Abstract
Psilocybin is a classic psychedelic with demonstrated preliminary clinical efficacy in a range of psychiatric disorders. Evaluating the impact of psilocybin on cognitive function is essential to unravel its potential benefits and risks. In this systematic review, we assessed psilocybin's effect on cognitive function through a comprehensive search of electronic databases from inception to January 2024, identifying 20 articles involving 2,959 participants. While 85% of studies were conducted in healthy volunteers, most of these studies (85%) used macrodoses, ranging from 45 μg/kg to 30 mg/70 kg. Various cognitive aspects were evaluated and yielded mixed results. Global cognitive function, and processing speed remained mostly unchanged in healthy individuals; However, a limited number of studies reported improvements in certain areas such as sustained attention, working memory, and executive function especially in patients with treatment‐resistant depression (TRD). Emotional processing was positively modified, particularly in TRD patients. Psilocybin was observed to enhance emotional empathy without significantly altering cognitive empathy and social cognition. Cognitive flexibility and creative cognition were noted to initially decline but could potentially improve over time. Additionally, with respect to learning and memory skills, psilocybin showed promise in improving specific memory types such as semantic associations and associative learning, while its effects on episodic and verbal memory have been less pronounced compared to other cognitive enhancers. The observed mixed findings underscore the complexity of psilocybin's cognitive influence. Further research is essential to provide a clearer understanding of psilocybin's impact on cognitive domains and to guide the development of safe and effective interventions.
Keywords: cognition, cognitive function, psilocybin, psychedelics, systematic review
Classic psychedelics include psilocybin {3‐(2‐[dimethylamino] ethyl)‐1H‐indol‐4‐yl dihydrogen phosphate}, N,N‐dimethyltryptamine and ergolines such as lysergic acid diethylamide. 1 Psilocybin was first discovered in mushrooms of the Psilocybe genus, and is dephosphorylated in the small intestines to its active metabolite, psilocin, which has a range of receptor interactions, including serotonin (5‐HT) receptors 1A, 2A, 2B and 2C agonist effects. 1 , 2 Of note, the activation of the 5‐HT2A receptor by psilocin is proposed to confer hallucinogenic effects. 1 , 2 Preliminary evidence supports the efficacy of psilocybin in several psychiatric conditions including major depressive disorder (MDD), treatment‐resistant depression (TRD), obsessive–compulsive disorder, alcohol and nicotine dependence, and cancer‐related anxiety. 2 , 3 , 4 , 5 , 6 Starting from the early to mid‐1960s, psilocybin found its application in psychiatric medicine and psychodynamic‐oriented psychotherapy. 7 Its initial isolation by Albert Hofmann in 1957, followed by synthesis in 1958 by Sandoz Pharmaceuticals: Basel, Switzerland, under the trade name Indocybin, played a crucial role in its development. 7 , 8 However, the progress of psilocybin research faced a significant setback when it was legally sanctioned and classified as a schedule 1 substance due to international legal restrictions. 9 Nonetheless, there has been renewed interest and resurgence in studying psilocybin's effects since the mid‐1990s. 10
Dosing with psilocybin results in an altered state of consciousness, characterized by shifts in emotional state and perception. 11 The psychological impacts of psilocybin encompass feelings of euphoria, hallucinations, dissociation, and altered cognitive states as well as impacting empathy, creativity, and subjective well‐being. 12 , 13 The therapeutic advancement of psilocybin for mental health disorders relies on targeting the neural mechanisms of cognitive and emotional processing. 14 Studies have revealed that serotonergic psychedelics can acutely alter activity within the neural circuits. 14 Importantly, psychedelic‐induced changes align with the concept of neuroplasticity, emphasizing the brain's capacity to undergo adaptive and transformative structural and functional alterations, thereby impacting cognition. 15
Cognitive deficits play a significant role in the development, progression, and treatment of mental disorders. 16 Many psychiatric disorders are characterized by impairments in cognitive function, which can significantly hinder a person's ability to function in real‐world situations. 17 The serotonin system, which is targeted by psychedelic compounds, may represent a promising target for pharmacological modulation of cognitive functioning and has been implicated in the pathophysiology of various mental health disorders. 17 , 18 Therefore, it is necessary to better understand the short‐ and long‐term effects of psilocybin on cognition.
In the realm of research with psilocybin, there exists a notable gap of knowledge regarding its effects on cognitive processes such as attention, memory, and executive functions in humans. Existing research, although limited, suggests the potential for psilocybin to influence cognition through its interaction with 5‐HT receptors and functionally downregulating default mode network (DMN) activity. 19 , 20 However, a comprehensive understanding of the specific cognitive mechanisms involved, the short‐ and long‐term effects, and potential therapeutic implications of psilocybin on cognitive function, is still lacking. Understanding psilocybin's impact on cognitive functions is crucial for elucidating its therapeutic potential and associated risks, essential for its safe and effective clinical use. Addressing this knowledge gap will inform evidence‐based decisions, optimizing psilocybin's application in treating psychological and cognitive conditions. This systematic review aims to comprehensively evaluate psilocybin's cognitive effects, guiding future research and clinical practice in psychedelic medicine.
Methods
Search strategy
We conducted a comprehensive search on three databases (MEDLINE, PsycINFO, Embase) through OVID from inception to January 2024. We adhered to Preferred Reporting Items for Systematic Review and Meta‐Analysis Statement for this systematic review. 21 Relevant articles were identified using the following keywords: (psilocybin or magic mushrooms or psilocybine or psilocibin or psiloc*) AND (cognition or cognitive or cognitive function or CI or cognitive impairment or cognitive disorder or memory or executive function or global impairment or neuropsychological test or speed or attention or processing or memory disorder or processing speed or executive control or cognitive processes or cognitive decline or cognition test). Further articles were identified by conducting searches on Google Scholar and manually examining the reference lists of relevant papers. No limitations were placed on publication date or language during this screening process.
The search for past and ongoing clinical trials was performed on ClinicalTrials.gov (https://clinicaltrials.gov/). The search terms ‘psilocybin’ and ‘cognition’ were utilized, without any limitations on the study status. The search was conducted on May 10, 2023.
Eligibility criteria
To streamline the screening process and eliminate duplicate articles, all search results were imported into the Covidence platform (covidence.org) for systematic review management. Two independent reviewers (S. M., T. J. T. G.) performed a comprehensive screening of all articles in two distinct stages. For the first level screening, the reviewers assessed the titles and abstracts of the articles. Subsequently, for the second‐level screening, full texts were thoroughly reviewed. Conflicts between two reviewers were resolved through discussion and consultation with V. B. Studies were included if they were: (i) original studies; (ii) evaluated the effect of psilocybin in humans with focus on cognitive function in both clinical or non‐clinical populations using at least one neurocognitive assessment as a primary or secondary outcome; (iii) included participants were not diagnosed with comorbid structural or biologically based brain disease or traumatic brain injury, mild cognitive impairment, or dementia; and (iv) in English. Any study designs (clinical trials, observational studies, and case reports/series) that captured the data on the effect of psilocybin on cognition were allowed. Systematic reviews, narrative reviews, meta analyses, animal studies, studies using the same dataset and letters to the editor were excluded.
Data extraction
Two reviewers independently (S. M., T. J. T. G.) reviewed the full text of eligible articles and extracted the following data: year of publication, author, study design, country, patient demographics (mean age, percentage female, sample size), outcome measure, intervention, study design, results, adverse events, therapy principles and conclusion. Furthermore, relevant information such as sample size, clinical diagnosis, study design, treatment administration method and dosage, as well as start, registration, and completion dates, were extracted from the registered clinical trials.
Quality assessment
For randomized trials, the Cochrane Collaboration's tool is used to assess the risk of bias. This tool examines five domains: bias from the randomization method, bias due to deviations from intended interventions, bias arising from missing outcome data, bias in outcome assessment, and bias in the selection of reported results. 22 , 23
Results
Search results
The systematic search resulted in 348 records. We removed duplicates (n = 4) and screened the title and abstract of the remaining records (n = 344). Three hundred and fourteen records were excluded based on our inclusion criteria. Subsequently, we reviewed the full‐text of 30 records. Out of 30 records, 10 records were excluded; six had no neurocognitive assessment and four did not report results pertaining to cognitive outcomes. Finally, 20 articles involving 2,959 participants met the inclusion criteria and were included in this systematic review. 12 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 Study selection details are indicated in Figure 1 and the quality assessment results are outlined in Fig. S1. We also included eight registered clinical trials; detailed characteristics can be found in Table S1 and Figs. S2 and S3.
Fig. 1.
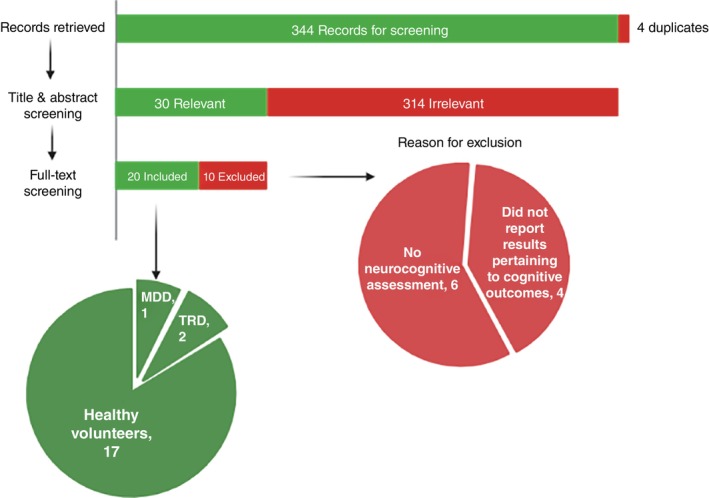
The Preferred Reporting Items for Systematic Review and Meta‐Analysis (PRISMA) flow diagram illustrates the systematic review process, indicating the number of studies included (shown in green) and excluded (shown in red) at each stage.
Characteristics of included published studies
The majority of studies enrolled healthy participants (85%) (Fig. 2), with most focusing on acute (short‐term) (12 studies [60%]) cognitive effects of psilocybin, rather than long‐term effects (Fig. 3a,b). While the majority of studies focused on healthy subjects (85%), three studies (15%) specifically assessed the impact of psilocybin on cognition in individuals with MDD 24 and TRD. 25 , 26 Several key distinctions among studies revolved around the doses administered, the targeted population (healthy individuals vs psychiatric disorders), and the timing of cognitive assessments relative to psilocybin administration. Most studies utilized doses ranging from 115 to 315 μg/kg, with only two studies opting for higher doses of 30 mg/70 kg (Fig. 4). 24 , 27 Among the six studies 12 , 25 , 26 , 27 , 28 , 30 employing graded psilocybin doses, four revealed a dose‐dependent gradient. Additionally, two studies investigated the cognitive effects of microdosing (0.5–0.7 g of dried psilocybin mushrooms). 32 , 33 In two of these studies, treatment involved two dosing sessions of psilocybin alongside psychotherapy. Variety of cognitive domains such as global cognitive function, attention, processing speed, working memory, emotional processing, social cognition, empathy, cognitive flexibility, creative cognition, memory, learning and executive function were evaluated in included studies. Detailed characteristics of included studies are summarized in Tables 1, 2, Figs. S4 and S5. Figure 5 indicates the overall psilocybin effect on different cognitive function domains.
Fig. 2.
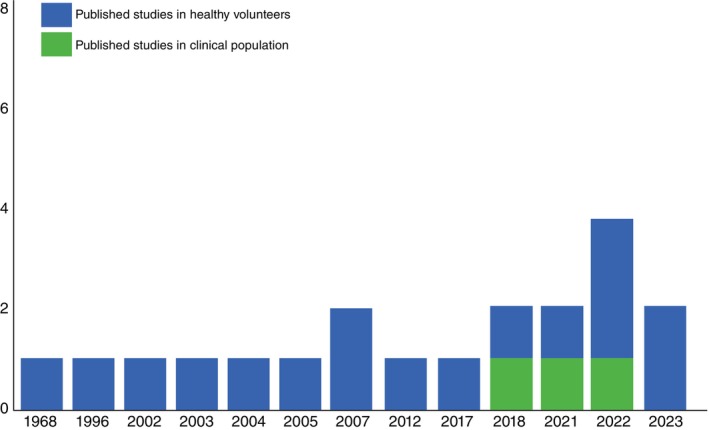
Publication year of the published studies until January 2024. Registration year of the included clinical trials, along with their corresponding completion status and reported reasons for trial withdrawal.
Fig. 3.
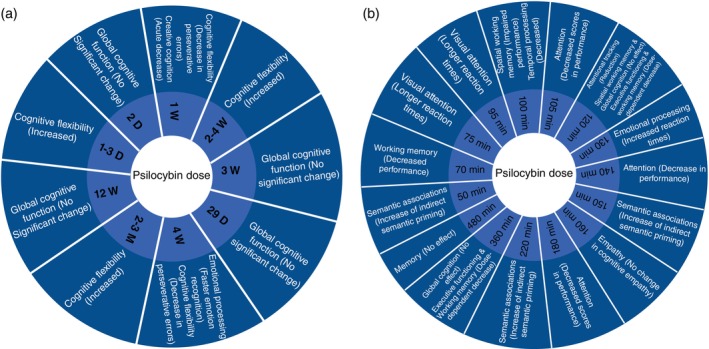
Psilocybin effect on different domains of cognitive function at assessed timepoints during: (a) Post‐acute. (b) Acute phases.
Fig. 4.
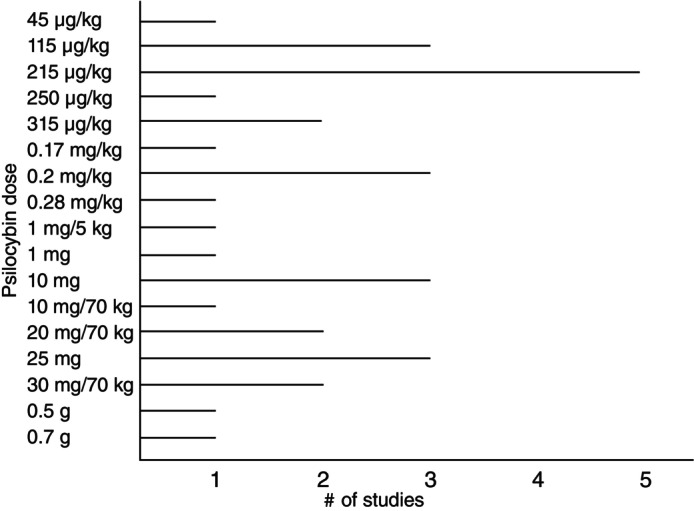
Psilocybin dose across included studies including macro and microdoses.
Table 1.
Characteristics of included studies
| Author | Country | Study design | Cognition domain | Participants | Intervention |
|---|---|---|---|---|---|
| Healthy volunteers | |||||
| Nikolič et al. 37 | Czech Republic | RCT | Memory | N = 20 (n = 10 females, mean age = 36, SD = 8.1) | 5 mg psilocybin (1 mg/5 kg) |
| Barrett et al. 27 | USA | RCT | Mental flexibility, associative learning, memory, executive functioning | N = 22 (n = 11 females, mean age 28.5 years) | Five drug administration sessions with 10, 20, and 30 mg/70 kg psilocybin; 400 mg/70 kg DXM; and placebo |
| Nayak et al. 42 | USA | Prospective, longitudinal | Cognitive flexibility | Sample sizes for each of the surveys: N = 8,006 (Survey 1), N = 2,833 (Survey 2; 2 weeks pre), N = 1,802 (Survey 3; 1 day pre), N = 1,551 (Survey 4; 1–3 days post), N = 1,182 (Survey 5; 2–4 weeks post), N = 657 (Survey 6; 2–3 months post. Mean age: 40 years | Dried psilocybin mushrooms (mean dose = 3.1 g) |
| Hasler et al. 28 | Switzerland | RCT | Attention | N = 8 (n = 4 females), mean age 29.5 years | Placebo and four different doses of psilocybin (very low dose = 45 μg/kg body weight; low dose = 115 μg/kg; medium dose = 215 μg/kg; and high dose = 315 μg/kg) on five experimental days at least 2 weeks apart |
| Vollenweider et al. 12 | Switzerland | RCT | Attention | N = 16 (n = 9 females, mean age 26.4 years) | Placebo and three doses of psilocybin: 115, 215, and 315 μg/kg separated by 4 weeks |
| Rucker et al. 30 | UK | RCT | Global cognitive function | Total N = 89 (n = 41 females), mean age 36.1 (SD = 9.06)/Control: N = 29 (n = 13 females), mean age 35.6 (SD = 7.69)/Psilocybin 25 mg: N = 30 (n = 13 females) mean age 36.6 (SD = 10.29) /Psilocybin 10 mg: N = 30 (n = 13 females) mean age 36.1 (SD = 9.25) | Single dose 25 mg psilocybin, 10 mg psilocybin or placebo |
| Cavanna et al. 32 | Argentina | RCT | Cognitive flexibility, attention, empathy, creative cognition | N = 34 (n = 11 females, mean age 31.26 ± 4.41 years) | Psilocybin mushrooms (Psilocybe cubensis) 0.5 g |
| Gouzoulis‐Mayfrank et al. 36 | Germany | RCT | Visual attention | N = 32 (n = 11 female, mean age 34 years) | Psilocybin capsules 0.2 mg/kg, MDE 2 mg/kg, or DMT 0.4 mg/kg |
| Carter et al. 34 | Switzerland | RCT | Attention and working memory | N = 8 (n = 3 female, mean age 27 years) | Psilocybin capsules 215 μg/kg and katanserin 50 mg. Four experimental days separated by at least 14 days |
| Marschall et al. 33 | Netherlands | RCT | Emotional processing | N = 52 (n = 19 females), mean age 29.75 | Repeated microdosing (0.7 g of dried psilocybin‐containing Galindoi truffles) over 3 weeks |
| Duke et al. 35 | USA | RCT | Attention and working memory | N = 8 male, mean age: NA | Psilocybin (0.2 mg/kg), dextro‐amphetamine (30 mg), placebo |
| Umbricht et al. 38 | Switzerland | RCT | Working memory | N = 18 (n = 8 females, mean age 25.1 ± 4.3 years) | Psilocybin (0.28 mg/kg) and a placebo session on two separate days |
| Pokorny et al. 40 | Switzerland | RCT | Empathy | N = 32 (n = 15 female, mean age 26.7 years) | Psilocybin 0.215 mg/kg. Placebo and psilocybin session, 10 days apart |
| Spitzer et al. 43 | Germany | RCT | Semantic associations | N = 8 (n = 0 female, mean age 39.4 years) | Psilocybin 0.2 mg/kg. Placebo and psilocybin session, one week apart |
| Wittmann et al. 31 | Switzerland | RCT | SWM | N = 12 (n = 6 female, mean age 26.8 years) | Psilocybin (115 μg/kg for medium dose and 250 μg/kg for high dose). Placebo, medium dose and high dose session at least 14 days apart |
| Kometer et al. 39 | Switzerland | RCT | Emotional processing | N = 17 (n = 6 female, mean age 26 years) |
Psilocybin 215 μg/kg and katanserin 50 mg. 4 experimental days, separated from each other by at least 2 weeks |
| Mason et al. 41 | Netherlands | RCT | Creative cognition | N = 60 (n = 25 female, mean age 23 years) | Psilocybin 0.17 mg/kg |
| Clinical populations | |||||
| Goodwin et al. 25 | USA | RCT | Global cognitive function | Psilocybin 25 mg N = 79 (n = 44 females) mean age 40.2 (SD = 12.19), psilocybin 10 mg N = 75 (n = 41 females) mean age 40.6 (SD = 12.76), Psilocybin 1 mg N = 79 (n = 36 females) mean age 38.7 (SD = 11.71) | A single dose of psilocybin 25 or 10, or 1 mg (control) |
| Stroud et al. 26 | UK | RCT | Emotional processing | N = 17 (n = 6 females) with TRD (mean age = 44.04, [SD = 11.51])/N = 16 (n = 5 females) control (mean age = 32, SD = 10.40) | Two dosing sessions, separated by 1 week. In the first session, patients received 10 mg of psilocybin and in the second, they received 25 mg |
| Doss et al. 24 | USA | RCT | Cognitive flexibility | N = 24 (n = 16 female, mean age of 39.8 years of age) randomized, N = 22 (n = 14 female) completed | Two sessions, first with 20 mg/70 psilocybin kg and the second with 30 mg/70 mg psilocybin |
| Therapy principles | Outcome measure | Protocol | Condition | Results | Conclusion |
|---|---|---|---|---|---|
| Healthy volunteers | |||||
| NA |
GMLT RAVLT |
Tests were administered at baseline, 8 h after dosing and before going to sleep | Healthy participants | The main effects of treatment and order were not found; participants did not make significantly more errors in the treatment group compared to the placebo group. anova test showed a significant interaction between treatment order (psilocybin first vs placebo first) and treatment (placebo × psilocybin; F[1,15] = 29.975, P < 0.001) | Psilocybin did not negatively affect memory consolidation in any of the tests used |
| NA | MMSE, letter N‐back, word encoding/recognition task, Stroop task, DSST | Stroop test: after 4 h, word encoding after 180 min and recall 360 min, DSST and MMSE after 2 h | Healthy participants | Functioning – Main effect of drug condition for attempted trials (F[4] = 23.52, P < 0.0001), accuracy (F[4] = 10.56, P < 0.0001), and substitution recall accuracy (F[12] = 2.07, P < 0.05). Working memory – Main effects of n‐back drug condition (F[4] = 3.47, P < 0.05) on discriminability, drug condition (F[4] = 6.75, P < 0.001) on response bias, drug condition (F[4] = 8.50, P < 0.0005) on response time | Dose‐dependent effects of psilocybin on cognition. Psilocybin exerted greater effects than DXM on measures of working memory, and DXM exerted selective effects on episodic memory, response inhibition, and executive control |
| NA | CFS | Surveys completed 2 weeks before, 1 day before, 1–3 days after, 2–4 weeks after, and 2–3 months after | Healthy participants | Cognitive flexibility mean total scores were 57.1 (7.8) for Survey 2, 59.0 (7.0) for Survey 5, and 59.1 (7.0) for Survey 6. The effect size (SMD [95% CI]) of CFS at 2–4 weeks was 0.23 [0.15, 0.31] (P < 0.001), and 0.22 [0.15, 0.30] (P < 0.001) at 2–3 months | Psilocybin significantly increased self‐reported cognitive flexibility from baseline to follow‐ups |
| NA | FAIR | FAIR was completed 140 min after drug administration | Healthy participants | Psilocybin exerted no significant influence on the FAIR scores MV (F[4,28] = 0.58, P = 0.687) and QV (F[4,28] = 1.39, P = 0.261). In contrast, administration of psilocybin led to a significant decrease in the FAIR scores PV (F[4,28] = 12.28, P < 0.00001) and CV (F[4,28] = 11.23, P < 0.00001) | The strongly impaired performance in the FAIR test under medium dose and high dose psilocybin is difficult to appraise |
| NA | FAIR | The FAIR task was conducted at 0, 105, 180, and 360 min after treatment | Healthy participants | Psilocybin impaired attentional performance on the FAIR task in a dose‐dependent manner. Psilocybin significantly reduced the FAIR attentional performance capacity score P and the FAIR score Q indexing the amount of attentively made decisions relative to the total decisions as well as the attentional continuity performance | Psilocybin‐induced impairments in sustained attention performance were positively correlated with reduced PPI at the 30 ms ISIs and not with the concomitant increases in PPI observed at long ISIs |
| Psychotherapy (preparation, dosing and integration sessions) | CANTAB, SWM‐Between Errors, SWM‐Strategy, Rapid Visual Information Processing A‐prime, PET; RMET; Scale of SSR; SVO; TEQ | Test was completed at baseline (day −1) to day 8 and 29 | Healthy participants | Trends of improved sustained attention, working memory, and executive function with psilocybin, but no significant differences between the doses or with placebo. Episodic memory and global safety outcomes showed no significant changes. Social cognition and emotional processing scores remained consistent across all groups and time points | Psilocybin did not have any detrimental short‐ or long‐term effects on cognitive functioning or emotional processing |
| NA | TMT, CFS, TECA | Participants completed self‐reported scales aimed 2 days before the first dosing day of each condition. Afterwards, they performed different tasks and activities on Wednesdays and Fridays, and completed a battery of scales on Fridays | Healthy participants | Decreased visibility of the second target with 300 ms lag in the attentional blink task, and increased RT in the Stroop task, both significant at P < 0.05 but only without correction for multiple comparisons. Significant increase in the time required for TMT‐A | Low doses of psilocybin mushrooms can result in noticeable subjective effects and altered EEG rhythms, but without evidence to support enhanced well‐being, creativity and cognitive function |
| NA | COVAT | COVAT was administered 1 h before drug ingestion and 75–95 min after drug ingestion | Healthy participants | Significant main effects of group (F = 12.83, P = 0.0001), cue (F = 33.77, P = 0.0001), SOA (F = 159.13, P = 0.0001) and time (pre‐drug/on‐drug: F = 30.47, P = 0.0001). The psilocybin group had significantly longer RTs than the placebo group in most types of trials | Psilocybin and MDE both caused overall slowing of RTs, although psychomotor retardation was less pronounced after MDE |
| NA | MOT, SST | The test was administered after 120 min of drug intake | Healthy participants | Attentional tracking was significantly affected by both drug (F[3,21] = 3.38, P < 0.05) and time (F[1,7] = 18.06, P < 0.01). Psilocybin (A = 2.24, s = 0.75) and the psilocybin plus ketanserin pretreatment (A = 2.35, s = 0.60) were both significantly (P < 0.05) reduced from placebo. No significant effect on the number of boxes remembered correctly in sequence ‘span length’ was found for drug [F(3,21) = 0.57, P = 0.64] or time [F(1,7) = 1.56, P = 0.12] | Psilocybin significantly reduced attentional tracking ability, but had no significant effect on SWM, suggesting a functional dissociation between the two tasks |
| NA | Emotional go/no‐go task | Test was completed 90 min after self‐administering their seventh dose | Healthy participants | Emotions had a significant effect on RTs, but the specific experimental conditions did not | No effect of psilocybin microdosing on interoceptive awareness, nor on emotion processing |
| NA | TMT‐A, B | After a sufficient time interval test was administered | Healthy participants | DA had a significantly greater effect on part A and part B than PL, and Ps had a significantly greater effect on part A and part B than DA | Both DA and psilocybin produce a deficit in performance as compared to placebo |
| NA | AX‐CPT | Subjects performed the AX‐CPT during the collection of ERP | Healthy participants | Psilocybin administration was associated with a significant decline of the hit rate at both ISIs (infusion × time interaction: F[1,15] = 23.92, P < 0.001). The infusion × time × ISI interaction was not significant, indicating no differential impairment of performance at the two ISIs (F[1,15] = 0.72, P = 0.4) | Psilocybin administration induced significant performance deficits in the AX‐CPT, but failed to reduce MMN generation significantly |
| NA | Multidimensional empathy test (MET) | Test was performed 160 min after administration of placebo or psilocybin | Healthy participants | Psilocybin increased explicit (F[1,30] = 7.74, P < 0.01) and implicit (F[1,30] = 4.77, P < 0.05) emotional empathy compared to placebo. No change in cognitive empathy and no significant main effect for drugs (F[1,30] = 1.45, P > 0.2) | Psilocybin significantly increased emotional, but not cognitive empathy compared to placebo, and the increase in implicit emotional empathy was significantly associated with psilocybin‐induced changed meaning of percepts |
| NA | Lexical decision paradigm | Test was performed before drug administration and at minutes 50, 150 and 220 | Healthy participants | No effect of psilocybin with the indirect priming effect. Significant increase of indirect semantic priming under psilocybin (t[7] = 2.82; P = 0.026) | Psilocybin increased indirect semantic priming which implies an increased availability of remote associations |
| NA | SST | Test was performed at 0, 100 and 360 min after capsule administration each session | Healthy participants | A significant difference for the contrast between placebo and HD psilocybin between t1 and t0 (P < 0.011) | At the peak of effects, high dose psilocybin (and not medium dose psilocybin) impaired spatial span task performance as indexed by span length |
| NA | Emotional go/no‐go task | Experiments started approximately 130 min after treatment | Healthy participants | Psilocybin increases RTs as a function of word valence. Specifically, psilocybin increased RT much more for negative and neutral than for positive words, which indicates a stronger response bias to positive versus negative words in psilocybin than in placebo condition | Psilocybin biases emotional processing towards positive relative to negative information, increased behavior towards positive relative to negative cues in the emotional go/no go task |
| NA |
PCT AUT |
Creativity tasks on three occasions: baseline, treatment day and 7 days after the drug administration. On drug testing day, PCT was administered 120 min post treatment and AUT 130 min post treatment | Healthy participants | Psilocybin influenced performance on the PCT in a time‐ and construct‐dependent manner (F[3,47] = 4.53, P = 0.007). Compared to placebo, psilocybin acutely decreased CT (d = 0.85), and measures of DT including fluency (d = 0.84), and originality (d = 0.65) | Psilocybin induces a time‐ and construct‐related differentiation of effects on creative thinking |
| Clinical populations | |||||
| 3 preparatory sessions + acute support + 2 integration sessions | DSST | DSST was administered at baseline, day 2, week 3, and week 12 | TRD | The difference in the least‐squares mean change from baseline to week 3 between the 25 mg group and 1 mg group were 1.5 (95% CI: −0.8, 3.8) and 0.5 (95% CI: −1.8, 2.8) | Treatment differences in measures of cognitive function were smaller but showed comparable trends for a greater effect of the 25 mg dose |
| Psychotherapy (preparation, acute support, integration) | Dynamic Emotional Expression Recognition Task (RT on correct trials) | Test was completed at baseline and 1 month later after two doses of psilocybin | TRD | Group × time interaction on speed of emotion recognition (P = 0.035). At baseline, patients were slower at recognizing facial emotions compared with controls (P < 0.001). After psilocybin, this difference was remediated (P = 0.208). Emotion recognition was faster at follow‐up compared with baseline in patients (P = 0.004) but not controls (P = 0.263) | Psilocybin with psychological support appears to improve processing of emotional faces in TRD, and this correlates with reduced anhedonia |
| Preparatory meetings (8 h), 2 psilocybin sessions 1–3 weeks apart and follow up sessions 2–3 h | Pen conditional exclusion test | Test was performed 4 weeks before the first psilocybin session and 1 and 4 weeks after the second psilocybin session | MDD | Perseverative errors on the PCET generally decreased from baseline to 1 week post‐psilocybin therapy with this effect sustained 4 weeks post‐treatment. This improvement in cognitive flexibility was supported by a main effect of time point | Psilocybin therapy was shown to increase cognitive and neural flexibility in patients with MDD |
AUT, alternative uses task; AX‐CPT, AX continuous performance test; CFS, cognitive flexibility scale; COVAT, covert orienting of attention task; DA, dextro‐amphetamine; DMT, dimethyltryptamine; DSST, digit symbol substitution test; DXM, dextromethorphan; ERP, event‐related potential; FAIR, Frankfurt Attention Inventory; GMLT, Groton Maze Learning Task; ISI, interstimulus intervals; MDD, major depressive disorder; MDE, 3,4‐methylenedioxyethamphetamine; MMN, mismatch negativity; MMSE, mini‐mental state examination; MOT, multiple object tracking; NA, not available; PCET, Penn conditional exclusion test; PCT, picture concept task; PET, pictorial empathy test; PPI, prepulse inhibition; RAVLT, Rey Auditory Verbal Learning Test; RCT, randomized clinical trial; RMET, reading the mind in the eyes test; RT, reaction time; SD, standard deviation; SSR, social responsibility; SST, spatial span test; SVO, social value orientation; SWM, spatial working memory; TECA, cognitive‐affective empathy test; TEQ, Toronto Empathy Questionnaire; TMT‐A, trail making test A; TMT‐B, trail making test B; TRD, treatment‐resistant depression.
Table 2.
Cognitive function results based on psilocybin dose and time of assessment
| Author | Participants | Psilocybin dose | Time of test | Assessment | Results | |
|---|---|---|---|---|---|---|
| Global cognitive function | ||||||
| Post‐acute | ||||||
| Rucker et al. 30 | 89 healthy participants | Single dose of 25 or 10 mg | Baseline (day −1), 8 and 29 days after psilocybin administration | CANTAB – Global composite score | No statistically significant effect | |
| Acute | ||||||
| Barrett et al. 27 | 22 healthy participants | Three sessions – 10, 20, and 30 mg/70 kg | 120–360 min after drug administration | MMSE | No statistically significant effect | |
| Attention | ||||||
| Acute | ||||||
| Hasler et al. 28 | 8 healthy participants | Four sessions – 0.045, 0.115, 0.215, 0.315 mg/kg | 140 min after drug administration | FAIR | Decrease in performance value (PV) and continuity value (CV) scores. No effect on efficiency and quality value scores | PV: P < 0.01 CV: P < 0.01 |
| Vollenweider et al. 12 | 16 healthy participants | Three sessions – 0.115, 0.215 and 0.315 mg/kg | 0, 105, 180, and 360 min after drug administration | FAIR | Dose‐dependent decreased scores in attentional performance and continuity. Decreased quality value score only after high dose. No effect on efficiency score | P < 0.00001 |
| Carter et al. 34 | 8 healthy participants | 0.215 mg/kg +/− ketanserine pretreatment | 120 min after drug administration | MOT | Reduction in number of dots correctly tracked | P < 0.05 |
| Duke et al. 35 | 8 healthy participants | 0.2 mg/kg | Not specified | TMT | Decreased performance and increased time needed to complete task | P < 0.05 |
| Gouzoulis‐Mayfrank et al. 36 | 32 healthy participants | 0.2 mg/kg | 0, 75 and 95 min after drug administration | COVAT (visual attention) | Longer RTs | P = 0.006 |
| Microdosing | ||||||
| Cavanna et al. 32 | 34 healthy participants | Psilocybe cubensis mushrooms, 0.5 g | Tests performed on dosing days | TMT | Significant increase in time required to complete task | Not specified |
| Post‐acute | ||||||
| Rucker et al. 30 | 89 healthy participants | Single dose of 25 or 10 mg | Baseline (day −1), 8 and 29 days after psilocybin administration | Rapid Visual Information Processing | Trend towards improved sustained attention | Not statistically significant |
| Working memory | ||||||
| Acute | ||||||
| Nikolič et al. 37 | 20 healthy participants | 1 mg/5 kg | Baseline, 8 h after dosing and before going to sleep |
RAVLT GMLT |
No statistically significant effect | |
| Carter et al. 34 | Eight healthy participants | 0.215 mg/kg +/− ketanserine pretreatment | 120 min after drug administration |
SWM SST |
No statistically significant effect | |
| Wittmann et al. 31 | 12 healthy participants | Two sessions – 0.115, 0.25 mg/kg | 0, 100 and 360 min after drug administration |
SWM SST |
Impaired SST performance with high dose. No effect with medium dose | P < 0.011 |
| Umbricht et al. 38 | 18 healthy participants | 0.28 mg/kg | 70 min after drug administration | AX CPT | Decreased performance; decreased hit rate and increased response to false alarms | P < 0.001 |
| Post‐acute | ||||||
| Rucker et al. 30 | 89 healthy participants | Single dose of 25 or 10 mg | Baseline (day −1), 8 and 29 days after psilocybin administration | SWM | Trends towards better performance for 25 mg dose compared to baseline, but no difference from placebo | Not statistically significant |
| Emotional processing | ||||||
| Acute | ||||||
| Kometer et al. 39 | 17 healthy participants | 0.215 mg/kg +/− ketanserine pretreatment | 130 min after drug administration | Emotional go/no‐go task | Increased RT for negative and neutral valence words and increased error rate depending on word valence | RT: P = 0.01, ER: P = 0.05 |
| Microdosing | ||||||
| Marschall et al. 33 | 52 healthy participants | Repeated microdosing (0.7 g of dried psilocybin‐containing truffles) over 3 weeks | 90 min after self‐administering their seventh dose | Emotional go/no‐go task | No statistically significant effect | |
| Post‐acute | ||||||
| Stroud et al. 26 | 17 participants with TRD | 10 mg on 1st session and 25 mg on second session separated by 1 week | 0 and 1 month after second psilocybin sessions | Dynamic Emotional Expression Recognition Task | Faster emotion recognition at follow up in subjects with TRD | P = 0.004 |
| Rucker et al. 30 | 89 healthy participants | Single dose of 25 or 10 mg | Baseline (day −1), 8 and 29 days after psilocybin administration | RMET | Not statistically significant | |
| Social cognition and empathy | ||||||
| Acute | ||||||
| Pokorny et al. 40 | 32 healthy participants | 0.215 mg/kg | 160 min after drug administration | Multidimensional empathy test | Increased explicit and implicit emotional empathy. No change in cognitive empathy | EE: P < 0.01 IE: P < 0.05 |
| Microdosing | ||||||
| Cavanna et al. 32 | 34 healthy participants | P. cubensis mushrooms, 0.5 g | Tests performed on dosing days | Cognitive‐affective empathy test | No statistically significant effect | |
| Post‐acute | ||||||
| Rucker et al. 30 | 89 healthy participants | Single dose of 25 or 10 mg | Baseline (day −1), 8 and 29 days after psilocybin administration | Pictorial empathy test (PET), TEQ, Scale of SSR, SVO | Not statistically significant | |
| Cognitive flexibility and creative cognition | ||||||
| Microdosing | ||||||
| Cavanna et al. 32 | 34 healthy participants | P. cubensis mushrooms, 0.5 g | Tests performed on dosing days | CFS, Remotes Associated Test, AUT and Wallach–Kogan test | No statistically significant effect | |
| Post‐acute | ||||||
| Mason et al. 41 | 60 healthy participants | 0.17 mg/kg | 0, 120–130 min after psilocybin administration, and 7 days after | Picture concept task | Acute decrease in convergent and divergent thinking. Sustained decrease in convergent thinking at the day 7 follow up | P = 0.007 |
| AUT | Acute decrease in fluency but increased scores of novelty at the 7 days follow up | P = 0.007 | ||||
| Doss et al. 24 | 22 participants with MDD | 20 mg/70 kg on 1st session and 30 mg/70 mg on second session 1–3 weeks apart | 0 and 1 and 4 weeks after the second psilocybin session | Penn conditional exclusion test | Decrease in perseverative errors sustained over 4 weeks compared to baseline | P < 0.001 |
| Nayak et al. 42 | 657 participants | Dried psilocybin mushrooms (mean dose = 3.1 g) | 1 to 3 days, 2–4 weeks, and 2–3 months after psilocybin administration | CFS | Increased self‐reported cognitive flexibility | P < 0.001 |
| Memory and learning | ||||||
| Acute | ||||||
| Rucker et al. 30 | 89 healthy participants | Single dose of 25 or 10 mg | Baseline (day −1), 8 and 29 days after psilocybin administration | Paired Associates Learning | Not statistically significant | |
| Post‐acute | ||||||
| Spitzer et al. 43 | Eight healthy participants | 0.200 mg/kg | 0, 50, 150 and 220 min after psilocybin administration | Lexical decision paradigm test (semantic associations) | Increase in indirect semantic priming | P = 0.026 |
| Barrett et al. 27 | 22 healthy participants | Three sessions – 10, 20, and 30 mg/70 kg | 120–360 min after psilocybin administration |
Word encoding/recognition task DSST |
Dose‐dependent decrease in word recall accuracy and increased response time | P < 0.0005 |
| Executive function | ||||||
| Acute | ||||||
| Barrett et al. 27 | 22 healthy participants | Three sessions – 10, 20, and 30 mg/70 kg | 120–360 min after psilocybin administration | DSST | Dose‐dependent decrease in attempted responses and decreased substitution recall accuracy | P < 0.0001 |
| Post‐acute | ||||||
| Goodwin et al. 25 | 233 participants with TRD | Single dose of 1, 25 or 10 mg | 0, day 2 and week 3 and 12 after psilocybin administration | DSST | Dose‐dependent trend towards increased scores in week 3 compared to baseline | Not statistically significant |
AUT, alternative uses task; AX‐CPT, AX continuous performance test; CANTAB, Cambridge Neuropsychological Test Automated Battery; CFS, cognitive flexibility scale; COVAT, covert orienting of attention task; DSST, digit symbol substitution test; FAIR, Frankfurt Attention Inventory; GMLT, Groton Maze Learning Task; MDD, major depressive disorder; MMSE, mini‐mental state examination; MOT, multiple object tracking; RAVLT, Rey Auditory Verbal Learning Test; RMET, reading the mind in the eyes test; RT, reaction time; SSR, social responsibility; SST, spatial span test; SVO, social value orientation; SWM, spatial working memory; TEQ, Toronto Empathy Questionnaire; TMT, trail making test; TRD, treatment‐resistant depression.
Fig. 5.
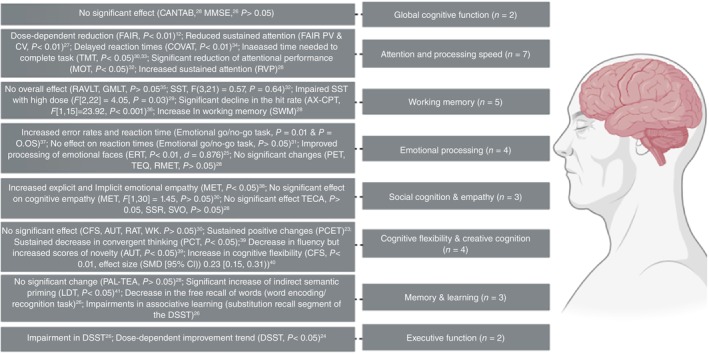
Summary of psilocybin effect on different domains of cognitive function. AUT, alternative uses task; AX‐CPT, AX continuous performance test; CFS, cognitive flexibility scale; COVAT, covert orienting of visual attention task; DSST, digit symbol substitution test; ERT, emotion recognition task; FAIR, Frankfurt Attention Inventory; GMLT, Groton Maze Learning Task; PCET, Penn conditional exclusion test; RAVLT, Rey Auditory Verbal Learning Test; TECA, cognitive‐affective empathy test.
Global cognitive function
Two studies investigated the effects of psilocybin on global cognitive function in healthy participants. Rucker et al. 30 studied the lasting effects of psilocybin on global cognitive function in 89 healthy subjects. Participants received either placebo or psilocybin (10 or 25 mg), along with psychotherapy sessions. Global cognitive function was measured using Cambridge Neuropsychological Test Automated Battery (CANTAB) global composite score at baseline (day −1), 8 and 29 days after treatment. The primary endpoint of this study was day 8. Overall, the CANTAB battery, when comparing psilocybin‐naïve and psilocybin‐experienced participants, yielded similar results.
Barrett et al. 27 studied the acute cognitive effects of psilocybin and the NMDA receptor antagonist dextromethorphan (DXM) among 22 healthy participants. The study measured global cognitive functioning using the mini‐mental status examination (MMSE). 27 Participants who received either 10, 20, or 30 mg/70 kg psilocybin, 400 mg/70 kg of DXM, or placebo completed the MMSE 2 h after each drug administration. None of the participants who ingested psilocybin, and all but one DXM participant, exhibited any discernible alterations in global cognitive function as assessed by the MMSE. 27
Overall, none of the two studies that investigated the effects of psilocybin on global cognitive function in healthy participants reported significant changes following the psilocybin administration.
Attention and processing speed
Seven studies evaluated psilocybin effects on processing speed and either overall attentional performance or specific aspects of attention, such as sustained attention and visuospatial attention through different tests in different settings. Hasler et al. evaluated sustained attention, the ability to maintain focus on a task, with the Frankfurt Attention Inventory (FAIR). In the study, the test was administered approximately 140 min after drug intake. Eight healthy participants received either placebo or different doses of psilocybin at least 2 weeks apart (very low dose: 45/kg, low dose: 115 μg/kg, medium dose: 215 μg/kg, high dose: 315 μg/kg). 28 Results of this study indicated that although the acute effects of psilocybin (i.e. within 2 h after drug administration) did not affect task clarity and decision making, cognitive processing and sustained attention were reduced. 28 Consistently, Vollenweider et al. 12 , in a randomized clinical trial (RCT) on psilocybin (three doses: 115, 215, 315 μg/kg) in healthy individuals, revealed a dose‐dependent reduction in sustained attention as indicated by reduction in FAIR attentional performance capacity, performance quality, and attentional continuity performance.
Carter et al. 34 conducted an RCT that measured effects of either 215 μg/kg psilocybin, placebo, the 5‐HT2A receptor antagonist ketanserin (50 mg), or psilocybin plus ketanserin on attention in eight healthy volunteers. Attention was measured using a multiple‐object tracking task which was administered 120 min after the drug intake. While there were no significant differences between the four drug conditions during the pretest phase, a significant reduction of attentional performance was reported following both psilocybin and psilocybin with ketanserin pretreatment.
Cavanna et al. 32 evaluated the effect of microdoses of psilocybin on attention in 34 healthy participants. Subjects received two doses of 0.5 g of dried Psilocybe cubensis separated by 2 days, and on the second day participants completed the tests. Consistent with the above‐mentioned studies, impaired attentional performance was observed as measured by trail making test (TMT) and attentional blink task. However, there was a notable increase in the time needed to complete part A of the TMT test. 32 Duke et al. 35 evaluated the effect of psilocybin (0.2 mg/kg) or dextro‐amphetamine (DA) (30 mg) on attention and working memory of healthy participants using TMT, part A and part B. Both DA and psilocybin significantly increased the time needed to complete the test, implying reduced working memory with both. However, DA had a significantly greater effect on part A and part B than placebo, and psilocybin had a significantly greater effect on part A and part B than DA. 35 Additionally, Gouzoulis‐Mayfrank et al. 36 , randomized 32 healthy individuals to four drug groups (psilocybin 0.2 mg/kg, 3,4‐methylenedioxyethamphetamine [MDE], dmethamphetamine, placebo, n = 8 each) and assessed visuo‐spatial attention 1 h before and between 75 and 95 min after the ingestion of the drug, using the covert orienting of attention task (COVAT). Whereas MDE did not affect reaction time (RT), low response inhibition and prolonged RT were reported in those receiving psilocybin. 36
Lastly, Rucker et al. 30 evaluated sustained attention using Rapid Visual Information Processing. A trend towards an increased sustained attention was observed for the 10 and 25 mg psilocybin doses by day 29 compared with baseline, which was not detected either against the placebo or between the two doses of psilocybin. 30
Overall, in the acute setting following psilocybin intake, both studies that administered the FAIR test showed decreased performance and continuity values, but not efficiency scores. 12 , 28 Two studies reported decreased performance in visual attention, measured through the COVAT and multiple object tracking tests. 34 , 36 Attentional performance measured by the trail‐making test also decreased, and a significant increase in the time needed to complete the task following a microdosing regimen was observed. 32 , 35 In the post‐acute setting, there was a non‐significant trend towards improved sustained attention. 30
Working memory
Five studies evaluated the effects of psilocybin on working memory on healthy participants. Nikolič et al. 37 conducted a study with 20 healthy volunteers to examine the impact of psilocybin on the consolidation of working and verbal memory. Although they found no evidence of cognitive decline in memory consolidation, they were also unable to demonstrate any improvement in memory consolidation from psilocybin using memory tests, including the Groton Maze Learning Task and Rey Auditory Verbal Learning Test. 37
Two studies assessed working memory with the spatial span test. 31 , 34 Carter et al. 34 investigated the impact of 215 μg/kg psilocybin on spatial working memory (SWM) in eight healthy volunteers. They discovered no significant influence on the correct recall of boxes in sequence (‘span length’) for both drug and time. This absence of effect persisted when comparing placebo and psilocybin conditions before and 120 min after drug intake in a 2 × 2 anova. Furthermore, the total number of errors in sequence order or location (‘total errors’) remained unaffected by drug administration or time of testing, further affirming the lack of psilocybin's impact on SWM performance. 34 Wittmann et al. 31 examined the impact of high (250 μg/kg) and medium (115 μg/kg) doses of psilocybin on the SWM of 12 healthy individuals using the same test. The test was accomplished at 0, 100 and 360 min after drug/placebo intake. The analysis showed no overall treatment effect, a significant effect over measurement time, and a significant interaction effect. Also, the analysis showed a significant interaction effect between measurement time and treatment group. A priori contrasts revealed a significant difference only in the contrast between placebo and high‐dose (HD) psilocybin between t1 and t0 (P < 0.011). Therefore, at the peak of effects, only HD psilocybin (not medium dose) impaired spatial span task performance, as indicated by span length. 31
Umbricht et al. 38 investigated the impact of psilocybin (0.28 mg/kg) on working memory using the AX continuous performance test in healthy individuals 70 min after psilocybin administration. The study revealed a substantial reduction in response accuracy and working memory during psilocybin administration which was independent of inter stimulus intervals. This effect was not attributed to a more liberal response bias induced by psilocybin. Finally, Rucker et al. 30 reported an increase in working memory using SWM.
In summary, five studies evaluated the impact of psilocybin on working memory, all of them worked with healthy subjects and only one evaluated the effects in the post‐acute setting. In the acute setting, two studies demonstrated impaired performance with doses over 0.25 mg/kg, 31 , 38 and two others showed no effect of psilocybin on working memory. 34 , 37 In the post‐acute setting, Rucker et al. 30 demonstrated a trend towards better performance for 25 mg dose compared to baseline, but no difference from placebo.
Emotional processing
Four studies evaluated the effect of psilocybin on emotional processing; three of these on healthy subjects 30 , 33 , 39 and one on subjects with TRD. 26 Kometer et al. 39 evaluated the acute effects of psilocybin, with or without ketanserin pretreatment, on emotional processing through the emotional go/no‐go task in 17 healthy participants 130 min after treatment. In this within subject design study, participants underwent four different experimental days, separated at least 2 weeks apart. Each session involved pretreatment with either placebo or ketanserin (50 mg) followed by placebo or psilocybin (215 g/kg) after 1 h. 39 The administration of psilocybin resulted in an increase in RT, with longer RTs observed for negative and neutral words compared to positive words, indicating a heightened response bias towards positive words. Moreover, psilocybin led to increased error rates based on word valence, specifically elevating error rates for neutral words while not affecting those for positive or negative words. These changes were observed irrespective of pretreatment with ketanserin. Using the same task, Marschall et al. 33 evaluated the effects of seven doses of approximately 1.5 mg psilocybin over 3 weeks on emotional processing in 52 healthy subjects. Contrary to the results by Kometer et al. the results showed that at 1.5 h after self‐administration of psilocybin, emotional processing had a significant effect on RTs, but the specific experimental conditions (placebo vs psilocybin) did not. 33
Interestingly, Stroud et al. 26 explored the lasting effect of psilocybin on emotional processing in 17 participants with TRD, and reported that psilocybin, along with psychotherapeutic support, appears to improve emotional processing in this indication. Patients participated in two dosing sessions, the first with 10 mg of psilocybin and 25 mg in the second, and received psychotherapy sessions including a preparation session, inner directed therapy during psilocybin treatment and an integration session after treatment. 26 One month after administration of psilocybin, participants completed the Dynamic Emotional Expression Recognition Task. Results showed that at pretreatment baseline, patients were slower at recognizing facial emotions compared with controls. After psilocybin, this difference was remediated. Emotion recognition was faster at follow‐up compared with baseline in patients but not controls. Lastly, Rucker et al. 30 reported no difference between either psilocybin group and placebo in the reading the mind in the eyes test scores relative to baseline.
The four studies assessing emotional processing had different experimental designs and studied various populations, yielding mixed results. Of the two studies employing the emotional go/no‐go task, the one using a microdosing regimen found no difference between the placebo and control groups. 33 In contrast, Kometer et al. 39 reported a significant difference with a 0.215 mg/kg dose, noting increased RTs and error rates depending on word valence. In the post‐acute setting with healthy participants, no significant effect of psilocybin was observed. 30 However, in the study involving participants with TRD, the authors found faster emotion recognition 1 month after psilocybin administration. 26
Social cognition and empathy
Regarding the effects of psilocybin on empathy levels, Pokorny et al. 40 compared the acute effects of psilocybin (0.215 mg/kg) with placebo in 32 healthy subjects using the multidimensional empathy test administered 160 min post treatment. Compared to placebo, psilocybin increased both explicit and implicit emotional empathy. However, no change in cognitive empathy and no significant main effect for psilocybin were indicated. Consistently, in the study by Cavanna et al. 32 , no significant changes were reported in cognitive empathy levels as measured by the cognitive‐affective empathy test. Lastly, Rucker et al. 30 reported similar empathy and social cognition scores across psilocybin and placebo groups and time points of the study assessed by the pictorial empathy test, scale of social responsibility, social value orientation and Toronto Empathy Questionnaire.
These three studies evaluated social cognition and empathy in both acute and post‐acute settings, as well as following a microdosing regimen. A significant difference between the placebo and psilocybin groups was observed only in the acute setting, with the psilocybin group showing increased explicit and implicit emotional empathy. 40 The other two studies found no difference between the placebo and psilocybin groups in social cognition and empathy tests in the post‐acute setting or after a microdosing regimen. 30 , 32
Cognitive flexibility and creative cognition
Inconsistent results were reported on the effects of psilocybin on cognitive flexibility and creative cognition, considering different psychiatric indications and various drug regimens used in these studies. Cavanna et al. 32 reported no significant effects of psilocybin microdosing (0.5 g of dried mushrooms) on cognitive flexibility and creative cognition in healthy subjects, assessed by the cognitive flexibility scale (CFS), the Remotes Associates Test (RAT; convergent thinking), the alternative uses task (AUT; divergent thinking), and the Wallach–Kogan Test (WK; divergent thinking). 32 However, in another study Mason et al. 41 reported a time‐related differentiation of effects on creative thinking by psilocybin. This study evaluated the acute and lasting effects of psilocybin (0.17 mg/kg psilocybin) on creativity in 60 healthy subjects utilizing the picture concept task and the AUT at three timepoints: baseline, treatment day and 7 days after the treatment. Psilocybin acutely decreased both convergent and divergent thinking compared to placebo, with convergent thinking still reduced at the 7‐day follow‐up. Additionally, psilocybin acutely decreased fluency and significantly increased novelty at the 7‐day follow‐up. Although aspects of divergent thinking were impaired during the acute psychedelic state, they were increased after 7 days, represented by the generation of a higher quantity of novel ideas for uses of an everyday object on the AUT compared to placebo. 41
Doss et al. 24 evaluated the lasting effects of psilocybin on cognitive flexibility in 22 participants with MDD. Participants received 20 mg/70 kg and 30 mg/70 kg of psilocybin across two sessions, 1–3 weeks apart. The cognitive flexibility was evaluated using the Penn conditional exclusion test 4 weeks before the first psilocybin administration, then 1 and 4 weeks after the second session. Sustained positive changes were shown in cognitive flexibility in patients with MDD, independent of changes in depressive symptoms as assessed by GRID‐Hamilton Depression Rating Scale. 24
Nayak et al. 42 investigated the effects of psilocybin on cognitive flexibility in healthy participants. The study comprised six sequential web‐based surveys, including the CFS. Results revealed a significant increase in self‐reported cognitive flexibility at 2–4 weeks and 2–3 months post‐psilocybin. The increase in cognitive flexibility was positively correlated with higher scores on the Mystical Experience Questionnaire at both time points, suggesting that individuals with more profound mystical experiences exhibited a greater rate of improvement in cognitive flexibility over time. 42
Overall, these studies demonstrated that psilocybin affects cognitive flexibility and creative cognition only in the post‐acute setting, not following a microdosing regimen. 24 , 32 , 41 , 42 In the post‐acute setting, a decrease in convergent thinking and increased novelty scores was observed 7 days after psilocybin administration. 41 Additionally, there was an increase in both objective and self‐reported cognitive flexibility in healthy participants as well as in subjects with depression. 24 , 42
Memory and learning
The main three investigated aspects of memory and learning in psilocybin studies were associative learning, semantic associations, and episodic memory. Rucker et al. 30 measured the effect of psilocybin on episodic memory using Paired Associates Learning‐Total Errors Adjusted (PAL‐TEA). The results of this study indicated no changes in PAL‐TEA following psilocybin administration. 30
Spitzer et al. 43 conducted an RCT in eight healthy subjects to evaluate the acute effects of psilocybin on semantic associations. Participants underwent two sessions a week apart, including one with 0.2 mg/kg psilocybin capsules and one placebo session. 43 Participants performed the lexical decision paradigm test before and at minutes 50, 150 and 220 after treatment. The results showed no effect of psilocybin with the indirect priming effect, but a significant increase of indirect semantic priming under psilocybin was indicated, which implies an increased availability of remote associations. 43
In a study by Barrett et al. 27 associative learning and short‐term memory in 22 healthy participants was assessed using a word encoding/recognition task and the substitution recall segment of the DSST. Psilocybin was administered in three doses 10, 20, and 30 mg/70 kg. Significant positive effects of the drug condition were noted on word recall accuracy and word recognition. 27 Planned comparisons revealed a notable difference between the DXM and 10 mg/70 kg psilocybin conditions for the familiarity index and the recollection index in the word recall task. Both psilocybin and DXM led to a decrease in the free recall of words, but DXM exhibited a more pronounced impact on free recall compared to psilocybin. Additionally, DXM selectively reduced recognition sensitivity and diminished the involvement of both familiarity and recognition processes compared to the 10 mg/70 kg dose of psilocybin. Regarding substitution recall in the DSST, both psilocybin and DXM induced impairments in associative learning. In summary, both drugs influenced incidental associative learning, and while DXM affected episodic memory selectively, psilocybin did not produce the same effect. 27
In summary, in the post‐acute setting, the study by Rucker et al. 30 showed no effect of psilocybin on episodic memory. On the other hand, in the acute setting, both studies showed an effect of psilocybin; Spitzer et al. 43 showed an increase in indirect semantic priming and Barrett et al. 27 showed a dose‐dependent decrease in word‐recall accuracy.
Executive function
Executive function was evaluated by two studies, both using the digit symbol substitution test (DSST). Barrett et al. 27 studied the acute cognitive effects of psilocybin in healthy volunteers. Executive function as measured by the DSST, showed a main effect of drug condition, as well as an interaction between drug condition and time point on the number of attempted trials. 27 Similarly, a main effect for drug condition on accuracy and interaction between drug condition and time point. Regarding the accuracy of attempted responses, psilocybin did induce a dose‐dependent decrease in attempted responses, indicating a successful speed‐accuracy trade‐off during the effects of psilocybin. In summary, both study drugs (psilocybin and DXM) caused impairment in DSST while acutely under the influence of drug. 27 However, in a study by Goodwin et al. 25 in patients with TRD, it was indicated that higher doses of psilocybin (25 and 10 mg) are associated with greater improvements in DSST scores compared to a lower dose (1 mg). This trial with 233 TRD participants administered DSST 3 weeks after treatment with a single dose of psilocybin (25, 10 or 1 mg). The difference in least‐squares mean change (standard error) in the DSST from baseline to week 3 for 25, 10 and 1 mg was 6.4 (0.84), 5.4 (0.87) and 4.8 (0.84), respectively. The difference between the 25 mg psilocybin group and the 1 mg control group was 1.5 (95% CI: −0.8, 3.8), and for the 10 mg group was 0.5 (95% CI: −1.8, 2.8). This study suggests a potential dose‐dependent improvement trend in cognitive performance 3 weeks post‐dosing. 25
In conclusion, in the acute setting, a dose‐dependent decrease in executive function performance was observed in the psilocybin group. Conversely, in the post‐acute setting, subjects with TRD exhibited a dose‐dependent, non‐significant trend towards increased scores at week 3 compared to baseline in the psilocybin group. 25
Discussion
In this review, we systematically identified, reviewed, and evaluated 20 published studies, which investigated the effect of psilocybin on cognitive function. Our systematic review on psilocybin's effect on cognitive function revealed nuanced findings. While global cognitive function in healthy individuals showed negligible effects, certain domains such as executive function exhibited potential improvements. Attention was consistently impaired by psilocybin in the acute phase, except in one study where sustained attention showed a non‐significant trend towards improvement 29 days after psilocybin administration. Working memory was not consistently impaired by psilocybin but showed deficits under specific conditions or doses. Psilocybin showed promise in modifying emotional processing, enhancing positive emotional bias and recognition of emotional expressions, especially in TRD patients, emphasizing the importance of therapeutic support. Furthermore, it can enhance emotional empathy without significantly altering cognitive empathy. Cognitive flexibility and creative cognition were initially impaired but transformed into improvements, particularly in settings involving significant psychological or mystical experiences.
Psilocybin's acute effects on cognition appear to follow a dose‐dependent gradient, with different doses showing distinct cognitive responses. Higher doses have been associated with both enhancements and impairments in cognitive performance, underscoring the importance of dosage optimization for desired outcomes. 12 , 25 , 27 , 28 , 30 , 31 The precise mechanisms underlying these dose dependent effects warrant further investigation. Understanding the temporal dynamics of psilocybin's cognitive effects is crucial for interpreting study findings accurately. Acute assessments conducted up to 24 h after psilocybin ingestion provide insights into immediate cognitive changes, whereas longer‐term assessments reveal the persistence and trajectory of these effects over time. The diverse range of study populations, including both healthy individuals and those with mood disorders such as MDD and TRD, offers valuable insights into the differential effects of psilocybin across distinct clinical contexts. While healthy participants may exhibit alterations in attention, working memory, and emotional processing, individuals with depression may experience cognitive improvements alongside mood enhancements following psilocybin‐assisted therapy. 25 , 26 Exploring the underlying neurobiological mechanisms mediating psilocybin's effects on cognition remains a frontier in psychedelic research. Neuroimaging studies have implicated alterations in brain connectivity, neurotransmitter systems, and neural plasticity as potential drivers of psilocybin‐induced cognitive changes. 34 , 39 Integrating psilocybin‐assisted therapy into existing treatment paradigms for depression, anxiety, and substance use disorders may offer a novel approach to addressing cognitive deficits and promoting overall psychological well‐being. Longitudinal studies examining the enduring cognitive effects of repeated psilocybin dosing and exploring potential biomarkers of treatment response are warranted.
Two studies have suggested that individuals may experience enhanced cognitive flexibility and creativity in the days and weeks following a psilocybin experience. 24 , 42 This post‐acute period, often referred to as the ‘afterglow’, is characterized by a heightened sense of novelty, openness to new ideas, and divergent thinking. Research has shown that psilocybin can facilitate the dissolution of rigid thought patterns and promote unconventional problem‐solving strategies, which may persist beyond the acute effects of the drug. 39 These findings have significant implications for harnessing psilocybin's potential as a catalyst for creativity enhancement and cognitive enrichment in both clinical and non‐clinical settings. The post‐acute phase following a psilocybin experience is also marked by shifts in mood and emotional resilience. 24 , 26 Many individuals report feelings of increased emotional openness, empathy, and connectedness with others, which may endure for days or even weeks after the acute effects have subsided. These changes in affective processing are thought to arise from alterations in 5‐HT receptor signaling and neuroplasticity induced by psilocybin, leading to a recalibration of emotional reactivity and regulation. 34 , 39 Moreover, the profound insights and spiritual experiences often reported during psilocybin sessions can foster a sense of existential meaning and psychological well‐being that persists beyond the immediate drug effects. 44 The post‐acute period serves as a critical window for the integration and consolidation of insights gained during the psychedelic experience. 45 Through reflective practices such as journaling, meditation, and psychotherapy, individuals can deepen their understanding of the transformative experiences catalyzed by psilocybin and integrate them into their sense of self and worldview. 46 This process of meaning‐making facilitates psychological integration, facilitates the resolution of existential concerns, and fosters a sense of coherence and authenticity in one's life narrative. 46 Moreover, integration practices play a vital role in mitigating potential adverse effects and maximizing the therapeutic benefits of psilocybin therapy. 47 Research into the mechanisms underlying these post‐acute effects can inform the development of novel therapeutic approaches targeting mood disorders, addiction, and existential distress.
Comparing the acute and post‐acute effects of psilocybin unveils a nuanced journey of cognitive and emotional transformation. In the acute phase, individuals undergo profound alterations in perception and consciousness, experiencing vivid hallucinations, ego dissolution, and heightened emotional intensity. 2 This period, lasting 4–6 h, often leads to enhanced introspection, insight, and altered sense of self. 2 In contrast, the post‐acute phase extends beyond the immediate experience, manifesting in improved cognitive flexibility, emotional resilience, and enduring changes in personality traits. 24 While acute effects are intense and transient, postacute effects are subtler but more enduring, influencing mood, cognition, and behavior over days, weeks, or even months. 48 Understanding the dynamic interplay between these phases is crucial for optimizing therapeutic outcomes, as integration practices during the post‐acute period can help individuals consolidate insights, and maximize the long‐term benefits of psilocybin‐assisted therapy.
The variability in results within the domain of cognitive function, even when studies focus on similar cognitive tasks such as attention, memory, or executive function, can be attributed to several factors. Firstly, differences in study design, including variations in sample size, participant demographics, dosing regimens of psilocybin, and the specific measures used to assess cognitive performance, contribute significantly. Studies may also vary in the timing of assessments post‐psilocybin administration, influencing the observed effects on cognitive domains. Moreover, the inclusion criteria and health status of participants (e.g. healthy volunteers vs individuals with psychiatric disorders) can introduce variability in baseline cognitive abilities and responsiveness to psilocybin. Additionally, the contextual factors during testing, such as the environment and psychological state of participants, could influence cognitive outcomes. These multifaceted factors highlight the complexity of studying psilocybin's effects on cognition and underscore the importance of comprehensive study design and analysis to better understand and interpret these varying results.
The exact mechanisms underlying how psilocybin may impact cognition are still subjects of ongoing investigation. One prominent theory proposes that psilocybin interacts with 5‐HT receptors in the brain, particularly the 5‐HT2A receptor. 49 This interaction leads to changes in neurotransmitter activity, with 5‐HT2A receptor activation modulating the release of key neurotransmitters such as dopamine and glutamate, which are integral to cognitive processes. 50 , 51 Other, recent preclinical studies have demonstrated that psilocin acts as an allosteric modulator of Tropomyosin receptor kinase B, the receptor for brain derived neurotrophic factor, a critical signaling molecule for the induction of synaptic protein synthesis. 52 As synaptic plasticity is known to be essential for cognition, and has been shown to be altered in mood disorders, this neurobiological mechanism provides insights into how psychedelics may exert their therapeutic efficacy. Furthermore, other studies have shown that treatment of cultured neurons with psychedelics is capable of increasing neuritogenesis and can induce spinogenesis when administered systemically. 53 Moreover, two‐photon imaging in live mice has shown increases in dendritic spines in the prefrontal cortex for up to 30 days following psilocybin administration. 54
While psilocybin preferentially activates the 5‐HT2A receptor, it also exerts an activating effect on the 5‐HT1A receptor. Studies that used ketanserin pretreatment, a preferential 5‐HT2A antagonist, did not show significant differences in emotional processing or attentional processing, suggesting the involvement of the 5‐HT1A receptor in modulating the cognitive changes observed. 34 , 39 Recent preclinical findings highlight the dual role of 5‐HT receptors in the actions of psychedelics. 55 These studies emphasize that while 5‐HT2A receptors predominantly mediate the perceptual and psychedelic effects, 56 5‐HT1A receptors may play a significant role in modulating cognitive and emotional processing, 57 potentially contributing to the therapeutic effects observed in clinical studies. Further research is warranted to elucidate the specific mechanisms and interactions between these receptor subtypes, which could inform the development of more targeted therapeutic approaches using psilocybin and related compounds. Furthermore, psilocybin‐induced alterations in neural connectivity and network dynamics, as observed using functional magnetic resonance imaging studies, may contribute to cognitive effects by temporarily disrupting the DMN. 58 , 59 This network is linked to self‐referential thoughts, and its transient suppression might foster a more flexible cognitive state. 58 , 59 Additionally, ongoing exploration of psilocybin's impact on neuroplasticity, dendritic remodeling, and synaptic pruning suggests a potential mechanism for cognitive benefits, promoting the growth of new neural connections and eliminating less efficient ones. 60 , 61 The psychedelic experience induced by psilocybin, marked by altered perceptions and increased introspection, is also considered a factor that might contribute to cognitive flexibility, empathy and creativity. 40 , 62 , 63 However, it is crucial to emphasize that these mechanistic hypotheses remain plausible and require further research for a comprehensive understanding of the intricate pathways through which psilocybin influences cognition. The evolving field of research, characterized by ongoing studies, aims to elucidate the complexities of psilocybin's effects on the brain and cognitive functions.
Stroud et al. 26 reported that a larger decrease in RT in the Dynamic Emotional Expression Recognition Task correlated with a greater reduction in anhedonia among depressed individuals receiving psilocybin. This correlation implies that as individuals become more proficient at recognizing and processing emotional expressions, they also experience a greater relief from anhedonia. Anhedonia, a hallmark symptom of depression characterized by a diminished ability to experience pleasure, often accompanies deficits in emotional processing. 64 Therefore, the observed association between improvements in emotional processing and reductions in anhedonia indicates that psilocybin may target underlying mechanisms involved in both emotional processing and anhedonic symptoms. One interpretation of this correlation is that psilocybin treatment enhances emotional processing capabilities, leading to a normalization of affective responses and ultimately reducing anhedonia. By facilitating more adaptive emotional responses to stimuli, psilocybin may alleviate anhedonic symptoms and restore the ability to experience pleasure. However, it is important to recognize that correlation does not imply causation. While the observed relationship between improved emotional processing and reduced anhedonia is intriguing, it does not establish a direct causal link between the two. Other factors, such as changes in mood, cognition, or neural circuitry, could mediate the observed correlation. Furthermore, the interpretation of this correlation should consider potential confounding variables and alternative explanations. Further research is needed to elucidate the causal relationships and underlying mechanisms involved, as well as to explore the clinical implications of these findings for the treatment of depression and related mood disorders.
While our review provides a comprehensive synthesis of studies on the changes in cognitive function following psilocybin, it is crucial to acknowledge several limitations. The marked heterogeneity across selected studies, encompassing variations in participant characteristics, study designs, and cognitive assessment methodologies, presents a substantial challenge for direct comparisons and generalizability. Furthermore, limited sample sizes in certain studies may compromise statistical power and the reliability of findings. The diverse range of psilocybin doses, administration protocols, and concurrent psychotherapy further complicates result interpretation. Additionally, the predominant focus on acute effects in existing research leaves a gap, with relatively few longitudinal studies examining sustained cognitive function changes. The use of varied assessment tools across studies presents a challenge in establishing a standardized framework for comprehensive understanding of psilocybin's impact. Moreover, the review is constrained by the relatively small number of available studies. This, in conjunction with the diverse methodologies employed, underscores the need for caution in drawing definitive conclusions. The observed heterogeneity in participant demographics, study designs, and cognitive assessment tools precludes the feasibility of conducting a meta‐analysis to quantitatively synthesize findings, emphasizing the current limitations and the imperative for further research in this growing field.
In conclusion, our comprehensive review synthesizes existing studies on the cognitive effects of psilocybin, providing insights into its multifaceted impact across various domains. The gathered evidence indicates potential alterations in cognition. Psilocybin's interaction with 5‐HT receptors, neural connectivity changes, and the psychedelic experience are hypothesized mechanisms influencing cognition. However, limitations present challenges. The absence of a standardized framework underscores the nascent stage of research in this field. Future studies should focus on further investigating specific cognitive domains, such as sustained attention, working memory, and executive functions, to address the inconsistencies observed. Methodological improvements are essential: larger sample sizes will enhance the reliability of findings, standardized cognitive assessments will ensure consistency, and longitudinal study designs will help track both acute and post‐acute effects. Additionally, exploring the effects of varying dosages, different contexts, and diverse population types, particularly in clinical settings, will provide a more comprehensive understanding of psilocybin's cognitive impacts.
Disclosure statement
V. B. is supported by an Academic Scholar Award from the University of Toronto Department of Psychiatry and has received research support from the Canadian Institutes of Health Research, Brain & Behavior Foundation, Ontario Ministry of Health Innovation Funds, Royal College of Physicians and Surgeons of Canada, Department of National Defense (Government of Canada), New Frontiers in Research Fund, Associated Medical Services Inc. Healthcare, American Foundation for Suicide Prevention, Roche Canada, Novartis, and Eisai. A. C. R. is Chief Innovation Officer at PurMinds NeuroPharma. R. J. is the CMO of Mydecine Innovation Group. D. E. is a paid advisor for Aya Biosciences, Lophora Aps, Clerkenwell Health, and Mindstate Design Lab.
Author contributions
V. B. conceptualized the article and contributed to the overall design. S. M. contributed to the concept, overall design, article selection, review, study quality appraisal, manuscript preparation and submission. T. J. T. G. contributed to article selection, study quality appraisal, and manuscript preparation. All authors contributed to review and editing. All authors have approved the final manuscript and note that this is our original work.
Supporting information
Figure S1. Quality assessment of included studies.
Figure S2. Registered trials study characteristics.
Figure S3. Cognitive domains and tests used in registered trials.
Figure S4. Map illustrating locations of included published studies.
Figure S5. Cognitive function tests used in included published studies.
Table S1. Characteristics of registered clinical trials.
[Correction added on 10 October 2024, after first online publication: The last name of the author, Eric Vermetten, has been corrected.]
Data availability statement
The excel sheet for the extracted variables is available upon request.
References
- 1. Passie T, Seifert J, Schneider U, Emrich HM. The pharmacology of psilocybin. Addict. Biol. 2002; 7: 357–364. [DOI] [PubMed] [Google Scholar]
- 2. Davis AK, Barrett FS, May DG et al. Effects of psilocybin‐assisted therapy on major depressive disorder: A randomized clinical trial. JAMA Psychiatry 2021; 78: 481–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Grob CS, Danforth AL, Chopra GS et al. Pilot study of psilocybin treatment for anxiety in patients with advanced‐stage cancer. Arch. Gen. Psychiatry 2011; 68: 71–78. [DOI] [PubMed] [Google Scholar]
- 4. Carhart‐Harris RL, Bolstridge M, Rucker J et al. Psilocybin with psychological support for treatment‐resistant depression: An open‐label feasibility study. Lancet Psychiatry 2016; 3: 619–627. [DOI] [PubMed] [Google Scholar]
- 5. Bogenschutz MP, Forcehimes AA, Pommy JA, Wilcox CE, Barbosa P, Strassman RJ. Psilocybin‐assisted treatment for alcohol dependence: A proof‐of‐concept study. J. Psychopharmacol. 2015; 29: 289–299. [DOI] [PubMed] [Google Scholar]
- 6. Moreno FA, Wiegand CB, Taitano EK, Delgado PL. Safety, tolerability, and efficacy of psilocybin in 9 patients with obsessive–compulsive disorder. J. Clin. Psychiatry 2006; 67: 1735–1740. [DOI] [PubMed] [Google Scholar]
- 7. Hofmann A, Heim R, Brack A, Kobel H. Psilocybin, a psychotropic substance from the Mexican mushroom Psilicybe mexicana Heim. Experientia 1958; 14: 107–109 (in German). [DOI] [PubMed] [Google Scholar]
- 8. Hofmann A, Heim R, Brack A et al. Psilocybin und psilocin, zwei psychotrope Wirkstoffe aus mexikanischen Rauschpilzen. Helv. Chim. Acta 1959; 42: 1557–1572 (in German). [Google Scholar]
- 9. Rucker J, Schnall J, D'Hotman D, King D, David T, Neill J. Medicinal Use of Psilocybin: Reducing Restrictions on Treatment and Research. Adam Smith Research Trust, London, 2020; 98. [Google Scholar]
- 10. Passie T. A history of the use of psilocybin in psychotherapy. In: Metzner R (ed.). Sacred Mushroom of Visions: Teonanácatl: A Sourcebook on the Psilocybin Mushroom. Park Street Press, Rochester, VT, 2004; 113–138. [Google Scholar]
- 11. Kraehenmann R, Preller KH, Scheidegger M et al. Psilocybin‐induced decrease in amygdala reactivity correlates with enhanced positive mood in healthy volunteers. Biol. Psychiatry 2015; 78: 572–581. [DOI] [PubMed] [Google Scholar]
- 12. Vollenweider FX, Csomor PA, Knappe B, Geyer MA, Quednow BB. The effects of the preferential 5‐HT2A agonist psilocybin on prepulse inhibition of startle in healthy human volunteers depend on interstimulus interval. Neuropsychopharmacology 2007; 32: 1876–1887. [DOI] [PubMed] [Google Scholar]
- 13. Irizarry R, Winczura A, Dimassi O, Dhillon N, Minhas A, Larice J. Psilocybin as a treatment for psychiatric illness: A meta‐analysis. Cureus 2022; 14: e31796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mertens LJ, Wall MB, Roseman L, Demetriou L, Nutt DJ, Carhart‐Harris RL. Therapeutic mechanisms of psilocybin: Changes in amygdala and prefrontal functional connectivity during emotional processing after psilocybin for treatment‐resistant depression. J. Psychopharmacol. 2020; 34: 167–180. [DOI] [PubMed] [Google Scholar]
- 15. Zhao X, Du Y, Yao Y et al. Psilocybin promotes neuroplasticity and induces rapid and sustained antidepressant‐like effects in mice. J. Psychopharmacol. 2024; 38: 489–499. [DOI] [PubMed] [Google Scholar]
- 16. Trivedi JK. Cognitive deficits in psychiatric disorders: Current status. Indian J. Psychiatry. 2006; 48: 10–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Arioli M, Crespi C, Canessa N. Social cognition through the lens of cognitive and clinical neuroscience. Biomed Res. Int. 2018; 2018: 4283427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jones DE, Greenberg M, Crowley M. Early social‐emotional functioning and public health: The relationship between kindergarten social competence and future wellness. Am. J. Public Health 2015; 105: 2283–2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gattuso JJ, Perkins D, Ruffell S et al. Default mode network modulation by psychedelics: A systematic review. Int. J. Neuropsychopharmacol. 2023; 26: 155–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Štrac DŠ, Pivac N, Mück‐Šeler D. The serotonergic system and cognitive function. Transl. Neurosci. 2016; 7: 35–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Page MJ, McKenzie JE, Bossuyt PM et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021; 372: n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Savović J, Weeks L, Sterne JAC et al. Evaluation of the Cochrane Collaboration's tool for assessing the risk of bias in randomized trials: Focus groups, online survey, proposed recommendations and their implementation. Syst. Rev. 2014; 3: 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Higgins J, Savović J, Page MJ, Elbers RG, Sterne J. Chapter 8: Assessing risk of bias in a randomized trial. Cochrane Training [Internet]. [Cited June 25 2024.] Available from URL: https://training.cochrane.org/handbook/current/chapter-08
- 24. Doss MK, Považan M, Rosenberg MD et al. Psilocybin therapy increases cognitive and neural flexibility in patients with major depressive disorder. Transl. Psychiatry 2021; 11: 574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Goodwin GM, Aaronson ST, Alvarez O et al. Single‐dose psilocybin for a treatment‐resistant episode of major depression. N. Engl. J. Med. 2022; 387: 1637–1648. [DOI] [PubMed] [Google Scholar]
- 26. Stroud JB, Freeman TP, Leech R et al. Psilocybin with psychological support improves emotional face recognition in treatment‐resistant depression. Psychopharmacology (Berl) 2018; 235: 459–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Barrett FS, Carbonaro TM, Hurwitz E, Johnson MW, Griffiths RR. Double‐blind comparison of the two hallucinogens psilocybin and dextromethorphan: Effects on cognition. Psychopharmacology (Berl) 2018; 235: 2915–2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hasler F, Grimberg U, Benz MA, Huber T, Vollenweider FX. Acute psychological and physiological effects of psilocybin in healthy humans: A double‐blind, placebo‐controlled dose‐effect study. Psychopharmacology (Berl) 2004; 172: 145–156. [DOI] [PubMed] [Google Scholar]
- 29. Johnson MW, Griffiths RR. Potential therapeutic effects of psilocybin. Neurother. 2017; 14: 734–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rucker JJ, Marwood L, Ajantaival RLJ et al. The effects of psilocybin on cognitive and emotional functions in healthy participants: Results from a phase 1, randomised, placebo‐controlled trial involving simultaneous psilocybin administration and preparation. J. Psychopharmacol. 2022; 36: 114–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wittmann M, Carter O, Hasler F et al. Effects of psilocybin on time perception and temporal control of behaviour in humans. J. Psychopharmacol. 2007; 21: 50–64. [DOI] [PubMed] [Google Scholar]
- 32. Cavanna F, Muller S, de la Fuente LA et al. Microdosing with psilocybin mushrooms: A double‐blind placebo‐controlled study. Transl. Psychiatry 2022; 12: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Marschall J, Fejer G, Lempe P et al. Psilocybin microdosing does not affect emotion‐related symptoms and processing: A preregistered field and lab‐based study. J. Psychopharmacol. 2022; 36: 97–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Carter OL, Burr DC, Pettigrew JD, Wallis GM, Hasler F, Vollenweider FX. Using psilocybin to investigate the relationship between attention, working memory, and the serotonin 1A and 2A receptors. J. Cogn. Neurosci. 2005; 17: 1497–1508. [DOI] [PubMed] [Google Scholar]
- 35. Duke RB, Keeler MH. The effects of psilocybin, dextro‐amphetamine and placebo on performance of the trail making test. J. Clin. Psychol. 1968; 24: 316–317. [DOI] [PubMed] [Google Scholar]
- 36. Gouzoulis‐Mayfrank E, Thelen B, Maier S et al. Effects of the hallucinogen psilocybin on covert orienting of visual attention in humans. Neuropsychobiology 2002; 45: 205–212. [DOI] [PubMed] [Google Scholar]
- 37. Nikolič M, Viktorin V, Zach P et al. Psilocybin intoxication did not affect daytime or sleep‐related declarative memory consolidation in a small sample exploratory analysis. Eur. Neuropsychopharmacol. 2023; 74: 78–88. [DOI] [PubMed] [Google Scholar]
- 38. Umbricht D, Vollenweider FX, Schmid L et al. Effects of the 5‐HT2A agonist psilocybin on mismatch negativity generation and AX‐continuous performance task: Implications for the neuropharmacology of cognitive deficits in schizophrenia. Neuropsychopharmacology 2003; 28: 170–181. [DOI] [PubMed] [Google Scholar]
- 39. Kometer M, Schmidt A, Bachmann R, Studerus E, Seifritz E, Vollenweider FX. Psilocybin biases facial recognition, goal‐directed behavior, and mood state toward positive relative to negative emotions through different serotonergic subreceptors. Biol. Psychiatry 2012; 72: 898–906. [DOI] [PubMed] [Google Scholar]
- 40. Pokorny T, Preller KH, Kometer M, Dziobek I, Vollenweider FX. Effect of psilocybin on empathy and moral decision‐making. Int. J. Neuropsychopharmacol. 2017; 20: 747–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mason NL, Kuypers KPC, Reckweg JT et al. Spontaneous and deliberate creative cognition during and after psilocybin exposure. Transl. Psychiatry 2021; 11: 209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Nayak SM, Jackson H, Sepeda ND et al. Naturalistic psilocybin use is associated with persisting improvements in mental health and wellbeing: Results from a prospective, longitudinal survey. Front. Psychiatry 2023; 14: 1199642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Spitzer M, Thimm M, Hermle L et al. Increased activation of indirect semantic associations under psilocybin. Biol. Psychiatry 1996; 39: 1055–1057. [DOI] [PubMed] [Google Scholar]
- 44. Belser AB, Agin‐Liebes G, Swift TC et al. Patient experiences of psilocybin‐assisted psychotherapy: An interpretative phenomenological analysis. J. Humanist. Psychol. 2017; 57: 354–388. [Google Scholar]
- 45. Ho JT, Preller KH, Lenggenhager B. Neuropharmacological modulation of the aberrant bodily self through psychedelics. Neurosci. Biobehav. Rev. 2020; 108: 526–541. [DOI] [PubMed] [Google Scholar]
- 46. Gorman I, Nielson EM, Molinar A, Cassidy K, Sabbagh J. Psychedelic harm reduction and integration: A transtheoretical model for clinical practice. Front. Psychol. 2021; 12: 645246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sloshower J, Guss J, Krause R et al. Psilocybin‐assisted therapy of major depressive disorder using acceptance and commitment therapy as a therapeutic frame. J. Contextual Behav. Sci. 2020; 15: 12–19. [Google Scholar]
- 48. Studerus E, Kometer M, Hasler F, Vollenweider FX. Acute, subacute and long‐term subjective effects of psilocybin in healthy humans: A pooled analysis of experimental studies. J. Psychopharmacol. 2011; 25: 1434–1452. [DOI] [PubMed] [Google Scholar]
- 49. Erkizia‐Santamaría I, Alles‐Pascual R, Horrillo I, Meana JJ, Ortega JE. Serotonin 5‐HT2A, 5‐HT2c and 5‐HT1A receptor involvement in the acute effects of psilocybin in mice. In vitro pharmacological profile and modulation of thermoregulation and head‐twich response. Biomed. Pharmacother. 2022; 154: 113612. [DOI] [PubMed] [Google Scholar]
- 50. López‐Giménez JF, González‐Maeso J. Hallucinogens and serotonin 5‐HT2A receptor‐mediated signaling pathways. Curr. Top. Behav. Neurosci. 2018; 36: 45–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Zhang G, Stackman RW. The role of serotonin 5‐HT2A receptors in memory and cognition. Front. Pharmacol. 2015; 6: 225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Cameron LP, Benetatos J, Lewis V et al. Beyond the 5‐HT2A receptor: Classic and nonclassic targets in psychedelic drug action. J. Neurosci. 2023; 43: 7472–7482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ly C, Greb AC, Cameron LP et al. Psychedelics promote structural and functional neural plasticity. Cell Rep. 2018; 23: 3170–3182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Shao LX, Liao C, Gregg I et al. Psilocybin induces rapid and persistent growth of dendritic spines in frontal cortex in vivo. Neuron 2021; 109: 2535–2544.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Warren AL, Lankri D, Cunningham MJ et al. Structural pharmacology and therapeutic potential of 5‐methoxytryptamines. Nature 2024; 6630: 237–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kometer M, Schmidt A, Jäncke L, Vollenweider FX. Activation of serotonin 2A receptors underlies the psilocybin‐induced effects on α oscillations, N170 visual‐evoked potentials, and visual hallucinations. J. Neurosci. 2013; 33: 10544–10551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Ögren SO, Eriksson TM, Elvander‐Tottie E et al. The role of 5‐HT(1A) receptors in learning and memory. Behav. Brain Res. 2008; 195: 54–77. [DOI] [PubMed] [Google Scholar]
- 58. Carhart‐Harris RL, Erritzoe D, Williams T et al. Neural correlates of the psychedelic state as determined by fMRI studies with psilocybin. Proc. Natl. Acad. Sci. U. S. A. 2012; 109: 2138–2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Grimm O, Kraehenmann R, Preller KH, Seifritz E, Vollenweider FX. Psilocybin modulates functional connectivity of the amygdala during emotional face discrimination. Eur. Neuropsychopharmacol. 2018; 28: 691–700. [DOI] [PubMed] [Google Scholar]
- 60. Raval NR, Johansen A, Donovan LL et al. A single dose of psilocybin increases synaptic density and decreases 5‐HT2A receptor density in the pig brain. Int. J. Mol. Sci. 2021; 22: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Du Y, Li Y, Zhao X et al. Psilocybin facilitates fear extinction in mice by promoting hippocampal neuroplasticity. Chin. Med. J. (Engl) 2023; 136: 2983–2992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Aday JS, Wood JR, Bloesch EK, Davoli CC. Psychedelic drugs and perception: A narrative review of the first era of research. Rev. Neurosci. 2021; 32: 559–571. [DOI] [PubMed] [Google Scholar]
- 63. Kometer M, Pokorny T, Seifritz E, Volleinweider FX. Psilocybin‐induced spiritual experiences and insightfulness are associated with synchronization of neuronal oscillations. Psychopharmacology (Berl) 2015; 232: 3663–3676. [DOI] [PubMed] [Google Scholar]
- 64. Germine LT, Garrido L, Bruce L, Hooker C. Social anhedonia is associated with neural abnormalities during face emotion processing. Neuroimage 2011; 58: 935–945. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Quality assessment of included studies.
Figure S2. Registered trials study characteristics.
Figure S3. Cognitive domains and tests used in registered trials.
Figure S4. Map illustrating locations of included published studies.
Figure S5. Cognitive function tests used in included published studies.
Table S1. Characteristics of registered clinical trials.
Data Availability Statement
The excel sheet for the extracted variables is available upon request.


