ABSTRACT
Peptic ulcer disease remains a prevalent gastrointestinal disorder worldwide. Current treatments often have limitations, sparking interest in alternative therapies from medicinal plants. This review examines the gastroprotective potential of 54 North African medicinal plants against peptic ulcers. An extensive literature search was conducted, focusing on plants with preclinical and clinical evidence of anti‐ulcer efficacy and documented use in North African traditional medicine. The review identified several promising plant species, such as licorice (Glycyrrhiza glabra), chamomile (Matricaria chamomilla), olive (Olea europaea), pomegranate (Punica granatum), Aloe vera, and black seed (Nigella sativa), along with their bioactive constituents, including flavonoids, tannins, and terpenoids. These compounds exhibit gastroprotective properties through multiple mechanisms, such as enhancing the gastric mucosal barrier, inhibiting acid secretion, displaying antioxidant and anti‐inflammatory effects, promoting ulcer healing, and combating Helicobacter pylori infection. The evidence presented includes in vitro assays, animal models, and some clinical studies. While many of the 53 plants reviewed demonstrated significant anti‐ulcer effects compared to standard drugs, further clinical research is needed to establish efficacy and safety in humans. The synergistic actions of phytochemical mixtures in medicinal plant extracts likely contribute to their therapeutic potential. This review highlights the role these North African medicinal plants may play in the prevention and treatment of peptic ulcers and identifies promising candidates for further research and development of evidence‐based botanical therapies.
Keywords: alternative therapies, gastroprotective effects, Helicobacter pylori, North African medicinal plants, peptic ulcer, phytochemical constituents
Peptic ulcer disease remains common globally, driving interest in alternative treatments. This review examines 53 North African medicinal plants, including licorice, chamomile, olive, pomegranate, Aloe vera, and black seed, for their anti‐ulcer effects. These plants contain compounds like flavonoids and tannins that protect the stomach lining, lower acid production, reduce inflammation, and combat Helicobacter pylori. Although promising, further clinical studies are needed to confirm their safety and effectiveness in humans.
1. Introduction
Peptic ulcers are painful sores in the lining of the stomach or duodenum, affecting nearly 10% of the global population (Majumdar and Looi 2024). The primary etiological factors are Helicobacter pylori infection and frequent NSAID use, contributing to 60%–90% and 30%–50% of ulcer cases, respectively (Hooi et al. 2017; Malfertheiner et al. 2017). Additional factors include excessive alcohol consumption, smoking, stress, gastric hyperacidity, diet, genetics, and radiotherapy (Sostres, Gargallo, and Lanas 2013).
Symptoms range from mild discomfort to severe pain and include nausea, vomiting, bloating, and heartburn (Lanas and Chan 2017). Complications such as gastrointestinal bleeding, perforation, and gastric outlet obstruction can arise if left untreated, increasing the risk of gastric cancer (Crew and Neugut 2006). Timely diagnosis and proper management are crucial.
Figure 1, under the heading “The symptom is classic for peptic ulcer,” presents a more comprehensive overview of the symptoms associated with peptic ulcers, detailing both the common and less frequent clinical manifestations.
FIGURE 1.
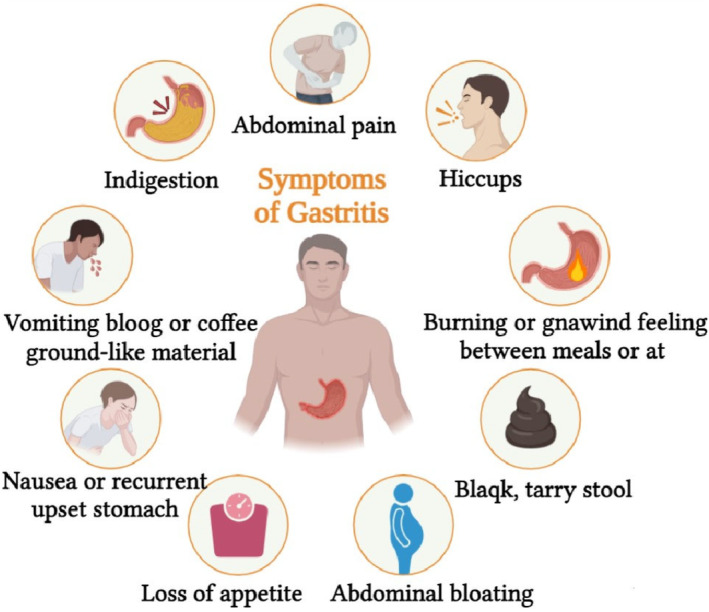
The symptom is classic for peptic ulcer.
Conventional treatments involve eradicating H. pylori and suppressing gastric acid secretion with antibiotics, PPIs, or H2 blockers (Malfertheiner et al. 2017). However, rising antibiotic resistance and long‐term side effects of PPIs, such as nutrient deficiencies and increased infection risks, highlight the need for alternative therapies (Fashner and Gitu 2015).
Traditional medicine offers a promising avenue, with North African medicinal plants gaining attention for their gastroprotective properties (Asnaashari, Dastmalchi, and Javadzadeh 2018). The rich biodiversity of North Africa, ranging from arid deserts to lush coastal regions, harbors numerous plants with a history of use in treating gastrointestinal disorders (Bouyahya et al. 2017; Ghanmi et al. 2014). Plants like olive leaf (Olea europaea), date palm (Phoenix dactylifera), and argan (Argania spinosa) have demonstrated anti‐ulcer effects through various mechanisms, including inhibition of gastric acid secretion and antioxidant activity (Rigacci and Stefani 2016; Silvan et al. 2021; Yasin, El‐Fawal, and Mousa 2015).
This review aims to summarize current scientific evidence on the gastroprotective efficacy of North African medicinal plants, examining their botanical sources, key phytochemical constituents, and mechanisms of action. By establishing the therapeutic potential of these traditional medicines, we hope to pave the way for novel botanical treatments for peptic ulcers.
2. Methods
A comprehensive literature search was conducted using PubMed, Scopus, ScienceDirect, Web of science, and Google Scholar, focusing on medicinal plants traditionally used in North Africa for treating gastrointestinal ulcers. Keywords included “anti‐ulcer plants,” “gastroprotective plants,” “peptic ulcer medicinal plants,” and “traditional anti‐ulcer herbs of North Africa.” References from relevant articles were also screened.
Inclusion criteria prioritized plants with robust evidence of gastroprotective properties demonstrated through preclinical in vivo and in vitro studies. We focused on botanicals with well‐established pharmacological validation and a documented history in North African ethnomedicine. Phytochemical studies were also reviewed to identify active compounds and their mechanisms of action.
This review synthesizes key findings from the literature, providing an overview of North African medicinal plants with potential anti‐ulcer effects, and aims to highlight their therapeutic viability as alternatives to conventional PUD treatments.
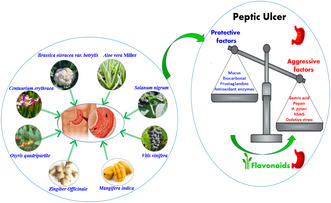
3. Medicinal Plants of North Africa
In the vast and varied landscapes of North Africa, traditional healing practices have flourished for centuries, deeply rooted in the rich biodiversity that thrives across the region. Indigenous communities, including the Tuareg, Bedouin, Amazigh (Berber), and Arab peoples, have cultivated an intimate relationship with the local flora, harnessing its medicinal properties to treat a wide array of ailments amid the challenges posed by the region's diverse environments (Alves‐Silva et al. 2017; Khalid et al. 2012; Miara et al. 2019). Spanning countries such as Morocco, Algeria, Tunisia, Libya, and Egypt, North Africa boasts a tapestry of ecosystems ranging from the arid expanses of the Sahara Desert to the fertile lands along the Mediterranean coast. Within this botanical mosaic, numerous plant families have emerged as prominent sources of medicinal remedies, including Asteraceae, Fabaceae, Apiaceae, Lamiaceae, Brassicaceae, Solanaceae, Apocynaceae, and Zygophyllaceae, among others (Benarba et al. 2015). The use of medicinal plants in North Africa is deeply intertwined with cultural traditions and indigenous knowledge systems. Passed down through generations, this wealth of botanical wisdom has enabled communities to address a wide range of health concerns, including gastrointestinal disorders, which have historically been prevalent in the region. The traditional healers of North Africa, often referred to as herbalists or healers, possess a deep understanding of the therapeutic properties of local plants and employ a holistic approach to healing that considers the interconnectedness of body, mind, and environment (Dinat, Orchard, and Van Vuuren 2023; Vale and Oleastro 2014). In the realm of gastrointestinal health, North African medicinal plants have garnered significant attention for their efficacy in treating conditions such as peptic ulcers, often caused by the bacterium H. pylori. This microorganism infects a substantial portion of the regional population and is implicated in the majority of duodenal and gastric ulcers. Research has identified several medicinal plants with potent anti‐H. pylori properties, including thyme (Thymus vulgaris), wild thyme (Coridothymus capitatus), rosemary (Rosmarinus officinalis), and turmeric (Curcuma longa) (Beladi Mousavi, Naghdifar, and Rafieian‐Kopaei 2016; Güvenir 2024; Reddy et al. 2023). These plants contain bioactive compounds that exhibit antibacterial activity against H. pylori, providing a natural alternative to conventional antibiotics. Furthermore, the berries of Pistacia lentiscus and Oleo gum resin from Cistus ladaniferus have demonstrated antibacterial effects against H. pylori, along with antioxidant properties that promote ulcer healing. Additionally, chamomile (Matricaria chamomilla) and licorice (Glycyrrhiza glabra) have been shown to exert gastroprotective effects by enhancing mucus production in the stomach lining or through anti‐secretory actions against gastric acid (Dinat, Orchard, and Van Vuuren 2023; Safavi, Shams‐Ardakani, and Foroumadi 2015).
However, the utilization of medicinal plants in North Africa is not without its challenges. Overexploitation and unsustainable harvesting practices threaten the existence of some valuable species, prompting calls for conservation efforts and sustainable management practices (Jamila and Mostafa 2014). Moreover, the lack of standardized dosages and potential interactions with conventional medications necessitate further research and clinical trials to validate their safety and efficacy in clinical settings (Naceiri Mrabti et al. 2021).
The table of the North African medicinal plants (Table 1) indicates a significant prominence of families such as Fabaceae, Asteraceae, and Lamiaceae, which are frequently utilized for their gastroprotective properties. Notable compounds including flavonoids (e.g., quercetin and kaempferol), tannins, alkaloids, and essential oils are commonly identified, and recognized for their anti‐inflammatory, antioxidant, and antimicrobial effects, all contributing to the treatment of peptic ulcers. The most utilized plant parts are the aerial parts, leaves, and roots, reflecting their high concentration of bioactive compounds.
TABLE 1.
Investigated the peptic ulcers medicinal plants of North Africa and identified possible bioactive compound.
| Scientific name | Family | Common names | Part(s) | Compound(s) present/isolated | Reference |
|---|---|---|---|---|---|
| Abelmoschus esculentus | Malvaceae | Okra or lady's finger | Aerial parts | Flavonoids (quercetin), carotenoids, alkaloids, saponins and tannins, starch, glycosides, and mucilage | Altaf et al. (2023); Naim et al. (2015) |
| Acacia Senegal | Fabaceae | Gum Arabic | The bark and roots | Ferulic acid, chlorogenic acid, quercetin, arabinogalactan, and polysaccharides | Khedr (2017); Mahamoud Saleh, El Sayed El Sahar, and Elnadi (2021) |
| Aegle marmelos | Rutaceae | Bael | Leaves and fruit | Essential oil, alkaloids, carbohydrates, glycosides, flavonoids, tannins, coumarins, sterols, and triterpenoid | Abdallah, Salem, and El‐Salam (2017); Ibrahim et al. (2015) |
| Aerva persica | Amaranthaceae | Cotton | Roots | Aervin, 3‐hydroxy‐4 methoxy benzaldehyde, ursolic acid, and (E)‐N‐(4‐hydroxy‐3‐methoxyphenethyl)‐3‐(4‐hydroxy‐3‐ethoxyphenyl) acryl amide | Asnaashari, Dastmalchi, and Javadzadeh (2018); Khan et al. (2012) |
| Alhagi camelorum | Leguminosae | Camelthorn | Aerial parts | Quercetin, kaempferol, isorhamnetin, β‐sitosterol, lupeol, phenolic acids, lignans, and alkaloids. | Shaker, Mahmoud, and Mnaa (2010) |
| Allium sativum | Amaryllidaceae | Toum or garlic | Bulb | Flavonoids, Tannins, Terpenoids, and gallic acid | Doukanı et al. (2021) |
| Aloe ferox | Aloaceae | Bitter aloe or Cape aloe | Leaves | Anthraquinones (aloin and aloe‐emodin), polysaccharides (acemannan), flavonoids, and saponins | Bentahar et al. (2016) |
| Aloe vera Miller | Xanthorrhoeaceae | Aloe | Aerial part (leaves) | Hydroxybenzoic acid, benzoic acid, catechin, gallic acid, chlorogenic acid, coumaric acid, epicatechin, rosmarinic acid, syringic acid, trans‐ferrulic acid; quercitin, cinamic acid, hesperidin, and sinapic acid | Fedoul et al. (2022) |
| Althaea officinalis | Malvaceae | Marshmallow | Roots, flowers, and leaves | Mucilage, quercetin, kaempferol, rutin, caffeic acid, and chlorogenic acid | Šutovská et al. (2009) |
| Artemisia herba‐alba | Asteraceae | White wormwood | Aerial parts | Essential oil; tricyclene, artemisia triene, α‐thujene, α‐pinene, camphene, sabinene, β‐pinene, cis‐pinane, myrcene, α‐phellandrene, α‐terpinene, ortho‐cymene, limonene, 1,8‐cineole, E‐ β‐ocimene, γ‐terpinene, artemisia ketone, α‐pinene oxide, sabinene trans hydrate, α‐thujone, β‐thujone, trans‐pinan‐2‐ol, chrysanthenone, terpinol, camphor, β‐pinene oxide, trans β‐dihydro terpineol, trans β‐terpineol, terpinen‐4‐ol, thuj‐3‐en‐10‐al, p‐cymen‐8‐ol, α‐terpineol, trans piperitol, p‐cymen‐9‐ol, trans chrysanthenyl acetate, piperitone, cis chrysanthenyl acetate, neo‐3‐ thujyl acetate, α‐terpinen‐7‐al, γ‐terpinen‐7‐al, α‐terpinyl acetate, β‐elemene, E‐caryophyllene, 9‐epi E–caryophyllene, and ô‐muurolene | Houti et al. (2023) |
| Berberis vulgaris | Berberidaceae | Zereshk | Aerial parts | Flavonoids, alkaloids, tannins, and saponins | |
| Brassica oleracea var. botrytis | Brassicaceae | Cauliflower | Aerial parts | Glucosinolates, vitamin C, vitamin K, quercetin, kaempferol, and sulforaphane | Kononenko, Mirzaliev, and Ostapets (2018); Sudharameshwari and Ayshwarya (2017) |
| Brassica oleracea var. capitata | Brassicaceae | Cabbage | Leaves | Gallic acid, chlorogenic acid, catechin hydrate, vanillic acid, (−) epicatechin, p‐coumaric acid, rutin hydrate, and rosmarinic acid | Rahman et al. (2022) |
| Carlina acanthifolia | Asteraceae | Baranbar | Roots | Sesquiterpene lactones, flavonoids, and triterpenes, | Ivancheva, Nikolova, and Tsvetkova (2006) |
| Centaurea chamaerhaponticum | Asteraceae | Pontic cornflower or Pontic knapweed | Aerial parts | Tannins, sesquiterpene lactones, and flavonoids | Bakhtaoui, Lakmichi, Chait, and Gadhi (2014) |
| Centaurium erythraea | Gentianaceae | Centaury | Aerial parts | Secoiridoid glycosides (sweroside and gentiopicroside), apigenin, luteolin, quercetin, mangiferin, and isomangiferin. | Bentahar et al. (2016); Đorđević et al. (2017) |
| Ceratonia siliqua | Fabaceae | Carob tree | Pods, seeds, and leaves | Gallic acid, condensed tannins, fiber, and proteins | Benmansour et al. (2020); Elaoufi et al. (2022) |
|
Cicorium intybus |
Asteraceae | Chicory | Roots and leaves | Sesquiterpene lactones, flavonoids, phenolic acids, chicoric acid, inulin, coumarins, inulin, and caffeic acid | Nouir et al. (2023); Saxena et al. (2011); Saxena, Sulakhiya, and Rathore (2014) |
| Cinnamomum verum | Lauraceae | Cinnamon | Bark | Essential oil; camphene, β‐pinene, sabinene, myrcene, 1,4‐cineole, limonene, cis‐β‐ocimene, trans‐β‐ocimene, p‐cymene, linalool, γ‐terpinene, α‐terpineol, piperitone, geraniol, (E)‐cinnamaldehyde, (Z)‐cinnamaldehyde, eugenol, (E)‐cinnamyl acetate, eugenyl acetate, and benzyl benzoate | Narayanankutty et al. (2021) |
| Globularia alypum | Globulariaceae | Globe daisy | Leaves, roots, and flowers | Alkaloids, flavonoids, and phenolic acids, iridoids, secoiridoids, and phenylethanoid glycosides | Nouir et al. (2023); Taghzouti et al. (2016) |
| Glycyrrhiza glabra | Leguminosae | Licorice | Rhizomes | Tannins, flavonoids, reducing compounds, saponins, coumarins, triterpenes, and sterols | Benbott et al. (2021) |
| Curcuma longa | Zingiberaceae | Turmeric | Rhizomes | Starch, glucosides, mucilage, and terpenoids | Benmeziane‐Derradji and Aoun (2022) |
| Helianthemum kahiricum | Cistaceae | Egyptian sunrose or Egyptian rockrose | Aerial parts | Kaempferol‐3‐D‐(6‐O‐trans‐p‐coumaroyl) glucopyranoside and kaempferol‐3‐O‐(3,6 ‐di‐O‐trans‐p‐coumaroyl) glucopyranoside | Ayoola et al. (2016); Mouffouk et al. (2023) |
| Helianthemum lippii | Cistaceae | Sunrose rockrose | Aerial parts | Flavonoids, phenolic acids, tannins, terpenoids, and alkaloids | Alsabri et al. (2014, 2013) |
| Juniperus communis | Cupressaceae | Common “juniper” or simply “juniper.” | Aerial parts | Alpha‐pinene, beta‐pinene, limonene, myrcene, quercetin, kaempferol, rutin, tannins, essential oils (monoterpenes and sesquiterpenes), and pectin | Bais et al. (2014); Pramanik et al. (2007) |
| Juniperus phoenicea | Cupressaceae | Red juniper | Leaves | Alpha‐pinene, beta‐pinene, limonene, and myrcene, flavonoids, tannins, and essential oils | Ali et al. (2015); Hoogerwerf and Pasricha (2006) |
| Lagenaria siceraria | Cucurbitaceae | Bottle gourd or calabash | Aerial parts | Coniferyl alcohol, ferulic acid, p‐coumaric acid, flavonoids, saponins, and tannins | Manchala (2019); Mehboob et al. (2022) |
| Lavandula stoechas | Lamiaceae | Spanish lavender or French lavender | Leaves | Camphor and fenchone | Ben Mansour et al. (2022) |
| Lawsonia inermis | Lythraceae | Henna | Leaves | 2‐hydroxy‐1,4‐naphthoquinone and suavissimoside R1 | Muheyuddeen et al. (2023) |
| Mangifera indica | Anacardiaceae | Mango | Leaves | Stigmasterol and β‐sitosterol, and pentagalloyl glucose | Kagbo and Aduku (2015); Mahmoud et al. (2022) |
| Matricaria chamomilla | Asteraceae | Chamomile | Flowers | Alkaloids, total tannins, condensed tannins, gallic tannins, mucilage, flavonoids, saponins, and glycosides | Online et al. (2018) |
| Mentha microphylla | Lamiaceae | Corsican mint or tiny‐leafed mint | Leaves | 1,2‐dihydroxy‐p‐menth‐4(8)‐en‐3‐one, 4‐hydroxy‐p‐mentha‐1,8‐dien‐3‐one, 4‐methoxy‐p‐mentha‐1,8‐dien‐3‐one, 3‐acetoxy‐6‐hydroxy‐p‐mentha‐1,8‐diene. | Mahmoud (2005) |
| Mentha spicata | Lamiaceae | Spearmint | Leaves | Caffeic, chlorogenic, rosmarinic, vanillic, syringic, p‐coumaric, ferulic, rosmarinic, 3,4‐dihydroxybenzoic, ferulic acid, and diosmetin, diosmin, diosmin‐7‐glucoside, thymonin, 5,6,4′‐Trihydroxy‐7,3′‐dimethoxyflavone, Eriocitrin, luteolin glucoside, isorhoifolin, luteolin, apigenin, quercetin, luteolin, scopoletin, catechin, epicatechin, rutin, myricetin, luteolin, apigenin, and naringenin | Fatiha et al. (2015) |
| Myrtus communis | Myrtaceae | Common myrtle or simply myrtle | Leaves | α‐Pinene, Limonene, 1,8‐cineol, Linalool oxide, Linalool, Fenchyl alcohol, α‐Terpineol, cis‐pinocarveol, Nerol, Mertenyl acetate, Geraniol, Linalyl acetate, P‐menth‐1‐enol, trans—Pinocarveyl acetete, α‐Terpinyl acetate, Neryl acetate, Methyl eugenol, Trans caryophyllene, α‐Humulene, α‐Pinene, Limonene, 1,8‐cineol, Linalool oxide, Linalool, Fenchyl alcohol, α‐Terpineol, cis‐pinocarveol, Nerol, Mertenyl acetate, Geraniol, Linalyl acetate, P‐menth‐1‐enol, trans—Pinocarveyl acetete, α‐Terpinyl acetate, Neryl acetate, Methyl eugenol, Trans caryophyllene, α‐Humulene, α‐Pinene, Limonene, 1,8‐cineol, Linalool oxide, Linalool, Fenchyl alcohol, α‐Terpineol, cis‐pinocarveol, Nerol, Mertenyl acetate, Geraniol, Linalyl acetate, P‐menth‐1‐enol, trans—Pinocarveyl acetete, α‐Terpinyl acetate, Neryl acetate, Methyl eugenol, Trans caryophyllene, α‐Humulene, α‐Pinene, limonene1,8‐cineol, linalool oxide, linalool, fenchyl alcohol, α‐terpineolcis‐pinocarveol, nerol, mertenyl acetate, geraniol, linalyl acetate, p‐menth‐1‐enol, trans—pinocarveyl aceteteα‐terpinyl acetate, neryl acetate, methyl eugenol, trans caryophyllene, and α‐humulene | Nassar et al. (2010) |
| Nigella sativa | Ranunculaceae | Black seed | Seeds | Volatile oil: p‐Cymene, α‐thujene, sabinene, thymoquinone, carvacrol, trans‐sabinene hydrate, γ‐terpinene, longifolene and α‐longipinene | Benkaci‐Ali, Baaliouamer, and Meklati (2005) |
| Olea europaea | Oleaceae | Olive | Leaves and fruit | Oleuropein, hydroxytyrosol, oleanolic acid, maslinic acid, and quercetin | Mahdavi et al. (2020); Musa et al. (2021) |
| Oreganum majorana | Lamiaceae | Sweet marjoram or simply marjoram | Aerial parts | Rosmarinic acid, apigenin, luteolin, caffeic acid, carvacrol, thymol, saponins, and essential oils | Dorman and Deans (2000); Mathias (1994) |
| Oreganum syriacum | Lamiaceae | Syrian oregano | Leaves | Rosmarinic acid, flavonoids, phenolic acids, carvacrol, thymol, essential oils, and saponins | El‐Meligy et al. (2017); Mesmar et al. (2022) |
|
Origanum compactum Benth |
Lamiaceae | Oroccan oregano or compact oregano | Leaves | Flavonoids, phenolic acids, carvacrol and thymol, saponins, and essential oils | Bakhtaoui, Lakmichi, Chait, and Gadhi (2014); Hayani et al. (2022) |
| Opuntia ficus‐indica | Cactaceae | Prickly pear or cactus pear | Fruit, pads (Cladodes), and seeds | Betacyanins, betaxanthins, dietary fiber, flavonoids, phenolic acids, and tannins | Trachtenberg and Mayer (1981) |
| Osyris quadripartite | Santalaceae | African sandalwood | Leaves, roots, stems, and bark, | Flavonoids, tannins, and saponins | Kale, Bambare, and Bhale (2023) |
| Phoenix dactylifera | Arecaceae | Date palm | Pulp and palm sap | Sugar, dietary fibers, sodium, potassium, magnesium, zinc, and iron | Abdellaziz et al. (2014) |
| Pistacia lentiscus | Anacardiaceae | Mastic tree | Aerial parts | Arachidonic acid, oleanolic acid, ursolic acid, masticadienonic acid, isomasticadienolic acid, and β‐sitosterol | Boutemine et al. (2018); Calder (2001) |
| Plantago major | Plantaginaceae | Broad leaf Plantain or Plantain | Leaf | Flavonoids, alkaloids, terpenoids, phenolic acid derivatives, iridoid glycosides, fatty acids, polysaccharides, and vitamins | Adom et al. (2017) |
|
Portulaca oleracea |
Portulacaceae | Purslane | Aerial parts | Kaempferol, apigenin, luteolin, myricetin, and quercetin, polysaccharides, and α‐linolenic acid | Karimi, Hosseinzadeh, and Ettehad (2004); Tijani, Temitayo, and Farombi (2022) |
|
Punica granatum |
Punicaceae | Pomegranate | Peel | Quinic acid, gallic acid, protocatechuic acid, catechin (+), caffeic acid, syringic acid, quercetin, kaempferol, and cirsiliol | Alimi et al. (2023) |
| Rhus tripartite | Anacardiaceae | Three‐leaved sumac or skunkbush sumac | Roots, stem, and leaves | Tannins, flavonoids, terpenoids, citric acid malic acid, and alkaloids | Benmansour et al. (2020); Shahat et al. (2016) |
| Solanum nigrum | Solanaceae | Black nightshade | Leaves, berries, roots, and stems | Saponins, flavonoids, alkaloids, phenolic acids, tannins, and steroids | Jainu and Devi (2004a); Zaghlool et al. (2019) |
| Syzygium aromaticum | Myrtaceae | Clove | Fleurs | Eugenol, gallic acid, β‐caryophyllene, vanillin, crategolic acid (maslinic acid), campesterol, kaempferol, rhamnetin, oleanolic acid, eugenitin, bicornin, eugenin, quercetin, and ellagic acid | Batiha et al. (2020) |
| Trigonella foenum‐graecum | Fabaceae | Fenugreek | Seed | Diosgenin, saponins, quercetin, apigenin, luteolin, galactomannans, and trigonelline | Jainu and Devi (2004b); Selmi et al. (2022) |
| Curcuma longa | Zingiberaceae | Turmeric | Rhizomes | Starch, glucosides, mucilage, and terpenoids | (Benmeziane–Derradji & Aoun, 2022) |
| Vitis vinifera | Vitaceae | Grapevine | Fruit, leaves, and seed | Quercetin, kaempferol, catechins, proanthocyanidins, resveratrol, tartaric acid, malic acid, citric acid, carotenoids, anthocyanins, proanthocyanidins, phenolic acids, and tannins. | Felicio, Santos, and Gonçalez (2001); Saadaoui et al. (2020) |
| Zingiber Officinale | Zingiberacea | Ginger | Rhizomes | Carbohydrates, volatile oils, sterols, triterpenoids, alkaloids, kaempferol, quercetin‐hexoside, quercetin‐3‐O‐rutinoside, syringic acid, (+)‐Catechin, ferulic acid, ellagic acid, quercetin, salicylic acid, gallic acid, quercetin‐hexoside, and kaempferol‐3‐O‐rutinoside | Saiah et al. (2018); Zaghlool et al. (2015) |
| Ziziphus lotus | Rhamnaceae | Lotus jujube or lote tree | Fruits, leaves, seeds, and bark | Tannins, betulinic acid, oleanolic acid, quercetin, kaempferol, saponins, arabinoxylans, and arabinogalactans | Bakhtaoui, Lakmichi, Megraud et al. (2014b); Wahida, Abderrahman, and Nabil (2007) |
4. Results
4.1. Medicinal Plants for Peptic Ulcer Disease
4.1.1. Abelmoschus esculentus
A. esculentus, commonly known as okra or lady's finger. A. esculentus has been traditionally used in certain herbal medicine systems for gastrointestinal health (Ortaç et al. 2018). A. esculentus contains mucilage, a gel‐like substance that is known for its demulcent properties. Mucilage can form a protective layer over the gastric mucosa, helping to soothe irritation and inflammation in the stomach lining (Naim et al. 2015). Additionally, several studies illustrated Okra is rich in antioxidants, including flavonoids, polyphenols, and vitamin C, which can help neutralize harmful free radicals in the body (Joshi et al. 2011). Oxidative stress is known to play a role in the development and progression of gastric ulcers, and the antioxidant compounds in okra may help protect the gastric mucosa from oxidative damage (Ortaç et al. 2018).
4.1.2. Acacia senegal L.
A. senegal (gum Arabic) was traditionally used to treat many disorders, including stomach illness. Researchers linked the constituents of gum Arabic and its efficacy in maintaining the normal status of gastric mucosa (Khedr 2017). The high percentage of polysaccharides in the aqueous solution of gum Arabic may explain its gastroprotective potential against gastritis and be a promising therapy in treating gastric cancer. Previous studies showed that oral gavage of 500 mg gum Arabic/kg to Wistar rats has reduced the ulcer index by 15% in the acute gastric ulcer model (Salama and Mariod 2018). Recent studies showed that oral administration of gum Arabic at doses of 500 mg/kg and 1000 mg/kg exhibits anti‐ulcer activity in rats ulcerated by ethanol. Scientists explained the efficacy of gum Arabic against ethanol gastritis in terms of the cytoprotective potency of gum Arabic to gastric cells as well as its free radical scavenging capacity (Salama and Mariod 2018).
4.1.3. Aegle marmelos
A. marmelos, commonly known as bael, is a tree from the Rutaceae family widely used in traditional ayurvedic medicine for gastrointestinal problems. Different parts of A. marmelos like the fruit, leaves, and roots have exhibited anti‐ulcer effects in animal models against gastric ulcers induced by ethanol and NSAIDs. Proposed gastroprotective mechanisms of bael include antisecretory activity to reduce gastric acid secretion and antioxidant properties from phenolic compounds that help prevent oxidative damage to gastric tissue (Venthodika et al. 2021). Specific phytochemicals isolated from bael like the coumarin marmin, obtained from leaves, can inhibit the growth of H. pylori and help protect gastric epithelial cells. Another key coumarin named Angeline, abundant in bael leaves, has shown anti‐secretory, cytoprotective, and antioxidant effects in the gastric mucosa in rats. However, despite the promising gastroprotective results in preliminary preclinical studies, rigorous randomized controlled trials in humans evaluating safety and efficacy are lacking for bael preparations. Further research warrants exploring its anti‐ulcer effects in clinically relevant models along with bioactivity‐guided fractionation to identify its active constituents (Zhang et al. 2020).
4.1.4. Aerva persica
A. persica, commonly known as desert cotton, has been used traditionally in various cultures for its medicinal properties diuretic, and demulcent properties (Chawla et al. 2013). Several biological activities have been reported from A. persica such as antioxidant, antibacterial, antifungal, hypoglycemic, and anti‐ulcer effects (Mehboob et al. 2022). Based on research into the anti‐ulcer effects of A. persica roots, it was found that administration of ethanol extract from these roots reduced ethanol‐induced hemorrhagic necrosis in the stomach of rats, as observed in histopathological evaluations of the gastric mucosa (Vasudeva et al. 2012).
4.1.5. Alhagi camelorum
A. camelorum belongs to the Leguminosae family and is used in folk medicine to treat some gastric diseases (Zarei, Ashtiyani, and Vaezi 2014). The whole plant has historically been used to treat a variety of ailments, including rheumatic, metabolic, GI, and liver issues, diuretics, migraines, fever, warts, and rash (Shaker, Mahmoud, and Mnaa 2010). There have been reports of this plant's anti‐ulcerogenic, gastroprotective, ureteral stone ejection, antidiarrheal, antinociceptive, and antioxidant properties. Previous research has demonstrated that an aqueous extract of A. camelorum greatly protected and inhibited secretory processes in rat stomach mucosa (Gharibn and Mard 2007). Additionally, rat stomach mucosa experimental research on A. maurorum extract showed strong mucosal protection and antisecretory activity (Shaker, Mahmoud, and Mnaa 2010).
4.1.6. Allium sativum
Garlic (Amaryllidaceae) has been used as a digestive stimulant and ulcer remedy in traditional medicine (Shang et al. 2019). Safavi, Shams‐Ardakani, and Foroumadi (2015) highlight its potential benefits, particularly in protecting against NSAID‐induced ulcers in rats. Aged garlic extract was found to safeguard against these ulcers by preventing the depletion of the important antioxidant glutathione and reducing lipid peroxidation. Moreover, it exhibited inhibitory effects on the growth of H. pylori bacteria and their adhesion to gastric epithelial cells in laboratory settings. The active compounds responsible for these gastroprotective effects were identified as organosulfur compounds, namely S‐allylcysteine and S‐allylmercaptocysteine, found in garlic. Despite promising preclinical findings, the efficacy of garlic in treating peptic ulcer disease (PUD) patients lacks substantial clinical validation. Thus, further clinical studies are warranted to elucidate the therapeutic potential of garlic in managing PUD (Shang et al. 2019).
4.1.7. Aloe ferox
A. ferox (Aloaceae), also known as bitter aloe or Cape aloe, has been traditionally used for various medicinal purposes, including potential therapeutic effects on gastric ulcers (Eamlamnam et al. 2006). A. ferox contains compounds such as anthraquinones, flavonoids, and polysaccharides, which have been shown to possess anti‐inflammatory properties. Chronic inflammation in the stomach lining can exacerbate gastric ulcers and A. ferox may help mitigate this inflammatory response (Bentahar et al. 2016). Additionally, the gel‐like sap (latex) obtained from the leaves of A. ferox has demulcent properties, meaning it forms a protective coating over the gastric mucosa. This protective barrier may help soothe irritation and inflammation in the stomach lining and promote the healing of gastric ulcers. Furthermore, some studies suggest that A. ferox may stimulate the production of gastric mucin, a glycoprotein that forms a protective layer over the stomach lining. By enhancing mucin production, Aloe ferox may help strengthen the gastric mucosal barrier and reduce the risk of ulcer formation (Borra, Lagisetty, and Mallela 2011).
4.1.8. Aloe vera Miller
The leaf pulp of the succulent A. vera plant (Xanthorrhoeaceae) has a long history of medicinal use for gastrointestinal problems in traditional medicine. In animal models, A. vera gel has exhibited gastroprotective effects against gastric ulcers through proposed mechanisms like antioxidant activity, increasing gastric mucus secretion, and stimulating the production of prostaglandins and bicarbonate (Martínez‐Burgos et al. 2022). Bioactive compounds identified in A. vera gel including polysaccharides like acemannan and anthraquinones such as aloin, can stimulate mucus cell proliferation and exert antioxidant effects to protect the gastric mucosa. Small clinical trials in humans have also revealed some beneficial effects of A. vera syrup in reducing abdominal pain, gastric acidity, and ulcer symptoms in peptic ulcer patients. However, larger‐scale randomized placebo‐controlled trials are required to conclusively validate the efficacy and safety of Aloe vera preparations as an adjunct or alternative therapy for peptic ulcers and gastritis (Ogidi and Enenebeaku 2023).
4.1.9. Althaea officinalis
A. officinalis, commonly known as marshmallow, has been used traditionally in herbal medicine for various gastrointestinal issues, including gastric ulcers (Ghavi 2015). The plant's roots, flowers, and leaves have been employed to treat a variety of illnesses including peptic ulcers, gastritis, ventricular ulcer, sore throat, asthma, bronchitis, and respiratory diarrhea. According to previous reports in the literature, aqueous extracts and polysaccharides in this plant present medicinal attributes (Šutovská et al. 2009). Furthermore, Marshmallow root contains high levels of mucilage, a gel‐like substance that forms a protective layer over the stomach lining. This mucilage may help soothe and protect the gastric mucosa, potentially reducing irritation and promoting ulcer healing. Additionally, Deters et al. have demonstrated that aqueous extracts and polysaccharides from A. officinalis roots can effectively stimulate epithelial cells, supporting the plant's traditional use as a tissue‐regenerating remedy for inflamed mucous membranes (Asnaashari, Dastmalchi, and Javadzadeh 2018; Deters et al. 2010).
4.1.10. Artemisia herba‐alba
A. herba‐alba, commonly referred to as white wormwood or desert wormwood, holds potential as a treatment for gastric ulcers. This plant, known for its medicinal properties, has been utilized in various cultures over time. Multiple studies have explored its ability to manage gastric ulcers, uncovering intriguing results (Gacem et al. 2020). Evidence suggests that A. herba‐alba possesses anti‐ulcerogenic properties, meaning it can help prevent the formation of ulcers in the stomach lining. Its effects are thought to stem from a blend of actions, including anti‐inflammatory, antioxidant, and cytoprotective mechanisms. Research has indicated that extracts derived from A. herba‐alba can mitigate the development of gastric ulcers induced by factors such as alcohol, nonsteroidal anti‐inflammatory drugs (NSAIDs), and stress in animal models. Moreover, its anti‐inflammatory attributes may aid in reducing the inflammation associated with gastric ulcers, thus facilitating the healing process (Abushwereb 2016). Additionally, the antioxidant compounds present in A. herba‐alba may shield the gastric mucosa from oxidative damage, a common factor implicated in the onset and progression of gastric ulcers (Abdel Jaleel, Abdallah, and Gomaa 2016).
Despite encouraging preliminary findings, further investigation, particularly clinical trials involving human subjects, is necessary to comprehensively assess the effectiveness and safety of A. herba‐alba in treating gastric ulcers. Moreover, delving into the specific bioactive compounds responsible for their anti‐ulcer effects and determining optimal dosage regimens would offer valuable insights into its therapeutic capabilities.
4.1.11. Berberis vulgaris
B. vulgaris (Berberidaceae) has been used in Ayurveda, Iranian, and Chinese medicine for treating gastrointestinal disorders (Shahdadian et al. 2023). Berberine, an alkaloid from Berberis vulgaris, exhibited anti‐ulcerogenic properties through inhibition of gastric acid secretion, stimulation of mucin secretion, and antioxidant effects in rats. Berberine also prevented NSAID‐induced intestinal injury by inhibiting inflammation and oxidative stress. Berberis's potential as an adjuvant to standard PUD therapy needs more investigation (Imenshahidi and Hosseinzadeh 2019).
4.1.12. Brassica oleracea var. botrytis
B. oleracea var. botrytis, commonly known as cauliflower, has shown therapeutic effects on gastric ulcers (Kononenko, Mirzaliev, and Ostapets 2018). Research indicates that extracts from B. oleracea, including var. botrytis, have gastroprotective properties against peptic ulcers (Kononenko, Mirzaliev, and Ostapets 2018). Studies have demonstrated that the aqueous fraction of B. oleracea botrytis offers high protection against gastric ulcers, with approximately 95% efficacy (Atta, Nasr, and Mouneir 2005; Kononenko, Mirzaliev, and Ostapets 2018). Additionally, the lipophilic extracts of B. oleracea have shown significant anti‐ulcerogenic activity, promoting the healing of gastric ulcers in animal models (Sudharameshwari and Ayshwarya 2017). These findings suggest that B. oleracea, along with other varieties of B. oleracea, could be beneficial in the management and treatment of gastric ulcers due to their gastroprotective and ulcer‐healing properties.
4.1.13. Brassica oleracea var. capitata
Both green and red cabbage varieties of B. oleracea have traditionally been used as part of folk remedies for healing stomach ulcers and gastrointestinal problems (Ray et al. 2021). Fresh cabbage juice has exhibited gastroprotective effects against ethanol, NSAID, stress, and cysteamine‐induced gastric ulcers and lesions in rat models through antioxidant mechanisms. Cabbage contains antioxidant flavonoids like kaempferol that help preserve the gastric mucosal barrier by stimulating mucus secretion and preventing oxidative damage to gastric tissue (Harsha et al. 2017; Hemmami et al. 2023). Another key flavonoid from cabbage, anthocyanin, has also demonstrated anti‐ulcer properties including antioxidant free radical scavenging, antisecretory effects on gastric acid, and cytoprotective activity in animal models. Crude extracts, juices, and purified bioactive compounds from cabbage thus seem to promote ulcer healing and gastritis treatment through combined antioxidant, antisecretory, mucoprotective, and anti‐inflammatory mechanisms. Further studies isolating and characterizing the specific phytochemicals responsible for cabbage's gastroprotective effects are warranted to understand its pharmaceutical potential for peptic ulcer disease (Kononenko et al. 2023).
4.1.14. Carlina acanthifolia
C. acanthifolia is a perennial plant that is common in Eastern Serbia's hills and mountains and belongs to the Asteraceae family (Leporatti and Ivancheva 2003). C. acanthifolia root is used for its diuretic, anti‐inflammatory therapy for the urinary tract, antioxidant, antimicrobial, and anti‐ulcer agent (Ivancheva, Nikolova, and Tsvetkova 2006). Traditionally have been used in herbal medicine for various gastrointestinal issues due to their potential anti‐inflammatory and gastroprotective properties. Previous studies on the efficacy of the root's essential oil showed a noteworthy dose‐dependent gastroprotective effect in rats that had completed a stress gastric ulcer test caused by ethanol (Đorđević et al. 2007).
4.1.15. Centaurea chamaerhaponticum Bal.
C. chamaerhaponticum, commonly known as Pontic cornflower or Pontic knapweed, is a species of flowering plant in the family Asteraceae (Kaunitz 1999). C. chamaerhaponticum belongs to the Centaurea genus, which includes several species known for their medicinal properties including ulcer gastric diseases. Some species within the Centaurea genus have been studied for their anti‐inflammatory, antioxidant, and gastroprotective effects, which could potentially have implications for the treatment or prevention of gastric ulcers. Previous studies' results showed that crude extracts of C. chamaerhaponticum possess significant gastroprotective and antisecretory properties which prevent gastric ulceration induced by different necrotizing agents (Bakhtaoui, Lakmichi, Chait, and Gadhi 2014).
4.1.16. Centaurium erythraea
C. erythraea, commonly known as common centaury, has been traditionally used in herbal medicine for various purposes including gastric ulcers (El Menyiy et al. 2021). Common centaury contains bitter principles, including iridoids such as gentiopicrin and amarogentin, which stimulate bitter receptors in the mouth and digestive tract. Bitter substances have been shown to enhance digestion by stimulating the secretion of saliva, gastric juices, and bile, which can aid in the breakdown and absorption of nutrients (Đorđević et al. 2017). Further, some studies have suggested that herbal remedies containing bitter principles may have gastroprotective effects, including promoting the secretion of protective mucus in the stomach lining and enhancing the integrity of the gastric mucosa (Bentahar et al. 2016). While direct evidence for C. erythraea gastroprotective effects is lacking, its bitter properties may contribute to similar mechanisms of action.
4.1.17. Ceratonia siliqua L.
C. siliqua, commonly known as carob tree or St. John's bread, is a species of flowering evergreen tree in the Fabaceae family, that exhibits therapeutic effects on gastric ulcers (Bakhtaoui, Lakmichi, Chait, and Gadhi 2014). Research has shown that the plant contains various beneficial compounds such as polyphenols, flavonoids, and phenolic acids, which contribute to its antioxidant, anti‐inflammatory, and anti‐ulcer properties (Elaoufi et al. 2022). Studies have demonstrated that the carob pods' extracts can inhibit hyaluronidase activity, reduce oxidative damage, and protect against gastric mucosal damage induced by HCl/ethanol (Rtibi et al. 2016). Additionally, C. siliqua extracts have been found to limit colonic damage in rats with ulcerative colitis, showcasing anti‐inflammatory and antioxidant effects (Lakkab et al. 2018). The plant's bioactive components also exhibit antibacterial, antifungal, and antidiabetic activities, suggesting its potential as a natural alternative for various health conditions, including gastrointestinal issues like gastric ulcers (Benmansour et al. 2020).
4.1.18. Cicorium intybus
C. intybus, commonly known as chicory exhibits therapeutic effects on gastric ulcers due to its gastroprotective properties. The plant's root extract demonstrates a gastroprotective effect by reducing gastric secretions and enhancing the defense barrier of the gastric mucosa in previous studies (Krylova et al. 2015). Various pharmacological studies confirm the plant's therapeutic value, showcasing activities such as anti‐ulcer, antioxidant, and free radical scavenging properties (Saxena, Sulakhiya, and Rathore 2014). Additionally, the hydroalcoholic extract of C. intybus roots show significant anti‐ulcer and antioxidant activities, reducing gastric juice volume and acidity while increasing pH levels, indicating its potential in ulcer treatment (Saxena et al. 2011). The plant's rich phytoconstituents, including inulin, flavonoids, sesquiterpene lactones, and vitamins, contribute to its diverse medicinal benefits (Das, Vasudeva, and Sharma 2016). Additionally, Chicory is rich in antioxidants, including vitamins A and C, flavonoids, and phenolic compounds. These antioxidants help neutralize free radicals and oxidative stress, which can damage the gastric mucosa and increase the risk of ulcer formation (Krylova et al. 2015).
4.1.19. Cinnamomum verum
C. verum, also known as true cinnamon, belongs to the Lauraceae family and is esteemed for its multifaceted medicinal properties. Across traditional healing practices, C. verum has been integral for centuries, notably in regions like South Asia and the Middle East, revered for its diverse therapeutic applications. The bark and essential oil of C. verum have been traditionally employed to address an array of health concerns, including gastrointestinal disorders such as indigestion, bloating, and diarrhea. These remedies often utilize decoctions or infusions derived from C. verum bark, renowned for its soothing and gastroprotective effects (Ranasinghe et al. 2013). Numerous scientific inquiries have illuminated the therapeutic potential of C. verum. Essential oils extracted from C. verum have demonstrated potent antimicrobial, antifungal, and antioxidant properties, suggesting their efficacy in combating various infectious and inflammatory conditions (Rao and Gan 2014). Additionally, research has underscored the anti‐ulcerative properties of C. verum extracts, particularly aqueous solutions, showcasing their potential to mitigate gastric ulceration in experimental models (Bandara, Uluwaduge, and Jansz 2012). Furthermore, C. verum has exhibited promising capabilities in modulating inflammatory pathways, exerting antisecretory effects, and bolstering the body's antioxidative defenses, indicative of its potential as a therapeutic agent for a spectrum of ailments (Sharma et al. 2022).
4.1.20. Globularia alypum L.
G. alypum, a member of the Globulariaceae family, is a common plant in the Mediterranean. G. alypum has long been utilized in traditional medicine to treat various disorders caused by oxidative stress (Ziyyat et al. 1997). The research on G. alypum highlights its gastroprotective efficacy along with various other beneficial properties. Studies have shown that extracts of G. alypum possess significant antioxidant activities, which can help protect against oxidative damage induced by radicals like AAPH (Ouffai et al. 2022). Additionally, the plant exhibits anti‐inflammatory properties, which can contribute to gastroprotection (Nouir et al. 2023). Furthermore, the phytochemical composition of G. alypum includes bioactive compounds like verbascoside, known for their potential gastroprotective effects (Hajji et al. 2021). Moreover, numerous studies have shown that G. alypum leaf and flower extracts are rich in secondary metabolites such as polyphenols and iridoids (Amessis‐Ouchemoukh et al. 2014; Taghzouti et al. 2016). Those compounds are well known for their antioxidant properties, anti‐ulceration, anti‐inflammation (Boutemak, Safta, and Ayachi 2015), and anticancer activities (Es‐Safi et al. 2006). These findings suggest that G. alypum has the potential to offer gastroprotective benefits, making it a promising candidate for further exploration in gastroprotection research. Nevertheless, further research, including preclinical and clinical studies, is needed to elucidate its efficacy and safety for the prevention and treatment of peptic ulcers and other gastrointestinal disorders.
4.1.21. Glycyrrhiza glabra
Licorice derived from the roots of G. glabra (Leguminosae) has a long history of use in traditional medicine systems as an expectorant and treatment for peptic ulcers and other gastrointestinal problems. Several deglycyrrhizinated licorice preparations containing glycyrrhizin, isoliquiritigenin, and polysaccharides have exhibited gastroprotective effects in animal models and human studies of peptic ulcer disease (Maqbool et al. 2023). Proposed mechanisms for licorice's anti‐ulcer properties include increasing the production of gastric mucus and prostaglandins, antioxidant effects, and inhibiting H. pylori colonization. The triterpene glycyrrhizin can bind to intracellular steroidal receptors leading to increased prostaglandin formation and cytoprotective effects in the gastric mucosa. Licorice flavonoids such as liquiritigenin can also stimulate mucin release from gastric epithelial cells through prostaglandin E2 pathways in vitro. Further research needs to definitively characterize the active anti‐ulcer constituents in licorice and elucidate their molecular mechanisms of gastroprotection. Nonetheless, the clinical studies combined with preclinical data suggest that licorice possesses therapeutic potential for peptic ulcer treatment warranting more rigorous randomized controlled trials to validate its efficacy and safety in humans (El‐Saber Batiha et al. 2020; Maqbool et al. 2023; Patel and Khetani 2021).
4.1.22. Helianthemum kahiricum L.
H. kahiricum is a species of flowering plant belonging to the family Cistaceae. H. kahiricum has shown therapeutic effects on gastric ulcers (Alsabri et al. 2014). Studies have highlighted its anti‐ulcer properties, including antioxidant effects and protection against ethanol‐induced ulcers (Mouffouk et al. 2023). Additionally, the plant exhibited significant anti‐inflammatory and anti‐microbial activities, contributing to its overall therapeutic potential (Ayoola et al. 2016; Olaitan Balogun et al. 2022). Chemical investigations have identified compounds in H. kahiricum that play a role in its medicinal properties, such as kaempferol derivatives with antimicrobial activity. Furthermore, the plant's extracts have demonstrated gastric ulcer healing effects, modulation of cytokines, and tissue regeneration, showcasing its potential in treating chronic gastric ulcers (Ayoola et al. 2016). Overall, H. kahiricum shows promise as a natural remedy for gastric ulcers due to its diverse pharmacological properties and therapeutic effects.
4.1.23. Helianthemum lippii L.
H. lippii commonly known as sunrose rockrose or white‐flowered cistus, is a flowering plant species belonging to the family Cistaceae (Ibtissam and Djahra 2022). H. lippii has been extensively studied for its therapeutic effects, including its anti‐ulcer properties. Research has shown that H. lippii methanolic extracts exhibit significant anti‐ulcer activity, with doses of 250 mg/kg and 500 mg/kg demonstrating substantial inhibition of gastric lesions in rats induced with ethanol, comparable to the standard anti‐ulcer drug ranitidine (Alsabri et al. 2013). The protective effect of H. lippii against ethanol‐induced gastric mucosal lesions is attributed to its increase in antioxidant activity, highlighting its potential in treating gastric ulcers (Alsabri et al. 2014). Additionally, the phytochemical screening of H. lippii revealed the presence of compounds like flavonoids and tannins, which contribute to its therapeutic properties, including anti‐ulcer effects (Alsabri et al. 2013). These findings position H. lippii as a promising natural remedy for gastric ulcers.
4.1.24. Juniperus communis
J. communis has been extensively studied for its therapeutic effects, including its anti‐ulcer properties. Research has shown that the leaf extract of J. communis significantly inhibits various types of gastric ulcerations in rats and promotes healing of ulcers, comparable to the standard drug ranitidine (Pramanik et al. 2007). Additionally, J. communis contains essential oils, phenolic compounds, and various chemical constituents that contribute to its pharmacological effects, such as anti‐inflammatory and analgesic properties (Al‐Snafi 2018). Traditionally used for its medicinal benefits, J. communis is known for its anti‐inflammatory, antiseptic, and astringent properties, making it potentially effective in treating abdominal disorders (Bais et al. 2014). Furthermore, the plant's extract and essential oils exhibit antibacterial, antioxidant, and anti‐inflammatory activities, highlighting its potential in treating various diseases, including ulcers (Fierascu et al. 2018).
4.1.25. Juniperus phoenicea L.
J. phoenicea also known as red juniper. In folk medicine, this plant is considered a remedy that is commonly used in many countries for the treatment of diarrhea, bronchitis, rheumatism, eczema, hemorrhoids, dysmenorrheal, and ulcer gastric (Mansour et al. 2023). The essential oil of J. phoenicea seeds has been identified as a potent source of natural antioxidants and antimicrobial agents, and ulcer gastric diseases. Consistent with this, different authors reported that essential oils have high curative characteristics and fewer side effects than chemical drugs, acting through the modulation of oxidative stress and the targeting of inflammatory parameters. Several studies illustrated, that the oral administration of the essential oil of J. phoenicea has potent anti‐ulcer activity, which justifies the ethnomedical claims about its significant gastroprotective effect, as evaluated by the significant antioxidant activity because it reduces the MDA level and increases the GSH, GST, GPx, CAT, and SOD levels (Ali et al. 2015; Hoogerwerf and Pasricha 2006). This effect may be related to an increase in gastric mucosal defense mechanisms. As a result, the effect of the essential oil and its low toxicity requires further study to elucidate the mechanism of action and isolate the active principle.
4.1.26. Lagenaria siceraria L.
L. siceraria, commonly known as bottle gourd or calabash, is a vine plant belonging to the gourd family (Cucurbitaceae) (Ahmed, Nedi, and Yimer 2022). The aqueous extracts of L. siceraria have shown therapeutic effects on gastric ulcers. Studies have demonstrated the anti‐gastric ulcer activity of L. siceraria, with significant reductions in gastric acidity, increased pH levels, and enhanced gastric wall mucus production, indicating gastroprotective potential (Saeed et al. 2022). Additionally, L. siceraria is known for its antioxidant properties due to compounds like flavonoids, saponins, and tannins, which contribute to its anti‐ulcer action (Mehboob et al. 2022). The anti‐ulcer efficacy of a methanolic extract of L. siceraria fruits was examined in Wistar rats using pylorus ligation, asprin, cold‐restraint stress, and ethanol ulcer models. MELS reduced stomach volume, free acidity, ulcer index, and total acidity significantly, indicating that L. siceraria fruit extract may have anti‐ulcer action. As a result of histological assessment investigations, it was shown that L. siceraria is both safe and effective in the treatment of stomach ulcers. Additionally, Bottle gourd is rich in dietary fiber, particularly soluble fiber. Soluble fiber can help regulate digestion, promote bowel regularity, and improve overall gastrointestinal health. Adequate fiber intake is associated with a reduced risk of certain gastrointestinal conditions, including gastric ulcers according to several studies (Manchala 2019).
4.1.27. Lavandula stoechas
L. stoechas, commonly known as Spanish lavender or French lavender, is a species of flowering plant in the Lavandula genus (Şahinler et al. 2022). L. stoechas has a long history of traditional medicinal use for various gastrointestinal issues, including stomachaches (Elrherabi et al. 2023). The aqueous extract demonstrated anti‐ulcer activity in ethanol‐induced gastric and duodenal ulcers in experimental animals, comparable to standard drugs like omeprazole and ranitidine. Additionally, the extract exhibited antimicrobial properties against Proteus Mirabilis, indicating its potential in combating ulcer‐related infections. Some studies suggest that lavender essential oil may have cytoprotective effects on the gastric mucosa. These effects involve enhancing the secretion of gastric mucus, increasing mucosal blood flow, and stimulating the production of prostaglandins, which help protect the stomach lining from harmful factors (Javed et al. 2020). Additionally, several studies illustrated the effects of anxiolytic and stress‐reducing L. stoechas chronic stress and anxiety are known risk factors for the development of peptic ulcers. Lavender has been traditionally used for its calming and stress‐relieving properties. By reducing stress and anxiety levels, lavender may indirectly help prevent ulcers and promote gastrointestinal health (Javed et al. 2020).
4.1.28. Lawsonia inermis L.
L. inermis, commonly known as henna, exhibits therapeutic effects on gastric ulcers. Studies have shown that L. inermis possesses anti‐ulcer properties (Elansary et al. 2020; Moutawalli et al. 2023). The plant's leaves, when administered in a nano formulation, demonstrated the ability to prevent ulcer formation in rats induced with aspirin, showcasing significant improvements in various parameters and biochemical activities (Muheyuddeen et al. 2023). Additionally, L. inermis contains active compounds like flavonoids, phenols, alkaloids, and tannins, which contribute to its pharmacological effects, including anti‐inflammatory, analgesic, and wound‐healing properties (Muheyuddeen et al. 2023). The plant's extracts have been found to have antioxidant activity and high flavonoid content, further supporting its potential therapeutic benefits for gastric ulcers. This collective evidence underscores the promising role of L. inermis in the management of gastric ulcers (Mohammed et al. 2022).
4.1.29. Mangifera indica
M. indica (Anacardiaceae), commonly known as mango, is being used in folk medicine for the treatment of various diseases including gastric ulcers (Sahu, Martha, and Pradhan 2017). Several studies, it was demonstrated that the ethanolic and aqueous extract of leaves of M. indica exhibited remarkable gastroprotective activity in pylorus ligation, aspirin plus pylorus ligation, and ethanol‐induced gastric ulcer models in experimental Wistar rats. In addition, Kagbo and Aduku (2015) illustrated that M. indica may have an equal extent of therapeutic effect gastroprotective effect with the largely used well‐known anti‐gastric ulcer drug Ranitidine. Moreover, other studies suggested glucosylxanthone from M. indica is a gastroprotective agent responsible for the anti‐ulcer effect (Carvalho et al. 2007).
4.1.30. Matricaria chamomilla
The dried flowers of M. chamomilla (Asteraceae) have been traditionally used as a digestive aid and treatment for gastritis and peptic ulcers (Srivastava and Gupta 2015). Apigenin, a bioactive flavone isolated from chamomile, has been shown to dose‐dependently reduce gastric injury and inflammation induced by H. pylori infection in vitro. It exhibits these gastroprotective effects by suppressing pro‐inflammatory cytokines like TNF‐α and IL‐8 while also inhibiting the activity of H. pylori cytotoxic virulence factors (El Joumaa and Borjac 2022). Beyond anti‐inflammatory effects, extracts of M. chamomilla flowers demonstrate direct antibacterial activity against H. pylori growth in vitro (Cvetanović et al. 2015). Animal studies have also revealed chamomile oil can accelerate the healing of acetic acid‐induced chronic gastric lesions in rats through antisecretory mechanisms and antioxidant effects. Specific constituents of chamomile flowers like the terpenoids bisabolol and chamazulene, and flavonoids like apigenin, quercetin, and patuletin have been postulated to mediate its gastroprotective properties, but require further investigation. Overall, chamomile possesses the broad therapeutic potential for the protection and treatment of peptic ulcers through its combination of antibacterial, anti‐inflammatory, antisecretory, mucoprotective, and antioxidant effects (Singh et al. 2023).
4.1.31. Mentha microphylla
M. microphylla, commonly known as Corsican mint or tiny‐leafed mint, is a low‐growing perennial herbaceous plant belonging to the Lamiaceae family (Brahmi et al. 2017). M. microphylla was originally used as a medicinal herb to treat stomachache, and it is commonly used in the form of tea as a home remedy to stimulate digestion, alleviate stomach pain, and treat biliary disorders, dyspepsia, enteritis, flatulence, gastritis, and gastric acidities. Some studies suggest that mint extracts can enhance the production of mucus in the stomach lining, which serves as a protective barrier against stomach acid and irritants. This mucosal protection may help prevent or alleviate gastrointestinal disorders. Moreover, several studies illustrated the aqueous fraction of M. microphylla showed a prominent protective effect (100% protection) in Wistar rats, which suggests the beneficial use of infusion or decoction teas of Mentha as protective against peptic ulcers (Thompson Coon and Ernst 2002). Additionally, certain compounds found in mint, such as menthol and menthone, have been investigated for their potential to prevent and heal ulcers in the stomach and intestines (Atta, Nasr, and Mouneir 2005). These compounds may help by reducing gastric acid secretion and enhancing mucosal defense mechanisms.
4.1.32. Mentha spicata
Studies have explored the potential of M. spicata, commonly known as spearmint, in treating peptic ulcer diseases, especially in Northern Africa where such ulcers are common and traditional remedies often include herbal treatments like spearmint (Anwar et al. 2019). Research indicates that Mentha spicata may offer benefits for individuals with peptic ulcers, as it is thought to have anti‐inflammatory and gastroprotective properties, potentially aiding in symptom relief and ulcer healing (Singh et al. 2023). Investigations in Northern Africa have shown promising results, demonstrating Spearmint's ability to reduce ulcer formation and alleviate symptoms in animal models. Furthermore, its historical use in traditional medicine in the region suggests its perceived effectiveness for gastrointestinal issues. However, further research, particularly well‐designed clinical trials involving human participants, is necessary to confirm the efficacy of M. spicata in managing peptic ulcer diseases in Northern Africa. Additionally, understanding its mechanisms of action and determining optimal dosages would enhance its therapeutic application in this context (Anwar et al. 2019).
4.1.33. Myrtus communis L.
M. communis, belonging to the Myrtaceae family, is commonly used in traditional medicine as a decoction to treat stomach and gastrointestinal disorders such as diarrhea, constipation, and peptic ulcer (Flamini et al. 2004). Moreover, distinct studies have previously demonstrated the disinfectant, antiseptic, antimicrobial, and antioxidant capacities of M. communis essential oils, as well as their potential to fight several diseases. In addition, a previous study highlighted the anti‐ulcer capacity of orally administered methanolic extracts of M. communis leaves (Alipour, Dashti, and Hosseinzadeh 2014), in experimental models of gastric ulceration (Ben Mansour et al. 2022). Further, several studies have demonstrated the positive effect of plant essential oils through the modulation of inflammatory mediators, antisecretory, and antioxidative stress defense. More importantly, the treatment of animals with MMEO successfully inhibited oxidative damage and reversed the impairment of the antioxidant system in the intestinal mucosa according to previous studies (Mansour et al. 2022).
4.1.34. Nigella sativa
N. sativa (black seed) is an annual herbaceous plant that belongs to the Ranunculaceae family. It grows up to 30–60 cm tall with finely divided leaves and small black seeds. The seeds of N. sativa have been utilized traditionally in North Africa for the treatment of various gastrointestinal disorders, including stomach ulcers. The therapeutic effects of N. sativa have been attributed to its various bioactive compounds, such as thymoquinone, thymohydroquinone, and thymol (Alam et al. 2023). Thymoquinone, a major active component of N. sativa, has been demonstrated to exhibit anti‐ulcer activity in several experimental models of gastric ulceration. In a study by Bukar et al. (2017), thymoquinone was found to protect against ethanol‐induced gastric mucosal injury in rats. Similarly, Zeren et al. (2016) reported that thymoquinone inhibited gastric acid secretion and increased mucus production in rats with indomethacin‐induced gastric ulcers. In addition, thymoquinone has been shown to exhibit antimicrobial activity against H. pylori, a bacterium commonly associated with stomach ulcers (Tabassum and Ahmad 2021). Other bioactive compounds in N. sativa, such as thymohydroquinone and thymol, have also been reported to possess anti‐ulcer activity. Thymohydroquinone has been shown to reduce gastric acid secretion and increase mucus production in rats with ethanol‐induced gastric ulcers (Paseban et al. 2020; Zakir et al. 2022). Moreover, thymol has been demonstrated to exhibit anti‐inflammatory and antioxidant activity, which may contribute to its gastroprotective effects (Liu et al. 2022). In vitro studies have also confirmed the anti‐ulcer activity of N. sativa extracts. For instance, Geetha and Anitha (2022) reported that an ethanolic extract of N. sativa inhibited the growth of H. pylori in vitro. Similarly, Bukar et al. (2017) found that an aqueous extract of N. sativa protected against ulceration in rats with acetic acid‐induced gastric lesions.
4.1.35. Olea europaea
O. europaea (Olive), commonly known as the olive tree, has been traditionally used for various medicinal purposes, including the treatment of gastric ulcers (Unissa et al. 2022). O. europaea exhibits therapeutic effects in treating gastric ulcers by demonstrating gastroprotective, anti‐ulcer, antioxidant, and anti‐inflammatory properties. Studies have shown that olive leaf extract significantly reduces ulcer index, protects the gastric mucosa, and regulates inflammatory cytokines, such as IL‐1β, IL‐2, IL‐4, IL‐6, IL‐10, and TNF‐α (Musa et al. 2021). Additionally, olive extract decreases histological changes in the small intestine, enhances gastrointestinal function, and reduces intestinal permeability, as indicated by decreased plasma D‐lactate concentration. Furthermore, olive oil extracted from Opuntia ficus‐indica seeds has been found to efficiently protect the gastric mucosa, stimulate mucus production, and accelerate the healing process of ethanol‐induced ulcers (Mahdavi et al. 2020; Musa et al. 2021).
4.1.36. Oreganum majorana
O. majorana, commonly known as sweet marjoram or simply marjoram, is an aromatic herb belonging to the mint family (Lamiaceae) (Al‐Howiriny et al. 2009). The aerial parts of marjoram or the oil are used for “strengthening of the stomach,” and to cure acute and chronic gastritis by containing various chemical constituents including phenolic glycosides, flavonoids, phenolic compounds, tannins, and essential oil (Dorman and Deans 2000; Mathias 1994). Several studies have demonstrated that pretreatment with marjoram leads to a significant improvement in the depleted levels of NP‐SH concentration, indicating the strong involvement of endogenous sulfhydryl compounds (SHs) in the gastroprotective effects of marjoram extract. NP‐SH compounds are known for their role in scavenging oxygen‐derived free radicals and regulating the production and quality of mucus. Chemical constituents found in marjoram, such as tannins, flavonoids, and volatile oils, are believed to prevent the loss of gastric mucus and NP‐SH. These compounds have been shown to eliminate free radicals generated as a result of ethanol‐induced mucosal ulceration. Tannins, flavonoids, and volatile oils have been associated with antioxidant properties in previous research (Allen et al. 1984; Andreo et al. 2006).
4.1.37. Oreganum syriacum L
O. syriacum, commonly known as Syrian oregano, is a herbaceous plant belonging to the mint family (Lamiaceae) (El‐Meligy et al. 2017). O. syriacum has demonstrated therapeutic effects on gastric ulcer. Several studies showed that oral administration of the ethanolic extract of O. syriacum significantly reduced gastric damage in experimental rats (Mesmar et al. 2022). In another study investigating the anti‐ulcerogenic activity of various plant extracts in prophylactic and curative models, the oral administration of O. syriacum ethanolic extract to rats with absolute‐ethanol‐induced gastric damage was shown to have a similar effect to the anti‐ulcer drug lansoprazole in the prophylactic model only (El‐Meligy et al. 2017). The ethanolic extract also exhibited high free‐radical scavenging activity, further supporting the antioxidant potential of O. syriacum and its protective and healing role in ethanol‐induced gastric ulceration. Furthermore, the extract from O. syriacum has been shown to protect the gastric mucosa from ethanol‐induced injury by inducing cyclooxygenase‐2 (COX‐2) expression and decreasing oxidative stress in the stomach.
4.1.38. Origanum compactum
O. compactum (Lamiaceae), commonly known as Moroccan oregano or compact oregano, is a species of oregano native to North Africa, particularly Morocco (Bouyahya et al. 2020). This Moroccan medicinal plant, also known as Zaatar, has been traditionally used to treat various ailments, including digestive issues (Bouyahya et al. 2016). Studies have highlighted the plant's antimicrobial, antioxidant, and anti‐inflammatory properties, which are crucial in addressing gastric ulcer pathophysiology (Hayani et al. 2022). The essential oils extracted from O. compactum have demonstrated significant antibacterial activity against various strains, including E. coli and S. aureus, indicating its potential in combating infections related to gastric ulcers (Bakhtaoui, Lakmichi, Chait, and Gadhi 2014). Additionally, the plant's bioactive compounds, such as thymol and carvacrol, contribute to its pharmacological mechanisms, supporting its efficacy in treating gastric ulcers and other related conditions. Further research on the therapeutic potential of O. compactum in gastric ulcer management is encouraged to explore its full range of benefits (Bakhtaoui, Lakmichi, Chait, and Gadhi 2014).
4.1.39. O. ficus‐indica
O. ficus‐indica, commonly known as prickly pear or cactus pear. The cladodes of O. ficus indica are used in folk medicine in the treatment of gastric mucosal diseases (Galati et al. 2001; Saag et al. 1975). Several researchers demonstrated in experimental Wistar rats, that the prophylactic therapy with lyophilized cladodes of O. ficus indica inhibits the ulcerative effect of ethanol. One could assume that the mucilages of O. ficus indica are responsible for the effects that have been noted. The mucilage could stop the necrotizing agent from penetrating the stomach mucosa. Maybe it creates a shield and prevents the deep necrotic lesions and the large‐scale ethanol‐induced surface epithelium exfoliation (Trachtenberg and Mayer 1981). It is likely that the mucilage, a high molecular weight acid polysaccharide primarily composed of arabinogalactan and galacturonic acid (Saag et al. 1975), can work in concert with the stomach mucosa's defense mechanisms.
4.1.40. Osyris quadripartita
O. quadripartita, commonly known as African sandalwood, is a species of flowering plant in the family Santalaceae (Abebaw, Mishra, and Gelayee 2017). O. quadripartita (OQ) has been scientifically validated for its therapeutic effect on gastric ulcers. Studies have shown that OQ possesses significant anti‐ulcer activity (Babu et al. 2020). The plant extract demonstrated a dose‐dependent and time‐dependent reduction in gastric ulcer index in both pylorus ligation‐induced and ethanol‐induced ulcer models, comparable to standard drugs like ranitidine and sucralfate. Additionally, OQ's oral median lethal dose (LD50) was estimated to be higher than 2000 mg/kg, indicating its safety profile (Kale, Bambare, and Bhale 2023). Furthermore, OQ's anti‐ulcer properties have been attributed to the presence of secondary metabolites like flavonoids, tannins, and saponins in the plant extract (Jainu and Devi 2004b). These findings validate the traditional use of OQ in Ethiopian folk medicine for treating peptic ulcers and highlight its potential as a natural remedy for gastric ulcer management.
4.1.41. Phoenix dactylifera L
P. dactylifera, commonly known as the date palm, is a member of the Arecaceae family and is renowned for its versatile medicinal properties. In traditional medicine, P. dactylifera has been utilized for centuries, particularly in the Middle East and North Africa, for its gastroprotective efficacy. Date palm pulp and pulp palm sap have been traditionally used as decoctions to alleviate various stomach and gastrointestinal disorders, including diarrhea, constipation, and peptic ulcers (Stielow et al. 2023).
Several studies have highlighted the therapeutic potential of P. dactylifera. Essential oils derived from P. dactylifera have demonstrated notable disinfectant, antiseptic, antimicrobial, and antioxidant properties, suggesting their potential efficacy in combating a range of diseases (Al‐Zoreky and Al‐Taher 2019). Additionally, research has indicated the anti‐ulcerative effects of orally administered aqueous extract of P. dactylifera, particularly in experimental models of gastric ulceration (Abdullahi and Chinedu 2022). Furthermore, P. dactylifera has shown promising results in modulating inflammatory mediators, exhibiting antisecretory effects, and enhancing the body's antioxidative stress defense mechanisms (Al‐Shahib and Marshall 2003).
4.1.42. Pistacia lentiscus L.
P. lentiscus (Anacardiaceae) is a flowering plant growing in the Mediterranean area. It is traditionally used in the treatment of gastrointestinal upsets and gastric ulcers (Al‐Habbal, Al‐Habbal, and Huwez 1984; Duru et al. 2003). The anti‐ulcer and anti‐inflammatory activities of PLFO could be due to its phytochemical compounds such as fatty acid, tocopherols, sterols, phenolic components, and terpenoids. The major fatty acids in PLFO can inhibit the activity of COX‐1 and COX‐2 (Ben Khedir et al. 2016). These enzymes are involved in the production of inflammatory mediators such as arachidonic acid‐derived eicosanoids (such as prostaglandins, thromboxanes, and leukotrienes) (Calder 2001). At sufficient levels, long‐chain n‐3 polyunsaturated fatty acids (PUFAs), such as α‐linoleic acid found in PLFO, inhibit arachidonic acid metabolism. PUFAs can also reduce the levels of pro‐inflammatory cytokines (such as IL‐6 and TNF‐α) and the expression of adhesion molecules implicated in the interactions between leukocytes and endothelial cells during the infiltration process. Moreover, Boutemine et al. (2018) highlighted its protective effect. As pre‐treatment, PLFO could be prescribed to patients predisposed to have a gastric ulcer such as people who consume a lot of alcohol and anti‐inflammatory drugs orally.
4.1.43. Plantago major
P. major, commonly referred to as greater plantain, has garnered attention for its potential to treat various gastrointestinal ailments, including peptic ulcer diseases. Particularly in regions like North Africa, where traditional herbal remedies hold significance, greater plantain has been historically utilized for its medicinal properties (Hemmami et al. 2023). Research indicates that P. major may possess anti‐inflammatory and mucoprotective properties, which could be advantageous in relieving symptoms and facilitating healing in individuals with peptic ulcers. Studies have demonstrated promising findings, suggesting that extracts derived from P. major can mitigate ulcer formation and shield the gastric mucosa from damage caused by substances like alcohol and NSAIDs (Ragheb, Ibrahem, and Shalaby 2021). Moreover, its wide availability and minimal toxicity enhance its appeal as an herbal treatment option. Nonetheless, further investigation, particularly through clinical trials involving human participants, is imperative to comprehensively grasp the effectiveness and optimal use of P. major in managing peptic ulcer diseases, not only in North Africa but also in other regions (Bahadeen Aref et al. 2023).
4.1.44. Portulaca oleracea
P. oleracea (Portulaceae, common name purslane). Kaempferol, apigenin, luteolin, myricetin, and quercetin are major flavonoids in P. oleracea that prevent gastrointestinal diseases (Farkhondeh and Samarghandian 2019). Studies have shown that P. oleracea extract and juice can effectively reduce ulcerative colitis (UC) symptoms by decreasing disease activity index (DAI) scores and inflammatory cell infiltration (Tijani, Temitayo, and Farombi 2022). Additionally, P. oleracea juice has been found to inhibit pyroptosis by reducing the expression of NLRP3 inflammasome, repairing intestinal barrier dysfunction, and upregulating tight junction proteins. Furthermore, the anti‐ulcer properties of P. oleracea have been linked to its ability to enhance the mucosa‐bicarbonate barrier, reduce gastric ulceration, and positively impact antioxidant defense systems (Karimi, Hosseinzadeh, and Ettehad 2004). Additionally, purslane contains omega‐3 fatty acids, flavonoids, and polysaccharides, which have demonstrated anti‐inflammatory properties. Chronic inflammation is a contributing factor to the development and progression of gastric ulcers. By reducing inflammation in the stomach lining, purslane may aid in the healing process of gastric ulcers (Kumar et al. 2010).
4.1.45. Punica granatum
Studies have explored the potential of P. granatum, or pomegranate, in treating and preventing stomach ulcers due to its traditional medicinal use and perceived health benefits. It is believed that P. granatum exhibits gastroprotective properties through various mechanisms, including its antioxidant, anti‐inflammatory, and mucoprotective actions (Muhialdin et al. 2023). Research indicates that extracts from this fruit can reduce the development of stomach ulcers induced by alcohol, NSAIDs, and stress in animal models. The high antioxidant content, particularly polyphenols like ellagic acid and punicalagin, may help protect the stomach lining from oxidative damage, a factor often linked to ulcer formation. Despite promising initial results, further investigations, including human clinical trials, are needed to fully comprehend the effectiveness of P. granatum in treating stomach ulcers. Additionally, identifying specific bioactive compounds and determining optimal dosage regimens would enhance its therapeutic application (Alimi et al. 2023).
4.1.46. Rhus tripartita
R. tripartita, also known as three‐leaved sumac or skunkbush sumac, is a shrub native to North Africa and parts of the Middle East. It is primarily known for its use in traditional medicine, particularly in North African traditional healing systems, specifically for ulcer gastric diseases (Shahat et al. 2016). Research indicates that R. tripartita may possess anti‐inflammatory, antioxidant, and cytoprotective effects properties, which could be advantageous in relieving symptoms and facilitating healing in individuals with peptic ulcers. Studies have demonstrated promising findings, suggesting that extracts derived from R. tripartita major can mitigate ulcer formation and shield the gastric mucosa from damage caused by substances like alcohol and NSAIDs (Ben Mansour et al. 2022). Nevertheless, further research, including clinical trials, is needed to validate the efficacy and safety of R. tripartita and related species for medicinal use in treating or preventing gastrointestinal disorders.
4.1.47. Solanum nigrum
S. nigrum, commonly known as black nightshade, is a plant that has been traditionally used in various cultures for its medicinal properties (Jainu and Devi 2006). Certain components of black nightshade, such as alkaloids and saponins, may possess gastroprotective properties (Zaghlool et al. 2019). These compounds may help strengthen the gastric mucosal barrier, inhibit gastric acid secretion, and promote tissue regeneration, thereby protecting against gastric ulcers. Previous reports indicated that S. nigrum fruits possess beneficial activity as an anti‐ulcer, and antioxidant‐promoting agent in rats (Jainu and Devi 2004a; Kumar et al. 2001). It has been reported earlier that aerial parts of S. nigrum are believed to offer their anti‐ulcer action through acid and peptic suppression in aspirin‐induced ulcerogenesis in rats (Akhtar and Munir 1989). The preliminary phytochemical screening of SNE revealed the presence of tannins, alkaloids, carbohydrates, saponins, volatile oils, and anthocyanins. Previous studies proved that anthocyanins possess significant antioxidant, anti‐ulcer, and ulcer healing activity in experimental ulcer models. Additionally, saponins, tannins, and volatile oils of some plants are also known to possess anti‐ulcer activity (Gohar and Zaki 2014).
4.1.48. Syzygium aromaticum
S. aromaticum, commonly known as clove, belongs to the Myrtaceae family and is esteemed for its diverse medicinal properties. Across traditional healing practices, S. aromaticum has been integral for centuries, particularly in regions like Southeast Asia and the Indian subcontinent, revered for its versatile therapeutic applications. The aromatic buds and essential oil of S. aromaticum have been traditionally employed to address an array of health concerns, including dental issues, digestive disorders, and respiratory ailments. These remedies often utilize clove infusions or decoctions derived from the buds, renowned for their soothing and antimicrobial effects (Batiha et al. 2020; Maggini et al. 2024). Numerous scientific inquiries have illuminated the therapeutic potential of S. aromaticum. Essential oils extracted from S. aromaticum have demonstrated potent antibacterial, antifungal, and antioxidant properties, suggesting their efficacy in combating various infectious and inflammatory conditions (Liñán‐Atero et al. 2024; Pandey et al. 2024). Additionally, research has underscored the anti‐ulcerogenic activity of polyphenol‐rich extract of clove buds, showcasing their potential in alleviating gastrointestinal disorders and ulcers (Issac et al. 2015). Furthermore, S. aromaticum has exhibited promising capabilities in modulating inflammatory pathways, antisecretory effects, and bolstering the body's antioxidative defenses, indicative of its potential as a therapeutic agent for a spectrum of ailments (Barboza et al. 2018; Okasha 2007).
4.1.49. Trigonella foenum‐graecum
Trigonella foenum‐graecum, commonly known as fenugreek, exhibits therapeutic effects on gastric ulcers (Jainu and Devi 2004b). Studies have shown that the aqueous and ethanolic extracts of Trigonella foenum‐graecum seeds significantly protect against ethanol‐induced gastric ulcers in rats, demonstrating gastroprotective properties comparable to omeprazole (Selmi et al. 2022). These extracts have been found to improve the condition of the gastric mucosa, enhance antioxidant enzyme activities like SOD and CAT, and reduce oxidative stress markers such as MDA and H2O2, thereby aiding in the prevention of gastric damage induced by harmful agents like ethanol (Afroz, Rahman, and Kamal 2017). Additionally, some studies have suggested that fenugreek may have mucoprotective effects on the gastric mucosa, helping to enhance the protective barrier and reduce the risk of ulcer formation. These effects could be attributed to the presence of polysaccharides and other bioactive compounds in fenugreek seeds (Bhat, Sharma, and Singh 2018).
4.1.50. Curcuma longa
Turmeric, derived from the rhizome of C. longa belonging to the Zingiberaceae family, has a long history of use in both Ayurvedic and Chinese medicinal practices for the treatment of gastric ulcers (Akaberi, Sahebkar, and Emami 2021). Within turmeric, the active compound curcumin has garnered attention for its remarkable gastroprotective properties. Studies have demonstrated its effectiveness against various ulcer‐inducing agents such as indomethacin, ethanol, stress, and pylorus ligation in rat models. Curcumin exerts its protective effects through multiple mechanisms including antioxidant activity, inhibition of gastric acid secretion, mucosal protection, and anti‐H. pylori activity (against H. pylori) (Mohammadi et al. 2022).
Additionally, curcumin has been found to work synergistically with conventional treatments such as omeprazole and probiotics like Lactobacillus acidophilus to enhance its anti‐ulcer effects. Despite its potential benefits, curcumin's poor bioavailability poses a challenge to its therapeutic application. Ongoing research focuses on developing innovative drug delivery systems to improve curcumin's bioavailability, thereby maximizing its efficacy as an anti‐ulcer agent (Beiranvand 2022).
4.1.51. Vitis vinifera L
V. vinifera commonly known as grapevine, is widely cultivated for its fruit. Vitis vinifera L. (Vitaceae) is one of the largest fruit crops in the world is a natural medicinal plant used since antiquity in traditional folk medicine in many countries in the world, including countries of northern Africa (Saadaoui et al. 2020). The leaves of V. vinifera L have been utilized traditionally in North Africa for the treatment of various gastrointestinal disorders, including stomach ulcers. it has been demonstrated that gastroprotective mechanisms are based on the ability to rapidly strengthen the main defensive mediators against gastric lesions which also can play a pivotal role in regulating gastric secretion and motility (Zhao et al. 2015). These gastroprotective mechanisms participate in gastric defense by inhibiting gastric acid secretion, stimulating the release of mucus and bicarbonate, and increasing blood flow on gastric mucosal (Matsuda et al. 2002; Wallace and Miller 2000). In a study by Saadaoui et al. (2020), acute pre‐treatment of rats with the grapevine leaves extracts or with omeprazole induced a significant reduction in the volume and acidity of gastric juice as well as the ulcer index. This gastroprotection seems to be related to, at least in part, antioxidant potential and also anti‐secretory acid effects. In addition, the presence of high rates of bioactive compounds certainly contributes to the installation of these activities such as organic acids, carotenoids, lipids, enzymes, vitamins, terpenes, reducing or non‐reducing sugars, resveratrol, other stilbene derivatives, phenolics acids, flavonoids including flavonols, anthocyanins, and proanthocyanidins (Bombardelli and Morazzoni 1995; Felicio, Santos, and Gonçalez 2001; Şendoğdu et al. 2006).
4.1.52. Zingiber Officinale
In North Africa, Z. officinale, commonly known as ginger, has garnered attention for its potential to treat peptic ulcer diseases, which are prevalent gastrointestinal ailments in the region. Traditional medicinal practices often incorporate herbs like ginger due to its perceived health benefits (Saiah et al. 2018). Studies suggest that ginger may possess anti‐inflammatory and gastroprotective properties, which could alleviate symptoms and aid in the healing of ulcers in the stomach and duodenum. Research conducted in North Africa has shown promising results, demonstrating ginger's ability to reduce ulcer formation and alleviate associated symptoms in animal models. However, further well‐designed clinical trials involving human participants are needed to confirm its efficacy in managing peptic ulcer diseases in the region. Additionally, exploring the mechanisms of action and determining optimal dosage regimens would provide valuable insights for its therapeutic use in this context (Bereksi et al. 2018; Zaghlool et al. 2015).
4.1.53. Ziziphus lotus L.
Z. lotus L., also known as lotus jujube or lote tree, is a plant species belonging to the genus Ziziphus. Some related species within the Ziziphus genus have been studied for their potential gastroprotective effects (Bakhtaoui, Lakmichi, Megraud et al. 2014). It has many therapeutic actions, anti‐inflammatory activity by compounds found in Ziziphus species, such as flavonoids and triterpenoids, possess anti‐inflammatory properties. These compounds may help reduce inflammation in the gastrointestinal tract and protect the gastric mucosa from damage. Further, Ziziphus species contain antioxidants that help scavenge free radicals and reduce oxidative stress. By protecting against oxidative damage, these antioxidants may contribute to the maintenance of gastric health and the prevention of gastric ulcers. Research indicates that Ziziphus extracts may exhibit anti‐ulcer activity by reducing gastric acid secretion, inhibiting the activity of enzymes involved in ulcer formation, and promoting the healing of gastric ulcers. These effects contribute to the overall gastroprotective properties of Ziziphus species (Wahida, Abderrahman, and Nabil 2007).
5. Role of Plant‐Derived Bioactive Compounds in the Management of Peptic Ulcers
Peptic ulcers, encompassing both gastric and duodenal ulcers, manifest as erosions in the lining of the gastrointestinal tract and are commonly associated with factors like H. pylori infection, the use of nonsteroidal anti‐inflammatory drugs (NSAIDs), alcohol intake, smoking, and stress (Lanas and Chan 2017). Traditional treatment approaches for peptic ulcers mainly revolve around suppressing acid, eradicating H. pylori, and safeguarding the mucosa. However, these methods sometimes fall short, prompting a growing interest in complementary or alternative therapies, notably those involving plant‐derived bioactive compounds (Boakye‐Yiadom et al. 2021).
The role of plant‐derived bioactive compounds can be delineated into several key aspects.
5.1. Anti‐Inflammatory Properties
Plant‐derived bioactive compounds, including flavonoids, curcumin, and polyphenols, exhibit notable anti‐inflammatory properties. These compounds have been extensively studied for their ability to modulate the inflammatory response by targeting various pathways involved in inflammation. One of the primary mechanisms through which they exert their anti‐inflammatory effects is by inhibiting the synthesis of pro‐inflammatory mediators such as cytokines and prostaglandins (Prayoga et al. 2024).
Flavonoids, which are abundantly found in fruits, vegetables, and herbs, possess strong anti‐inflammatory activity. They interfere with the signaling pathways that lead to the production of inflammatory cytokines, such as tumor necrosis factor‐alpha (TNF‐α) and interleukins (IL), thereby attenuating the inflammatory response in the gastrointestinal mucosa. Additionally, flavonoids inhibit the activity of enzymes involved in the synthesis of prostaglandins, such as cyclooxygenase (COX), which further contributes to their anti‐ulcerogenic effects (Kononenko, Mirzaliev, and Ostapets 2018; Zhang et al. 2020).
Curcumin, a bioactive compound derived from turmeric, is renowned for its potent anti‐inflammatory properties. It exerts its effects by modulating multiple molecular targets involved in inflammation, including transcription factors, cytokines, and enzymes. Curcumin inhibits the activation of nuclear factor‐kappa B (NF‐κB), a key regulator of inflammation, and downregulates the expression of pro‐inflammatory cytokines like TNF‐α, IL‐1β, and IL‐6. Moreover, curcumin suppresses the activity of COX and lipoxygenase (LOX), enzymes responsible for the synthesis of prostaglandins and leukotrienes, respectively (Akaberi, Sahebkar, and Emami 2021; Mohammadi et al. 2022).
Polyphenols, another class of plant‐derived compounds found in foods such as green tea, berries, and nuts, possess potent anti‐inflammatory properties. They exert their effects by modulating various signaling pathways involved in inflammation, including the mitogen‐activated protein kinase (MAPK) pathway and the NF‐κB pathway. Polyphenols inhibit the activation of inflammatory transcription factors, leading to reduced expression of pro‐inflammatory genes. Additionally, they enhance the activity of endogenous antioxidant enzymes, thereby mitigating oxidative stress‐induced inflammation (Hemmami et al. 2023).
By targeting multiple pathways involved in inflammation, plant‐derived bioactive compounds effectively attenuate the inflammatory response within the gastrointestinal mucosa. This anti‐inflammatory activity plays a crucial role in facilitating the healing of peptic ulcers by reducing mucosal inflammation and promoting tissue repair. Moreover, by alleviating inflammation, these compounds also help alleviate symptoms associated with peptic ulcers, such as pain and discomfort, thereby improving the overall quality of life for affected individuals (Gündoğdu, Özbayer, and Kar 2023; Silvan et al. 2021).
5.2. Antioxidant Capacity
Oxidative stress is a key contributor to the pathogenesis of peptic ulcers, leading to mucosal damage and impaired healing. Reactive oxygen species (ROS) and free radicals generated during oxidative stress can cause oxidative damage to biomolecules such as lipids, proteins, and DNA, resulting in cellular dysfunction and tissue injury. In the gastrointestinal tract, oxidative stress disrupts the delicate balance between mucosal defense mechanisms and harmful factors, predisposing the mucosa to ulcer formation and delaying the healing process (Gündoğdu, Özbayer, and Kar 2023).
Plant‐derived antioxidants, including flavonoids, phenolic acids, and carotenoids, play a crucial role in combating oxidative stress and promoting ulcer recovery. These bioactive compounds possess the ability to neutralize free radicals and reactive oxygen species, thereby preventing oxidative damage to cellular components. Additionally, they reinforce cellular antioxidant mechanisms by enhancing the activity of endogenous antioxidant enzymes such as superoxide dismutase (SOD), catalase, and glutathione peroxidase (Silvan et al. 2021).
Flavonoids, abundant in various fruits, vegetables, and beverages such as tea and red wine, are potent antioxidants that scavenge free radicals and inhibit oxidative stress‐induced cellular damage. These compounds exert their antioxidant effects through multiple mechanisms, including direct radical scavenging, metal chelation, and modulation of antioxidant enzyme activity (Silvan et al. 2021; Zhang et al. 2020).
Phenolic acids, found in foods such as berries, nuts, and whole grains, also exhibit strong antioxidant properties. They effectively neutralize free radicals and inhibit lipid peroxidation, a process that contributes to mucosal damage and ulcer formation. Additionally, phenolic acids enhance the activity of endogenous antioxidant enzymes, further bolstering the cellular defense against oxidative stress (Alimi et al. 2023; Geetha and Anitha 2022).
Carotenoids, pigments present in colorful fruits and vegetables, act as potent antioxidants by scavenging free radicals and singlet oxygen species. These compounds help protect the gastric mucosa from oxidative harm and promote ulcer recovery by preventing lipid peroxidation and preserving cellular integrity (Pol et al. 2023).
By scavenging free radicals and bolstering cellular antioxidant defenses, plant‐derived antioxidants shield the gastric mucosa from oxidative damage and facilitate ulcer recovery. Their ability to counteract oxidative stress represents a promising therapeutic approach for the management of peptic ulcers, offering potential benefits in terms of both prevention and treatment. Incorporating antioxidant‐rich foods or supplements into the diet may help reduce oxidative stress and promote mucosal healing in individuals with peptic ulcers (Gündoğdu, Özbayer, and Kar 2023).
5.3. Cytoprotective Attributes
Certain mucilaginous compounds found in specific plants, notably licorice, and marshmallow, exhibit notable cytoprotective attributes. These compounds, known for their gel‐like consistency when exposed to water, play a crucial role in forming a protective barrier over the gastric mucosa (Maqbool et al. 2023; Zaghlool et al. 2015).
This protective barrier acts as a physical shield, effectively shielding the gastric mucosa from corrosive agents such as gastric acid and pepsin. By preventing direct contact between these aggressive factors and the delicate mucosal lining, mucilaginous compounds mitigate mucosal injury and reduce the risk of ulcer development (Zakir et al. 2022).
Licorice, for instance, contains glycyrrhizin and glycyrrhetinic acid, compounds known for their mucoprotective properties. These substances form a viscous coating over the gastric mucosa, providing a barrier against gastric acid and enhancing mucosal integrity. Similarly, marshmallows contain mucilage, a gel‐forming substance that adheres to the mucosal surface, forming a protective layer that helps prevent ulcer formation and promotes healing (Maqbool et al. 2023).
The cytoprotective effects of these mucilaginous compounds are particularly beneficial in conditions where mucosal integrity is compromised, such as in peptic ulcers. By bolstering the natural defense mechanisms of the gastric mucosa, these compounds contribute to the maintenance of mucosal health and facilitate ulcer healing. Overall, the cytoprotective attributes of mucilaginous compounds derived from plants like licorice and marshmallow represent a promising therapeutic approach for the management of peptic ulcers. By forming a protective barrier over the gastric mucosa, these compounds help prevent mucosal injury and promote ulcer healing, offering potential benefits in the prevention and treatment of peptic ulcer disease (Asnaashari, Dastmalchi, and Javadzadeh 2018).
5.4. Antimicrobial Potential
Several plant‐derived compounds demonstrate potent antimicrobial properties, specifically targeting H. pylori, a major contributor to peptic ulceration. Compounds like berberine and allicin have garnered attention for their effectiveness in inhibiting the growth of H. pylori and are considered potential adjunctive therapies in eradication protocols for this bacterium (Majumdar and Looi 2024).
Berberine, a naturally occurring alkaloid found in various medicinal plants such as goldenseal and barberry, exhibits significant antimicrobial activity against H. pylori. Berberine disrupts crucial microbial processes within H. pylori cells, including interference with cell wall synthesis and inhibition of essential enzymes, ultimately leading to growth inhibition and bactericidal effects (Imenshahidi and Hosseinzadeh 2019).
Allicin, a sulfur‐containing compound derived from garlic, is another potent antimicrobial agent effective against H. pylori. Allicin exerts its antimicrobial effects by disrupting multiple cellular processes in H. pylori, including inhibition of key enzymes involved in energy metabolism and cellular respiration. This disruption impairs bacterial growth and viability, making allicin a promising candidate for adjunctive therapy in H. pylori eradication regimens (Shang et al. 2019).
By targeting H. pylori, these plant‐derived compounds offer a complementary approach to conventional antibiotic therapy in the management of peptic ulcers associated with H. pylori infection. Incorporating these compounds into treatment protocols may enhance the efficacy of H. pylori eradication and reduce the risk of ulcer recurrence, thereby improving clinical outcomes for affected individuals (Harsha et al. 2017; Hemmami et al. 2023).
5.5. Facilitation of Gastric Mucosal Healing
Plant‐derived compounds such as epigallocatechin gallate (EGCG) play a pivotal role in facilitating the healing process of the gastric mucosa. EGCG, a polyphenol abundant in green tea, possesses remarkable properties that stimulate reparative mechanisms within the gastric mucosa, thereby expediting ulcer healing and reducing the likelihood of recurrent ulcers. One of the primary mechanisms through which EGCG facilitates gastric mucosal healing is by augmenting the proliferation of epithelial cells. Epithelial cells play a crucial role in maintaining the integrity of the gastric mucosa and are essential for the repair of damaged tissue. EGCG promotes the proliferation of these cells, accelerating the regeneration of the mucosal lining and facilitating the closure of ulcerative lesions (Ahmed, Nedi, and Yimer 2022; Zhang et al. 2020).
In addition to enhancing epithelial cell proliferation, EGCG also fosters angiogenesis, the process of new blood vessel formation. Angiogenesis is vital for supplying oxygen and nutrients to the site of injury, facilitating tissue repair and regeneration. By promoting angiogenesis, EGCG enhances the blood supply to the ulcerated area, thereby facilitating the delivery of essential nutrients and growth factors necessary for mucosal healing (Zhang et al. 2020).
Furthermore, EGCG possesses anti‐inflammatory and antioxidant properties, which contribute to its ability to promote gastric mucosal healing. By reducing inflammation and oxidative stress within the gastric mucosa, EGCG creates a favorable environment for tissue repair and regeneration, thereby expediting the healing process. Overall, plant‐derived compounds like EGCG play a crucial role in facilitating gastric mucosal healing by promoting epithelial cell proliferation, fostering angiogenesis, and mitigating inflammation and oxidative stress. By accelerating ulcer healing and restoring the structural integrity of the gastric mucosa, these compounds offer promising therapeutic benefits in the management of peptic ulcers, reducing the likelihood of ulcer recurrence and improving clinical outcomes for affected individuals (Rodríguez‐Negrete et al. 2024).
5.6. Acid Secretion Reduction
Certain plant‐derived compounds, such as deglycyrrhizinated licorice (DGL), play a crucial role in reducing gastric acid secretion, thereby alleviating symptoms associated with peptic ulcers and creating an environment conducive to mucosal healing. DGL, a form of licorice from which the compound glycyrrhizin has been removed, exerts its effects by modulating the activity of proton pumps in the stomach (Patel and Khetani 2021; Singh et al. 2023).
Proton pumps are responsible for the secretion of hydrochloric acid into the gastric lumen, contributing to the acidic environment necessary for digestion. By modulating the activity of these proton pumps, DGL effectively reduces the production of gastric acid, leading to decreased acidity within the stomach. The reduction in gastric acid secretion achieved by DGL offers several benefits in the management of peptic ulcers. Firstly, it helps alleviate symptoms such as abdominal pain, heartburn, and indigestion, which are often exacerbated by excessive gastric acid secretion. By lowering acid levels, DGL provides symptomatic relief, improving the overall quality of life for individuals with peptic ulcers. Moreover, the reduction in gastric acid secretion creates a more favorable environment for mucosal healing. Excessive gastric acid can exacerbate mucosal injury and delay the healing process by further damaging the already compromised gastric mucosa. By lowering acid levels, DGL helps mitigate mucosal damage and promotes ulcer healing, facilitating the restoration of mucosal integrity (El‐Saber Batiha et al. 2020; Singh et al. 2023).
Overall, specific plant‐derived compounds like DGL offer a targeted approach to reducing gastric acid secretion, providing symptomatic relief, and fostering an environment conducive to mucosal healing in individuals with peptic ulcers. By modulating proton pump activity, DGL represents a valuable adjunctive therapy in the management of peptic ulcers, offering benefits in both symptom management and ulcer healing.
The table of the North African medicinal plants indicates a significant prominence of families such as Fabaceae, Asteraceae, and Lamiaceae, which are frequently utilized for their gastroprotective properties. Notable compounds including flavonoids (e.g., quercetin and kaempferol), tannins, alkaloids, and essential oils are commonly identified, recognized for their anti‐inflammatory, antioxidant, and antimicrobial effects, all contributing to the treatment of peptic ulcers. The most utilized plant parts are the aerial parts, leaves, and roots, reflecting their high concentration of bioactive compounds.
6. Mechanisms of Action
Gastric ulcers are commonly associated with H. pylori bacteria or overuse of nonsteroidal anti‐inflammatory drugs (NSAIDs) (Usta and Urganci 2015). Botanical compounds have demonstrated therapeutic potential in treating this gastrointestinal condition (Boakye‐Yiadom et al. 2021). Multiple plant‐derived metabolites including flavonoids, tannins, and terpenoids can modulate gastric acid output, reinforce the gastric mucosal barrier, and suppress H. pylori proliferation (Guevara and Cogdill 2020; Miri et al. 2022). These phytochemicals owe their gastroprotective effects to several mechanisms: inhibition of gastric acid secretion by blocking H+/K+‐ATPase action (Sachs et al. 1995), stimulation of mucus and bicarbonate output to reinforce the gastric mucus‐bicarbonate barrier (Zatorski 2017), elimination of H. pylori via antibacterial action (Guevara and Cogdill 2020; Miri et al. 2022), antioxidative action to counter inflammation and oxidative damage (Gündoğdu, Özbayer, and Kar 2023; Zaghlool et al. 2019), and enhancement of endogenous anti‐ulcer prostaglandins and nitric oxide while inhibiting pro‐ulcer leukotrienes and cytokines (Sánchez‐Mendoza et al. 2010). Through one or more of these mechanisms, plant bioactive can decrease ulcer index and increase curative ratio in chemically induced ulcer animal models (Yoo et al. 2022). The multi‐target synergistic effects of the phytochemical mixtures in medicinal plant extracts seemingly underlie their efficacy in protecting against gastric ulceration as well as enhancing healing of existing ulcers. Further pharmacodynamic and preclinical research is warranted to elucidate the most potent anti‐ulcer plant extracts and compounds for standardized botanical therapies (Yoo et al. 2022) (Figure 2).
FIGURE 2.
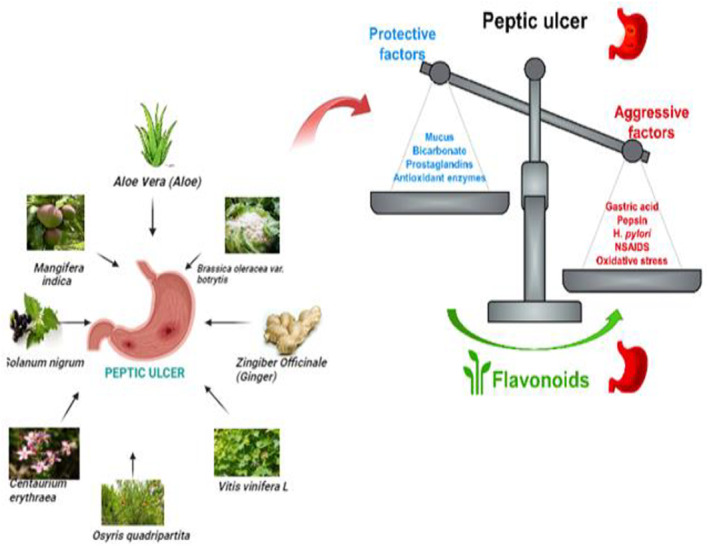
A figure summarizing how the mentioned medicinal plants affect peptic ulcers.
7. Future Perspectives
The promising findings on North African medicinal plants for peptic ulcer treatment pave the way for exciting developments in both research and practical applications. Future studies should prioritize rigorous randomized controlled trials to establish the efficacy and safety of key plant extracts such as Glycyrrhiza glabra, Matricaria chamomilla, and Nigella sativa in human populations. Optimizing dosages, exploring potential synergies between plant compounds, and developing standardized extraction methods are crucial next steps. For instance, combining the antioxidant properties of Olea europaea with the mucoprotective effects of Aloe vera could yield enhanced therapeutic benefits. Long‐term studies on traditionally consumed plants like Phoenix dactylifera and Opuntia ficus‐indica may reveal their potential in ulcer prevention. Translating this research into practical applications could lead to a variety of product forms, including standardized herbal extracts in capsules, therapeutic teas blending multiple gastroprotective plants, topical gels containing Aloe vera or olive leaf extracts, and functional foods fortified with pomegranate or date palm extracts. The development of such products, alongside continued research, holds promise for more effective, natural approaches to managing peptic ulcers, potentially improving quality of life for millions affected by this condition.
8. Conclusion
In conclusion, this review highlights the gastroprotective efficacy and therapeutic potential of 54 North African medicinal plants for treating peptic ulcers. Notable plants such as licorice (Glycyrrhiza glabra), chamomile (Matricaria chamomilla), olive (Olea europaea), pomegranate (Punica granatum), Aloe vera, and black seed (Nigella sativa) demonstrated significant anti‐ulcer effects in preclinical studies, attributed to their bioactive compounds, including flavonoids, tannins, and terpenoids. These phytochemicals exhibit gastroprotective properties through various mechanisms, such as enhancing the gastric mucosal barrier, inhibiting acid secretion, displaying antioxidant and anti‐inflammatory effects, promoting ulcer healing, and combating Helicobacter pylori infection. The reviewed studies provide valuable insights into the potential of these medicinal plants as complementary or alternative therapies for peptic ulcer disease. However, it is important to acknowledge the limitations of the current evidence, which primarily consists of in vitro and animal studies, with limited human clinical trials. Additionally, variations in plant extraction methods, dosages, and study designs may impact the comparability and reproducibility of the findings. Future research should focus on conducting well‐designed, randomized, placebo‐controlled human trials to establish the safety and efficacy of these medicinal plants in preventing and treating peptic ulcers. Furthermore, investigations into the optimal dosage, formulation, and potential drug interactions are necessary to develop standardized therapeutic protocols. Elucidating the precise mechanisms of action and identifying the key bioactive compounds responsible for the gastroprotective effects will also aid in the development of targeted, evidence‐based interventions. While this review comprehensively covers the gastroprotective effects of North African medicinal plants, it may be beneficial to consider the potential for developing multi‐herb formulations that leverage synergistic effects between different plant compounds. Many traditional medicine systems utilize complex herbal formulas rather than single herbs. Given the diverse mechanisms of action highlighted for various plants in this review (antioxidant, anti‐inflammatory, mucus‐protective, etc.), there may be opportunities to create more potent and multi‐targeted gastroprotective preparations by strategically combining complementary herbs. This approach could potentially enhance efficacy while allowing for lower doses of individual herbs, potentially reducing side effects. Additionally, such formulations may better address the multifactorial nature of peptic ulcer disease. Overall, this review underscores the promising role of North African medicinal plants in the management of peptic ulcers and highlights the need for continued research to bridge the gap between traditional knowledge and modern therapeutic applications.
Author Contributions
Nezar Cherrada: conceptualization (equal), formal analysis (equal), project administration (equal). Ahmed Elkhalifa Chemsa: resources (equal). Noura Gheraissa: validation (equal). Ibtissam Laib: investigation (equal). Zakia Gueboudji: software (equal). Mohamed EL‐Shazly: investigation (equal). Abdelmalek Zaater: validation (equal). Asma Abid: formal analysis (equal). Sherouk Hussein Sweilam: formal analysis (equal). Talha Bin Emran: visualization (equal). Sadok Nani: formal analysis (equal). Bilal Benamor: resources (equal). Djilani Ghemam Amara: resources (equal). Ayomide Victor Atoki: resources (equal). Mohammed Messaoudi: resources (equal).
Ethics Statement
The authors have nothing to report.
Conflicts of Interest
The authors declare no conflicts of interest.
Acknowledgments
The authors wish to express their profound gratitude to Mr. Cherrada Djilani and Mrs. Hammi Meriam, for their invaluable guidance and unwavering support throughout this research.
Funding: The authors received no specific funding for this work.
Data Availability Statement
The data that support the findings of this study are included in the article. This encompasses all the original data generated during the research and any secondary data utilized in the analyses. For further inquiries or detailed information, the corresponding author can be contacted.
References
- Abdallah, I. , Salem I., and El‐Salam N.. 2017. “Evaluation of Antidiabetic and Antioxidant Activity of Aegle marmelos L. Correa Fruit Extract in the Diabetic Rats.” Egyptian Journal of Hospital Medicine 67: 731–741. 10.12816/0037829. [DOI] [Google Scholar]
- Abdel Jaleel, G. A. R. , Abdallah H. M. I., and Gomaa N. E. L. S.. 2016. “Pharmacological Effects of Ethanol Extract of Egyptian Artemisia Herba‐Alba in Rats and Mice.” Asian Pacific Journal of Tropical Biomedicine 6, no. 1: 44–49. 10.1016/j.apjtb.2015.10.005. [DOI] [Google Scholar]
- Abdellaziz, S. , Hichem S., Kaïs R., et al. 2014. “Effects of Dates Pulp Extract and Palm Sap (Phoenix dactylifera L.) on Gastrointestinal Transit Activity in Healthy Rats.” Journal of Medicinal Food 17, no. 7: 782–786. 10.1089/jmf.2013.0112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdullahi, L. , and Chinedu O.. 2022. “Effect of Date (Phoenix dactylifera L.) on Stimulated Gastric Acid Secretion in Wistar Rats.” Annals of Basic and Medical Sciences 3: 313–317. 10.51658/ABMS.202231.13. [DOI] [Google Scholar]
- Abebaw, M. , Mishra B., and Gelayee D. A.. 2017. “Evaluation of Anti‐Ulcer Activity of the Leaf Extract of Osyris Quadripartita Decne (Santalaceae) in Rats.” Journal of Experimental Pharmacology 9: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abushwereb, H. 2016. “Gastroprotective Activity of Artemisia herba‐alba Aqueous Extract on Aspirin‐Induced Gastric Lesions in Albino Rats.” Journal of Pharmaceutical and Applied Chemistry 3: 1–11. 10.18576/jpac/020303. [DOI] [Google Scholar]
- Adom, M. B. , Taher M., Mutalabisin M. F., et al. 2017. “Chemical Constituents and Medical Benefits of Plantago major .” Biomedicine & Pharmacotherapy 96: 348–360. 10.1016/j.biopha.2017.09.152. [DOI] [PubMed] [Google Scholar]
- Afroz, R. , Rahman K., Kamal A. M., et al. 2017. “The Gastro Protective Effect of Trigonella foenum graecum Seed (Methi) and Omeprazole in Experimentally Induced Gastric Ulcer in Rats.” Journal of Dhaka Medical College 26, no. 2: 126–131. [Google Scholar]
- Ahmed, O. , Nedi T., and Yimer E. M.. 2022. “Evaluation of Anti‐Gastric Ulcer Activity of Aqueous and 80% Methanol Leaf Extracts of Urtica Simensis in Rats.” Metabolism Open 14: 100172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akaberi, M. , Sahebkar A., and Emami S. A.. 2021. “Turmeric and Curcumin: From Traditional to Modern Medicine.” Advances in Experimental Medicine and Biology 1291: 15–39. 10.1007/978-3-030-56153-6_2. [DOI] [PubMed] [Google Scholar]
- Akhtar, M. S. , and Munir M.. 1989. “Evaluation Op the Gastric Antiulcerogenic Effects of Solanum nigrum, Brassica oleracea and Ocimum basilicum in Rats.” Journal of Ethnopharmacology 27, no. 1–2: 163–176. [DOI] [PubMed] [Google Scholar]
- Alam, M. , Bhat S. A., Rather S. A., et al. 2023. “A Comprehensive Review of Nigella sativa (Kalonji) From the Unani Perspective.” Journal of Drug Delivery and Therapeutics 13, no. 10: 121–128. [Google Scholar]
- Al‐Habbal, M. J. , Al‐Habbal Z., and Huwez F. U.. 1984. “A Double‐Blind Controlled Clinical Trial of Mastic and Placebo in the Treatment of Duodenal Ulcer.” Clinical and Experimental Pharmacology and Physiology 11, no. 5: 541–544. [Record #1241 is using a reference type undefined in this output style]. [DOI] [PubMed] [Google Scholar]
- Al‐Howiriny, T. , Alsheikh A., Alqasoumi S., Al‐Yahya M., Eltahir K., and Rafatullah S.. 2009. “Protective Effect of Origanum majorana L. ‘Marjoram’ on Various Models of Gastric Mucosal Injury in Rats.” American Journal of Chinese Medicine 37, no. 3: 531–545. [DOI] [PubMed] [Google Scholar]
- Ali, M. J. B. , Guesmi F., Harrath A. H., et al. 2015. “Investigation of Antiulcer and Antioxidant Activity of Juniperus phoenicea L. (1753) Essential Oil in an Experimental Rat Model.” Biological and Pharmaceutical Bulletin 38, no. 11: 1738–1746. [DOI] [PubMed] [Google Scholar]
- Alimi, H. , Mabrouk F. H., Zouari N., Sakly M., and Rhouma K. B.. 2023. “Lc–Esi–Ms. Phenolic Contents Assessment, Antioxidant, and Protective Ability of Punica granatum Root Bark Extract Against Ethanol‐Induced Gastric Ulcer in Rats: In Silico H+, K+‐Atpase Inhibitory Pathway Study.” Toxicology Research 12, no. 2: 189–200. 10.1093/toxres/tfad006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alipour, G. , Dashti S., and Hosseinzadeh H.. 2014. “Review of Pharmacological Effects of Myrtus communis L. And Its Active Constituents.” Phytotherapy Research 28, no. 8: 1125–1136. [DOI] [PubMed] [Google Scholar]
- Allen, A. , Cunliffe W., Pearson J., Sellers L., and Ward R.. 1984. “Studies on Gastrointestinal Mucus.” Scandinavian Journal of Gastroenterology. Supplement 93: 101–113. [PubMed] [Google Scholar]
- Alsabri, S. G. , Ebsaim M. A., Mohamed S. S., et al. 2014. “Antiulcer Property of Libyan Helianthemum kahiricum Plant.” Journal of Pharmacognosy and Phytochemistry 2, no. 5: 80–84. [Google Scholar]
- Alsabri, S. G. , Rmeli N. B., Zetrini A. A., et al. 2013. “Phytochemical, Anti‐Oxidant, Anti‐Microbial, Anti‐Inflammatory, and Anti‐Ulcer Properties of Helianthemum lippii .” Journal of Pharmacognosy and Phytochemistry 2, no. 2: 86–96. [Google Scholar]
- Al‐Shahib, W. , and Marshall R. J.. 2003. “The Fruit of the Date Palm: Its Possible Use as the Best Food for the Future?” International Journal of Food Sciences and Nutrition 54, no. 4: 247–259. 10.1080/09637480120091982. [DOI] [PubMed] [Google Scholar]
- Al‐Snafi, A. E. 2018. “Medical Importance of Juniperus communis‐a Review.” Indo American Journal Of Pharmaceutical Sciences 5, no. 3: 1779–1792. [Google Scholar]
- Altaf, S. , Abbas R. Z., Akhtar T., et al. 2023. “Antioxidant Rich Medicinal Plants as a Potential Candidate to Treat Gastric Ulcer.” Boletin Latinoamericano Y Del Caribe De Plantas Medicinales Y Aromáticas 22, no. 5: 560–580. [Google Scholar]
- Alves‐Silva, J. M. , Romane A., Efferth T., and Salgueiro L.. 2017. “North African Medicinal Plants Traditionally Used in Cancer Therapy.” Frontiers in Pharmacology 8: 383. 10.3389/fphar.2017.00383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al‐Zoreky, N. S. , and Al‐Taher A. Y.. 2019. “In Vitro and In Situ Inhibition of Some Food‐Borne Pathogens by Essential Oils From Date Palm (Phoenix dactylifera L.) Spathe.” International Journal of Food Microbiology 299: 64–70. 10.1016/j.ijfoodmicro.2019.03.018. [DOI] [PubMed] [Google Scholar]
- Amessis‐Ouchemoukh, N. , Abu‐Reidah I. M., Quirantes‐Piné R., et al. 2014. “Tentative Characterisation of Iridoids, Phenylethanoid Glycosides and Flavonoid Derivatives From Globularia alypum L.(Globulariaceae) Leaves by Lc‐Esi‐Qtof‐Ms.” Phytochemical Analysis 25, no. 5: 389–398. [DOI] [PubMed] [Google Scholar]
- Andreo, M. A. , Ballesteros K. V. R., Hiruma‐Lima C. A., Da Rocha L. R. M., Brito A. R. M. S., and Vilegas W.. 2006. “Effect of Mouriri Pusa Extracts on Experimentally Induced Gastric Lesions in Rodents: Role of Endogenous Sulfhydryls Compounds and Nitric Oxide in Gastroprotection.” Journal of Ethnopharmacology 107, no. 3: 431–441. [DOI] [PubMed] [Google Scholar]
- Anwar, F. , Abbas A., Mehmood T., Gilani A. H., and Rehman N. U.. 2019. “Mentha: A Genus Rich in Vital Nutra‐Pharmaceuticals‐a Review.” Phytotherapy Research 33, no. 10: 2548–2570. 10.1002/ptr.6423. [DOI] [PubMed] [Google Scholar]
- Asnaashari, S. , Dastmalchi S., and Javadzadeh Y.. 2018. “Gastroprotective Effects of Herbal Medicines (Roots).” International Journal of Food Properties 21, no. 1: 902–920. [Google Scholar]
- Atta, A. , Nasr S. M., and Mouneir S. M.. 2005. “Antiulcerogenic Effect of Some Plants Extracts.”
- Ayoola, M. , Akinlolu A., Adeboga S., and Otulana J.. 2016. “Gastroprotective Activities of the N‐Hexane Fraction of Heliotropium Indicum on Gastric Ulceration.” Nigerian Journal of Basic and Applied Sciences 24, no. 1: 7–13. [Google Scholar]
- Babu, D. , Akila C., Kumar S., and Vinaya B.. 2020. “Anti‐Ulcer Mechanism of the Aqueous Extract of Osyris quadripartite .” International Journal of Research in Phytochemistry and Pharmacology 10, no. 4: 71–74. [Google Scholar]
- Bahadeen Aref, B. , Fakhri S., Morovati M. R., et al. 2023. “Evaluating the Protective Effects of Aqueous and Hydroalcoholic Extracts of Plantago major Leaf in a Rat Model of Ethanol‐Induced Peptic Ulcer.” Journal of Medicinal Plants and By‐Products 13, no. 3: 605–613. 10.22034/jmpb.2023.362108.1548. [DOI] [Google Scholar]
- Bais, S. , Gill N. S., Rana N., and Shandil S.. 2014. “A Phytopharmacological Review on a Medicinal Plant: Juniperus communis .” International Scholarly Research Notices 2014: 634723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakhtaoui, F.‐Z. , Lakmichi H., Chait A., and Gadhi C. A.. 2014. “In Vivo Gastro‐Protective Effects of Five Moroccan Medicinal Plants Against Gastric Ulcer.” American Journal of Phytomedicine and Clinical Therapeutics 2: 1262–1276. [Google Scholar]
- Bakhtaoui, F.‐Z. , Lakmichi H., Megraud F., Chait A., and Gadhi C.‐E. A.. 2014. “Gastro‐Protective, Anti‐Helicobacter Pylori and, Antioxidant Properties of Moroccan Zizyphus lotus L.” Journal of Applied Pharmaceutical Science 4, no. 10: 81–87. [Google Scholar]
- Bandara, T. , Uluwaduge I., and Jansz E. R.. 2012. “Bioactivity of Cinnamon With Special Emphasis on Diabetes Mellitus: A Review.” International Journal of Food Sciences and Nutrition 63, no. 3: 380–386. 10.3109/09637486.2011.627849. [DOI] [PubMed] [Google Scholar]
- Barboza, J. N. , da Silva Maia Bezerra Filho C., Silva R. O., Medeiros J. V. R., and de Sousa D. P.. 2018. “An Overview on the Anti‐Inflammatory Potential and Antioxidant Profile of Eugenol.” Oxidative Medicine and Cellular Longevity 2018: 3957262. 10.1155/2018/3957262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batiha, G. E. , Alkazmi L. M., Wasef L. G., Beshbishy A. M., Nadwa E. H., and Rashwan E. K.. 2020. “ Syzygium aromaticum L. (Myrtaceae): Traditional Uses, Bioactive Chemical Constituents, Pharmacological and Toxicological Activities.” Biomolecules 10, no. 2: 119–128. 10.3390/biom10020202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beiranvand, M. 2022. “A Review of the Most Common In Vivo Models of Stomach Ulcers and Natural and Synthetic Anti‐Ulcer Compounds: A Comparative Systematic Study.” Phytomedicine Plus 2, no. 2: 100264. 10.1016/j.phyplu.2022.100264. [DOI] [Google Scholar]
- Beladi Mousavi, S. , Naghdifar S., and Rafieian‐Kopaei M.. 2016. “Treatment of Helicobacter pylori Infection by Herbal Drugs; a Review on Current Data.” Journal of Preventive Epidemiology 1, no. 1: e06. [Google Scholar]
- Ben Khedir, S. , Mzid M., Bardaa S., Moalla D., Sahnoun Z., and Rebai T.. 2016. “In Vivo Evaluation of the Anti‐Inflammatory Effect of Pistacia lentiscus Fruit Oil and Its Effects on Oxidative Stress.” Evidence‐based Complementary and Alternative Medicine 2016: 6108203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben Mansour, R. , Wasli H., Serairi‐Beji R., et al. 2022. “In Vivo Gastroprotective Effect and Biological Potentialities of Six Tunisian Medicinal Plants Using Multivariate Data Treatment.” Plant Biosystems‐An International Journal Dealing With all Aspects of Plant Biology 156, no. 1: 152–163. [Google Scholar]
- Benarba, B. , Belabid L., Righi K., et al. 2015. “Ethnobotanical Study of Medicinal Plants Used by Traditional Healers in Mascara (North West of Algeria).” Journal of Ethnopharmacology 175: 626–637. 10.1016/j.jep.2015.09.030. [DOI] [PubMed] [Google Scholar]
- Benbott, A. , Karouche S., Mosbah C., Boukeria S., and Yahia A.. 2021. “Phytochemical, Free Radical Scavenging and Antimicrobial Activities of Glycyrrhiza glabra L. Rhizomes Collected From Algeria.” Journal of Biochemical Technology 12, no. 4: 78–83. 10.51847/0ShxsdgtLC. [DOI] [Google Scholar]
- Benkaci‐Ali, F. , Baaliouamer A., and Meklati B.. 2005. “Comparative Study of Chemical Composition of Nigella sativa Linn. Originated From Ouargla (Algeria) Extracted by Microwaves and Hydro‐Distillation.” Journal of Nature 17: 3–12. [Google Scholar]
- Benmansour, N. , Cherif H., El Hanballi F., and Akssira M.. 2020. “Study of the Biological Activities of the Seeds of the Plant Ceratonia siliqua/I L. Recovered in the Bejaia Region.” Medical Technologies Journal 4, no. 1: 520–521. [Google Scholar]
- Benmeziane‐Derradji, F. , and Aoun S.. 2022. “Characterization of Algerian Turmeric and Ginger Based on Their Physicochemical, Functional and Biological Properties: Characterisation of Algerian Turmeric and Ginger.” Journal of Spices and Aromatic Crops 31, no. 2: 143–156. 10.25081/josac.2022.v31.i2.7776. [DOI] [Google Scholar]
- Bentahar, A. , Khennouf S., Bouaziz A., et al. 2016. “Polyphenols Content and Antioxidant Activities of Selected Algerian Plants Used for Gastro‐Duodenal Ulcers.” Der Pharma Chemica 8, no. 12: 88–99. [Google Scholar]
- Bereksi, M. , Hassaine H., Bekhechi C., and Abdelouahid D.. 2018. “Evaluation of Antibacterial Activity of Some Medicinal Plants Extracts Commonly Used in Algerian Traditional Medicine Against Some Pathogenic Bacteria.” Pharmacognosy Journal 10: 507–512. 10.5530/pj.2018.3.83. [DOI] [Google Scholar]
- Bhat, G. A. , Sharma P., and Singh R.. 2018. “Therapeutic Effects of Trigonella foenum‐graecum Extract on Fasting Blood Glucose, Tissue Glycogen, Glycosylated Haemoglobin, Plasma Concentrations of Insulin and Glp‐1 in Healthy and Diabetic Subjects.” International Journal of Biological & Medical Research 9, no. 1: 6205–6211. [Google Scholar]
- Boakye‐Yiadom, M. , Kumadoh D., Adase E., and Woode E.. 2021. “Medicinal Plants with Prospective Benefits in the Management of Peptic Ulcer Diseases in Ghana.” BioMed Research International 2021, no. 1: 557404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bombardelli, E. , and Morazzoni P.. 1995. “Vitis vinifera L.”
- Borra, S. K. , Lagisetty R. K., and Mallela G. R.. 2011. “Anti‐Ulcer Effect of Aloe vera in Non‐Steroidal Anti‐Inflammatory Drug Induced Peptic Ulcers in Rats.” African Journal of Pharmacy and Pharmacology 5, no. 16: 1867–1871. [Google Scholar]
- Boutemak, K. , Safta B., and Ayachi N.. 2015. “Study of the Anti‐Inflammatory Activity of Flavonic Extract of Globularia alypum L.” Acta Physica Polonica A 128, no. 2B: B239–B240. [Google Scholar]
- Boutemine, I.‐M. , Amri M., Amir Z.‐C., et al. 2018. “Gastro‐Protective, Therapeutic and Anti‐Inflammatory Activities of Pistacia lentiscus L. Fatty Oil Against Ethanol‐Induced Gastric Ulcers in Rats.” Journal of Ethnopharmacology 224: 273–282. [DOI] [PubMed] [Google Scholar]
- Bouyahya, A. , Abrini J., Et‐Touys A., Bakri Y., and Dakka N.. 2017. “Indigenous Knowledge of the Use of Medicinal Plants in the North‐West of Morocco and Their Biological Activities.” European Journal of Integrative Medicine 13: 9–25. 10.1016/j.eujim.2017.06.004. [DOI] [Google Scholar]
- Bouyahya, A. , Jamal A., Edaoudi F., Et‐Touys A., Bakri Y., and Dakka N.. 2016. “ Origanum compactum Benth: A Review on Phytochemistry and Pharmacological Properties.” Medicinal and Aromatic Plants 5, no. 4: 252. [Google Scholar]
- Bouyahya, A. , Zengin G., Belmehdi O., et al. 2020. “ Origanum compactum Benth., From Traditional Use to Biotechnological Applications.” Journal of Food Biochemistry 44, no. 8: e13251. [DOI] [PubMed] [Google Scholar]
- Brahmi, F. , Khodir M., Mohamed C., and Pierre D.. 2017. “Chemical Composition and Biological Activities of Mentha Species.” Aromatic and Medicinal Plants‐Back to Nature 10: 47–79. [Google Scholar]
- Bukar, M. A. , Ishaya H. B., Dibal N. I., and Attah M. O.. 2017. “Gastroprotective Effect of Nigella sativa Seed on Ethanol‐Induced Gastric Ulcer in Rats.” Libyan Journal of Medical Sciences 1, no. 3: 63–67. [Google Scholar]
- Calder, P. C. 2001. “Polyunsaturated Fatty Acids, Inflammation, and Immunity.” Lipids 36, no. 9: 1007–1024. [DOI] [PubMed] [Google Scholar]
- Carvalho, A. C. S. , Guedes M. M., De Souza A. L., et al. 2007. “Gastroprotective Effect of Mangiferin, a Xanthonoid From Mangifera indica, Against Gastric Injury Induced by Ethanol and Indomethacin in Rodents.” Planta Medica 73, no. 13: 1372–1376. [DOI] [PubMed] [Google Scholar]
- Chawla, A. , Chawla P., Vasudeva N., Sharma S. K., and Baghel U.. 2013. “Pharmacognostic Standardization of the Stem of Aerva persica (Burm. F) Merrill (Amaranthaceae).” Journal of Medicinal Plant Research 7, no. 11: 637–644. [Google Scholar]
- Crew, K. D. , and Neugut A. I.. 2006. “Epidemiology of Gastric Cancer.” World Journal of Gastroenterology: WJG 12, no. 3: 354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cvetanović, A. , Švarc‐Gajić J., Zeković Z., et al. 2015. “Comparative Analysis of Antioxidant, Antimicrobiological and Cytotoxic Activities of Native and Fermented Chamomile Ligulate Flower Extracts.” Planta 242, no. 3: 721–732. 10.1007/s00425-015-2308-2. [DOI] [PubMed] [Google Scholar]
- Das, S. , Vasudeva N., and Sharma S.. 2016. “ Cichorium intybus: A Concise Report on Its Ethnomedicinal, Botanical, and Phytopharmacological Aspects.” Drug Development and Therapeutics 7, no. 1: 1. [Google Scholar]
- Deters, A. , Zippel J., Hellenbrand N., Pappai D., Possemeyer C., and Hensel A.. 2010. “Aqueous Extracts and Polysaccharides From Marshmallow Roots (Althea officinalis L.): Cellular Internalisation and Stimulation of Cell Physiology of Human Epithelial Cells In Vitro.” Journal of Ethnopharmacology 127, no. 1: 62–69. [DOI] [PubMed] [Google Scholar]
- Dinat, S. , Orchard A., and Van Vuuren S.. 2023. “A Scoping Review of African Natural Products Against Gastric Ulcers and Helicobacter Pylori.” Journal of Ethnopharmacology 301: 115698. [DOI] [PubMed] [Google Scholar]
- Đorđević, M. , Mihailović M., Jovanović J. A., et al. 2017. “ Centaurium erythraea Methanol Extract Protects Red Blood Cells From Oxidative Damage in Streptozotocin‐Induced Diabetic Rats.” Journal of Ethnopharmacology 202: 172–183. [DOI] [PubMed] [Google Scholar]
- Đorđević, S. , Petrović S., Dobrić S., et al. 2007. “Antimicrobial, Anti‐Inflammatory, Anti‐Ulcer and Antioxidant Activities of Carlina acanthifolia Root Essential Oil.” Journal of Ethnopharmacology 109, no. 3: 458–463. [DOI] [PubMed] [Google Scholar]
- Dorman, H. D. , and Deans S. G.. 2000. “Antimicrobial Agents From Plants: Antibacterial Activity of Plant Volatile Oils.” Journal of Applied Microbiology 88, no. 2: 308–316. [DOI] [PubMed] [Google Scholar]
- Doukanı, K. , Bouhennı H., Hanganu D., Olah N.‐K., Şekeroğlu N., and Gezici S.. 2021. “Analysis of Bioactive Compounds and Antioxidant Activities of Cultivated Garlic (Allium sativum L.) and Red Onion (Allium cepa L.) in Algeria.” International Journal of Agriculture Environment and Food Sciences 5, no. 4: 550–560. 10.31015/jaefs.2021.4.15. [DOI] [Google Scholar]
- Duru, M. , Cakir A., Kordali S., et al. 2003. “Chemical Composition and Antifungal Properties of Essential Oils of Three Pistacia Species.” Fitoterapia 74, no. 1: 170–176. [DOI] [PubMed] [Google Scholar]
- Eamlamnam, K. , Patumraj S., Visedopas N., and Thong‐Ngam D.. 2006. “Effects of Aloe vera and Sucralfate on Gastric Microcirculatory Changes, Cytokine Levels and Gastric Ulcer Healing in Rats.” World Journal of Gastroenterology: WJG 12, no. 13: 2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Joumaa, M. M. , and Borjac J. M.. 2022. “ Matricaria chamomilla: A Valuable Insight Into Recent Advances in Medicinal Uses and Pharmacological Activities.” Phytochemistry Reviews 21, no. 6: 1913–1940. 10.1007/s11101-022-09817-0. [DOI] [Google Scholar]
- El Menyiy, N. , Guaouguaou F.‐E., El Baaboua A., et al. 2021. “Phytochemical Properties, Biological Activities and Medicinal Use of Centaurium erythraea Rafn.” Journal of Ethnopharmacology 276: 114171. [DOI] [PubMed] [Google Scholar]
- Elansary, H. O. , Szopa A., Kubica P., Ekiert H., Al‐Mana F., and Al‐Yafrsi M. A.. 2020. “Antioxidant and Biological Activities of Acacia Saligna and Lawsonia inermis Natural Populations.” Plants 9, no. 7: 908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elaoufi, M. M. , Bouterfas K., Djebbar A. A., et al. 2022. “Chemical Composition, Anti‐Ulcer and Anti‐Inflammatory Effects of Carob Pods (Ceratonia siliqua L.) Polyphenols From Ain Temouchent.” Journal of Microbiology, Biotechnology and Food Sciences 12, no. 2: e5427. [Google Scholar]
- El‐Meligy, R. M. , Awaad A. S., Soliman G. A., Kenawy S. A., and Alqasoumi S. I.. 2017. “Prophylactic and Curative Anti‐Ulcerogenic Activity and the Possible Mechanisms of Action of Some Desert Plants.” Saudi Pharmaceutical Journal 25, no. 3: 387–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elrherabi, A. , Bouhrim M., Abdnim R., et al. 2023. “Phenolic Content, Antioxidant, Hemidiaphragm Glucose Consumption, and Hemoglobin Glycosylation Inhibitory Activities of Lavandula stoechas L. Aqueous Extract.” Journal of Natural Remedies 23, no. 2: 653–660. [Google Scholar]
- El‐Saber Batiha, G. , Magdy Beshbishy A., El‐Mleeh A., Abdel‐Daim M. M., and Prasad Devkota H.. 2020. “Traditional Uses, Bioactive Chemical Constituents, and Pharmacological and Toxicological Activities of Glycyrrhiza glabra L. (Fabaceae).” Biomolecules 10, no. 3: 352. 10.3390/biom10030352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Es‐Safi, N.‐E. , Khlifi S., Kollmann A., Kerhoas L., El Abbouyi A., and Ducrot P.‐H.. 2006. “Iridoid Glucosides From the Aerial Parts of Globularia alypum L. (Globulariaceae).” Chemical and Pharmaceutical Bulletin 54, no. 1: 85–88. [DOI] [PubMed] [Google Scholar]
- Farkhondeh, T. , and Samarghandian S.. 2019. “The Therapeutic Effects of Portulaca oleracea L. In Hepatogastric Disorders.” Gastroenterología y Hepatología (English Edition) 42, no. 2: 127–132. [DOI] [PubMed] [Google Scholar]
- Fashner, J. , and Gitu A. C.. 2015. “Diagnosis and Treatment of Peptic Ulcer Disease and H. pylori Infection.” American Family Physician 91, no. 4: 236–242. [PubMed] [Google Scholar]
- Fatiha, B. , Didier H., Naima G., et al. 2015. “Phenolic Composition, In Vitro Antioxidant Effects and Tyrosinase Inhibitory Activity of Three Algerian Mentha Species: M. spicata (L.), M. pulegium (L.) and M. rotundifolia (L.) Huds (Lamiaceae).” Industrial Crops and Products 74: 722–730. 10.1016/j.indcrop.2015.04.038. [DOI] [Google Scholar]
- Fedoul, F. F. , Meddah B., Larouci M., Touil A. T., Merazi Y., and Cakmak Y. S.. 2022. “Phenolic Profile and Biological Activities of Aloe barbadensis (Miller) From Western Algeria.” European Journal of Biological Research 12, no. 4: 282–293. https://jbrodka.com/index.php/ejbr/article/view/24. [Google Scholar]
- Felicio, J. , Santos R. D. S., and Gonçalez E.. 2001. “Chemical Constituents From Vitis vinifera (Vitaceae).” Arquivos do Instituto Biológico 68, no. 1: 47–50. [Google Scholar]
- Fierascu, I. , Ungureanu C., Avramescu S. M., et al. 2018. “Genoprotective, Antioxidant, Antifungal and Anti‐Inflammatory Evaluation of Hydroalcoholic Extract of Wild‐Growing Juniperus communis L.(Cupressaceae) Native to Romanian Southern Sub‐Carpathian Hills.” BMC Complementary and Alternative Medicine 18: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flamini, G. , Cioni P. L., Morelli I., Maccioni S., and Baldini R.. 2004. “Phytochemical Typologies in Some Populations of Myrtus communis L. on Caprione Promontory (East Liguria, Italy).” Food Chemistry 85, no. 4: 599–604. [Google Scholar]
- Gacem, M. A. , Ould El Hadj‐Khelil A., Boudjemaa B., and Gacem H.. 2020. “Phytochemistry, Toxicity and Pharmacology of Pistacia Lentiscus, Artemisia herba‐alba and Citrullus colocynthis .” In Sustainable Agriculture Reviews 39, edited by Lichtfouse E., 57–93. Berlin: Springer International Publishing. [Google Scholar]
- Galati, E. M. , Monforte M. T., Tripodo M. M., D'aquino A., and Mondello M. R.. 2001. “Antiulcer Activity of Opuntia Ficus indica (L.) Mill.(Cactaceae): Ultrastructural Study.” Journal of Ethnopharmacology 76, no. 1: 1–9. [DOI] [PubMed] [Google Scholar]
- Geetha, K. M. , and Anitha K. N.. 2022. “Protective Effect of Nigella sativa Seeds Extract Against Ethanol‐Induced Gastric Ulcer in Rats: Possible Soluble Epoxide Hydrolase Inhibition.” Indian Journal of Pharmaceutical Sciences 84, no. 1: 41–48. [Google Scholar]
- Ghanmi, M. , Aafi A., Satrani B., Aberchane M., Khia A., and Yakhlef S. E. B.. 2014. “Aromatic and Medicinal Plants in North Africa: Opportunities, Constraints and Prospects.” In Novel Plant Bioresources, 409–423. Hoboken, NJ: John Wiley & Sons. [Google Scholar]
- Gharibn, A. M. , and Mard S.. 2007. “Gastroprotective Effect of Alhagi camelorum on Experimental Gastric Ulcer in Rats.” Physiology and Pharmacology 10: 243–249. [Google Scholar]
- Ghavi, P. P. 2015. “The Extraction Process Optimization of Antioxidant Polysaccharides From Marshmallow (Althaea officinalis L.) Roots.” International Journal of Biological Macromolecules 75: 51–57. [DOI] [PubMed] [Google Scholar]
- Gohar, A. A. , and Zaki A. A.. 2014. “Assessment of Some Herbal Drugs for Prophylaxis of Peptic Ulcer.” Iranian Journal of Pharmaceutical Research: IJPR 13, no. 3: 1081. [PMC free article] [PubMed] [Google Scholar]
- Guevara, B. , and Cogdill A. G.. 2020. “ Helicobacter pylori: A Review of Current Diagnostic and Management Strategies.” Digestive Diseases and Sciences 65: 1917–1931. [DOI] [PubMed] [Google Scholar]
- Gündoğdu, A. Ç. , Özbayer C., and Kar F.. 2023. “Boric Acid Alleviates Gastric Ulcer by Regulating Oxidative Stress and Inflammation‐Related Multiple Signaling Pathways.” Biological Trace Element Research 202: 2124–2132. [DOI] [PubMed] [Google Scholar]
- Güvenir, M. 2024. “ Conehead thyme (Thymus capitatus) and Evidence‐Based Usage in Eradicating: Helicobacter pylori .” In Ancient and Traditional Foods, Plants, Herbs and Spices Used in the Middle East, 135–142. Boca Raton, FL: CRC Press. [Google Scholar]
- Hajji, N. , Hudik E., Iovino P., et al. 2021. “ Globularia alypum L. Modulates Inflammatory Markers in Human Colon and Shows a Potential Antioxidant Role in Myeloid Leukemic Cells.” Translational Medicine@ UniSa 24, no. 1: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harsha, C. , Banik K., Bordoloi D., and Kunnumakkara A. B.. 2017. “Antiulcer Properties of Fruits and Vegetables: A Mechanism Based Perspective.” Food and Chemical Toxicology 108: 104–119. 10.1016/j.fct.2017.07.023. [DOI] [PubMed] [Google Scholar]
- Hayani, M. , Bencheikh N., Ailli A., et al. 2022. “Quality Control, Phytochemical Profile, and Antibacterial Effect of Origanum compactum Benth. Essential Oil From Morocco.” International Journal of Plant Biology 13, no. 4: 546–560. [Google Scholar]
- Hemmami, H. , Seghir B. B., Zeghoud S., et al. 2023. “Desert Endemic Plants in Algeria: A Review on Traditional Uses, Phytochemistry, Polyphenolic Compounds and Pharmacological Activities.” Molecules 28, no. 4: 1834. 10.3390/molecules28041834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoogerwerf, W. A. , and Pasricha P. J.. 2006. “Pharmacotherapy of Gastric Acidity, Peptic Ulcers, and Gastroesophageal Reflux Disease.” Pharmacological Basis of Therapeutics 11: 967–981. [Google Scholar]
- Hooi, J. K. , Lai W. Y., Ng W. K., et al. 2017. “Global Prevalence of Helicobacter pylori Infection: Systematic Review and Meta‐Analysis.” Gastroenterology 153, no. 2: 420–429. [DOI] [PubMed] [Google Scholar]
- Houti, H. , Ghanmi M., Satrani B., et al. 2023. “Moroccan Endemic Artemisia herba‐alba Essential Oil: Gc‐Ms Analysis and Antibacterial and Antifungal Investigation.” Separations 10, no. 1: 59. https://www.mdpi.com/2297‐8739/10/1/59. [Google Scholar]
- Ibrahim, N. A. , El‐Sakhawy F. S., Mohammed M. M., Farid M. A., AbdelWahed N. A., and Deabes D. A.. 2015. “Chemical Composition, Antimicrobial and Antifungal Activities of Essential Oils of the Leaves of Aegle marmelos (L.) Correa Growing in Egypt.” Journal of Applied Pharmaceutical Science 5, no. 2: 1–5. [Google Scholar]
- Ibtissam, L. , and Djahra A. B.. 2022. “Phytochemical Investigation of Helianthemum lippii L. Aerial Dum. Cours Part and Evaluation for Its Antioxidant Activities.” International Journal of Secondary Metabolite 9, no. 2: 229–237. [Google Scholar]
- Imenshahidi, M. , and Hosseinzadeh H.. 2019. “Berberine and Barberry (Berberis vulgaris): A Clinical Review.” Phytotherapy Research 33, no. 3: 504–523. 10.1002/ptr.6252. [DOI] [PubMed] [Google Scholar]
- Issac, A. , Gopakumar G., Kuttan R., Maliakel B., and Krishnakumar I. M.. 2015. “Safety and Anti‐Ulcerogenic Activity of a Novel Polyphenol‐Rich Extract of Clove Buds (Syzygium aromaticum L).” Food & Function 6, no. 3: 842–852. 10.1039/c4fo00711e. [DOI] [PubMed] [Google Scholar]
- Ivancheva, S. , Nikolova M., and Tsvetkova R.. 2006. “Pharmacological Activities and Biologically Active Compounds of Bulgarian Medicinal Plants.” Phytochemistry: Advances in Research 37661: 87–103. [Google Scholar]
- Jainu, M. , and Devi C. S.. 2004a. “Antioxidant Effect of Methanolic Extract of Solanum Nigrum Berries on Aspirin Induced Gastric Mucosal Injury.” Indian Journal of Clinical Biochemistry 19: 57–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jainu, M. , and Devi C. S.. 2004b. “Effect of Cissus Quadrangularis on Gastric Mucosal Defensive Factors in Experimentally Induced Gastric Ulcer—A Comparative Study With Sucralfate.” Journal of Medicinal Food 7, no. 3: 372–376. [DOI] [PubMed] [Google Scholar]
- Jainu, M. , and Devi C. S. S.. 2006. “Antiulcerogenic and Ulcer Healing Effects of Solanum nigrum (L.) on Experimental Ulcer Models: Possible Mechanism for the Inhibition of Acid Formation.” Journal of Ethnopharmacology 104, no. 1–2: 156–163. [DOI] [PubMed] [Google Scholar]
- Jamila, F. , and Mostafa E.. 2014. “Ethnobotanical Survey of Medicinal Plants Used by People in Oriental Morocco to Manage Various Ailments.” Journal of Ethnopharmacology 154, no. 1: 76–87. 10.1016/j.jep.2014.03.016. [DOI] [PubMed] [Google Scholar]
- Javed, M. A. , Khan M. A., Arshad H., et al. 2020. “Pharmacological Evaluation of Lavandula stoechas L. for Ethanol‐Induced Gastric Mucosal Ulcer.” RADS Journal of Pharmacy and Pharmaceutical Sciences 8, no. 1: 47–57. [Google Scholar]
- Joshi, S. V. , Kedar K. A., Markana U. V., et al. 2011. “Alteration of Gastric Mucus Secretion in Rats Treated With Abelmoschus Esculentus Seed Mucilage.” Der Pharmacia Lettre 3, no. 5: 183–188. [Google Scholar]
- Kagbo, H. , and Aduku O.. 2015. “Evaluation of Anti‐Ulcer Effects of the Methanol Extract of Mangifera indica L Stem Bark.” European Journal of Medicinal Plants 7, no. 1: 1–6. [Google Scholar]
- Kale, B. S. , Bambare A. S., and Bhale M. S.. 2023. “Pharmacological, Phytochemistry and Ethnomedicinal Profiles of Osyris Quadripartita Salz. Ex Decne: A Critical Review.” South Asian Journal of Experimental Biology 13, no. 3: 189–196. [Google Scholar]
- Karimi, G. , Hosseinzadeh H., and Ettehad N.. 2004. “Evaluation of the Gastric Antiulcerogenic Effects of Portulaca oleracea L. Extracts in Mice.” Phytotherapy Research: An International Journal Devoted to Pharmacological and Toxicological Evaluation of Natural Product Derivatives 18, no. 6: 484–487. [DOI] [PubMed] [Google Scholar]
- Kaunitz, J. D. 1999. “Barrier Function of Gastric Mucus.” Keio Journal of Medicine 48, no. 2: 63–68. [DOI] [PubMed] [Google Scholar]
- Khalid, H. , Abdalla W., Abdelgadir A., Opatz T., and Efferthc T.. 2012. “Gems From Traditional North‐African Medicine: Medicinal and Aromatic Plants From Sudan.” Natural Products and Bioprospecting 2: 92–103. 10.1007/s13659-012-0015-2. [DOI] [Google Scholar]
- Khan, A. W. , Jan S., Parveen S., et al. 2012. “Phytochemical Analysis and Enzyme Inhibition Assay of Aerva Javanica for Ulcer.” Chemistry Central Journal 6: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khedr, A. 2017. “Antiulcer Protective Activity of Gum Arabic (Acacia senegal) in Adult Rats.” Bulletin of the National Nutrition Institute of the Arab Republic of Egypt 49, no. 1: 1–28. [Google Scholar]
- Kononenko, N. , Chikitkina V., Karabut L., Matviichuk O., and Vislous O.. 2023. “Study of the Antiulcer Activity of Garden Cabbage Extract on the Chronic Acetic Ulcer Model in Rats.” ScienceRise: Biological Science 2, no. 35: 12–17. 10.15587/2519-8025.2023.285891. [DOI] [Google Scholar]
- Kononenko, N. , Mirzaliev M., and Ostapets M.. 2018. “The Effect of the Brassica oleracea Extract on the Structural Components of Mucous Cells of the Stomach in Its Ulcerative Damage.” Вісник фармації 1: 54–57. [Google Scholar]
- Krylova, S. , Vymyatnina Z., Zueva E., Amosova E., Razina T., and Litvinenko V.. 2015. “Effects of Cichorium intybus L. Root Extract on Secretory Activity of the Stomach in Health and Ulcer Disease.” Bulletin of Experimental Biology and Medicine 159, no. 5: 638–641. [DOI] [PubMed] [Google Scholar]
- Kumar, A. , Sharma A., Vijayakumar M., and Rao C.. 2010. “Antiulcerogenic Effect of Ethanolic Extract of Portulaca oleracea Experimental Study.” Pharmacology 1: 417–432. [Google Scholar]
- Kumar, V. P. , Shashidhara S., Kumar M., and Sridhara B.. 2001. “Cytoprotective Role of Solanum Nigrum Against Gentamicin‐Induced Kidney Cell (Vero Cells) Damage In Vitro.” Fitoterapia 72, no. 5: 481–486. [DOI] [PubMed] [Google Scholar]
- Lakkab, I. , Hajaji H. E., Lachkar N., Bali B. E., Lachkar M., and Ciobica A.. 2018. “Phytochemistry, Bioactivity: Suggestion of Ceratonia siliqua L. As Neurodegenerative Disease Therapy.” Journal of Complementary and Integrative Medicine 15, no. 4: 20180013. [DOI] [PubMed] [Google Scholar]
- Lanas, A. , and Chan F. K.. 2017. “Peptic Ulcer Disease.” Lancet 390, no. 10094: 613–624. [DOI] [PubMed] [Google Scholar]
- Leporatti, M. L. , and Ivancheva S.. 2003. “Preliminary Comparative Analysis of Medicinal Plants Used in the Traditional Medicine of Bulgaria and Italy.” Journal of Ethnopharmacology 87, no. 2–3: 123–142. [DOI] [PubMed] [Google Scholar]
- Liñán‐Atero, R. , Aghababaei F., García S. R., et al. 2024. “Clove Essential Oil: Chemical Profile, Biological Activities, Encapsulation Strategies, and Food Applications.” Antioxidants (Basel) 13, no. 4: 488. 10.3390/antiox13040488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, Y. , Yan H., Yu B., et al. 2022. “Protective Effects of Natural Antioxidants on Inflammatory Bowel Disease: Thymol and Its Pharmacological Properties.” Antioxidants 11, no. 10: 1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggini, V. , Semenzato G., Gallo E., Nunziata A., Fani R., and Firenzuoli F.. 2024. “Antimicrobial Activity of Syzygium aromaticum Essential Oil in Human Health Treatment.” Molecules 29, no. 5: 999. https://www.mdpi.com/1420‐3049/29/5/999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahamoud Saleh, E. M. , El Sayed El Sahar E. S. G., and Elnadi A. F.. 2021. “Comparative Study on the Effectiveness of Gum Arabic Acacia Senegal and Moringa oleifera Extracts on Prevention of Peptic Ulcer in the Experimental Rats.” African Journal of Biological Sciences 17, no. 1: 63–72. [Google Scholar]
- Mahdavi, F. S. , Mardi P., Mahdavi S. S., et al. 2020. “Therapeutic and Preventive Effects of Olea europaea Extract on Indomethacin‐Induced Small Intestinal Injury Model in Rats.” Evidence‐based Complementary and Alternative Medicine 2020, no. 1: 6669813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmoud, A. A. 2005. “New Monoterpenes From Mentha microphylla .” Planta Medica 71, no. 8: 782–784. [DOI] [PubMed] [Google Scholar]
- Mahmoud, M. F. , Nabil M., Hasan R. A., El‐Shazly A. M., El‐Ansari M. A., and Sobeh M.. 2022. “Pentagalloyl Glucose, a Major Compound in Mango Seed Kernel, Exhibits Distinct Gastroprotective Effects in Indomethacin‐Induced Gastropathy in Rats Via Modulating the no/Enos/Inos Signaling Pathway.” Frontiers in Pharmacology 13: 800986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majumdar, D. , and Looi S.. 2024. “ Helicobacter pylori Infection and Peptic Ulcers.” Medicine 52, no. 3: 152–160. 10.1016/j.mpmed.2023.12.006. [DOI] [Google Scholar]
- Malfertheiner, P. , Megraud F., O'Morain C., et al. 2017. “Management of Helicobacter pylori Infection—The Maastricht V/Florence Consensus Report.” Gut 66, no. 1: 6–30. [DOI] [PubMed] [Google Scholar]
- Manchala, P. 2019. “Evaluation of Anti‐Ulcer Activity of Lagenaria siceraria Chloroform Extracts in Pylorus Ligated Rats.” Electronic Journal of Biology 15: 27–37. [Google Scholar]
- Mansour, R. B. , Beji R. S., Wasli H., et al. 2022. “Gastroprotective Effect of Microencapsulated myrtus communis Essential Oil Against Ethanol/Hcl‐Induced Acute Gastric Lesions.” Molecules 27, no. 5: 1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansour, R. B. , Wasli H., Bourgou S., et al. 2023. “Insights on Juniperus phoenicea Essential Oil as Potential Anti‐Proliferative, Anti‐Tyrosinase, and Antioxidant Candidate.” Molecules 28, no. 22: 7547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maqbool, Z. , Amir M., Zereen A., Abid G., and Wahab S.. 2023. “Licorice.” In Essentials of Medicinal and Aromatic Crops, 763–787. Berlin: Springer. [Google Scholar]
- Martínez‐Burgos, W. J. , Serra J. L., Marsigliaf R. M., et al. 2022. “ Aloe vera: From Ancient Knowledge to the Patent and Innovation Landscape – A Review.” South African Journal of Botany 147: 993–1006. 10.1016/j.sajb.2022.02.034. [DOI] [Google Scholar]
- Mathias, M. E. 1994. “Magic, Myth and Medicine.” Economic Botany 48: 3–7. [Google Scholar]
- Matsuda, H. , Pongpiriyadacha Y., Morikawa T., Kashima Y., Nakano K., and Yoshikawa M.. 2002. “Protective Effects of Polygodial and Related Compounds on Ethanol‐Induced Gastric Mucosal Lesions in Rats: Structural Requirements and Mode of Action.” Bioorganic & Medicinal Chemistry Letters 12, no. 3: 477–482. [DOI] [PubMed] [Google Scholar]
- Mehboob, M. , Naureen I., Saleem A., and Amanat A.. 2022. “Medicinal and Nutritional Importance of Lagenaria siceraria (Lauki).” Saudi Journal of Biomedical Research 7, no. 2: 67–73. [Google Scholar]
- Mesmar, J. , Abdallah R., Badran A., Maresca M., and Baydoun E.. 2022. “ Origanum syriacum Phytochemistry and Pharmacological Properties: A Comprehensive Review.” Molecules 27, no. 13: 4272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miara, M. D. , Teixidor‐Toneu I., Sahnoun T., Bendif H., and Ait Hammou M.. 2019. “Herbal Remedies and Traditional Knowledge of the Tuareg Community in the Region of Illizi (Algerian sahara).” Journal of Arid Environments 167: 65–73. 10.1016/j.jaridenv.2019.04.020. [DOI] [Google Scholar]
- Miri, A. H. , Kamankesh M., Llopis‐Lorente A., et al. 2022. “The Potential Use of Antibiotics Against Helicobacter pylori Infection: Biopharmaceutical Implications.” Frontiers in Pharmacology 13: 917184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammadi, A. , Khanbabaei H., Zandi F., et al. 2022. “Curcumin: A Therapeutic Strategy for Targeting the Helicobacter pylori‐Related Diseases.” Microbial Pathogenesis 166: 105552. 10.1016/j.micpath.2022.105552. [DOI] [PubMed] [Google Scholar]
- Mohammed, D. M. , Ahmed K. A., Desoukey M. A., and Sabry B. A.. 2022. “Assessment of the Antiulcer Properties of Lawsonia inermis L. Leaves and Its Nano‐Formulation Against Prolonged Effect of Acute Ulcer in Rats.” Toxicology Reports 9: 337–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouffouk, S. , Mouffouk C., Mouffouk S., and Haba H.. 2023. “Medicinal, Pharmacological and Biochemical Progress on the Study of Genus Helianthemum: A Review.” Current Chemical Biology 17, no. 3: 147–159. [Google Scholar]
- Moutawalli, A. , Benkhouili F. Z., Doukkali A., Benzeid H., and Zahidi A.. 2023. “The Biological and Pharmacologic Actions of Lawsonia inermis L.” Phytomedicine Plus 3: 100468. [Google Scholar]
- Muheyuddeen, G. , Rav D. S., Verma S., Gautam S. K., and Gupta S. K.. 2023. “ Lawsonia inermis Linnaeus: Pharmacological Peculiarity and Modern Progression.” Research Journal of Pharmacognosy and Phytochemistry 15, no. 1: 63–76. [Google Scholar]
- Muhialdin, A. J. , Alamri Z. Z., Hussein A. M., et al. 2023. “Gastro‐Protective and Therapeutic Effect of Punica granatum Against Stomach Ulcer Caused by Helicobacter pylori .” Cellular and Molecular Biology (Noisy‐le‐Grand, France) 69, no. 1: 48–53. 10.14715/cmb/2022.69.1.9. [DOI] [PubMed] [Google Scholar]
- Musa, A. , Shady N. H., Ahmed S. R., et al. 2021. “Antiulcer Potential of Olea europea L. Cv. Arbequina Leaf Extract Supported by Metabolic Profiling and Molecular Docking.” Antioxidants 10, no. 5: 644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naceiri Mrabti, H. , Bouyahya A., Naceiri Mrabti N., Jaradat N., Doudach L., and Faouzi M. E. A.. 2021. “Ethnobotanical Survey of Medicinal Plants Used by Traditional Healers to Treat Diabetes in the Taza Region of Morocco.” Evidence‐based Complementary and Alternative Medicine 2021: 5515634. 10.1155/2021/5515634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naim, Z. , Billah M., Ibrahim M., et al. 2015. “Anti‐Inflammatory, Analgesic and Anti‐Nociceptive Efficacy of Peel of Abelmoschus esculentus Fruits in Laboratory Animal.” Current Drug Therapy 10, no. 2: 113–121. [Google Scholar]
- Narayanankutty, A. , Kunnath K., Alfarhan A., Rajagopal R., and Ramesh V.. 2021. “Chemical Composition of Cinnamomum verum Leaf and Flower Essential Oils and Analysis of Their Antibacterial, Insecticidal, and Larvicidal Properties.” Molecules 26, no. 20: 6303. 10.3390/molecules26206303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nassar, M. , Aboutabl E.‐S., Ahmed R., El‐Khrisy E.‐D., Ibrahim K., and Sleem A.. 2010. “Secondary Metabolites and Bioactivities of Myrtus Communis .” Pharmacognosy Research 2: 325–329. 10.4103/0974-8490.75449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nouir, S. , Dbeibia A., Bouhajeb R., et al. 2023. “Phytochemical Analysis and Evaluation of the Antioxidant, Antiproliferative, Antibacterial, and Antibiofilm Effects of Globularia alypum (L.) Leaves.” Molecules 28, no. 10: 4019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogidi, O. I. , and Enenebeaku U. E.. 2023. “Medicinal Potentials of Aloe vera (Aloe barbadensis Miller): Technologies for the Production of Therapeutics.” In Sustainable Utilization and Conservation of Africa's Biological Resources and Environment, edited by Izah S. C. and Ogwu M. C., 295–321. Singapore: Springer Nature. [Google Scholar]
- Okasha, M. A. 2007. “Anti‐Ulcerogenic and Anti‐Secretory Activity of the N‐ Butanol Portion of Syzygium aromaticum in Rat.”
- Olaitan Balogun, S. , Sabino Damazo A., Pavan E., et al. 2022. “Evidence for the Involvement of Cytokines Modulation and Prokinetic Properties in Gastric Ulcer Healing Effects of Helicteres sacarolha A. St.‐Hil. A. Juss.” Chemistry & Biodiversity 19, no. 12: e202200322. [DOI] [PubMed] [Google Scholar]
- Online, B. K. , Ait Slimane‐Ait Kaki S., Oulebsir‐Mohandkaci H., and Amel B.. 2018. “Phytochemical Characterization and Antimicrobial Potentialities of Two Medicinal Plants, Chamaemelum Nobile (L.) All and Matricaria Chamomilla (L.).” International Journal of Innovative Approaches in Science Research 2: 126–139. 10.29329/ijiasr.2018.173.2. [DOI] [Google Scholar]
- Ortaç, D. , Cemek M., Karaca T., et al. 2018. “In Vivo Anti‐Ulcerogenic Effect of Okra (Abelmoschus esculentus) on Ethanol‐Induced Acute Gastric Mucosal Lesions.” Pharmaceutical Biology 56, no. 1: 165–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouffai, K. , Rachid A., Abboou F., and Lahfa F. B.. 2022. “Antihemolytic and Antioxidant Activities of Aerial Parts Extracts of Zygophyllum album L. and Globularia alypum L. From Algeria.” Journal of Natural Product Research and Applications 1, no. 3: 41–55. [Google Scholar]
- Pandey, V. K. , Srivastava S., Ashish D. K. K., et al. 2024. “Bioactive Properties of Clove (Syzygium aromaticum) Essential Oil Nanoemulsion: A Comprehensive Review.” Heliyon 10, no. 1: e22437. 10.1016/j.heliyon.2023.e22437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paseban, M. , Niazmand S., Soukhtanloo M., et al. 2020. “The Therapeutic Effect of Nigella sativa Seed on Indomethacin‐Induced Gastric Ulcer in Rats.” Current Nutrition & Food Science 16, no. 3: 276–283. [Google Scholar]
- Patel, V. , and Khetani M.. 2021. “A Pharmacognostic Review of Liquorice: Pharmacological Actions, Current Uses and Future Prospects.” Himalayan Journal of Health Sciences 6, no. 2: 38–45. 10.22270/hjhs.v6i2.99. [DOI] [Google Scholar]
- Pol, M. , Surana K., Patil D., Ahire E., and Kshirsagar S.. 2023. “Approaches for Treating Ulcer With Functional Foods.” In Applications of Functional Foods in Disease Prevention, 191–210. Palm Bay, FL: Apple Academic Press. [Google Scholar]
- Pramanik, K. C. , Biswas R., Bandyopadhyay D., Mishra M., Ghosh C., and Chatterjee T.. 2007. “Evaluation of Anti‐Ulcer Properties of the Leaf Extract of Juniperus communis L. in Animals.” Journal of Natural Remedies 7: 207–213. [Google Scholar]
- Prayoga, D. K. , Aulifa D. L., Budiman A., and Levita J.. 2024. “Plants With Anti‐Ulcer Activity and Mechanism: A Review of Preclinical and Clinical Studies.” Drug Design, Development and Therapy 18: 193–213. 10.2147/dddt.S446949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragheb, E. M. , Ibrahem E. S., and Shalaby R. A.. 2021. “Potential Protective Effects of Plantago major Extracts Against Indomethacin‐Induced Gastric Ulcer in Rats.” Egyptian Journal of Nutrition and Health 16, no. 1: 1–20. 10.21608/ejnh.2021.194452. [DOI] [Google Scholar]
- Rahman, M. M. , Abdullah A. T. M., Sharif M., et al. 2022. “Relative Evaluation of In‐Vitro Antioxidant Potential and Phenolic Constituents by Hplc‐Dad of Brassica Vegetables Extracted in Different Solvents.” Heliyon 8, no. 10: e10838. 10.1016/j.heliyon.2022.e10838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranasinghe, P. , Pigera S., Premakumara G. A., Galappaththy P., Constantine G. R., and Katulanda P.. 2013. “Medicinal Properties of 'True' Cinnamon (Cinnamomum zeylanicum): A Systematic Review.” BMC Complementary and Alternative Medicine 13: 275. 10.1186/1472-6882-13-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao, P. V. , and Gan S. H.. 2014. “Cinnamon: A Multifaceted Medicinal Plant.” Evidence‐based Complementary and Alternative Medicine 2014: 642942. 10.1155/2014/642942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray, L. R. , Alam M. S., Junaid M., et al. 2021. “ Brassica Oleracea Var. Capitata F. alba: A Review on Its Botany, Traditional Uses, Phytochemistry and Pharmacological Activities.” Mini Reviews in Medicinal Chemistry 21, no. 16: 2399–2417. 10.2174/1389557521666210111150036. [DOI] [PubMed] [Google Scholar]
- Reddy, K. V. , Sree N. R. S., Ranjit P., et al. 2023. “Essential Oils, Herbal Extracts and Propolis for Alleviating Helicobacter pylori Infections: A Critical View.” South African Journal of Botany 157: 138–150. 10.1016/j.sajb.2023.03.056. [DOI] [Google Scholar]
- Rigacci, S. , and Stefani M.. 2016. “Nutraceutical Properties of Olive Oil Polyphenols. An Itinerary From Cultured Cells Through Animal Models to Humans.” International Journal of Molecular Sciences 17, no. 6: 843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez‐Negrete, E. V. , Morales‐González Á., Madrigal‐Santillán E. O., et al. 2024. “Phytochemicals and Their Usefulness in the Maintenance of Health.” Plants 13, no. 4: 523. https://www.mdpi.com/2223‐7747/13/4/523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rtibi, K. , Jabri M.‐A., Selmi S., et al. 2016. “Preventive Effect of Carob (Ceratonia siliqua L.) in Dextran Sulfate Sodium‐Induced Ulcerative Colitis in Rat.” RSC Advances 6, no. 24: 19992–20000. [Google Scholar]
- Saadaoui, N. , Weslati A., Barkaoui T., et al. 2020. “Gastroprotective Effect of Leaf Extract of Two Varieties Grapevine (Vitis vinifera L.) Native Wild and Cultivar Grown in North of Tunisia Against the Oxidative Stress Induced by Ethanol in Rats.” Biomarkers 25, no. 1: 48–61. [DOI] [PubMed] [Google Scholar]
- Saag, L. M. K. , Sanderson G. R., Moyna P., and Ramos G.. 1975. “Cactaceae Mucilage Composition.” Journal of the Science of Food and Agriculture 26, no. 7: 993–1000. [Google Scholar]
- Sachs, G. , Shin J. M., Briving C., Wallmark B., and Hersey S.. 1995. “The Pharmacology of the Gastric Acid Pump: The H+, K+ Atpase.” Annual Review of Pharmacology and Toxicology 35, no. 1: 277–305. [DOI] [PubMed] [Google Scholar]
- Saeed, M. , Khan M. S., Amir K., et al. 2022. “ Lagenaria siceraria Fruit: A Review of Its Phytochemistry, Pharmacology, and Promising Traditional Uses.” Frontiers in Nutrition 9: 927361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safavi, M. , Shams‐Ardakani M., and Foroumadi A.. 2015. “Medicinal Plants in the Treatment of Helicobacter pylori Infections.” Pharmaceutical Biology 53, no. 7: 939–960. 10.3109/13880209.2014.952837. [DOI] [PubMed] [Google Scholar]
- Şahinler, S. Ş. , Yilmaz B. S., Sarikürkcü C., and Bektaş T.. 2022. “The Importance of Lavandula stoechas L. In Pharmacognosy and Phytotherapy.” International Journal of Secondary Metabolite 9, no. 3: 360–376. [Google Scholar]
- Sahu, P. K. , Martha S. K., and Pradhan D.. 2017. “Gastro‐Protective Activity of Leaves of Mangifera indica in Ulcer Induced Albino Rats.” World Journal of Pharmaceutical Research 6: 685–693. [Google Scholar]
- Saiah, W. , Halzoune H., Djaziri R., Tabani K., Koceir E. A., and Omari N.. 2018. “Antioxidant and Gastroprotective Actions of Butanol Fraction of Zingiber officinale Against Diclofenac Sodium‐Induced Gastric Damage in Rats.” Journal of Food Biochemistry 42, no. 1: e12456. 10.1111/jfbc.12456. [DOI] [Google Scholar]
- Salama, S. M. , and Mariod A. A.. 2018. “Gastroprotective Activity of Gum Arabic: A Review.” Gum Arabic: 305–312. [Google Scholar]
- Sánchez‐Mendoza, M. E. , Reyes‐Trejo B., Sánchez‐Gómez P., et al. 2010. “Bioassay‐Guided Isolation of an Anti‐Ulcer Chromene From Eupatorium Aschenbornianum: Role of Nitric Oxide, Prostaglandins and Sulfydryls.” Fitoterapia 81, no. 1: 66–71. [DOI] [PubMed] [Google Scholar]
- Saxena, R. , Belemkar S., Apte K., Saxena R., and Bharti M.. 2011. “Antiulcerogenic and Antioxidant Activity of Hydro Alcoholic Extract of the Root of Cichorium intybus L. in Experimentally Induced Ulcer in Rats.” Ethnopharmacology 2: 1–4. [Google Scholar]
- Saxena, R. , Sulakhiya K. B., and Rathore M.. 2014. “ Cichorium intibus Linn: A Review of Pharmacological Profile.” International Journal of Current Pharmaceutical Research 6, no. 4: 11–15. [Google Scholar]
- Selmi, S. , Alimi D., Rtibi K., et al. 2022. “Gastroprotective and Antioxidant Properties of Trigonella foenum graecum Seeds Aqueous Extract (Fenugreek) and Omeprazole Against Ethanol‐Induced Peptic Ulcer.” Journal of Medicinal Food 25, no. 5: 513–522. [DOI] [PubMed] [Google Scholar]
- Şendoğdu, N. , Aslan M., Orhan D. D., Ergun F., and Yeşilada E.. 2006. “Antidiabetic and Antioxidant Effects of Vitis vinifera L. Leaves in Streptozotocin‐Diabetic Rats.” Turkish Journal of Pharmaceutical Sciences 3, no. 1: 7–18. [Google Scholar]
- Shahat, A. A. , Alsaid M. S., Rafatullah S., et al. 2016. “Treatment With Rhus Tripartita Extract Curtails Isoproterenol‐Elicited Cardiotoxicity and Oxidative Stress in Rats.” BMC Complementary and Alternative Medicine 16: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahdadian, F. , Bagherniya M., Askari G., Kesharwani P., Rajendram R., and Sahebkar A.. 2023. “Barberry (Berberis Vulgaris): Composition and Use of Extracts in Cancer Studies.” In Ancient and Traditional Foods, Plants, Herbs and Spices Used in Cancer, 113–128. Boca Raton, FL: CRC Press. [Google Scholar]
- Shaker, E. , Mahmoud H., and Mnaa S.. 2010. “Anti‐Inflammatory and Anti‐Ulcer Activity of the Extract From Alhagi maurorum (Camelthorn).” Food and Chemical Toxicology 48, no. 10: 2785–2790. [DOI] [PubMed] [Google Scholar]
- Shang, A. , Cao S. Y., Xu X. Y., et al. 2019. “Bioactive Compounds and Biological Functions of Garlic (Allium sativum L).” Food 8, no. 7: 246. 10.3390/foods8070246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma, V. K. , Prateeksha G. S. C., Singh B. N., Rao C. V., and Barik S. K.. 2022. “ Cinnamomum Verum‐Derived Bioactives‐Functionalized Gold Nanoparticles for Prevention of Obesity Through Gut Microbiota Reshaping.” Materials Today Bio 13: 100204. 10.1016/j.mtbio.2022.100204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvan, J. M. , Guerrero‐Hurtado E., Gutiérrez‐Docio A., Alarcón‐Cavero T., Prodanov M., and Martinez‐Rodriguez A. J.. 2021. “Olive‐Leaf Extracts Modulate Inflammation and Oxidative Stress Associated With Human H. pylori Infection.” Antioxidants 10, no. 12: 2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh, N. , Sharma U., Mishra B., Kandalkar A. M., and Jain S. K.. 2023. “Herbs and Herbal Formulations for the Management and Prevention of Gastrointestinal Diseases.” In Herbal Medicine Phytochemistry: Applications and Trends, edited by Izah S. C., Ogwu M. C., and Akram M., 1–35. Berlin: Springer International Publishing. [Google Scholar]
- Sostres, C. , Gargallo C. J., and Lanas A.. 2013. “Nonsteroidal Anti‐Inflammatory Drugs and Upper and Lower Gastrointestinal Mucosal Damage.” Arthritis Research & Therapy 15, no. 3: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava, J. K. , and Gupta S.. 2015. “Chamomile: A Herbal Agent for Treatment of Diseases of the Elderly.” In Foods and Dietary Supplements in the Prevention and Treatment of Disease in Older Adults, edited by Watson R. R., 171–183. Cambridge, MA: Academic Press. [Google Scholar]
- Stielow, M. , Witczyńska A., Kubryń N., Fijałkowski Ł., Nowaczyk J., and Nowaczyk A.. 2023. “The Bioavailability of Drugs—The Current State of Knowledge.” Molecules 28, no. 24: 8038. https://www.mdpi.com/1420‐3049/28/24/8038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudharameshwari, K. , and Ayshwarya M.. 2017. “Evaluation of Antiulcerogenic Activity of Methanol Extracts of Brassica oleracea Var. Capitata Rubra on Albino Rat Gastric Ulceration.” Evaluation 10, no. 3: 314–317. [Google Scholar]
- Šutovská, M. , Nosáľová G., Šutovský J., Fraňová S., Prisenžňáková L., and Capek P.. 2009. “Possible Mechanisms of Dose‐Dependent Cough Suppressive Effect of Althaea officinalis Rhamnogalacturonan in Guinea Pigs Test System.” International Journal of Biological Macromolecules 45, no. 1: 27–32. [DOI] [PubMed] [Google Scholar]
- Tabassum, H. , and Ahmad I. Z.. 2021. “Molecular Docking and Dynamics Simulation Analysis of Thymoquinone and Thymol Compounds From Nigella sativa L. That Inhibit Cag a and Vac a Oncoprotein of Helicobacter pylori: Probable Treatment of H. pylori Infections.” Medicinal Chemistry 17, no. 2: 146–157. [DOI] [PubMed] [Google Scholar]
- Taghzouti, O. K. , Balouirib M., Ouedrhiric W., Chahadd A., and Romanea A.. 2016. “In Vitro Evaluation of the Antioxidant and Antimicrobial Effects of Globularia alypum L. Extracts.” Journal of Materials and Environmental Science 7: 1988–1995. [Google Scholar]
- Thompson Coon, J. , and Ernst E.. 2002. “Herbal Medicinal Products for Non‐Ulcer Dyspepsia.” Alimentary Pharmacology & Therapeutics 16, no. 10: 1689–1699. [DOI] [PubMed] [Google Scholar]
- Tijani, A. S. , Temitayo M. J., and Farombi O. E.. 2022. “Cytoprotective Effect of Methanol Extract of Laportea aestuans on Acidified Ethanol‐Induced Gastric Ulcer in Male Wistar Rats.” Trends in Sciences 19, no. 13: 4648. [Google Scholar]
- Trachtenberg, S. , and Mayer A. M.. 1981. “Composition and Properties of Opuntia ficus‐indica Mucilage.” Phytochemistry 20, no. 12: 2665–2668. [Google Scholar]
- Unissa, R. , Humera B., Alrahef S. S., et al. 2022. “Anti‐Ulcer Properties, Cytokines, and Apoptosis Regulatory Effects of Olea europaea Leaves From Hail Province, Saudi Arabia.” Notulae Botanicae Horti Agrobotanici Cluj‐Napoca 50, no. 3: 12891. [Google Scholar]
- Usta, M. , and Urganci N.. 2015. “Upper Gastrointestinal Bleeding in Children: The Role of Helicobacter pylori Infection and Non‐Steroidal Anti‐Inflammatory Drug Use.” West Indian Medical Journal 64, no. 2: 113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vale, F. F. , and Oleastro M.. 2014. “Overview of the Phytomedicine Approaches Against Helicobacter pylori .” World Journal of Gastroenterology: WJG 20, no. 19: 5594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasudeva, N. , Sethi P., Sharma S. K., Kumar S., and Sharma S.. 2012. “Antiulcer Potential of the Ethanolic Extract of Aerva persica Merrill Root in Rats.” Journal of Acupuncture and Meridian Studies 5, no. 2: 80–86. [DOI] [PubMed] [Google Scholar]
- Venthodika, A. , Chhikara N., Mann S., Garg M. K., Sofi S. A., and Panghal A.. 2021. “Bioactive Compounds of Aegle marmelos L., Medicinal Values and Its Food Applications: A Critical Review.” Phytotherapy Research 35, no. 4: 1887–1907. 10.1002/ptr.6934. [DOI] [PubMed] [Google Scholar]
- Wahida, B. , Abderrahman B., and Nabil C.. 2007. “Antiulcerogenic Activity of Zizyphus lotus (L.) Extracts.” Journal of Ethnopharmacology 112, no. 2: 228–231. [DOI] [PubMed] [Google Scholar]
- Wallace, J. L. , and Miller M. J.. 2000. “Nitric Oxide in Mucosal Defense: A Little Goes a Long Way.” Gastroenterology 119, no. 2: 512–520. [DOI] [PubMed] [Google Scholar]
- Yasin, B. R. , El‐Fawal H. A., and Mousa S. A.. 2015. “Date (Phoenix dactylifera) Polyphenolics and Other Bioactive Compounds: A Traditional Islamic Remedy's Potential in Prevention of Cell Damage, Cancer Therapeutics and Beyond.” International Journal of Molecular Sciences 16, no. 12: 30075–30090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo, C.‐Y. , Son H.‐U., Kim S.‐K., Kim S.‐O., and Lee S.‐H.. 2022. “Improved Image Analysis for Measuring Gastric Ulcer Index in Animal Models and Clinical Diagnostic Data.” Diagnostics 12, no. 5: 1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaghlool, S. S. , Abo‐Seif A. A., Rabeh M. A., Abdelmohsen U. R., and Messiha B. A.. 2019. “Gastro‐Protective and Anti‐Oxidant Potential of Althaea officinalis and Solanum nigrum on Pyloric Ligation/Indomethacin‐Induced Ulceration in Rats.” Antioxidants 8, no. 11: 512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaghlool, S. S. , Shehata B. A., Abo‐Seif A. A., and Abd El‐Latif H. A.. 2015. “Protective Effects of Ginger and Marshmallow Extracts on Indomethacin‐Induced Peptic Ulcer in Rats.” Journal of Natural Science, Biology and Medicine 6, no. 2: 421–428. 10.4103/0976-9668.160026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakir, F. , Mishra H., Azharuddin M., Mirza M. A., Aggarwal G., and Iqbal Z.. 2022. “Gastrointestinal Abnormalities and Nigella sativa: A Narrative Review of Preclinical and Clinical Studies.” In Black Seeds (Nigella Sativa), 355–386. Amsterdam, The Netherlands: Elsevier. [Google Scholar]
- Zarei, A. , Ashtiyani S. C., and Vaezi G. H.. 2014. “A Study on the Effects of the Hydroalcholic Extract of the Aerial Parts of Alhagi camelorum on Prolactin and Pituitary‐Gonadal Activity in Rats With Hypercholesterolemia.” Archivio Italiano di Urologia, Andrologia 86, no. 3: 188–192. [DOI] [PubMed] [Google Scholar]
- Zatorski, H. 2017. “Pathophysiology and Risk Factors in Peptic Ulcer Disease.” Introduction to Gastrointestinal Diseases 2: 7–20. [Google Scholar]
- Zeren, S. , Bayhan Z., Kocak F. E., et al. 2016. “Gastroprotective Effects of Sulforaphane and Thymoquinone against Acetylsalicylic Acid–Induced Gastric Ulcer in Rats.” Journal of Surgical Research 203, no. 2: 348–359. [DOI] [PubMed] [Google Scholar]
- Zhang, W. , Lian Y., Li Q., et al. 2020. “Preventative and Therapeutic Potential of Flavonoids in Peptic Ulcers.” Molecules 25, no. 20: 4626. 10.3390/molecules25204626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, Z. , Gong S., Wang S., and Ma C.. 2015. “Effect and Mechanism of Evodiamine Against Ethanol‐Induced Gastric Ulcer in Mice by Suppressing Rho/Nf‐кB Pathway.” International Immunopharmacology 28, no. 1: 588–595. [DOI] [PubMed] [Google Scholar]
- Ziyyat, A. , Legssyer A., Mekhfi H., Dassouli A., Serhrouchni M., and Benjelloun W.. 1997. “Phytotherapy of Hypertension and Diabetes in Oriental Morocco.” Journal of Ethnopharmacology 58, no. 1: 45–54. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are included in the article. This encompasses all the original data generated during the research and any secondary data utilized in the analyses. For further inquiries or detailed information, the corresponding author can be contacted.


