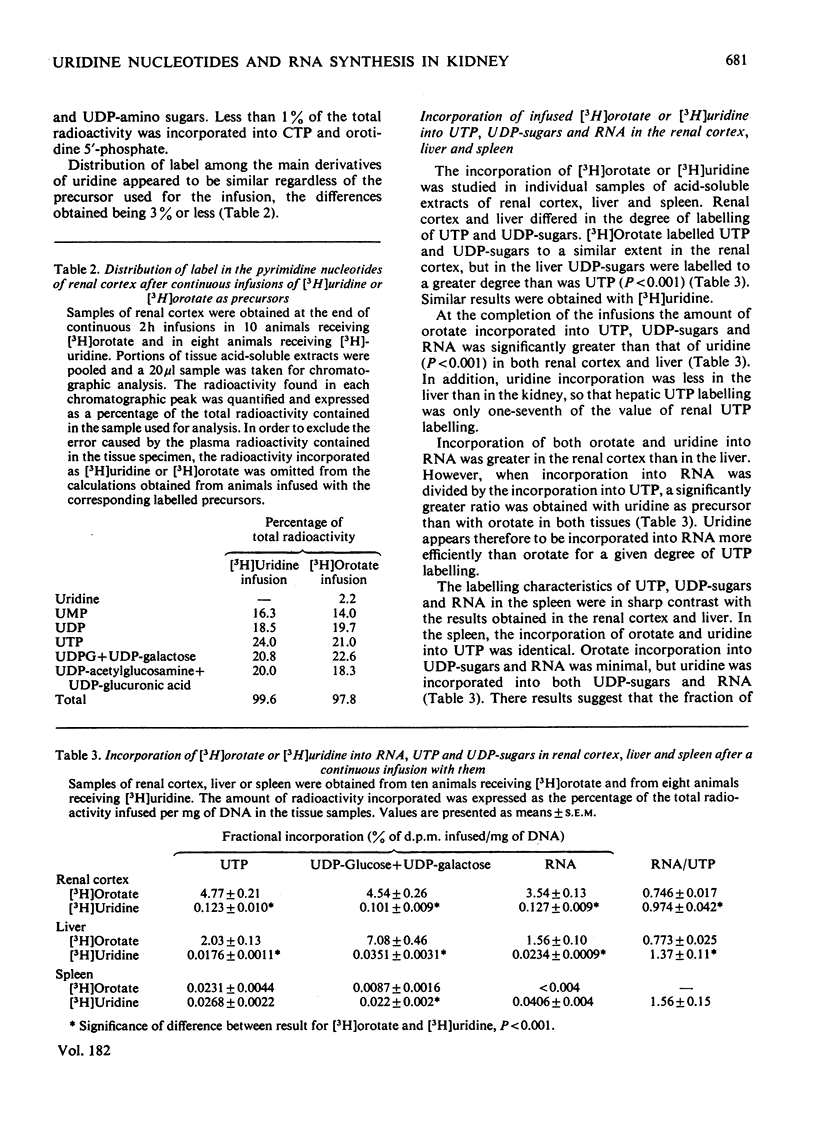
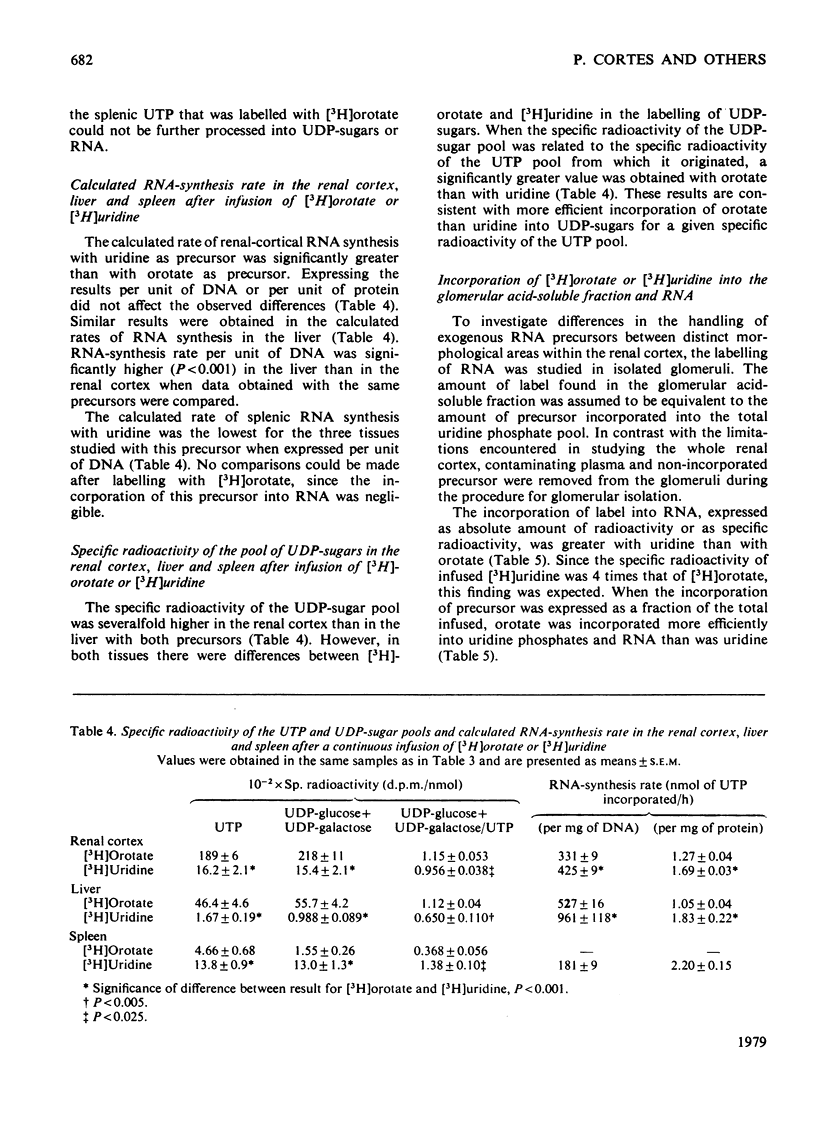
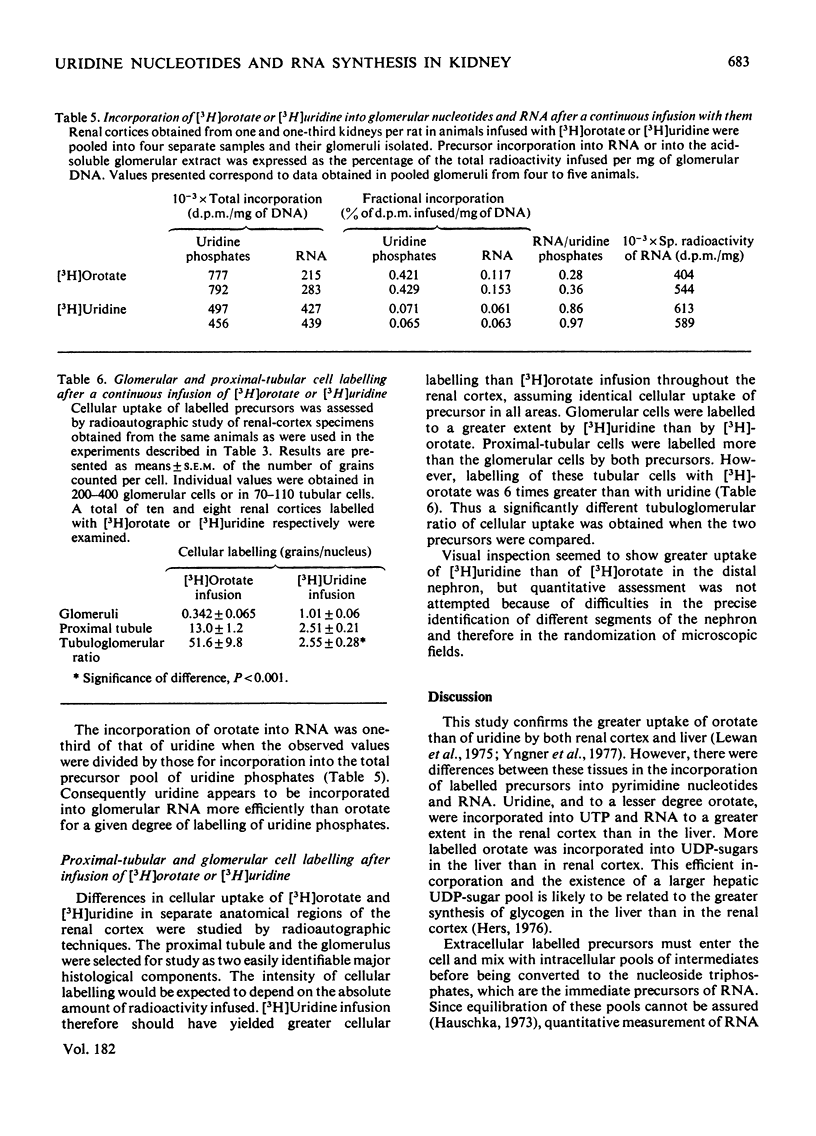
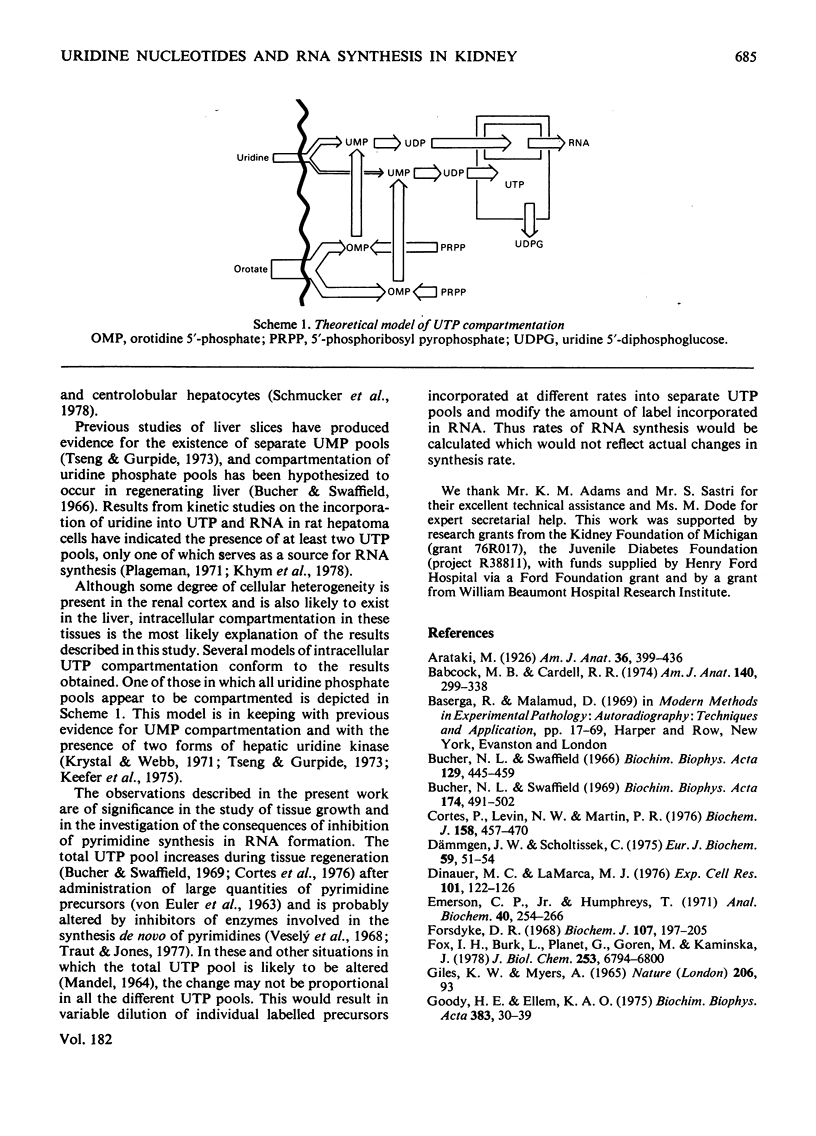
Abstract
The possibility of compartmentation of UTP in vivo was investigated in the renal cortex of unanaesthetized rats. In addition, liver and spleen were studied in order to compare tissues with different utilization of precursors for pyrimidine nucleotide synthesis. After continuous 2h infusions of [3H]uridine or [3H]orotate, their incorporation into UTP, UDP-sugars and RNA was quantified. Rates of RNA synthesis were calculated by dividing the incorporation of precursor into RNA by the average specific radioactivity of the UTP pool. Although similar RNA-synthesis rates might have been expected with the two precursors, higher rates were found with uridine than with orotate. The relative incorporation into UDP-sugars of these precursors was also different. Similar results were obtained in the liver. In the spleen, equal amounts of both precursors were incorporated into UTP, but [3H]orotate incorporation did not lead to labelling of RNA. To evaluate the heterogeneity of cells with respect to the metabolism of pyrimidines, precursor incorporation was studied in isolated glomeruli and by radioautography. Incorporation into glomeruli was qualitatively similar to but quantitatively different from results in the renal cortex. Although there is obvious tissue heterogeneity, compartmentation of UTP pools is the most credible explanation for the results obtained with the renal cortex and liver. Consequently RNA and UDP-sugars may originate from two different UTP pools. Tissue heterogeneity is the likely explanation for the results obtained in the spleen. Studies of synthesis of pyrimidine and RNA, particularly in relation to growth and regeneration, must take into consideration the precursor used, the apparent existence of UTP compartmentation and the degree of cellular heterogeneity.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babcock M. B., Cardell R. R., Jr Hepatic glycogen patterns in fasted and fed rats. Am J Anat. 1974 Jul;140(3):299–337. doi: 10.1002/aja.1001400302. [DOI] [PubMed] [Google Scholar]
- Bucher N. L., Swaffield M. N. Nucleotide pools and [6-14C]orotic acid incorporation in early regenerating rat liver. Biochim Biophys Acta. 1966 Dec 21;129(3):445–459. doi: 10.1016/0005-2787(66)90060-8. [DOI] [PubMed] [Google Scholar]
- Bucher N. L., Swaffield M. N. Ribonucleic acid synthesis in relation to precursor pools in regenerating rat liver. Biochim Biophys Acta. 1969 Feb 18;174(2):491–502. doi: 10.1016/0005-2787(69)90278-0. [DOI] [PubMed] [Google Scholar]
- Cortes P., Levin N. W., Martin P. R. Ribonucleic acid synthesis in the renal cortex at the initiation of compensatory growth. Biochem J. 1976 Aug 15;158(2):457–470. doi: 10.1042/bj1580457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinauer M. C., LaMarca M. J. The size of the guanosine triphosphate pool active in the synthesis of stable RNA by stage 6 oocytes of Xenopus laevis. Exp Cell Res. 1976 Aug;101(1):122–126. doi: 10.1016/0014-4827(76)90420-1. [DOI] [PubMed] [Google Scholar]
- Dämmgen J. W., Scholtissek C. Cellular RNA and influenza-virion RNA are synthesized from different pyrimidine-nucleoside-triphosphate pools in chick-embryo cells. Eur J Biochem. 1975 Nov 1;59(1):51–54. doi: 10.1111/j.1432-1033.1975.tb02423.x. [DOI] [PubMed] [Google Scholar]
- Emerson C. P., Jr, Humphreys T. A simple and sensitive method for quantitative measurement of cellular RNA synthesis. Anal Biochem. 1971 Apr;40(2):254–266. doi: 10.1016/0003-2697(71)90384-8. [DOI] [PubMed] [Google Scholar]
- Forsdyke D. R. Studies on the incorporation of [5-3H] uridine during activation and transformation of lymphocytes induced by phytohaemagglutinin. Dependence on the incorporation rate on uridine concentration at certain critical concentrations. Biochem J. 1968 Mar;107(2):197–205. doi: 10.1042/bj1070197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox I. H., Burk L., Planet G., Goren M., Kaminska J. Pyrimidine nucleotide biosynthesis. A study of normal and purine enzyme-deficient cells. J Biol Chem. 1978 Oct 10;253(19):6794–6800. [PubMed] [Google Scholar]
- Goody H. E., Ellem K. A. Nutritional effects on precursor uptake and compartmentalization of intracellular pools in relation to RNA synthesis. Biochim Biophys Acta. 1975 Feb 24;383(1):30–39. doi: 10.1016/0005-2787(75)90243-9. [DOI] [PubMed] [Google Scholar]
- HARBERS E., CHAUDHURI N. K., HEIDELBERGER C. Studies on fluorinated pyrimidines. VIII. Further biochemical and metabolic investigations. J Biol Chem. 1959 May;234(5):1255–1262. [PubMed] [Google Scholar]
- Hartwick R. A., Brown P. R. The performance of microparticle chemically-bonded anion-exchange resins in the analysis of nucleotides. J Chromatogr. 1975 Oct 29;112:650–662. doi: 10.1016/s0021-9673(00)99994-1. [DOI] [PubMed] [Google Scholar]
- Hauschka P. V. Analysis of nucleotide pools in animal cells. Methods Cell Biol. 1973;7:361–462. doi: 10.1016/s0091-679x(08)61787-2. [DOI] [PubMed] [Google Scholar]
- Hers H. G. The control of glycogen metabolism in the liver. Annu Rev Biochem. 1976;45:167–189. doi: 10.1146/annurev.bi.45.070176.001123. [DOI] [PubMed] [Google Scholar]
- Hill J. M. Ribosomal RNA metabolism during renal hypertrophy. Evidence of decreased degradation of newly synthesized ribosomal RNA. J Cell Biol. 1975 Jan;64(1):260–265. doi: 10.1083/jcb.64.1.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalra J., Wheldrake J. F. Evidence that the stimulation of precursor incorporation into RNA of rat kidney by aldosterone is mainly an effect on uptake. FEBS Lett. 1972 Sep 15;25(2):298–300. doi: 10.1016/0014-5793(72)80508-8. [DOI] [PubMed] [Google Scholar]
- Katz N., Teutsch H. F., Sasse D., Jungermann K. Heterogeneous distribution of glucose-6-phosphatase in microdissected periportal and perivenous rat liver tissue. FEBS Lett. 1977 Apr 15;76(2):226–230. doi: 10.1016/0014-5793(77)80157-9. [DOI] [PubMed] [Google Scholar]
- Kaukel E., Fuhrmann U., Hilz H. Divergent action of cAMP and dibutyryl cAMP on macromolecular synthesis in HeLa S3 cultures. Biochem Biophys Res Commun. 1972 Sep 26;48(6):1516–1524. doi: 10.1016/0006-291x(72)90886-8. [DOI] [PubMed] [Google Scholar]
- Khym J. X., Jones M. H., Lee W. H., Regan J. D., Volkin E. On the question of compartmentalization of the nucleotide pool. J Biol Chem. 1978 Dec 25;253(24):8741–8746. [PubMed] [Google Scholar]
- Krystal G., Webb T. E. Multiple forms of uridine kinase in normal and neoplastic rat liver. Biochem J. 1971 Oct;124(5):943–947. doi: 10.1042/bj1240943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lewan L., Petersen I., Yngner T. Incorporation of orotic acid into nucleotides and RNA in mouse organs during 60 minutes. Hoppe Seylers Z Physiol Chem. 1975 Apr;356(4):425–429. doi: 10.1515/bchm2.1975.356.1.425. [DOI] [PubMed] [Google Scholar]
- Liberti J. P., Kline E. S. Differential protein and RNA synthesis of rat kidney cortex and medulla. Life Sci. 1974 Nov 15;15(10):1815–1826. doi: 10.1016/0024-3205(74)90183-0. [DOI] [PubMed] [Google Scholar]
- Lieu T. S., Hudson R. A., Brown R. K., White B. C. Transport of pyrimidine nucleosides across human erythrocyte membranes. Biochim Biophys Acta. 1971 Sep 14;241(3):885–893. [PubMed] [Google Scholar]
- Loeb J. N., Yeung L. L. Synthesis and degradation of ribosomal RNA in regenerating liver. J Exp Med. 1975 Sep 1;142(3):575–587. doi: 10.1084/jem.142.3.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MASTER R. W. POSSIBLE SYNTHESIS OF POLYRIBONUCLEOTIDES OF KNOWN BASE-TRIPLET SEQUENCES. Nature. 1965 Apr 3;206:93–93. doi: 10.1038/206093b0. [DOI] [PubMed] [Google Scholar]
- MCKAY E. PENTOSE ESTIMATION BY THE ORCINOL METHOD, WITH PARTICULAR REFERENCE TO PLASMA PENTOSE. Clin Chim Acta. 1964 Oct;10:320–329. doi: 10.1016/0009-8981(64)90062-2. [DOI] [PubMed] [Google Scholar]
- Mandel P. Free nucleotides in animal tissues. Prog Nucleic Acid Res Mol Biol. 1964;3:299–334. doi: 10.1016/s0079-6603(08)60744-8. [DOI] [PubMed] [Google Scholar]
- Melvin W. T., Kumar A., Malt R. A. Conservation of ribosomal RNA during compensatory renal hypertrophy. A major mechanism in RNA accretion. J Cell Biol. 1976 Jun;69(3):548–556. doi: 10.1083/jcb.69.3.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misra R. P. Isolation of glomeruli from mammalian kidneys by graded sieving. Am J Clin Pathol. 1972 Aug;58(2):135–139. doi: 10.1093/ajcp/58.2.135. [DOI] [PubMed] [Google Scholar]
- Munro H. N. The determination of nucleic acids. Methods Biochem Anal. 1966;14:113–176. doi: 10.1002/9780470110324.ch5. [DOI] [PubMed] [Google Scholar]
- Ouellett A. J., Malt R. A. Accumulation and decay of messenger ribonucleic acid in mouse kidney. Biochemistry. 1976 Jul 27;15(15):3358–3361. doi: 10.1021/bi00660a029. [DOI] [PubMed] [Google Scholar]
- Pickard M. A., Paterson A. R. Nucleoside transport in human erythrocytes: equilibrium exchange diffusion of uridine. Can J Biochem. 1972 Jun;50(6):704–705. doi: 10.1139/o72-096. [DOI] [PubMed] [Google Scholar]
- Plagemann P. G., Marz R., Wohlhueter R. M. Uridine transport in Novikoff rat hepatoma cells and other cell lines and its relationship to uridine phosphorylation and phosphorolysis. J Cell Physiol. 1978 Oct;97(1):49–72. doi: 10.1002/jcp.1040970107. [DOI] [PubMed] [Google Scholar]
- Plagemann P. G. Nucleotide pools of Novikoff rat hepatoma cells growing in suspension culture. II. Independent nucleotide pools for nucleic acid synthesis. J Cell Physiol. 1971 Apr;77(2):241–248. doi: 10.1002/jcp.1040770213. [DOI] [PubMed] [Google Scholar]
- Preisig R., Bircher J., Paumgartner G. Physiologic and pathophysiologic aspects of the hepatic hemodynamics. Prog Liver Dis. 1972;4:201–216. [PubMed] [Google Scholar]
- Ross J. S., Malamud D., Caulfield J. A., Malt R. A. Differential labeling with orotic acid and uridine in compensatroy renal hypertrophy. Am J Physiol. 1975 Oct;229(4):952–954. doi: 10.1152/ajplegacy.1975.229.4.952. [DOI] [PubMed] [Google Scholar]
- SMITH L. H., Jr, BAKER F. A. Pyrimidine metabolism in man. I. The biosynthesis of orotic acid. J Clin Invest. 1959 May;38(5):798–809. doi: 10.1172/JCI103862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmucker D. L., Mooney J. S., Jones A. L. Stereological analysis of hepatic fine structure in the Fischer 344 rat. Influence of sublobular location and animal age. J Cell Biol. 1978 Aug;78(2):319–337. doi: 10.1083/jcb.78.2.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soeiro R., Ehrenfeld E. Cytoplasmic and nuclear pyrimidine ribonucleotide pools in HeLa cells. J Mol Biol. 1973 Jun 15;77(1):177–187. doi: 10.1016/0022-2836(73)90371-9. [DOI] [PubMed] [Google Scholar]
- Solomon S. Developmental changes in nephron number, proximal tubular length and superficial nephron glomerular filtration rate of rats. J Physiol. 1977 Nov;272(3):573–589. doi: 10.1113/jphysiol.1977.sp012061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TSANEV R., MARKOV G. G. Substances interfering with spectrophotometric estimation of nucleic acids and their elimination by the two-wavelength method. Biochim Biophys Acta. 1960 Aug 26;42:442–452. doi: 10.1016/0006-3002(60)90822-2. [DOI] [PubMed] [Google Scholar]
- Toback F. G., Smith P. D., Lowenstein L. M. Effect of hyperosmolality on renal uridine metabolism. Am J Physiol. 1974 Jun;226(6):1474–1479. doi: 10.1152/ajplegacy.1974.226.6.1474. [DOI] [PubMed] [Google Scholar]
- Toback F. G., Smith P. D., Lowenstein L. M. Enhancement of uridine uptake in the kidney papilla by urea. Proc Soc Exp Biol Med. 1974 May;146(1):222–224. doi: 10.3181/00379727-146-38074. [DOI] [PubMed] [Google Scholar]
- Traut T. W., Jones M. E. Inhibitors of orotate phosphoribosyl-transferase and orotidine-5'-phosphate decarboxylase from mouse Ehrlich ascites cells: a procedure for analyzing the inhibition of a multi-enzyme complex. Biochem Pharmacol. 1977 Dec 1;26(23):2291–2296. doi: 10.1016/0006-2952(77)90293-3. [DOI] [PubMed] [Google Scholar]
- Tseng J. K., Gurpide E. Compartmentalization of uridine and uridine 5'-monophosphate in rat liver slices. J Biol Chem. 1973 Aug 25;248(16):5634–5640. [PubMed] [Google Scholar]
- VON EULER L. H., RUBIN R. J., HANDSCHUMACHER R. E. Fatty livers induced by orotic acid. II. Changes in nucleotide metabolism. J Biol Chem. 1963 Jul;238:2464–2469. [PubMed] [Google Scholar]
- Veselý J., Cihák A., Sorm F. Biochemical mechanism of drug resistance. VII. Inhibition of orotic acid metabolism by 5-azacytidine in leukemic mice sensitive and resistant to 5-azacytidine. Biochem Pharmacol. 1968 Apr;17(4):519–524. doi: 10.1016/0006-2952(68)90267-0. [DOI] [PubMed] [Google Scholar]
- Welsh F. A. Changes in distribution of enzymes within the liver lobule during adaptive increases. J Histochem Cytochem. 1972 Feb;20(2):107–111. doi: 10.1177/20.2.107. [DOI] [PubMed] [Google Scholar]
- Wiegers U., Kramer G., Klapproth K., Hilz H. Separate pyrimidine-nucleotide pools for messenger-RNA and ribosomal-RNA synthesis in HeLa S3 cells. Eur J Biochem. 1976 May 1;64(2):535–540. doi: 10.1111/j.1432-1033.1976.tb10333.x. [DOI] [PubMed] [Google Scholar]
- Witschi H. A comparative study of in vivo RNA and protein synthesis in rat liver and lung. Cancer Res. 1972 Aug;32(8):1686–1694. [PubMed] [Google Scholar]
- Wu R. S., Soeiro R. Turnover of nuclear RNA in HeLa cells: evidence for a single ribonucleotide pool. J Mol Biol. 1971 Jun 14;58(2):481–487. doi: 10.1016/0022-2836(71)90365-2. [DOI] [PubMed] [Google Scholar]
- Yu F. L., Feigelson P. Effects of cortisone on orotic acid transport and RNA synthesis in rat liver. Arch Biochem Biophys. 1970 Dec;141(2):662–667. doi: 10.1016/0003-9861(70)90186-4. [DOI] [PubMed] [Google Scholar]
- de Rouffignac C., Monnens L. Functional and morphologic maturation of superficial and juxtamedullary nephrons in the rat. J Physiol. 1976 Oct;262(1):119–129. doi: 10.1113/jphysiol.1976.sp011588. [DOI] [PMC free article] [PubMed] [Google Scholar]


