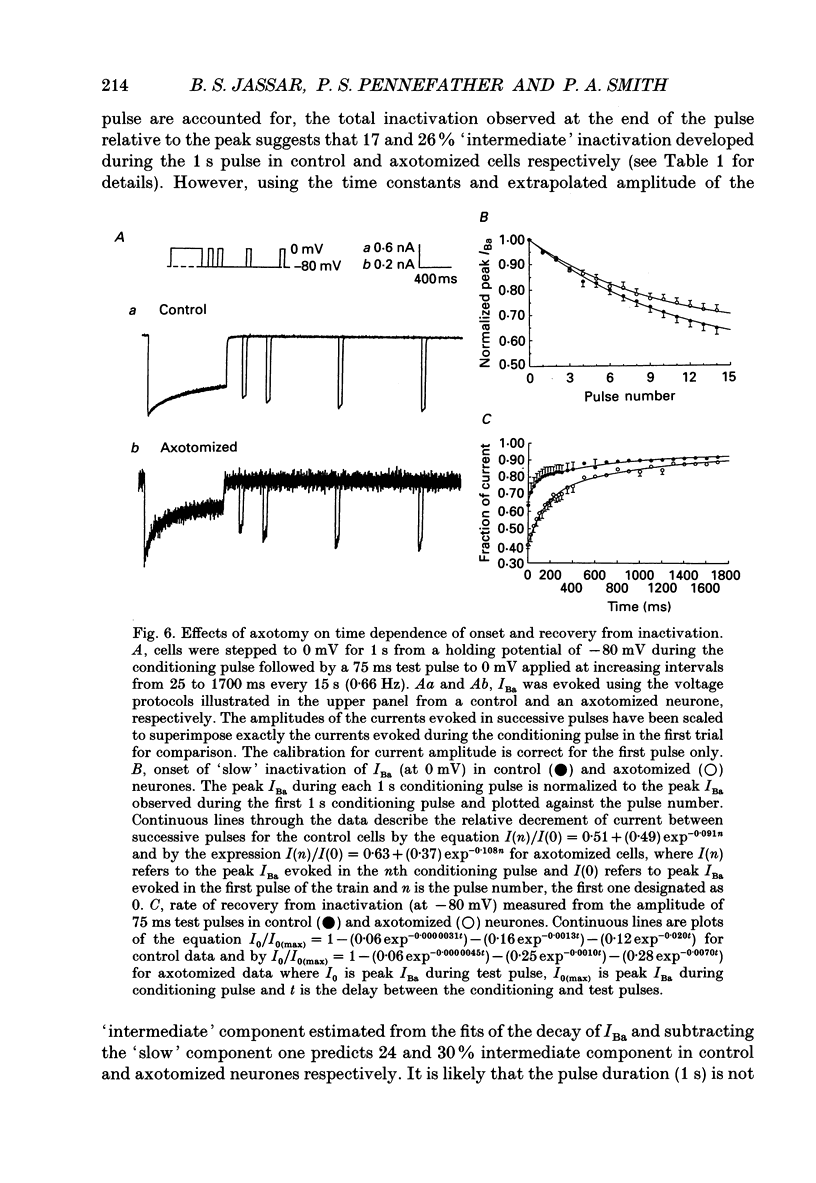
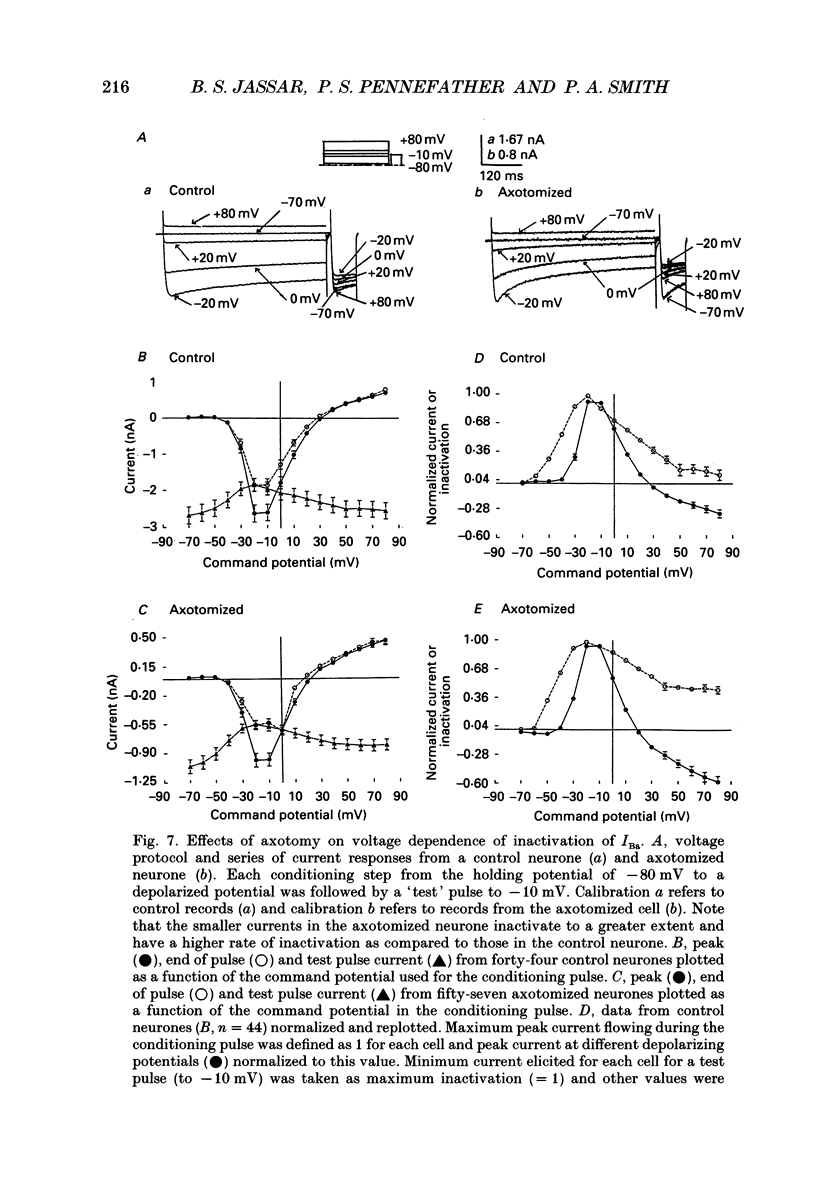
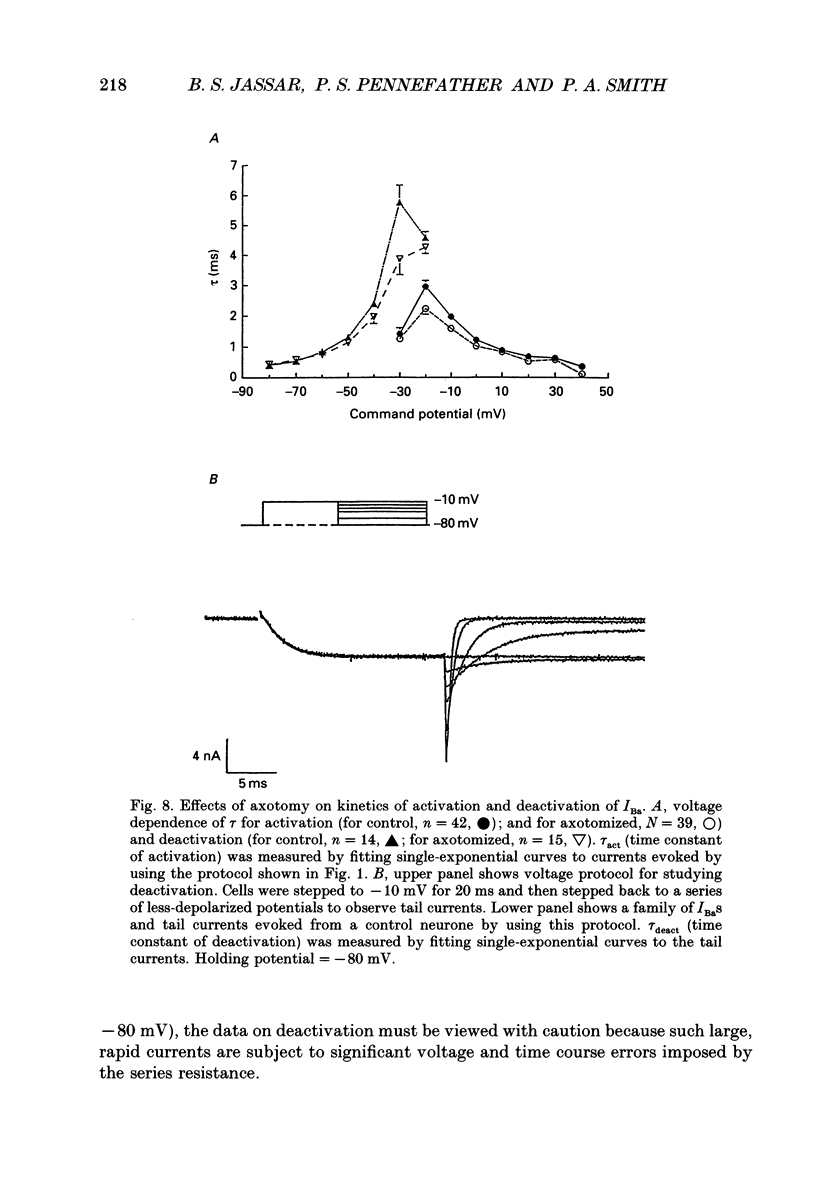
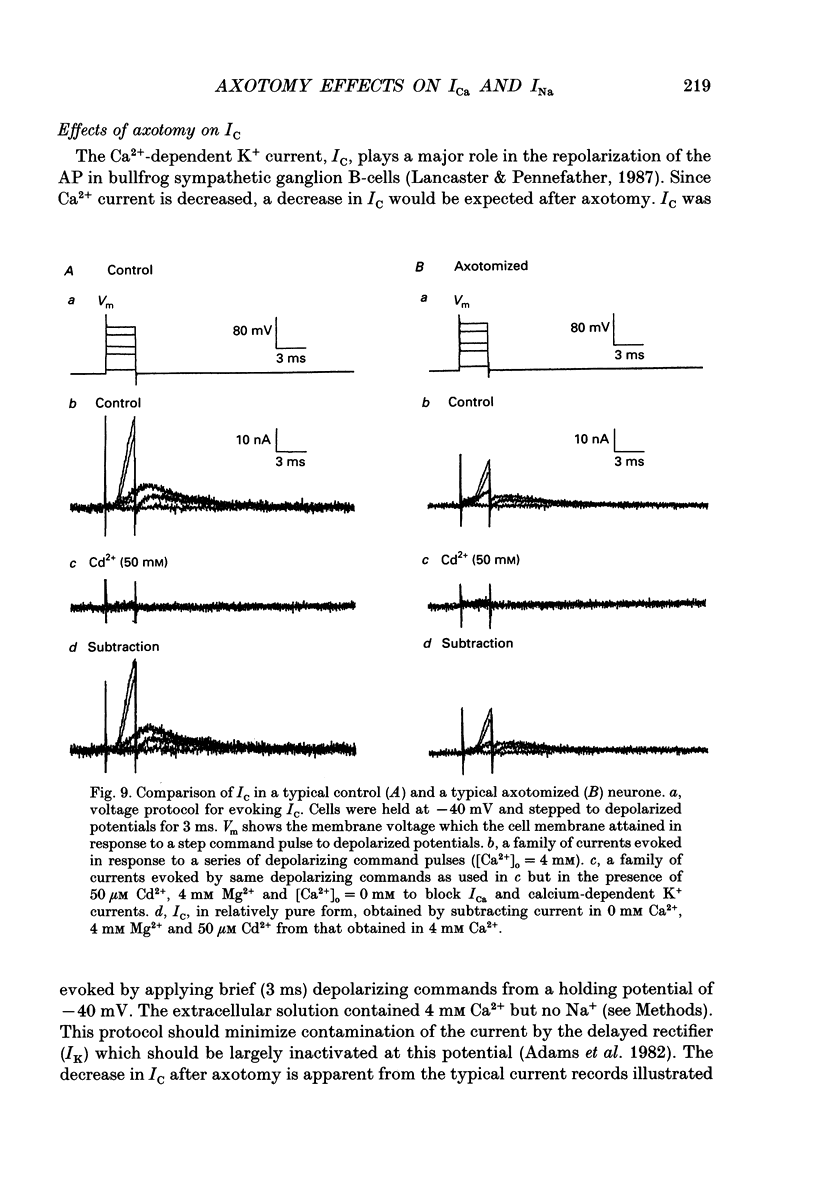
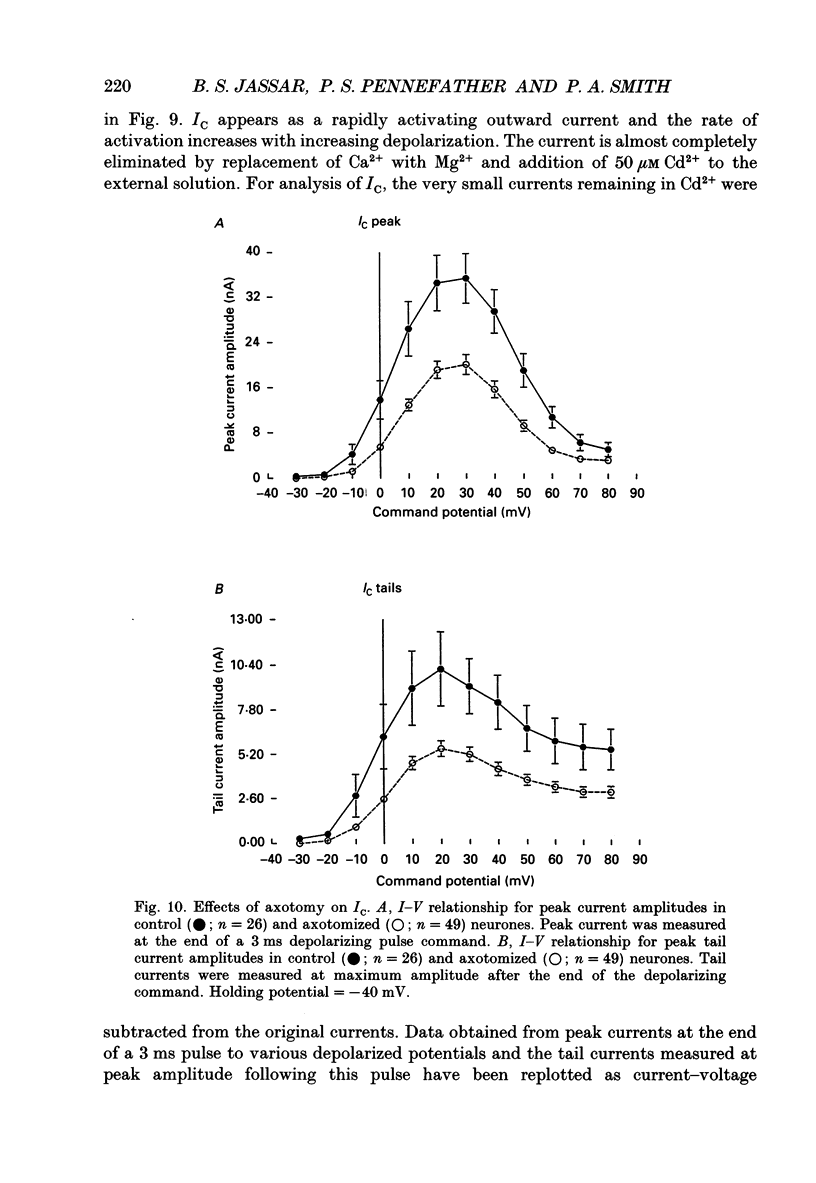
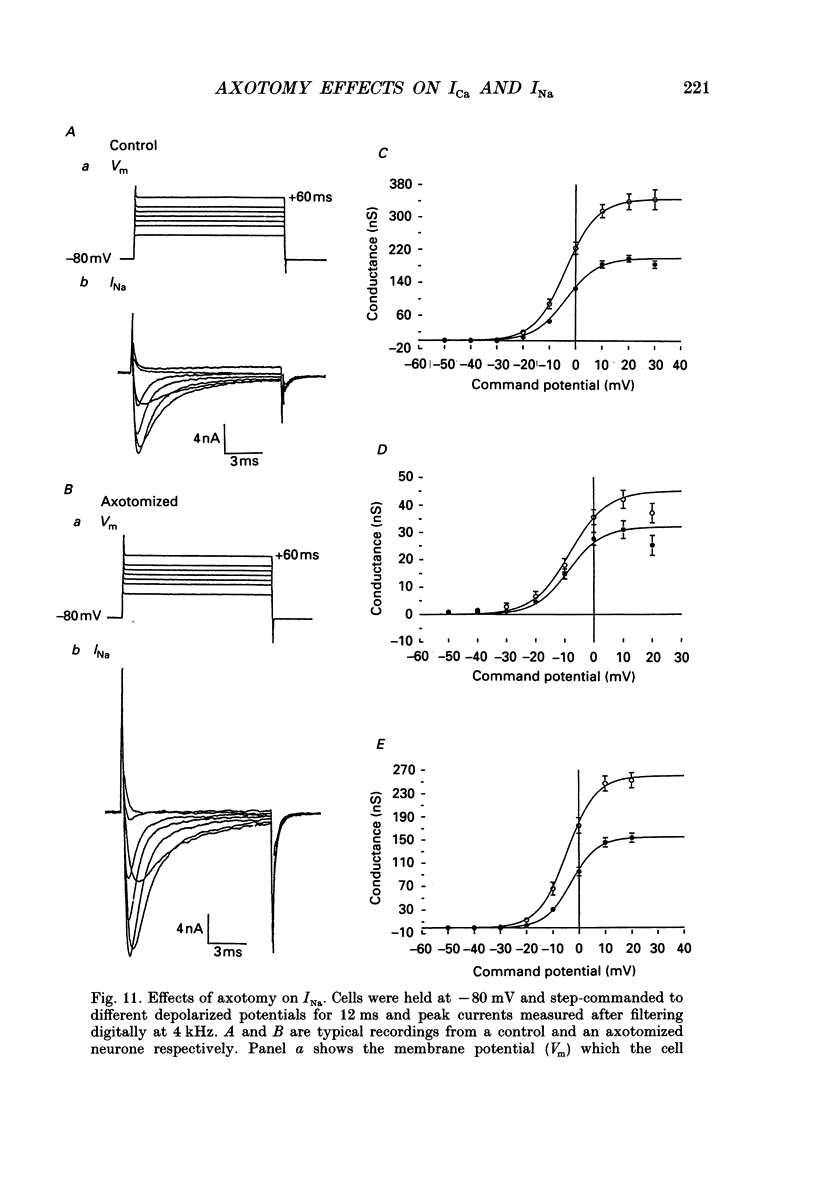
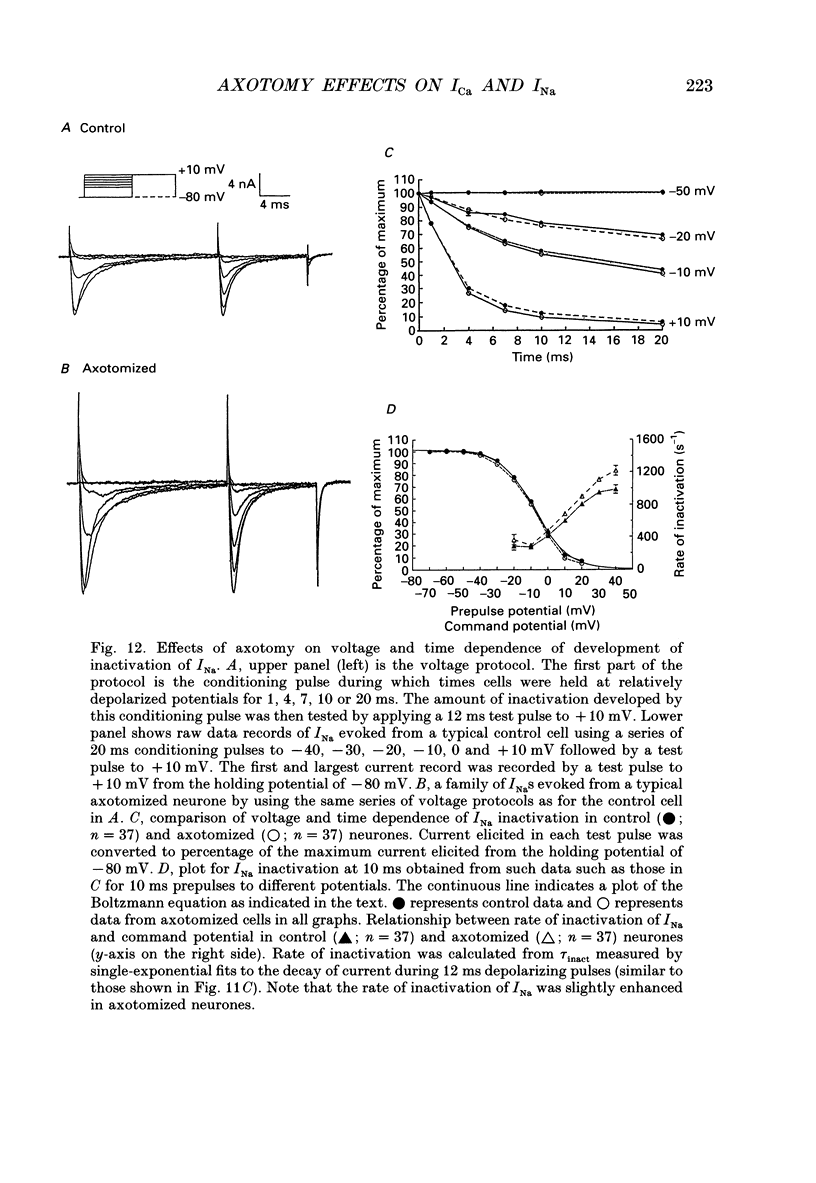
1. Currents mediated by Ca2+ channels using Ba2+ as a charge carrier (IBa), Na+ currents (INa) and voltage- and Ca(2+)-dependent K+ currents (IC) were recorded from bullfrog paravertebral sympathetic ganglion B-cells using whole-cell patch-clamp recording techniques. Currents recorded from control cells were compared with those from axotomized cells 13-15 days after transection of the postganglionic nerve. 2. Axotomy reduced peak IBa at -10 mV (holding potential = -80 mV) from 3.3 +/- 0.3 nA (n = 42) to 1.7 +/- 0.1 nA (n = 39, P < 0.001). Tail IBa at -40 mV following a step to +70 mV from a holding potential of -80 mV was also reduced in axotomized neurones (9.7 +/- 0.6 nA for forty-two control neurones and 5.2 +/- 0.3 nA for thirty-nine axotomized neurones; P < 0.001). Minimal changes were observed in the kinetics of activation and deactivation. 3. Pharmacological experiments using 1,4-dihydro-2,6-dimethyl-3-nitro-4-(2- trifluoromethylphenyl)-pyridine-5-carboxylic acid methyl ester (BayK 8644), nifedipine and omega-conotoxin showed that axotomy predominantly affected the N-type Ca2+ channels which carry the majority of ICa in these neurones. L-type Ca2+ current was little affected and T-type Ca2+ currents were not observed in control or axotomized cells. 4. Development of inactivation of 0 mV and recovery from inactivation of IBa at -80 mV exhibited three distinct components in both control and axotomized neurones: 'fast', 'intermediate' and 'slow'. The relative proportions of both the 'fast' and 'intermediate' components of inactivation at 0 mV were almost doubled after axotomy (fast component was 15% in control and 29% in axotomized neurones; intermediate component was 17% in control and 26% in axotomized neurones). 'Fast' and 'intermediate' inactivation tended to develop more rapidly and recover more slowly after axotomy. The rate of onset of 'slow' inactivation was unaffected by axotomy but the steady-state level at -40 mV was increased. Most of the change in IBa properties may be secondary to enhanced inactivation associated with axotomy. 5. Axotomy reduced IC (measured at the end of a 3 ms step from -40 to +20 mV) from 34.5 +/- 4.9 (n = 26) to 19.2 +/- 1.5 nA (n = 49, P < 0.005). This reduction may be secondary to the reduction in calcium channels available for activation from -40 mV following axotomy. 6. The TTX-sensitive and TTX-insensitive components of peak Na+ conductance (GNa) were both increased after axotomy. Total GNa was increased from 184.9 +/- 8.4 to 315.2 +/- 16.4 nS (n = 37 for both P < 0.001). Most of the kinetic and steady-state properties of INa were unchanged after axotomy.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams P. R., Brown D. A., Constanti A. M-currents and other potassium currents in bullfrog sympathetic neurones. J Physiol. 1982 Sep;330:537–572. doi: 10.1113/jphysiol.1982.sp014357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barish M. E. Differentiation of voltage-gated potassium current and modulation of excitability in cultured amphibian spinal neurones. J Physiol. 1986 Jun;375:229–250. doi: 10.1113/jphysiol.1986.sp016114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belmonte C., Gallego R., Morales A. Membrane properties of primary sensory neurones of the cat after peripheral reinnervation. J Physiol. 1988 Nov;405:219–232. doi: 10.1113/jphysiol.1988.sp017330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsen R. C., Kiff J., Ryugo K. Suppression of the cell body response in axotomized frog spinal neurons does not prevent initiation of nerve regeneration. Brain Res. 1982 Feb 18;234(1):11–25. doi: 10.1016/0006-8993(82)90469-3. [DOI] [PubMed] [Google Scholar]
- Czéh G., Kudo N., Kuno M. Membrane properties and conduction velocity in sensory neurones following central or peripheral axotomy. J Physiol. 1977 Aug;270(1):165–180. doi: 10.1113/jphysiol.1977.sp011944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodd J., Horn J. P. A reclassification of B and C neurones in the ninth and tenth paravertebral sympathetic ganglia of the bullfrog. J Physiol. 1983 Jan;334:255–269. doi: 10.1113/jphysiol.1983.sp014493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmslie K. S., Kammermeier P. J., Jones S. W. Calcium current modulation in frog sympathetic neurones: L-current is relatively insensitive to neurotransmitters. J Physiol. 1992 Oct;456:107–123. doi: 10.1113/jphysiol.1992.sp019329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkel A. S., Redman S. Theory and operation of a single microelectrode voltage clamp. J Neurosci Methods. 1984 Jun;11(2):101–127. doi: 10.1016/0165-0270(84)90029-3. [DOI] [PubMed] [Google Scholar]
- Fox A. P., Nowycky M. C., Tsien R. W. Kinetic and pharmacological properties distinguishing three types of calcium currents in chick sensory neurones. J Physiol. 1987 Dec;394:149–172. doi: 10.1113/jphysiol.1987.sp016864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda J., Kameyama M., Yamaguchi K. Breakdown of cytoskeletal filaments selectively reduces Na and Ca spikes in cultured mammal neurones. Nature. 1981 Nov 5;294(5836):82–85. doi: 10.1038/294082a0. [DOI] [PubMed] [Google Scholar]
- Gallego R., Ivorra I., Morales A. Effects of central or peripheral axotomy on membrane properties of sensory neurones in the petrosal ganglion of the cat. J Physiol. 1987 Oct;391:39–56. doi: 10.1113/jphysiol.1987.sp016724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goh J. W., Kelly M. E., Pennefather P. S. Electrophysiological function of the delayed rectifier (IK) in bullfrog sympathetic ganglion neurones. Pflugers Arch. 1989 Mar;413(5):482–486. doi: 10.1007/BF00594177. [DOI] [PubMed] [Google Scholar]
- Gordon T., Kelly M. E., Sanders E. J., Shapiro J., Smith P. A. The effects of axotomy on bullfrog sympathetic neurones. J Physiol. 1987 Nov;392:213–229. doi: 10.1113/jphysiol.1987.sp016777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottmann K., Dietzel I. D., Lux H. D., Huck S., Rohrer H. Development of inward currents in chick sensory and autonomic neuronal precursor cells in culture. J Neurosci. 1988 Oct;8(10):3722–3732. doi: 10.1523/JNEUROSCI.08-10-03722.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurtu S., Smith P. A. Electrophysiological characteristics of hamster dorsal root ganglion cells and their response to axotomy. J Neurophysiol. 1988 Feb;59(2):408–423. doi: 10.1152/jn.1988.59.2.408. [DOI] [PubMed] [Google Scholar]
- Gustafsson B., Pinter M. J. Effects of axotomy on the distribution of passive electrical properties of cat motoneurones. J Physiol. 1984 Nov;356:433–442. doi: 10.1113/jphysiol.1984.sp015474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol. 1952 Aug;117(4):500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jassar B. S., Smith P. A. Slow frequency-dependence of action potential afterhyperpolarization in bullfrog sympathetic ganglion neurones. Pflugers Arch. 1991 Nov;419(5):478–485. doi: 10.1007/BF00370792. [DOI] [PubMed] [Google Scholar]
- Jones S. W., Jacobs L. S. Dihydropyridine actions on calcium currents of frog sympathetic neurons. J Neurosci. 1990 Jul;10(7):2261–2267. doi: 10.1523/JNEUROSCI.10-07-02261.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones S. W., Marks T. N. Calcium currents in bullfrog sympathetic neurons. I. Activation kinetics and pharmacology. J Gen Physiol. 1989 Jul;94(1):151–167. doi: 10.1085/jgp.94.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones S. W., Marks T. N. Calcium currents in bullfrog sympathetic neurons. II. Inactivation. J Gen Physiol. 1989 Jul;94(1):169–182. doi: 10.1085/jgp.94.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones S. W. Sodium currents in dissociated bull-frog sympathetic neurones. J Physiol. 1987 Aug;389:605–627. doi: 10.1113/jphysiol.1987.sp016674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly M. E., Gordon T., Shapiro J., Smith P. A. Axotomy affects calcium-sensitive potassium conductance in sympathetic neurones. Neurosci Lett. 1986 Jun 18;67(2):163–168. doi: 10.1016/0304-3940(86)90391-5. [DOI] [PubMed] [Google Scholar]
- Laiwand R., Werman R., Yarom Y. Electrophysiology of degenerating neurones in the vagal motor nucleus of the guinea-pig following axotomy. J Physiol. 1988 Oct;404:749–766. doi: 10.1113/jphysiol.1988.sp017317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster B., Pennefather P. Potassium currents evoked by brief depolarizations in bull-frog sympathetic ganglion cells. J Physiol. 1987 Jun;387:519–548. doi: 10.1113/jphysiol.1987.sp016587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman A. R. The axon reaction: a review of the principal features of perikaryal responses to axon injury. Int Rev Neurobiol. 1971;14:49–124. doi: 10.1016/s0074-7742(08)60183-x. [DOI] [PubMed] [Google Scholar]
- Lipscombe D., Madison D. V., Poenie M., Reuter H., Tsien R. Y., Tsien R. W. Spatial distribution of calcium channels and cytosolic calcium transients in growth cones and cell bodies of sympathetic neurons. Proc Natl Acad Sci U S A. 1988 Apr;85(7):2398–2402. doi: 10.1073/pnas.85.7.2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nerbonne J. M., Gurney A. M. Development of excitable membrane properties in mammalian sympathetic neurons. J Neurosci. 1989 Sep;9(9):3272–3286. doi: 10.1523/JNEUROSCI.09-09-03272.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirchio M., Lightowler S., Crunelli V. Postnatal development of the T calcium current in cat thalamocortical cells. Neuroscience. 1990;38(1):39–45. doi: 10.1016/0306-4522(90)90372-b. [DOI] [PubMed] [Google Scholar]
- Plummer M. R., Hess P. Reversible uncoupling of inactivation in N-type calcium channels. Nature. 1991 Jun 20;351(6328):657–659. doi: 10.1038/351657a0. [DOI] [PubMed] [Google Scholar]
- Sala F. Activation kinetics of calcium currents in bull-frog sympathetic neurones. J Physiol. 1991 Jun;437:221–238. doi: 10.1113/jphysiol.1991.sp018592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson S. M., Wong R. K. Development of calcium current subtypes in isolated rat hippocampal pyramidal cells. J Physiol. 1991 Aug;439:671–689. doi: 10.1113/jphysiol.1991.sp018687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titmus M. J., Faber D. S. Axotomy-induced alterations in the electrophysiological characteristics of neurons. Prog Neurobiol. 1990;35(1):1–51. doi: 10.1016/0301-0082(90)90039-j. [DOI] [PubMed] [Google Scholar]
- Varadi G., Lory P., Schultz D., Varadi M., Schwartz A. Acceleration of activation and inactivation by the beta subunit of the skeletal muscle calcium channel. Nature. 1991 Jul 11;352(6331):159–162. doi: 10.1038/352159a0. [DOI] [PubMed] [Google Scholar]


