Abstract
An obstacle to developing a vaccine against human respiratory syncytial virus (RSV) is that natural infection typically does not confer solid immunity to reinfection. To investigate methods to augment the immune response, recombinant RSV (rRSV) was constructed that expresses murine granulocyte-macrophage colony-stimulating factor (mGM-CSF) from a transcription cassette inserted into the G-F intergenic region. Replication of rRSV/mGM-CSF in the upper and lower respiratory tracts of BALB/c mice was reduced 23- to 74- and 5- to 588-fold, respectively, compared to that of the parental rRSV. Despite this strong attenuation of replication, the level of RSV-specific serum antibodies induced by rRSV/mGM-CSF was comparable to, or marginally higher than, that of the parental rRSV. The induction of RSV-specific CD8+ cytotoxic T cells was moderately reduced during the initial infection, which might be a consequence of reduced antigen expression. Mice infected with rRSV/mGM-CSF had elevated levels of pulmonary mRNA for gamma interferon (IFN-γ) and interleukin 12 (IL-12) p40 compared to animals infected by wild-type rRSV. Elevated synthesis of IFN-γ could account for the restriction of RSV replication, as was observed previously with an IFN-γ-expressing rRSV. The accumulation of total pulmonary mononuclear cells and total CD4+ T lymphocytes was accelerated in animals infected with rRSV/mGM-CSF compared to that in animals infected with the control virus, and the level of IFN-γ-positive or IL-4-positive pulmonary CD4+ cells was elevated approximately twofold. The number of pulmonary lymphoid and myeloid dendritic cells and macrophages was increased up to fourfold in mice infected with rRSV/mGM-CSF compared to those infected with the parental rRSV, and the mean expression of major histocompatibility complex class II molecules, a marker of activation, was significantly increased in the two subsets of dendritic cells. Enhanced antigen presentation likely accounts for the maintenance of a strong antibody response despite reduced viral replication and would be a desirable property for a live attenuated rRSV vaccine.
Respiratory syncytial virus (RSV) is the most important viral etiologic agent of serious pediatric respiratory tract disease worldwide. RSV also is receiving increasing recognition as an important cause of respiratory tract disease in the elderly; in immunocompromised patients, such as bone marrow transplant recipients; and in the general population (reviewed in reference 16). Reinfection by RSV is common, although disease is less severe. A licensed RSV vaccine is not available, but passive immunoprophylaxis with RSV-neutralizing antibody is now available for high-risk infants (2).
RSV is a nonsegmented negative-strand RNA virus of the family Paramyxoviridae. RSV encodes 11 proteins (reviewed in references 8, 15, and 16), namely, three transmembrane surface proteins (G, F, and SH); the virion matrix protein M; the nucleocapsid and polymerase proteins N, P, M2-1, and L; the putative transcription-replication regulatory factor M2-2; and two nonstructural proteins, NS1 and NS2, that have been implicated as antagonists of the type I interferon antiviral state (31). While many components of the innate and adaptive immune systems contribute to restricting and resolving an RSV infection, the increased resistance to reinfection that is conferred by prior infection appears to be mediated primarily by RSV-specific secretory and serum antibodies (16, 17, 27). The F and G glycoproteins are the major RSV neutralization antigens (17).
In the 1960s, a formalin-inactivated RSV vaccine evaluated in infants and children was not protective and, paradoxically, was associated with increased frequency and severity of disease during subsequent natural RSV infection (26). It is now understood that the initial vaccination primed for an immune-mediated pathogenic response that occurred upon reexposure to viral antigen during the subsequent natural infection, although the details of this phenomenon have not been completely elucidated. One element appears to involve the disproportionate induction of the Th2 subset of CD4+ T helper lymphocytes, characterized by increased secretion of interleukin 4 (IL-4) and IL-10 (18). Subunit vaccines consisting of the RSV F and G proteins also have been associated with enhanced pulmonary pathology upon RSV challenge in experimental animals (22, 28, 35). Other studies indicate that the disproportionate induction of CD4+ T lymphocytes by RSV antigen is abrogated by the concurrent induction of CD8+ T lymphocytes, suggesting that the latter have a regulatory role (23, 32). Thus, the imbalanced CD4+-T-cell response observed with RSV protein vaccines might be due to their inefficiency in stimulating CD8+ T lymphocytes. In contrast, the strong CD8+-T-cell response associated with natural RSV infection could provide a regulatory role that accounts for the lack of immune-mediated disease enhancement during natural reinfection (35). This would be an important advantage for a vaccine strategy based on infection with an attenuated RSV (36).
The peak of serious pediatric RSV disease is between 2 and 9 months of life, and thus, an RSV vaccine should be administered before this time. Unfortunately, this age group exhibits reduced immune responses due to immunologic immaturity and to immunosuppression mediated by maternally derived RSV-specific serum immunoglobulin G (IgG) (36). An additional challenge for developing a successful live attenuated RSV vaccine is to achieve an appropriate level of attenuation and safety while retaining sufficient immunogenicity. Recent clinical studies of RSV vaccine candidates suggest that this goal can be achieved (36). However, since even natural infection does not confer solid immunity, we have been interested in the possibility of modifying the immune response by expression of one or more cytokines or chemokines from one or more genes inserted into the viral genome. Two possible desirable outcomes would be augmentation of the immune response and attenuation of the virus. An additional possibility is that infection early in life might have a general beneficial effect in “educating” the immature immune system (30), and this might be augmented by expression of one or more appropriate cytokines or chemokines.
We previously described a recombinant RSV (rRSV) that expressed murine IFN-γ from a supernumerary gene placed in the RSV genome between the G and F genes, and we demonstrated that this cytokine mediated attenuation of the virus and augmentation of the immune response (11). A comparable rRSV expressing murine IL-2 had similar but smaller effects (12). In the present study, we constructed a comparable rRSV that expresses murine granulocyte-macrophage colony-stimulating factor (mGM-CSF). This cytokine was of particular interest because it mediates the differentiation and maturation of dendritic cells and macrophages, which are important to both innate and adaptive immunity. Several groups have suggested using GM-CSF as a vaccine adjuvant expressed from a plasmid during DNA immunization or supplied as soluble protein (1, 5, 20, 21, 25, 29, 37). The development of reverse genetics for negative-strand RNA viruses allowed us to explore the approach of expressing GM-CSF as a supernumerary gene inserted into live rRSV. In the present study of mice, expression of mGM-CSF by rRSV was associated with attenuation of virus replication, augmented proliferation and activation of pulmonary antigen-presenting cells, and augmented stimulation of pulmonary CD4+ T lymphocytes.
MATERIALS AND METHODS
Construction and propagation of the recombinant viruses.
We purchased a cDNA, flanked by MluI and BstBI sites, that encodes the mature, 124-amino-acid form of mGM-CSF lacking the 17-amino-acid signal peptide (R&D Systems, Minneapolis, Minn.). The upstream end of this cDNA was modified by insertion into the HindIII-MluI window immediately upstream of the mGM-CSF coding sequence of a synthetic DNA containing, in order, an XmaI site, an RSV transcriptional gene start signal, and the coding sequence for the signal peptide in frame with the GM-CSF coding sequence. This synthetic DNA fragment was formed by annealing the following two oligonucleotides: AGCTTCCCGGGATGGGGCAAATATGTGGCTGCAGAATTTACTTTTCCTGGGCATTGTGGTCTACAGCCTCTCAGCTCCGA and CGCGTCGGAGCTGAGAGGCTGTAGACCACAATGCCCAGGAAAAGTAAATTCTGCAGCCACATATTTGCCCCATCCCGGGA (the XmaI restriction endonuclease sites are italicized, the RSV gene start sequences are underlined, the signal peptide coding sequences begin immediately after the underlined sequences, and nucleotides that contribute to the HindIII and MluI restriction sites are in boldface). The downstream end of the cDNA was modified to add an RSV gene end signal and XmaI site by inserting into the BstBI-BamHI window a synthetic double-stranded DNA formed by annealing the following two oligonucleotides: CGAATGCAAAAAACCAGTCCAAAAATAAAGTTATTAAAAATTCCCGG and GATCCCGGGAATTTTTAATAACTTTATTTTTGGACTGGTTTTTTGCATT (the RSV gene end signals are underlined, the XmaI restriction sites are italicized, and the sticky ends of BstBI and BamHI restriction sites are in boldface). The sequence of the engineered cDNA was confirmed in full.
The XmaI fragment containing the mGM-CSF transcription cassette was inserted into a cDNA of the RSV antigenome modified to contain a unique XmaI site between the G and F genes (9) (Fig. 1). This increased the length of the encoded antigenomic RNA from 15,223 to 15,688 nucleotides. The encoded recombinant virus, designated rRSV/mGM-CSF, was recovered by cotransfection of the antigenomic plasmid with plasmids expressing the N, P, L, and M2-1 support proteins into HEp-2 cells infected with a vaccinia virus recombinant expressing T7 RNA polymerase (14).
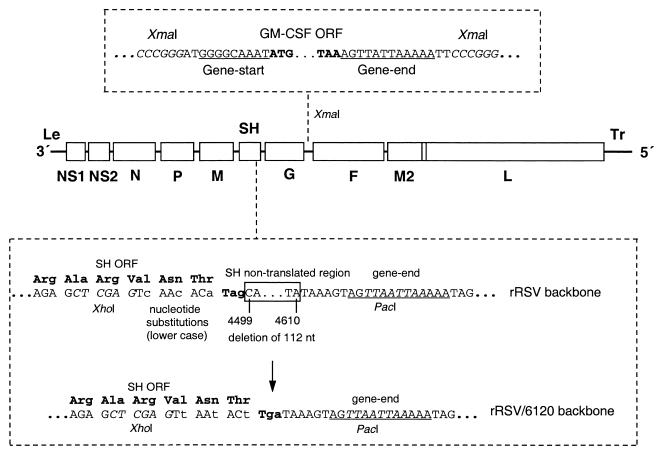
FIG. 1.
Diagram of the genomic RNAs of two versions of rRSV expressing mGM-CSF, namely, rRSV/mGM-CSF and rRSV/6120/mGM-CSF. In each virus, a transcription cassette consisting of the mGM-CSF ORF under the control of RSV gene start and gene end transcription signals (upper box) was inserted into the XmaI site in the G-F intergenic region of rRSV. The start and stop codons of the GM-CSF ORF are in boldface, XmaI restriction endonuclease sites are in italics, and RSV gene start and gene end signals are underlined. The two viruses differ only in the SH gene of the respective rRSV backbones, as illustrated in the lower box. The backbone of the rRSV/mGM-CSF is that of the previously described wt rRSV (14) modified to contain an XmaI site in the G-F intergenic region (9). In the backbone of rRSV/6120/mGM-CSF, the SH gene was modified (i) to contain five translationally silent nucleotide substitutions in the last four codons of the SH ORF and (ii) to delete 112 nucleotides (nt; positions 4499 to 4610) of the complete antigenomic sequence) from the downstream nontranslated region of the SH gene (lower box). The XhoI and PacI sites used in the construction (Materials and Methods) are italicized and labeled, the SH gene end signal is underlined, the SH codons are shown as triplets, nucleotide substitutions are in lowercase, and the deleted sequence is boxed with the sequence positions indicated.
We also constructed a second version of the mGM-CSF recombinant using an antigenomic cDNA that was modified so that most of the downstream noncoding region of the SH gene was deleted without a change at the amino acid level. Specifically, the 141-bp XhoI-PacI window that runs from the end of the SH open reading frame (ORF) to the SH gene end signal (Fig. 1) was replaced with a synthetic DNA formed from the following two oligonucleotides: TCGAGTtAAtACtTgaTAAAGTAGTTAAT and TAACTACTTTAtcAaGTaTTaAC (parts of the XhoI and PacI restriction sites are in boldface, the nucleotides of the SH ORF and termination codon are underlined, and silent nucleotide changes are indicated by lowercase). The encoded virus has silent nucleotide substitutions in the last three codons and the termination codon of the SH ORF and has a deletion of 112 nucleotides from the SH downstream nontranslated region that leaves the gene end signal intact. The mGM-CSF expression cassette was inserted into this backbone exactly as described above for rRSV/mGM-CSF. The resulting recovered recombinant virus was designated rRSV/6120/mGM-CSF, and its parental counterpart was designated rRSV/6120.
The viruses were propagated in HEp-2 cells in Optim-Mem medium (Life Technologies) containing 5% fetal bovine serum (FBS; Summit Biotechnology). rRSV/mGM-CSF was used in the experiment shown in Fig. 2 (see also Fig. 5 for another experiment) with wild-type (wt) rRSV as the control virus; for an animal study (see Fig. 5), virus was concentrated by differential centrifugation in an SW28 rotor (Beckman Coulter, Fullerton, Calif.) at 25,000 rpm for 1 h at 4°C. rRSV/6120/GM-CSF was used in the experiments shown in Fig. 3 and 4 (and in other experiments [see Fig. 6 to 8]) with the parental rRSV/6120 as the control virus.
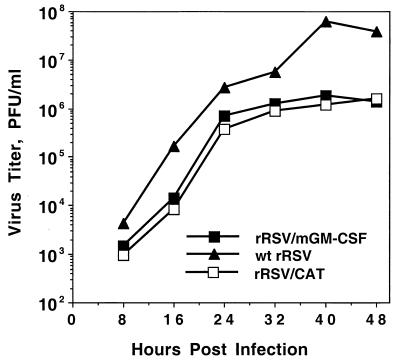
FIG. 2.
Growth kinetics of rRSV/mGM-CSF, rRSV/CAT, and wt rRSV in HEp-2 cells. Cell monolayers were infected with 2 PFU per cell (two replicate wells per virus), and 200-μl aliquots of medium were taken and replaced with fresh medium at the indicated time points. These samples were flash frozen, and the titer of infectious virus was determined later by plaque assay. The average of the two monolayers is shown for each time point.
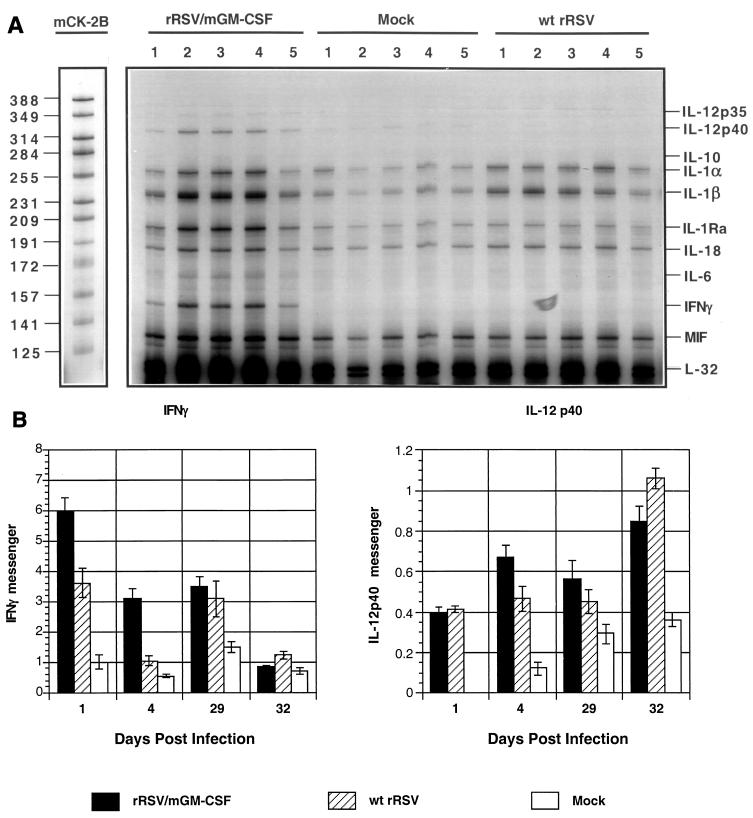
FIG. 5.
Detection of pulmonary cytokine and chemokine mRNAs by an RNase protection assay. Animals (20 per group) were mock infected or infected with 106 PFU of rRSV/mGM-CSF or wt rRSV per animal on day 0. Five mice per group per time point were sacrificed on days 1 and 4, and total pulmonary RNA was isolated and analyzed by an RNase protection using mixed probes specific to selected cytokines and chemokines. The remaining animals were challenged by infection with 106 PFU of wt RSV on day 28; five mice per group per time point were sacrificed on days 29 and 32, and total pulmonary RNA was isolated and analyzed in the same way. (A) Autoradiogram showing the results for one probe kit, mCK-2B, for mRNA isolated on day 4, with each gel lane representing an individual mouse and unhybridized probe mix run in parallel as a marker, with the nucleotide lengths of individual probes shown on the left. Protected probes corresponding to individual cytokines or the housekeeping gene L-32 are identified on the right. IL-1Ra, IL-1 receptor antagonist; MIF, macrophage migration inhibitory factor; IL-12 p35 and p40, constitutive and inducible monomers of IL-12, respectively. (B) Accumulation of pulmonary mRNA for IFN-γ and the inducible p40 monomer of dimeric IL-12. Autoradiograms such as those shown in panel A were quantified by phosphorimagery and calculated as a percentage of the amount of L-32 housekeeping gene mRNA in the same sample. Mean values of five mice per virus per day (with SE) are shown. Note that each y axis has a different scale.
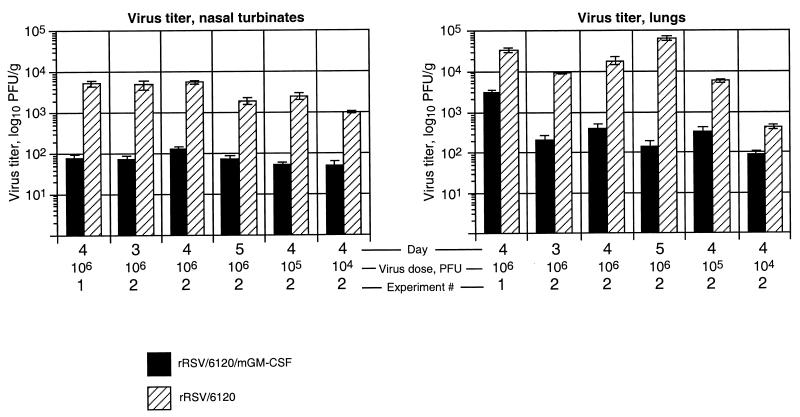
FIG. 3.
Replication of rRSV/6120/mGM-CSF and rRSV/6120 in BALB/c mice. The mice were inoculated intranasally with the indicated dose (104, 105, or 106 PFU per animal) of rRSV/6120/mGM-CSF or rRSV/6120 in two separate experiments (1 and 2). Six animals per time point were sacrificed on day 3, 4, or 5, as indicated; lung and nasal turbinate tissues were harvested; and virus titers were determined by plaque titration. The error bars indicate standard error.
FIG. 4.
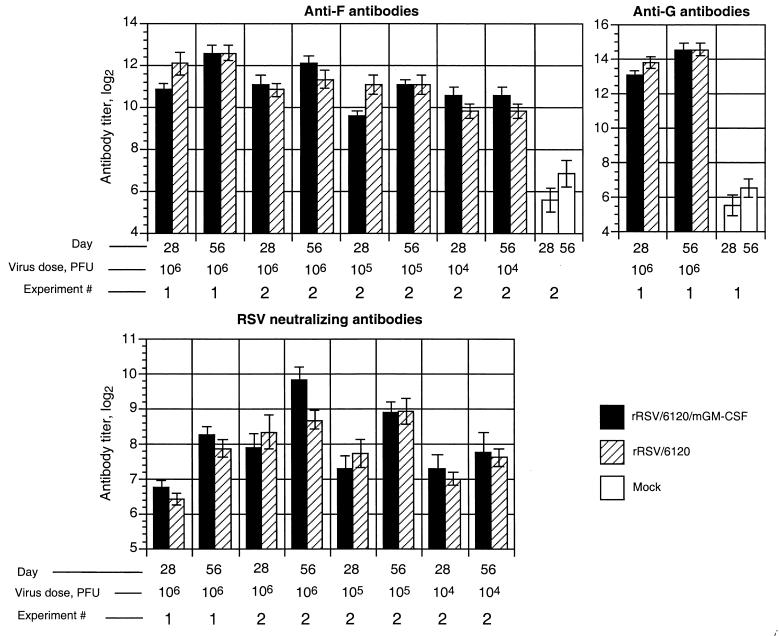
Titer of RSV-specific serum antibodies in BALB/c mice infected with rRSV/6120/mGM-CSF or rRSV/6120. The mice were mock infected (administered medium alone) or infected intranasally with the indicated dose (104, 105, or 106 PFU per animal) of rRSV/6120/mGM-CSF or rRSV/6120 as part of the two experiments (1 and 2) shown in Fig. 3. Serum samples were collected 28 and 56 days later and analyzed for IgG specific to the F or G glycoprotein by glycoprotein-specific ELISA and for RSV-neutralizing antibodies using a 60% plaque reduction assay in the presence of complement. Mean titers are shown. Each ELISA titer is the mean (±SE) for eight animals, and the neutralization titers are the means for six to eight animals.
FIG. 6.
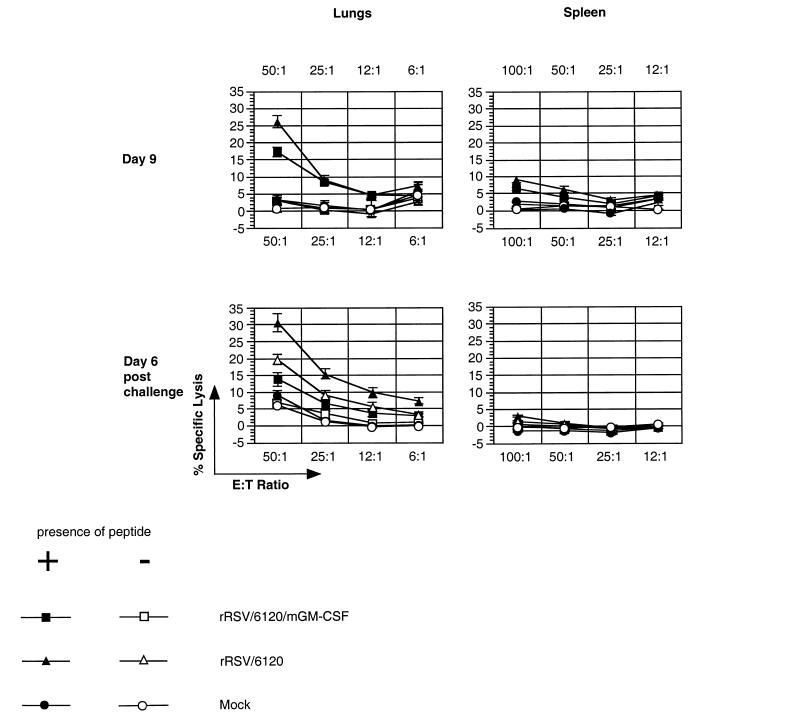
Analysis of the RSV-specific CTL response in BALB/c mice infected with rRSV/6120/mGM-CSF or rRSV/6120. Groups of mice were infected with the indicated virus or mock infected on day 0, and two (mock-infected) or five (virus-infected) animals per group were sacrificed on days 5 (not shown), 9, and 21 (not shown) and processed for CTL analysis. The remaining animals were challenged by intranasal infection with wt rRSV on day 31, and two (mock-infected) or four (virus-infected) animals were sacrificed 6 days later for CTL analysis. Mononuclear cells were isolated from lungs (left) and spleens (right), stimulated in vitro with M2–182–90 peptide, and assayed for cytotoxic activity against P815 target cells that had been incubated with M2–182–90 peptide (+) or did not receive peptide (−).
FIG. 8.
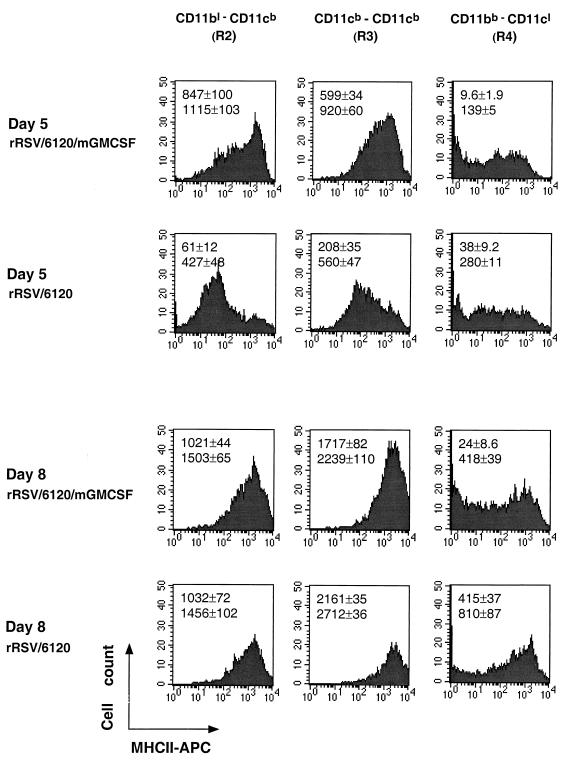
Flow cytometry analysis of the three fractions of PMC, namely, regions R2, R3, and R4 shown in Fig. 7B, for the expression of MHCII molecules. Results are shown for days 5 and 8 postinfection for a single mouse from each group of four animals. The median (upper value) and mean (lower value) level of MHCII expression (±SE) for each group of four are indicated.
Measurement of GM-CSF produced in HEp-2 cells.
Duplicate monolayers were infected with 2 PFU per cell of rRSV/mGM-CSF, rRSV/6120/mGM-CSF, an rRSV expressing the chloramphenicol acetyltransferase (CAT) gene (rRSV/CAT [9]), or wt rRSV. Samples from the medium were taken at intervals over a 96-h period, and the concentration of mGM-CSF was determined by enzyme-linked immunoadsorbent assay (ELISA) using the Quantikine M Mouse GM-CSF Immunoassay (R&D Systems).
Virus replication and immunogenicity in vivo
Eleven-week-old respiratory-pathogen-free female BALB/c mice were infected intranasally under light methoxyflurane anesthesia on day 0 with rRSV/6120/mGM-CSF, rRSV/6120, or medium alone. In experiment 1, the mice received 106 PFU per animal in a 0.1-ml inoculum, and six animals per group were sacrificed with CO2 on day 4. The nasal turbinates and lungs were harvested and assayed for infectious RSV by plaque titration (28). In experiment 2, groups of mice received doses of 106, 105, or 104 PFU of either virus. Six mice per group were sacrificed on days 3 (106 group only), 4 (all groups), and 5 (106 group only), and nasal turbinates and lung tissues were harvested and assayed for infectious RSV by plaque titration. To monitor serum antibody responses, each experiment described above also included (i) an uninfected control group and (ii) eight additional animals in each experimental group from which serum samples were taken on days 0, 28, and 56. Serum antibodies specific to the RSV F or G protein were measured by glycoprotein-specific ELISA (28), and RSV-neutralizing antibodies were measured by a 60% plaque reduction assay in the presence of complement (13).
Detection of mGM-CSF mRNA in mouse lungs by reverse transcription (RT)-PCR.
Total RNA isolated from lungs of BALB/c mice on day 4 after infection with rRSV/mGM-CSF or wt rRSV was reverse transcribed using the primer AGAAAGGTTTTAAGGCTGTC, specific for positions 537 to 556 of the GM-CSF mRNA sequence (GenBank accession number X02333). PCR was performed using the above-mentioned oligonucleotide as a direct primer and the reverse primer R1 (GTGGTCTACAGCCTCTCAGC), specific for GM-CSF mRNA positions 207 to 226, or R2 (GTGGTCTACAGCCTCTCAGCT), which has the same sequence as the primer R1 except that it has an additional T base at the 3′ end (boldface) which is specific for the silent mutation in the synthetic GM-CSF cDNA (R&D Systems) used in the construct. An initial 2-min denaturation step was performed, during which the Taq DNA polymerase was added, and then 30 cycles of PCR were performed (denaturation, 1 min at 94°C; annealing, 1 min at 45°C; elongation, 2 min at 72°C). The products were analyzed on a 2.5% agarose gel.
Analysis of pulmonary cytokine mRNAs.
BALB/c mice in groups of 20 were infected intranasally with 106 PFU of rRSV/mGM-CSF or wt rRSV or were mock infected with Opti-MEM medium. On days 1 and 4 after infection, five mice from each group were sacrificed by CO2 asphyxiation, and lung tissues were harvested and processed for purification of total lung RNA by homogenization and extraction with Trizol (Life Technologies). The remaining 10 animals per group were challenged on day 28 by the intranasal administration of 106 PFU of wt rRSV per animal. On days 1 and 4 postchallenge (29 and 32 days following the initial infection), five animals from each group were sacrificed and total lung RNA was harvested. Material from each mouse was processed separately. Cytokine mRNAs were quantitated by an RNase protection method using the RiboQuant Multi-Probe RNase Protection Assay System (PharMingen) according to the instruction manual. Briefly, mouse cytokine mRNA-specific RNA probes labeled with [32P]UTP were synthesized using the multiprobe template sets mCK-1 and mCK-2B, and each probe set was hybridized separately overnight at 56°C with pulmonary mRNA or control mouse total RNA and yeast RNA and treated with RNase A followed by proteinase K. The remaining RNA was purified with phenol and chloroform and electrophoresed on 5% polyacrylamide sequencing gel in parallel with untreated probe as a marker. Radioactive bands were quantitated using a PhosphorImager 445 SI, the background was subtracted, and each species was expressed as a percentage of the L-32 housekeeping gene mRNA in the same RNA sample.
Isolation of pulmonary and spleen mononuclear cells (PMC and SMC).
Lungs and spleens were removed following sacrifice, with material from each mouse processed separately. The lungs were rinsed, minced, and digested with 3,500 Dornase U of DNase I (Calbiochem)/ml and 75 U of collagenase (Life Technologies)/ml at 37°C for 2 h, adjusted to 0.01 M EDTA, chilled on ice, and filtered through 100-μm-pore-size nylon monofilament cloth (PGS). The cells were pelleted, resuspended, and subjected to centrifugation in Ficoll-Paque Plus solution (Amersham Pharmacia Biotech) at 400 × g and 20°C for 30 min. The PMC interface was collected, washed twice, and resuspended in 5 ml of RPMI 1640 medium (Life Technologies) containing 10% FBS, 100 U of penicillin/ml, and 100 μg of streptomycin sulfate/ml. For purification of SMC, the spleens were rinsed, mashed, washed, and subjected to differential centrifugation in Ficoll-Paque Plus solution as described above.
Assay of RSV-specific pulmonary and spleen cytotoxic T lymphocytes (CTL).
BALB/c mice (five mice per group per day) were infected with 106 PFU of rRSV/6120/mGM-CSF or rRSV/6120 or were mock infected. PMC and SMC were isolated as described above on days 5, 9, and 21 postinfection and assayed as described below. On day 31, the remaining mice were challenged with 106 PFU of wt rRSV, and the cells were isolated on day 37 (6 days postchallenge) and assayed. Cytolytic activity was measured after the freshly isolated cells were incubated overnight (7) with the peptide SYIGSINNI, representing amino acids 82 to 90 of the M2-1 protein (27). 51Cr-labeled P815 target cells were pulsed for 2 h with 1 μM RSV-specific peptide. The peptide-labeled or unlabeled target cells were mixed with PMC or SMC and incubated for 4 h at 37°C. The assays were performed in triplicate, and the percent specific lysis was calculated as follows: [(experimental release − spontaneous release)/(maximum release − spontaneous release)] × 100. Maximum release was determined from supernatants of cells that were lysed by addition of 5% Triton X-100. Spontaneous release was determined from the target cells incubated without effector cells (6).
Intracellular cytokine staining and flow cytometric analysis of pulmonary CD4+ cells.
To stimulate intracellular cytokine accumulation, freshly isolated PMC (separately from each mouse) were incubated at 37°C for 4 h in the presence of 2.5 ng of phorbol 12-myristate 13-acetate (Sigma)/ml, 250 ng of ionomycin (Sigma)/ml, and 6.5 μl of GolgiStop reagent (PharMingen)/ml. DNase I was added to a final concentration of 3,500 Dornase U/ml, and incubation was continued for an additional 10 min. The cells were pelleted and resuspended in cold Cytostain Buffer (FBS) (PharMingen). To block Fc receptors, cells at 106 per 100 μl were combined with 1 μg of purified rat anti-mouse CD16/CD32 (FcγIII/II receptor) and incubated for 15 min at 4°C. Then, the cells were diluted, pelleted, and washed, and 1 μg of Tri-Color-conjugated rat IgG2a against the mouse CD4 clone CT-CD4 (Caltag Laboratories) was added. The cells were incubated for 30 min at 4°C, washed, and simultaneously fixed and permeabilized by resuspension in 250 μl of Cytofix/Cytoperm solution (PharMingen), incubation for 20 min at 4°C, and double washing with PermWash solution (PharMingen). To detect the accumulated cytokines, the cells were resuspended in 100 μl of PermWash, and the following two fluorochrome-labeled antibodies were added in the amounts which had been optimized in preliminary experiments: (i) fluorescein isothiocyanate (FITC)-conjugated rat IgG1 against the mouse IFN-γ clone XMG1.2 (PharMingen) and (ii) R-phycoerythrin (R-PE)-conjugated rat IgG2b against the mouse IL-4 clone BVD4-1D11 (PharMingen). The cells were incubated for 30 min at 4°C in the dark, washed successively with PermWash and Cytostain buffer (FBS), and resuspended in 500 μl of Cytostain buffer (FBS) each. The specificity of staining was confirmed by isotype control and by blocking performed by 30 min of preincubation (4°C) with 5 μg of an unconjugated preparation of the same antibody. The isotype control for the FITC-conjugated IFN-γ-specific IgG1 rat antibody was FITC-conjugated rat IgG1 clone R3-34 (PharMingen), and the control for the Tri-Color-conjugated CD4-specific rat IgG2a antibody was conjugated rat IgG2a clone LO-DNP-16 (Caltag Laboratories). Flow cytometry analysis was performed using a FACSCalibur flow cytometer (Becton Dickinson), and lymphocytes were gated as described previously (24). A total of 60,000 lymphocytes were analyzed per sample, excluding dead cells.
Flow cytometric analysis of pulmonary dendritic cells and macrophages.
PMC from individual mice were treated to block Fc receptors as described above and combined with predetermined optimal amounts of the following fluorochromes added simultaneously: (i) FITC-labeled anti-mouse CD11b (clone M1/70; PharMingen), (ii) R-PE-labeled anti-mouse CD11c (clone HL3; PharMingen), and (iii) biotin-labeled anti-mouse I-Ad/I-Ed (clone 2G9; PharMingen). The cells were incubated for 30 min at 4°C in the dark, allophycocyanin-labeled streptavidin (PharMingen) was added, and the cells were incubated for a further 30 min at 4°C in the dark, washed twice with Hanks balanced salt solution–2% FBS, fixed by resuspension in 250 μl of Cytofix buffer (PharMingen) and incubation for 20 min at 4°C, and washed two more times. Flow cytometry analysis was performed, with 100,000 cells (excluding dead cells) analyzed per sample.
RESULTS
Construction and in vitro characterization of rRSV expressing mGM-CSF.
A cDNA encoding mGM-CSF was placed under the control of RSV transcription gene start and gene end signals and inserted into the G-F intergenic region of an RSV antigenomic cDNA clone (Fig. 1). Recombinant virus was recovered and designated rRSV/mGM-CSF.
The growth of rRSV/mGM-CSF in HEp-2 cells was examined and was found to be delayed and reduced compared to that of its wt rRSV parent examined in parallel, with a maximum difference of 34-fold at 40 h postinfection (Fig. 2). However, the growth kinetics of rRSV/mGM-CSF in vitro were indistinguishable from those of rRSV/CAT, a previously described recombinant that contains a 735-nucleotide transcriptional unit encoding bacterial CAT (compared to 465 nt for the present mGM-CSF insert) inserted into the same genome locus. We have previously noted that the presence of a foreign insert attenuates RSV replication in vitro irrespective of its encoded protein (9–12). The basis for this attenuation has not been characterized in detail but appears to be increased genome length.
Northern blot hybridization was used to monitor the expression of the inserted mGM-CSF gene as well as those of the RSV G, F, and L genes (data not shown). This confirmed that the mGM-CSF gene was expressed as a separate, abundant mRNA of the expected size. Transcription of the other genes was not affected significantly by the additional gene. In addition, RT-PCR analysis of viral RNA isolated from infected HEp-2 cells on the 10th passage in vitro did not reveal any evidence of deletion within the insert (data not shown), suggesting that the insert was stable, as has typically been observed for inserts in recombinant mononegaviruses. The concentration of mGM-CSF secreted into the medium of infected HEp-2 cells was approximately 1 μg/ml by 48 h postinfection (data not shown), a level of expression that was similar to those of IFN-γ and IL-2 in previous studies (11, 12).
The reduced growth of the rRSV/mGM-CSF virus in vitro impeded the preparation of a stock of sufficient titer for inoculation of mice. For one experiment (Fig. 5), rRSV/mGM-CSF was concentrated by centrifugation, but this resulted in considerable loss of overall titer due to the lability of RSV infectivity. While this work was in progress, we prepared another version of rRSV, called rRSV/6120, in which most of the downstream noncoding region of the SH gene was deleted without any change at the amino acid level (see Materials and Methods). rRSV/6120 replicated approximately fivefold more efficiently in vitro than its full-length rRSV parent, presumably due to the 112-nucleotide reduction in length. Therefore, we inserted the mGM-CSF transcription cassette into the rRSV/6120 backbone and recovered the encoded virus, rRSV/6120/mGM-CSF. This virus is identical to rRSV/mGM-CSF except for the above-mentioned noncoding deletion in the SH gene. As expected, it replicated approximately fivefold more efficiently in vitro than rRSV/mGM-CSF (not shown), and the concentration of mGM-CSF secreted into the cell medium was exactly the same as for rRSV/mGM-CSF (not shown). rRSV/6120/mGM-CSF was used for in vivo experiments (Fig. 3, 4, 6, 7, and 8 and Table 1), with the parental rRSV/6120 as the control virus.
FIG. 7.
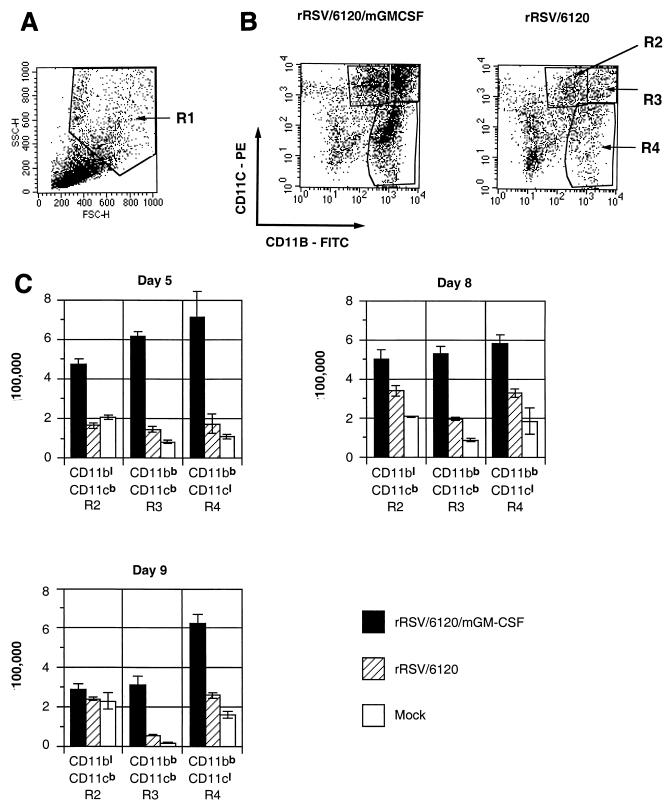
Flow cytometry analysis of dendritic cells and macrophages in the lungs of BALB/c mice infected with rRSV/6120/mGM-CSF or rRSV/6120 by flow cytometry of total PMC. (A) Cellular profile of total PMC on forward-side scatter, showing the R1 region, which is enriched for macrophage- and monocyte-like cells and depleted of lymphocytes. (B) Dot plot analysis of the R1 fraction of PMC isolated from mice on days 5, 8, and 9 postinfection with rRSV/6120/GM-CSF (left) or rRSV/6120 (right) based on the expression of CD11c versus CD11b. Three regions were identified: CD11blow/CD11cbright (region R2; lymphoid dendritic cells), CD11bbright/CD11cbright (region R3; myeloid dendritic cells), and CD11bbright/CD11clow (region R4; macrophages) (4, 33, 34). On each day, cells of four mice per group were processed and analyzed separately; each plot represents analysis of cells isolated from one mouse from each group. (C) Total number (per mouse) in regions R2, R3, and R4 of total PMC isolated on days 5, 8, and 9 from mice following infection with rRSV/6120/GM-CSF or rRSV/6120 or mock infection, as analyzed in panel B. This was calculated by multiplying the percentage of each cell population by the total number of PMC from each mouse. The results are means (±SE) based on four mice per group infected with rRSV/6120/mGM-CSF or rRSV/6120 and two mice per group for mock infection. The differences between rRSV/6120/mGM-CSF and rRSV/6120 are statistically significant (P < 0.05) for all three cell populations on all 3 days except for CD11blow/CD11cbright cells on day 9.
TABLE 1.
Flow cytometry analysis of total pulmonary CD4+ cells isolated from mice mock infected or infected with rRSV/6120/mGM-CSF or rRSV/6120a
| Day | rRSV/6120/mGM-CSF
|
rRSV/6120
|
Mock
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PMCb | CD4+
|
PMC | CD4+
|
PMC | CD4+
|
|||||||
| Totalc | IFN-γ+d | IL-4+d | Total | IFN-γ+ | IL-4+ | Total | IFN-γ+ | IL-4+ | ||||
| 5 | 151.2 ± 0.08* | 79.08 ± 2.00* | 2.44 ± 0.34† | 1.58 ± 0.08* | 55.60 ± 0.15 | 17.55 ± 1.73 | 0.57 ± 0.06 | 0.16 ± 0.02 | 12.0 | 2.51 ± 0.49 | 0.03 ± 0.00 | 0.02 ± 0.00 |
| 10 | 204.7 ± 16.99 | 86.24 ± 8.49 | 9.21 ± 1.16‡ | 0.93 ± 0.15§ | 265.6 ± 65.5 | 80.84 ± 17.33 | 5.40 ± 1.35 | 0.44 ± 0.09 | 61.0 ± 11 | 18.05 ± 3.55 | 0.23 ± 0.05 | 0.08 ± 0.02 |
| 32 | 119.5 ± 21.1 | 56.86 ± 8.26 | 4.11 ± 0.37 | 1.60 ± 0.58 | 105.5 ± 0.60 | 50.27 ± 5.62 | 3.35 ± 0.36 | 1.36 ± 0.14 | 57.0 | 11.97 | 0.31 | 0.17 |
| 38 | 47.5 ± 7.8 | 20.24 ± 2.82 | 4.93 ± 0.67 | 0.20 ± 0.04 | 51.0 ± 4.0 | 21.73 ± 1.35 | 5.48 ± 0.41 | 0.16 ± 0.01 | 77.0 ± 20.0 | 18.28 ± 4.03 | 1.85 ± 0.41 | 0.36 ± 0.09 |
Mice were infected intranasally on day 0 with 106 PFU of the indicated virus per animal or were mock infected. Animals from each group were harvested on days 5 and 10. The remaining animals were challenged on day 28 with 106 PFU of wt RSV per animal and sacrificed on days 32 and 38. The average number of isolated PMC was calculated. Total PMC were analyzed by flow cytometry with immunostaining for CD4, IFN-γ, and IL-4. Four animals per group per day were used except for the mock group, for which two, two, one, and three animals were used on days 5, 10, 32, and 38, respectively. Statistical significance was calculated by Student's t test compared to the rRSV/6120 control: *, P < 0.001; †, P < 0.01; ‡, P < 0.1; §, P < 0.05.
Mean number of total PMC per mouse (in units of 100,000) with SE.
Mean number of pulmonary CD4+ cells per mouse (in units of 100,000) with SE.
Mean number of CD4+ lymphocytes positive for IFN-γ or IL-4 following in vitro stimulation (in units of 100,000) with SE.
Replication of rRSV/6120/mGM-CSF in mice is significantly attenuated compared to that of rRSV/6120.
The replication of rRSV/6120/mGM-CSF in vivo was evaluated in parallel with that of its wt equivalent, rRSV/6120, by intranasal infection of BALB/c mice, after which the animals were sacrificed and virus titers in the lungs and nasal turbinates were determined by plaque assay (Fig. 3). In one experiment (Fig. 3, experiment 1), animals were each infected with 106 PFU of virus and the tissues were harvested on day 4. In a second experiment (Fig. 3, experiment 2), groups of animals received the same dose, 106 PFU, and were sacrificed for viral quantitation on day 3, 4, or 5 to examine the time course of infection. Other groups of animals received 105 or 104 PFU and were sacrificed for virus titration on day 4 in order to examine the extent of viral replication following a lower inoculum.
The titer of rRSV/6120/mGM-CSF was significantly lower than that of rRSV/6120 in both the upper and lower respiratory tract at all time points and all doses (P < 0.001 at any time point and any dose). For example, at the dose of 106 PFU, the difference between the two viruses was 10- to 74-fold depending on the location, time point, and particular experiment, with the exception of the lungs on day 5, when it was 588-fold. We previously showed that the presence of a foreign insert of this size at this site does not attenuate RSV in vivo by itself (11); therefore, the attenuation observed here is associated with expression of mGM-CSF.
RT-PCR was used to confirm the expression of mGM-CSF mRNA in the lungs of animals infected with rRSV/mGM-CSF (not shown). Under the conditions of the assay, mGM-CSF mRNA was not detected in mock-infected or rRSV-infected animals but was readily detected in animals infected with rRSV/mGM-CSF (not shown).
The induction of RSV-specific antibodies by rRSV/6120/mGM-CSF was not affected by its attenuation.
Mice were infected with rRSV/6120/mGM-CSF or wt rRSV/6120 at a dose of 104, 105, or 106 PFU per animal, and serum samples were taken on days 28 and 56. The titer of RSV-specific serum IgG was measured by glycoprotein-specific ELISA using purified G and F proteins, and the titer of virus-neutralizing antibodies was measured by plaque reduction assay (Fig. 4). Despite the substantial degree of attenuation of rRSV/6120/mGM-CSF, its immunogenicity was very similar to that of its wt RSV counterpart for each viral inoculum.
Increased accumulation of pulmonary IFN-γ and IL-12 p40 mRNA in response to rRSV/mGM-CSF.
Mice were infected with rRSV/mGM-CSF or wt rRSV, animals from each group were harvested 1 and 4 days later, and total pulmonary RNA was isolated. The remaining animals were challenged with wt RSV and harvested for pulmonary RNA isolation on days 29 and 32 (1 and 4 days postchallenge, respectively). The RNA samples were analyzed by an RNase protection assay with a commercially obtained kit using the probes specific to IL-1α, IL-1β, IL-3, IL-4, IL-5, IL-6, IL-9, IL-10, IL-12 p35, IL-12 p40, IL-13, IL-15, IFN-γ, IL-1 receptor antagonist, and macrophage migration inhibitory factor. An example of an autoradiogram showing direct data is shown in Fig. 5A. The most pronounced increases involved IFN-γ and IL-12 p40 mRNA, and they are summarized in Fig. 5B. The level of IFN-γ mRNA was substantially increased in response to rRSV/mGM-CSF compared to that in response to wt rRSV on days 1 and 4 postinfection, by 65% (P < 0.02) and threefold (P < 0.001), respectively (Fig. 5). No significant difference was detected after the challenge. The level of IL-12 p40 mRNA was increased by 42% (P < 0.05) in mice immunized with rRSV/mGM-CSF compared to the level in mice immunized with wt rRSV on day 4, whereas there was no significant difference on day 1. On day 29, there was no statistically significant difference, and on day 32, the level of IL-12 p40 mRNA was slightly greater (25%) in mice immunized with wt rRSV than in those immunized with rRSV/mGM-CSF (P < 0.05). IL-6 mRNA exhibited a marginal increase on day 4 in response to rRSV/6210/mGM-CSF. The remaining cytokine mRNAs were not affected by infection with either virus under these conditions.
The CTL response to rRSV/6120/mGM-CSF was moderately diminished compared to the response to rRSV/6120.
We next compared the abilities of rRSV/mGM-CSF and rRSV/6120 to stimulate RSV-specific CTL. Groups of 20 mice each were mock infected or infected with rRSV/6120/mGM-CSF or rRSV/6120. Five animals from each group were sacrificed on days 5, 9, and 21 postinfection, and the levels of RSV-specific primary spleen and pulmonary CTL were assayed using target cells that had been prepared by incubation with a synthetic peptide containing amino acids 82 to 90 of the RSV M2-1 protein. We previously showed that this sequence contains an immunodominant epitope for major histocompatibility complex class I (MHCI) (H2d)-restricted CD8+ CTL in BALB/c mice (27). The remaining five animals in each group were challenged intranasally with rRSV on day 31 and sacrificed for CTL assay on day 37 (6 days postchallenge).
RSV-specific CTL activity was undetectable for any group in the mononuclear cell fraction isolated from either the lung or the spleen on days 5 and 21 (data not shown). On day 9, pulmonary CTL activity was detected from mice infected with rRSV/6120/mGM-CSF or rRSV/6120, with the level of specific activity (percent lysis of pulse-labeled cells − percent lysis of unlabeled cells) associated with the former being somewhat lower than that for the latter: 14.24% (standard error [SE], 1.29) versus 23.05% (SE, 2.39), respectively (P < 0.02) (here and below, the effector-to-target ratio was 50:1) (Fig. 6). In the spleen at this same time point, there was a low level of RSV-specific CTL activity, which was not statistically significant between the two viruses.
RSV-specific pulmonary CTL also were detected on day 37 (6 days postchallenge) (Fig. 6). The level of CTL in mice that had received rRSV/6120/mGM-CSF in the initial inoculation was somewhat reduced compared to the level in those that had received rRSV/6120: 6.81% (SE, 1.59) versus 10.86% (SE, 1.36), however, this difference was not statistically significant. The level of RSV-specific CTLs in the spleen at this time point was negligible. Thus, expression of GM-CSF was associated with a moderate reduction of the CTL response during the initial infection.
Expression of mGM-CSF by rRSV results in increased levels of PMC, total pulmonary CD4+ T lymphocytes, and IFN-γ-positive and IL-4-positive CD4+ T lymphocytes.
Groups of BALB/c mice were infected with 106 PFU of rRSV/6120/mGM-CSF or wt rRSV/6120 or were mock infected. Animals from each group were sacrificed on days 5 and 10, and lungs were harvested and processed to isolate PMC. The remaining mice were challenged intranasally on day 28 with 106 PFU of wt rRSV and sacrificed on days 32 and 38 (4 and 10 days postchallenge), and PMC were isolated. Fresh cells were counted, stimulated in vitro to accumulate intracellular cytokines, stained immunologically for CD4, IFN-γ, and IL-4, and analyzed by flow cytometry (Table 1).
Infection with wt rRSV/6120 resulted in a substantial increase in the total number of PMC on days 5 and 10 (4.6- and 4.3-fold) compared to the mock-infected control. Following the challenge on day 28, the number of PMC was elevated on day 32, although less than for the primary infection, and diminished by day 38. The response to infection with rRSV/6120/mGM-CSF was similar, except that the increase in the number of PMC occurred more rapidly (2.7-fold greater on day 5 compared to infection with rRSV/6120).
The accumulation of pulmonary CD4+ T lymphocytes followed a similar pattern. Specifically, in wt rRSV/6120-infected mice, the numbers of pulmonary CD4+ T cells were 7.0-, 4.5-, and 4.2-fold greater on days 5, 10, and 32, respectively, than in mock-infected animals. In animals infected with rRSV/6120/mGM-CSF, this increase occurred more rapidly, such that the number of CD4+ cells on day 5 was 4.4-fold greater than in animals infected with rRSV/6120. Thus, rRSV/6120/mGM-CSF appeared to induce a more rapid increase in total PMC and CD4+ T lymphocytes during the initial infection than rRSV/6120, although the magnitudes of the responses at their peaks were similar for the two viruses.
The CD4+-T-lymphocyte response was analyzed further by intracellular cytokine staining for IFN-γ and IL-4, which are markers for the Th1 and Th2 subsets, respectively. This showed that infection with wt rRSV/6120 resulted in large increases in cells positive for IFN-γ or IL-4 (23.5- and 5.5-fold increases, respectively, on day 10 compared to the mock-infected animals). As is characteristic of this model, the number of IL-4-positive cells was substantially less than the number of IFN-γ-positive cells. Also, most of the CD4+ T lymphocytes were not positive for either cytokine. Similar results were observed in response to rRSV/6120/mGM-CSF, except that the numbers of cells positive for IFN-γ or IL-4 were higher on days 5 and 10 than those in mice infected with the rRSV/6120 parent. For example, on day 5, there were 4.2- and 9.9-fold more IFN-γ-positive and IL-4-positive CD4+ cells, respectively, in animals infected with rRSV/6120/mGM-CSF than in those infected with rRSV/6120, and on day 10 the differences were 1.7- and 2.1-fold, respectively. Thus, the total magnitude of the response of each of the two CD4+ subsets, as well as the rapidity of the response, was greater in animals infected with rRSV/6120/mGM-CSF.
These results showed that expression of mGM-CSF during RSV infection was associated with a more rapid increase in PMC, total pulmonary CD4+ T lymphocytes, IFN-γ-positive pulmonary CD4+ T cells, and IL-4-positive CD4+ T cells during the initial infection. The proportionate stimulations of the Th1 and Th2 subsets were similar. The response to the wt rRSV challenge did not appear to be greatly different whether the initial infecting virus was rRSV/6120 or rRSV/6120/GM-CSF.
Increased pulmonary dendritic cells and macrophages in response to expression of mGM-CSF.
Groups of mice were infected with rRSV/6120/mGM-CSF or rRSV/6120 (four mice per group per day) or mock infected (two mice per group per day). Total PMC were isolated on days 5, 8, and 9 postinfection; labeled for CD11b, CD11c, and MHCII molecules; and analyzed by flow cytometry. The nonlymphoid fraction of freshly isolated PMC was identified by forward-side scatter characteristics (region R1) (Fig. 7A) and was analyzed further on the basis of expression of CD11b and CD11c (Fig. 7B) to identify three populations, namely, resident lung lymphoid dendritic cells (CD11blow/CD11cbright; region R2), myeloid dendritic cells (CD11bbright/CD11cbright; region R3), and macrophages (CD11bbright/CD11clow; region R4). Dot plots for representative individual mice are shown in Fig. 7B, and the calculated mean number of cells of each population is shown in Fig. 7C.
Infection with rRSV/6120 did not significantly increase the accumulation of any of these cell populations on any day compared to the mock-infected control, although for each population on each day the mean cell number was marginally greater for the rRSV/6120-infected animals. In contrast, infection with rRSV/6120/GM-CSF resulted in a large increase (up to fourfold) in each cell population on each day, with the exception of R2 on day 9.
Activation of pulmonary dendritic cells in mice infected with rRSV/6120/mGM-CSF.
We further analyzed the R2 (lymphoid dendritic cell), R3 (myeloid dendritic cell), and R4 (macrophage) cell subpopulations isolated on days 5, 8, and 9 postinfection to quantify the expression of MHCII molecules as a measure of activation. The results for days 5 and 8 are shown in Fig. 8. On day 5, the mean expression of MHCII was increased for the lymphoid and myeloid dendritic cells (R2 and R3 regions, respectively) from mice infected with rRSV/6120/mGM-CSF compared to those infected with wt rRSV/6120 (2.6- and 1.6-fold greater mean fluorescence). In contrast, the mean fluorescence of MHCII molecules in the macrophage subpopulation (R4 region) was 2.0-fold lower in animals infected with rRSV/6120/mGM-CSF. However, since the overall number of macrophages in these animals was increased 4.0-fold, the increase in numbers of MHCII-positive cells may compensate for that. On days 8 (Fig. 8) and 9 (not shown), the mean levels of expression of MHCII on lymphoid dendritic cells (R2 region) were similar for mice infected with the two viruses, but that for myeloid dendritic cells (R3) and macrophages (R4) was somewhat lower for mice infected with wt rRSV/6120/mGM-CSF versus rRSV/6120, although as indicated above, this was offset by the greater number of dendritic cells and macrophages in animals infected with rRSV/6120/mGM-CSF.
DISCUSSION
A promising strategy for a pediatric RSV vaccine involves immunization by intranasal infection with live attenuated RSV (36). rRSV can be attenuated by the staged introduction of known attenuating mutations via the cDNA intermediate, and various candidates are under clinical evaluation. However, all of the attenuating mutations that have been identified to date reduce the magnitude of viral replication in vivo. Unfortunately, this also appears to reduce the immunogenicity of the virus, probably due to a decreased number of infected cells and decreased antigen expression. For example, infection of chimpanzees with the attenuated vaccine candidate cpts248/404 induced a titer of RSV-neutralizing serum antibodies that was reduced 9.2-fold compared to that with wt RSV (19). A second problem associated with any pediatric RSV vaccine is the fact that the immune response of young infants, the vaccine target population, is reduced due to immunologic immaturity and the immunosuppressive effects of maternally derived RSV-specific serum antibodies present in that age group. To test the possibility of improving the immunogenicity of RSV, we constructed an rRSV expressing mGM-CSF and evaluated its replication and immunogenicity in mice. GM-CSF is well known to be a major stimulatory cytokine for dendritic cells, representing the most efficient antigen-presenting cells, and for macrophages, which have important roles in innate and adaptive immunity as effector cells, as cytokine-secreting cells, and in antigen presentation.
Replication of rRSV/6120/mGM-CSF in the respiratory tracts of mice was reduced approximately 50-fold compared to the parental virus, an effect that was specific to mGM-CSF. Thus, expression of this cytokine attenuated the virus. Despite this high degree of attenuation, rRSV/6120/mGM-CSF was at least as immunogenic as the control virus with regard to the induction of RSV-binding and RSV-neutralizing serum antibodies. As described below, this probably reflects the increased accumulation and activation of pulmonary antigen-presenting cells. On the other hand, there was a modest reduction in the induction of RSV-specific CD8+ CTL during the initial infection. This might be a consequence of reduced antigen expression due to attenuation of the virus.
Infection with rRSV/mGM-CSF was associated with an increased accumulation of pulmonary mRNA for IFN-γ and IL-12 p40 compared to infection with wt rRSV. The increased production of IFN-γ likely reflects increased stimulation of CD4+ T lymphocytes as detected by flow cytometry. NK cells can be activated by GM-CSF, and they represent another likely source of IFN-γ, particularly on day 1 postinfection. Monocytes, macrophages, and dendritic cells probably were the source for the increased expression of IL-12 p40 mRNA, either as a direct effect of mGM-CSF or due to the increased CD4+ T cell help and expression of IFN-γ. Interestingly, the magnitude of expression of pulmonary IFN-γ mRNA in response to rRSV/mGM-CSF was very similar to that described previously for a comparable rRSV expressing mIFN-γ (11) and thus represents a high level. Indeed, the level of attenuation of rRSV/mGM-CSF was very similar to that observed previously for rRSV/mIFN-γ, suggesting that attenuation in each case was mediated in large part by the antiviral effects of IFN-γ.
We also examined the accumulation and activation of PMC, and in particular of subpopulations representing CD4+ T lymphocytes, lymphoid dendritic cells, myeloid dendritic cells, and macrophages. Infection with rRSV/6120 resulted in a large, rapid increase in total PMC compared to mock-infected controls, and this increase occurred more rapidly during infection with rRSV/6120/mGM-CSF (2.7-fold greater on day 5 than during infection with rRSV/6120). Similarly, infection with rRSV/6120 resulted in a large, rapid increase in the number of CD4+ T lymphocytes, and this also occurred more rapidly during infection with rRSV/6120/mGM-CSF (4.5-fold greater on day 5 than in infection with rRSV/6120). The number of CD4+ T lymphocytes expressing IFN-γ (Th1 cells) or IL-4 (Th2 cells) also was increased with rRSV/6120/mGM-CSF compared to wt rRSV/6120, and this difference was observed on both days 5 and 10 and thus involved both a more rapid and a higher-magnitude response. Thus, infection with RSV resulted in a substantial increase in pulmonary CD4+ lymphocytes that involved both the Th1 and Th2 subsets and was not strongly biased towards either, and these effects were augmented by the expression of mGM-CSF. In contrast, the CD4+ response to the wt RSV challenge was the same whether the initial infection involved rRSV/6120 or rRSV/6120/mGM-CSF. As indicated by this finding, the cytokines that we have expressed from rRSV to date do not appear to skew the immune response to subsequent exposure to RSV antigens, which probably is a desirable property.
The most striking effect associated with expression of mGM-CSF was the large increase in pulmonary lymphoid and myeloid dendritic cells and pulmonary macrophages (up to fourfold, compared to rRSV/6120). This difference was greatest on day 5 but also was apparent on days 8 and 9 following infection. These cells also exhibited increased expression of MHCII molecules, a marker of activation, in response to rRSV/6120/mGM-CSF compared to that in response to rRSV/6120: on day 5 the level of MHCII expression was greater for the dendritic cells on the basis of both mean expression and number of positive cells, whereas for macrophages and myeloid dendritic cells on days 8 and 9, the mean expression was somewhat reduced, but the greater number of positive cells may compensate for that. These results indicate that mGM-CSF produced by the rRSV acted to increase the accumulation, proliferation, and activation of pulmonary dendritic cells and macrophages. This likely resulted in a higher level of antigen presentation in the context of MHCII molecules and increased stimulation of CD4+ T lymphocytes and B cells, accounting for the high level of RSV-specific antibodies despite the reduced level of virus replication.
It should be noted that the mouse is only semipermissive to RSV replication. As indicated in Fig. 3, the peak levels of virus replication are not high, and the duration of replication peaks at day 4 or 5 and rapidly declines (3). The greatest number of dendritic cells and macrophages, and the highest level of activation, was observed at that time. The slightly lower levels of activation observed on days 8 and 9 occurred at a time when virus shedding is not detected and infection has largely been resolved. This might explain the reduced level of MHCII expression for myeloid dendritic cells and macrophages on those days. If this is so, it might be that the short-lived nature of the infection in BALB/c mice under these conditions results in an incomplete evaluation of the effects of expression of mGM-CSF. For example, the rapid resolution of infection would result in a rapid cessation of mGM-CSF production, which might limit the recruitment, proliferation, and activation of the dendritic cells and macrophages. It will be important to evaluate the effect of expression of immune modulatory molecules such as GM-CSF in primates, where RSV infection is more long-lived and closely resembles infection in humans.
ACKNOWLEDGMENTS
We thank Myron Hill, Kim Tran, Ernie Williams, and Fatemah Dawoodi for technical assistance. We also thank David Stephany of the NIAID Flow Cytometry Section for skilled assistance, advice, and the use of equipment.
This work is part of a continuing program of research and development with Wyeth Lederle Vaccines through CRADA grants AI-000087 and AI-000099.
REFERENCES
- 1.Ahlers J D, Dunlop N, Alling D W, Nara P L, Berzofsky J A. Cytokine-in-adjuvant steering of the immune response phenotype to HIV-1 vaccine constructs: granulocyte-macrophage colony-stimulating factor and TNF-alpha synergize with IL-12 to enhance induction of cytotoxic T lymphocytes. J Immunol. 1997;158:3947–3958. [PubMed] [Google Scholar]
- 2.American Academy of Pediatrics Committee on Infectious Diseases and Committee on Fetus and Newborn. Prevention of respiratory syncytial virus infections: indications for the use of palivizumab and update on the use of RSV-IGIV. Pediatrics. 1998;102:1211–1216. doi: 10.1542/peds.102.5.1211. [DOI] [PubMed] [Google Scholar]
- 3.Anderson J J, Norden J, Saunders D, Toms G L, Scott R. Analysis of the local and systemic immune responses induced in BALB/c mice by experimental respiratory syncytial virus infection. J Gen Virol. 1990;71:1561–1570. doi: 10.1099/0022-1317-71-7-1561. [DOI] [PubMed] [Google Scholar]
- 4.Anjuere F, Martin P, Ferrero I, Fraga M, del Hoyo G, Wright N, Ardavin C. Definition of dendritic cell subpopulations present in the spleen, Peyer's patches, lymph nodes, and skin of the mouse. Blood. 1999;93:590–598. [PubMed] [Google Scholar]
- 5.Belyakov I M, Ahlers J D, Clements J D, Strober W, Berzofsky J A. Interplay of cytokines and adjuvants in the regulation of mucosal and systemic HIV-specific CTL. J Immunol. 2000;165:6454–6462. doi: 10.4049/jimmunol.165.11.6454. [DOI] [PubMed] [Google Scholar]
- 6.Belyakov I M, Derby M A, Ahlers J D, Kelsall B L, Earl P, Moss B, Strober W, Berzofsky J A. Mucosal immunization with HIV-1 peptide vaccine induces mucosal and systemic cytotoxic T lymphocytes and protective immunity in mice against intrarectal recombinant HIV-vaccinia challenge. Proc Natl Acad Sci USA. 1998;95:1709–1714. doi: 10.1073/pnas.95.4.1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Belyakov, I. M., J. Wang, R. Koka, J. D. Ahlers, J. T. Snyder, R. Tse, J. Cox, J. S. Gibbs, D. Margulies, and J. A. Berzofsky. Activating CTL precursors to reveal the CTL repertoire without skewing caused by in vitro stimulation. Eur. J. Immunol, in press. [DOI] [PubMed]
- 8.Bermingham A, Collins P L. The M2-2. protein of human respiratory syncytial virus is a regulatory factor involved in the balance between RNA replication and transcription. Proc Natl Acad Sci USA. 1999;96:11259–11264. doi: 10.1073/pnas.96.20.11259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bukreyev A, Camargo E, Collins P L. Recovery of infectious respiratory syncytial virus expressing an additional, foreign gene. J Virol. 1996;70:6634–6641. doi: 10.1128/jvi.70.10.6634-6641.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bukreyev A, Murphy B R, Collins P L. Respiratory syncytial virus can tolerate an intergenic sequence of at least 160 nucleotides with little effect on transcription or replication in vitro and in vivo: J. Virol. 2000;74:11017–11026. doi: 10.1128/jvi.74.23.11017-11026.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bukreyev A, Whitehead S S, Bukreyeva N, Murphy B R, Collins P L. Interferon gamma expressed by a recombinant respiratory syncytial virus attenuates virus replication in mice without compromising immunogenicity. Proc Natl Acad Sci USA. 1999;96:2367–2372. doi: 10.1073/pnas.96.5.2367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bukreyev A, Whitehead S S, Prussin C, Murphy B R, Collins P L. Effect of coexpression of interleukin-2 by recombinant respiratory syncytial virus on virus replication, immunogenicity, and production of other cytokines. J Virol. 2000;74:7151–7157. doi: 10.1128/jvi.74.15.7151-7157.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coates H V, Alling D W, Chanock R M. An antigenic analysis of respiratory syncytial virus isolates by a plaque reduction neutralization test. Am J Epidemiol. 1966;83:299–313. doi: 10.1093/oxfordjournals.aje.a120586. [DOI] [PubMed] [Google Scholar]
- 14.Collins P L, Hill M G, Camargo E, Grosfeld H, Chanock R M, Murphy B R. Production of infectious human respiratory syncytial virus from cloned cDNA confirms an essential role for the transcription elongation factor from the 5′ proximal open reading frame of the M2 mRNA in gene expression and provides a capability for vaccine development. Proc Natl Acad Sci USA. 1995;92:11563–11567. doi: 10.1073/pnas.92.25.11563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Collins P L, Hill M G, Cristina J, Grosfeld H. Transcription elongation factor of respiratory syncytial virus, a nonsegmented negative-strand RNA virus. Proc Natl Acad Sci USA. 1996;93:81–85. doi: 10.1073/pnas.93.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Collins P L, McIntosh K, Chanock R M. Respiratory syncytial virus. In: Fields B N, Knipe D M, Howley P M, Chanock R M, Melnick J L, Monath T P, Roizman B, Straus S E, editors. Fields virology. 3rd ed. Vol. 2. Philadelphia, Pa: Lippincott-Raven; 1996. pp. 1313–1352. [Google Scholar]
- 17.Connors M, Collins P L, Firestone C Y, Murphy B R. Respiratory syncytial virus (RSV) F, G, M2 (22K), and N proteins each induce resistance to RSV challenge, but resistance induced by M2 and N proteins is relatively short-lived. J Virol. 1991;65:1634–1637. doi: 10.1128/jvi.65.3.1634-1637.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Connors M, Giese N A, Kulkarni A B, Firestone C Y, Morse H C R, Murphy B R. Enhanced pulmonary histopathology induced by respiratory syncytial virus (RSV) challenge of formalin-inactivated RSV-immunized BALB/c mice is abrogated by depletion of interleukin-4 (IL-4) and IL-10. J Virol. 1994;68:5321–5325. doi: 10.1128/jvi.68.8.5321-5325.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Crowe J E, Jr, Bui P T, Davis A R, Chanock R M, Murphy B R. A further attenuated derivative of a cold-passaged temperature-sensitive mutant of human respiratory syncytial virus retains immunogenicity and protective efficacy against wild-type challenge in seronegative chimpanzees. Vaccine. 1994;12:783–790. doi: 10.1016/0264-410x(94)90286-0. [DOI] [PubMed] [Google Scholar]
- 20.Disis M L, Bernhard H, Shiota F M, Hand S L, Gralow J R, Huseby E S, Gillis S, Cheever M A. Granulocyte-macrophage colony-stimulating factor: an effective adjuvant for protein and peptide-based vaccines. Blood. 1996;88:202–210. [PubMed] [Google Scholar]
- 21.Dranoff G, Jaffee E, Lazenby A, Golumbek P, Levitsky H, Brose K, Jackson V, Hamada H, Pardoll D, Mulligan R C. Vaccination with irradiated tumor cells engineered to secrete murine granulocyte-macrophage colony-stimulating factor stimulates potent, specific, and long-lasting anti-tumor immunity. Proc Natl Acad Sci USA. 1993;90:3539–3543. doi: 10.1073/pnas.90.8.3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hancock G E, Speelman D J, Heers K, Bortell E, Smith J, Cosco C. Generation of atypical pulmonary inflammatory responses in BALB/c mice after immunization with the native attachment (G) glycoprotein of respiratory syncytial virus. J Virol. 1996;70:7783–7791. doi: 10.1128/jvi.70.11.7783-7791.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hussell T, Baldwin C J, O'Garra A, Openshaw P J. CD8+ T cells control Th2-driven pathology during pulmonary respiratory syncytial virus infection. Eur J Immunol. 1997;27:3341–3349. doi: 10.1002/eji.1830271233. [DOI] [PubMed] [Google Scholar]
- 24.Hussell T, Spender L C, Georgiou A, O'Garra A, Openshaw P J. Th1 and Th2 cytokine induction in pulmonary T cells during infection with respiratory syncytial virus. J Gen Virol. 1996;77:2447–2455. doi: 10.1099/0022-1317-77-10-2447. [DOI] [PubMed] [Google Scholar]
- 25.Iwasaki A, Stiernholm B J, Chan A K, Berinstein N L, Barber B H. Enhanced CTL responses mediated by plasmid DNA immunogens encoding costimulatory molecules and cytokines. J Immunol. 1997;158:4591–4601. [PubMed] [Google Scholar]
- 26.Kim H W, Canchola J G, Brandt C D, Pyles G, Chanock R M, Jensen K, Parrott R H. Respiratory syncytial virus disease in infants despite prior administration of antigenic inactivated vaccine. Am J Epidemiol. 1969;89:422–434. doi: 10.1093/oxfordjournals.aje.a120955. [DOI] [PubMed] [Google Scholar]
- 27.Kulkarni A B, Collins P L, Bacik I, Yewdell J W, Bennink J R, Crowe J E, Jr, Murphy B R. Cytotoxic T cells specific for a single peptide on the M2 protein of respiratory syncytial virus are the sole mediators of resistance induced by immunization with M2 encoded by a recombinant vaccinia virus. J Virol. 1995;69:1261–1264. doi: 10.1128/jvi.69.2.1261-1264.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Murphy B R, Sotnikov A V, Lawrence L A, Banks S M, Prince G A. Enhanced pulmonary histopathology is observed in cotton rats immunized with formalin-inactivated respiratory syncytial virus (RSV) or purified F glycoprotein and challenged with RSV 3 to 6 months after immunization. Vaccine. 1990;8:497–502. doi: 10.1016/0264-410x(90)90253-i. [DOI] [PubMed] [Google Scholar]
- 29.Okada E, Sasaki S, Ishii N, Aoki I, Yasuda T, Nishioka K, Fukushima J, Miyazaki J, Wahren B, Okuda K. Intranasal immunization of a DNA vaccine with IL-12- and granulocyte-macrophage colony-stimulating factor (GM-CSF)-expressing plasmids in liposomes induces strong mucosal and cell-mediated immune responses against HIV-1 antigens. J Immunol. 1997;159:3638–3647. [PubMed] [Google Scholar]
- 30.Openshaw P J, Hewitt C. Protective and harmful effects of viral infections in childhood on wheezing disorders and asthma. Am J Respir Crit Care. 2000;162:S40–S43. doi: 10.1164/ajrccm.162.supplement_1.maic-11. [DOI] [PubMed] [Google Scholar]
- 31.Schlender J, Bossert B, Buchholz U, Conzelmann K K. Bovine respiratory syncytial virus nonstructural proteins NS1 and NS2 cooperatively antagonize alpha/beta interferon-induced antiviral response. J Virol. 2000;74:8234–8242. doi: 10.1128/jvi.74.18.8234-8242.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Srikiatkhachorn A, Braciale T J. Virus-specific CD8+ T lymphocytes downregulate T helper cell type 2 cytokine secretion and pulmonary eosinophilia during experimental murine respiratory syncytial virus infection. J Exp Med. 1997;186:421–432. doi: 10.1084/jem.186.3.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vremec D, Lieschke G, Dunn A, Robb L, Metcalf D, Shortman K. The influence of granulocyte/macrophage colony-stimulating factor on dendritic cell levels in mouse lymphoid organs. Eur J Immunol. 1997;27:40–44. doi: 10.1002/eji.1830270107. [DOI] [PubMed] [Google Scholar]
- 34.Wang J, Snider D, Hewlett B, Lukacs N, Gauldie J, Liang H, Xing Z. Transgenic expression of granulocyte-macrophage colony-stimulating factor induces the differentiation and activation of a novel dendritic cell population in the lung. Blood. 2000;95:2337–2345. [PubMed] [Google Scholar]
- 35.Waris M E, Tsou C, Erdman D D, Day D B, Anderson L J. Priming with live respiratory syncytial virus (RSV) prevents the enhanced pulmonary inflammatory response seen after RSV challenge in BALB/c mice immunized with formalin-inactivated RSV. J Virol. 1997;71:6935–6939. doi: 10.1128/jvi.71.9.6935-6939.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wright P, Karron R, Belshe R, Thompson J, Crowe J, Jr, Boyce T, Halburnt L, Reed G, Whitehead S, Anderson E, Wittek A, Casey R, Eichelberger M, Thumar B, Randolph V, Udem S, Chanock R, Murphy B. Evaluation of a live, cold-passaged, temperature-sensitive, respiratory syncytial virus vaccine candidate in infancy. J Infect Dis. 2000;182:1331–1342. doi: 10.1086/315859. [DOI] [PubMed] [Google Scholar]
- 37.Xiang Z, Ertl H C. Manipulation of the immune response to a plasmid-encoded viral antigen by coinoculation with plasmids expressing cytokines. Immunity. 1995;2:129–135. doi: 10.1016/s1074-7613(95)80001-8. [DOI] [PubMed] [Google Scholar]